Abstract
Bortezomib is a potent inhibitor of proteasomes currently used to eliminate malignant plasma cells in multiple myeloma patients. It is also effective in depleting both alloreactive plasma cells in acute Ab-mediated transplant rejection and their autoreactive counterparts in animal models of lupus and myasthenia gravis (MG).
In this study, we demonstrate that bortezomib at 10 nM or higher concentrations killed long-lived plasma cells in cultured thymus cells from 9 early-onset MG patients and consistently halted their spontaneous production not only of autoantibodies against the acetylcholine receptor but also of total IgG. Surprisingly, lenalidomide and dexamethasone had little effect on plasma cells. After bortezomib treatment, they showed ultrastructural changes characteristic of endoplasmic reticulum stress after 8 hours, and were no longer detectable at 24 hours. Bortezomib therefore appears promising for treating MG and possibly other antibody-mediated autoimmune or allergic disorders, especially if given in short courses at modest doses before the standard immunosuppressive drugs have taken effect.
Keywords: bortezomib, plasma cells, myasthenia gravis, proteasome inhibition, autoimmunity
Introduction
Myasthenia gravis (MG) with Abs against the muscle acetylcholine receptor (AChR) is one of the best understood of the numerous autoimmune neurological diseases now recognized (1). It is generally agreed that the patients’ autoAbs are pathogenic, as they decrease AChR numbers by antigenic modulation and complement-mediated damage (2, 3). Patients with early-onset MG (EOMG; before age 45) are an unusually well-defined subgroup, with strong female and HLA-B8 biases (4), and characteristic lymph node-like infiltrates in the thymic medulla (5-7).
Treatment of MG relies primarily on glucocorticoids, often combined with broad-spectrum immunosuppressants such as azathioprine or mycophenolate mofetil, or rituximab (8). However, their efficacy and side-effects vary greatly between patients, and they reduce autoAb titers and restore muscle strength only after delays as long as 4 - 15 months (9, 10). In addition, drug-resistant AChR-MG patients treated with rituximab (anti-CD20) showed no reduction in either AChR Ab titers or IgG levels, despite complete elimination of circulating B-cells (10). In such patients, long-lived plasma cells, which are CD20 negative, are likely to be the main producers of the autoAbs. Moreover, they are probably responsible for the delayed responses of most MG patients to immunosuppressants, which mainly act by preventing generation of new plasma cells from B-cells and by impairing the activation and proliferation of T-helper cells (11-13).
Plasma cells are high-rate Ab-secreting cells (>10,000 molecules per cell per second (14, 15)). They are terminally differentiated and do not divide. Among the B-cell lineage, they are uniquely radio-resistant. Whereas some are short-lived, others persist for many months (or even years) (16) in special survival niches in bone marrow (17) and lymphoid tissues (18). They are the main producers of circulating IgG, and are clearly key players in chronic Ab-mediated autoimmune diseases. Their resistance to both standard immunosuppressants and rituximab therefore necessitates a different pharmacological approach.
Many recent studies have focused on drugs that target the neoplastic plasma cells in multiple myelomas (MM). Partly because of their high rate of protein synthesis and dependence on protective unfolded protein responses, MM cells are very susceptible to proteasome inhibitors (19). These rapidly induce apoptosis by activating the terminal unfolded protein response (20) and inhibiting the transcription factor NF-κB (21). Proteasome inhibition has similar effects on non-neoplastic plasma cells in vivo (22, 23). Bortezomib, the first clinically approved proteasome inhibitor, is widely used for treating MM. In addition, it is now used to prevent acute Ab-mediated rejection of solid organ transplants (24). It is also showing promise in Ab-mediated autoimmune diseases such as systemic lupus erythematosus (SLE) and thrombotic thrombocytopenic purpura (TTP) (18, 25). In autoimmune animal models of SLE, ANCA-induced glomerulonephritis and MG, it depleted both plasma cells and autoAbs (22, 23, 26, 27).
Non-neoplastic plasma cells may also be susceptible to other anti-myeloma drugs, for example, the thalidomide derivative lenalidomide, which is frequently combined with dexamethasone in non-pregnant MM patients, and appears relatively safe. Lenalidomide inhibits the proliferation of several MM cell lines, and disrupts the stromal support in their survival niches (28). Since it reduces IgM and IgG responses to PWM (29), it must affect earlier B-lineage cells too.
In most EOMG patients, the thymic infiltrates include numerous germinal centers (5-7), many of them AChR-specific, and autoreactive T- and B-cells along with terminal plasma cells (30). In our experience, some degree of thymic hyperplasia is observed in >80% of steroid-naïve EOMG patients (30, 31). In primary cultures of cells from EOMG, but not from control thymi, autoreactive plasma cells spontaneously secrete AChR autoAbs, with titers and epitope specificities very similar to those in the patients’ sera (30, 31). They do so for several weeks (at least) – even after irradiation (31) – implying that many of them are long-lived. This longevity and radiation resistance contrasts strikingly with the majority of thymic subsets, e.g. immature thymocytes and T-cells, which have a very high turnover in vivo (32, 33) and die rapidly in culture (31, 34).
Thymectomy is part of standard management of EOMG in many centers (8). Thus the tissue removed is an almost uniquely accessible source of long-lived human autoimmune plasma cells. Here, we have used it to test their susceptibility to drugs, as assessed by their ultra-structure and production of IgG and AChR autoAbs. We demonstrate that very low concentrations of bortezomib are cytotoxic for total and autoimmune human plasma cells, and thereby prevent production and release of autoAbs, whereas lenalidomide and dexamethasone had little effect.
Patients, Materials and Methods
Patients
The EOMG patients’ clinical information is shown in Table 1. Thymus tissue was obtained with their informed consent and Ethics Committee approval. None of the patients had been pre-treated with glucocorticoids; otherwise, they were selected only because of high serum anti-AChR titers, correspondingly high productivity of these Abs by their thymic cells in culture (31), and availability of irradiated cells. Thymi were removed in London between 1983-1990, when enzymatically-dispersed cell suspensions were cryostored in liquid nitrogen (now at the Biobank of Oxford University (31, 35)); one more thymus was tested fresh in Maastricht in 2013, again after mechanical and enzymatic dispersion immediately after thymectomy. All thymi showed follicular hyperplasia.
Table 1.
Information of EOMG patients.
| Patient* | Sex | MG grade | Age at MG onset (yrs) | MG duration at thymectomy (yrs) | Anti-AChR (nM) |
|---|---|---|---|---|---|
|
| |||||
| MG-1 | F | 2A | 20 | 2.5 | >730 |
| MG-2 | F | 3 | 16 | 0.6 | 180 |
| MG-3 | F | 3 | 24 | 0.4 | 252 |
| MG-4 | F | 2B | 39 | 1.8 | 71 |
| MG-5 | F | 2A | 30 | 1.9 | 156 |
| MG-6 | F | 2B | 21 | 0.7 | 72 |
| MG-7 | F | 2A | 34 | 0.5 | 79 |
| MG-8 | F | 2A | 25 | 13.8 | 500 |
| MG-9 | M | 2B | 36 | 0.8 | 23 |
| MG-10 | F | 2A | 20 | 0.7 | >500 |
MG-1 - MG-9 were thymectomized in London (UK) and their thymic cell suspensions stored in liquid nitrogen at the Neuroscience Group Biobank, Oxford University; MG-10 was thymectomized in Maastricht (the Netherlands) and her cells were tested fresh. None of the patients received immunosuppressive drugs before thymectomy and all thymi showed follicular hyperplasia.
Cell culture and experimental design
Thymic cells were cultured as described (35). Briefly, enzymatically dispersed thymic cell suspensions were washed (and some aliquots irradiated with 1,250 rads from a 60Co source) and cryo-stored within a few hours of thymectomy. Subsequently, they were thawed carefully, and cultured at 6× 105 - 1× 106 cells per well in 96 well round-bottomed plates, without added stimulants, in 200 μL of RPMI medium containing 15% fetal bovine serum (Bodinco, the Netherlands), 50 U/mL penicillin, 50 U/mL streptomycin and 1 mM sodium pyruvate, at 37°C in humidified air with 5% CO2. Every 2 - 3 days, we removed (and stored) 90 μL of supernatant from each well, and replaced it with 100 μL of fresh medium ± any test drugs. Thymic cells were pre-cultured for 3 - 7 days, to allow recovery from the thawing procedure, adaptation to culture conditions and for measuring baseline Ab production before addition of test drugs.
We dissolved lyophilized bortezomib (Velcade, Janssen-Cilag B.V., Belgium) in sterile saline, dexamethasone (D4902; Sigma-Aldrich) in absolute ethanol, and lenalidomide (Santa Cruz Biotechnology; sc-218656) in dimethyl sulfoxide.
AutoAb and total IgG assays
In a standard radio-immunoprecipitation assay, we incubated 20 μL of culture supernatant overnight at 4°C with 12.5 μL of TE671 human rhabdomyosarcoma cell membrane-extract (containing approximately 3 fmol of human AChR). The AChR was labeled with excess 125I-α-bungarotoxin (125I-α-BT, NEX126, 3.4 TBq/mmol; PerkinElmer), and normal human serum was used as carrier. Any immune complexes were precipitated by addition of 150 μL of goat anti-human IgG and incubation for 4 hours at 4°C. We also used a standard curve after assaying serial dilutions of the anti-AChR mAb 637 (36) in parallel. Results are expressed as nanomoles of α-BT binding sites/L culture medium/day (nM/day), normalized as % of those in untreated cultures.
We measured total IgG in the diluted culture supernatants with a standard sandwich ELISA as described (26), capturing with goat F(ab′)2 anti-human IgG-Fcγ (109-006-008; Jackson Immuno-Research; diluted 1:200) and detecting with horseradish peroxidase-conjugated goat F(ab′)2 anti-human IgG-Fcγ (109-036-008; Jackson Immuno-Research; diluted 1:20,000). Results were expressed as ng of total IgG secreted per mL of supernatant/day, normalized as above. The newly synthesized AChR Ab and total IgG were quantitated as their present concentrations (in 200 μL) minus the concentration in the previous sample (in 100 μL) / time interval.
Immunofluorescence staining and enumeration of plasma cells by microscopy
Cultured cells (5× 104 - 5× 105) were cytocentrifuged onto poly-L-lysine-coated slides (for 5 minutes at ~120 g; Cyto-Tek centrifuge model 4332; Sakura Finetek, Japan). Slides were air-dried for 1 hour at 22°C and then fixed in 4% paraformaldehyde at 4°C for 10 minutes. Subsequently, they were blocked with 2% bovine serum albumin in PBS and incubated with Hoechst 33342 solution (2 μg/mL, Cat. B2261; Sigma-Aldrich) to stain DNA. We stained for plasma cells with mouse anti-human CD138 mAb (1:250, Clone MI15, Dako), donkey anti-mouse IgG Alexa 594 (1:300, A21203; Molecular Probes-Invitrogen) and goat anti-human IgG Alexa 488 (1:500, A11013; Molecular Probes-Invitrogen). Slides were mounted in 80% glycerol-TBS and stored at 4°C in the dark. All washing and incubation steps were performed with TBSTriton X-100 (0.03%). We counted the plasma cells from each well, in a blinded fashion, on a fluorescence microscope (Olympus BX51), identifying them by their distinctive size, shape (extensive cytoplasm and eccentric nuclei), and positive staining for internal IgG and/or surface CD138. Absolute numbers of plasma cells per well are given in Table 2 and shown in the Figures as the % of those in untreated cultures. Total numbers of recovered cells were measured by automated counting of trypan blue-excluding cells (TC20 Automated Cell Counter, BioRad).
Table 2.
Absolute values (average ± standard deviation) for the different parameters analyzed in thymic cell control cultures (without experimental drugs). These averages were used for normalization of data in Figs. 2, 3 and 4. All measured values are from day 14 except for anti-AChR production which are from day 11. N/A: not analyzed; Irrad: irradiated.
| Patient | IgG [ng/ml/day] | Anti-AChR [nM/day] | Plasma cells [N°/well] |
Cell numbers / well / 1000 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Irrad. | Irrad. | Non-Irrad. | Irrad. | Non-Irrad. | Irrad. | Total viable (PI−) | CD3+ T-cells | CD20+ B-cells | |
| MG-1 | 77.9 ± 19.6 | 65.9 ± 62.6 | 2.8 ± 0.7 | 2.0 ± 0.9 | 96 ± 17 | 89 ± 8 | 28.12 ± 13.67 | 18.28 ± 8.88 | 2.43 ± 1.13 |
| MG-2 | 191.9 ± 35.3 | 17.8 ± 12.8 | 1.2 ± 0.6 | ~0.2 | 236 ± 16 | 16 ± 8 | 78.07 ± 22.68 | 57.42 ± 18.66 | 6.04 ± 0.80 |
| MG-3 | 57.7 ± 6.0 | 4.0 ± 1.5 | 1.3 ± 0.2 | <0.1 | 61 ± 37 | 1 ± 1 | 37.35 ± 12.98 | 20.91 ± 7.74 | 3.26 ± 1.11 |
| MG-4 | 298.0 ± 75.0 | N/A | 1.0 ± 0.1 | N/A | 84 ± 53 | N/A | 147.01 ± 13.48 | 64.33 ± 3.85 | 4.08 ± 0.82 |
| MG-5 | 83.8 ± 18.0 | 14.6 ± 7.2 | 1.1 ± 0.4 | <0.1 | 165 ± 31 | 21 ± 6 | 130.53 ± 19.47 | 85.86 ± 16.29 | 4.81 ± 1.57 |
| MG-6 | 9.0 ± 2.8 | 27.7 ± 10.4 | <0.1 | <0.1 | N/A | 21 ± 12 | 26.12 ± 9.94 | 17.54 ± 6.28 | 0.32 ± 0.20 |
| MG-7 | 325.0 ± 158.6 | N/A | <0.1 | N/A | N/A | N/A | N/A | N/A | N/A |
| MG-8 | 43.8 ± 17.6 | N/A | ~0.2 | N/A | 157 ± 68 | N/A | N/A | N/A | N/A |
| MG-9 | N/A | N/A | N/A | N/A | N/A | N/A | 16.94 ± 2.73 | N/A | N/A |
| MG-10 | 42.3 ± 10.6 | N/A | 1.3 ± 0.4 | N/A | N/A | N/A | N/A | N/A | N/A |
FACS analysis of B-, T- and plasma cells, and membrane integrity
For B- and T-lymphocyte identification, cells were incubated for 30 minutes at 4°C in FACS buffer (2% fetal calf serum and 0.1% sodium azide in PBS) with FITC mouse anti-human CD3 (BD 555339) and PE mouse anti-human CD19 (BD 555413), all diluted 1:100 in FACS buffer. The samples were washed twice, kept at 4°C in the dark, and analyzed within 2 hours. Dead cells were identified by propidium iodide (PI) counter-staining (Apoptosis detection kit; BD).
To identify plasma cells, we incubated for 15 minutes at 22°C in the dark with the following mix of anti-human mAbs: CD138 PE (IBL M2287; 1:25), CD19 PerCy5.5 (BD 332780; 1:40), CD38 APC (BD 345807; 1:40), CD3 V450 (BD 560365; 1:20) and CD45 V500 (560777; 1:20). Cells were then resuspended in 1x Cytofix/Cytoperm buffer (BD 554722) for fixation and permeabilization, and later incubated for 30 minutes at 4°C in the dark with an antibody mix of anti-human Kappa-LC FITC (BD, 555791) + anti-human Lambda-LC FITC (BD 347247, clone 1-155-2), each diluted 1:35 in perm/wash buffer (BD 554723). We used either a FACSCalibur (for B- and T-cells) or a FACSCanto II (for plasma cells), plus CellQuest Software (all from BD) for data acquisition, and analyzed results using the Flowing Software (version 2.5.1; Turku Center for Biotechnology, Finland). Samples were gated to exclude cell debris and aggregates; at least 10,000 events per sample were measured for B- and T-cell analyses, and 150,000 events for plasma cells. Results are expressed as the absolute number of cells/well, normalized as the % of B-, T- and total viable cells in untreated cultures.
Electron microscopy
Cultured thymic cells were collected, pelleted (7 min, 220 g, 4°C) and fixed with 3% glutaraldehyde + 1.4% sucrose buffered in 0.09 M KH2PO4 at pH 7.4. They were then washed in 0.09 M KH2PO4 buffer with 7.5% sucrose and transferred to a 1% OsO4 + 1.5% ferrocyanide solution buffered with veronal at pH 7.4 for subsequent immersion fixation for 1 hour at 4°C. After washing in veronal buffer with 7% sucrose at pH 7.4, dehydration was carried out rapidly in graded ethanol series. Samples were then incubated overnight in propylene oxide and Epon (1:1), and subsequently embedded in Epon. Serial 80 nm sections were stained with uranyl acetate, lead citrate, and coded. We used a Philips CM100 electron microscope to count plasma cells and examine their ultrastructure in five representative sections for each sample.
Statistics
GraphPad Prism 4 was used for statistical analyses. We compared normally distributed values using 1- or 2-way ANOVA analyses, and Bonferroni post-hoc tests. A two-sided probability value of 0.05 or lower was considered significant. Values are expressed as means ± standard error of the mean (SEM) unless stated otherwise. We used Spearman (non-parametric) correlation coefficients (ρ).
Results
Culturing EOMG thymic cells
Plasma cells were identified by their characteristic ultra-structural morphology (Fig. 1), intense internal IgG staining and surface CD138 expression (Fig. 2A), though the latter was a dim and not totally consistent marker (as previously noted for cryo-stored cells (37)). They were frequently found in clumps of 3-5 cells (or sometimes more), in close contact with extracellular matrix and other cell types (Fig. 2A), as in their survival niches in the spleen or bone marrow (38).
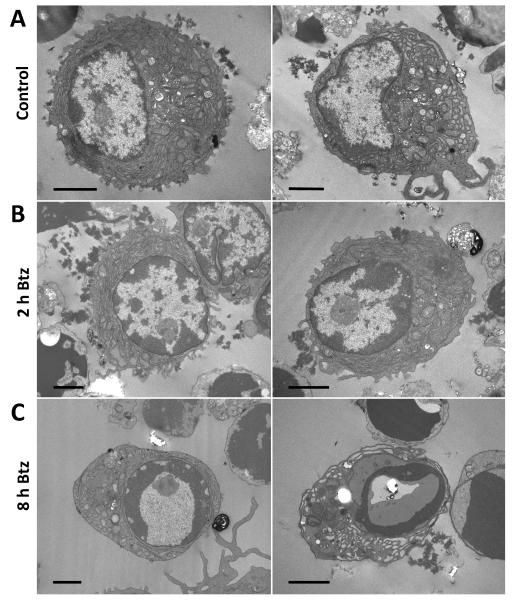
Figure 1.
Ultrastructural changes in plasma cells incubated with bortezomib. A. Normal plasma cells in control samples. Note the typical morphology, with elaborate endoplasmic reticulum (ER) and eccentric nuclei (with a cart-wheel heterochromatin configuration) B. Most plasma cells also appeared largely normal after 2 hours of treatment with 2.5 μM bortezomib. C. After 8 hours of treatment with bortezomib, most plasma cells appeared apoptotic, with heterochromatin condensed around the perimeter of the nucleus and distension of the ER lumen. After 24 hours with bortezomib, no plasma cells could be detected. Coded cell samples were post-fixed with osmium tetroxide and counter-stained with uranyl acetate and lead citrate. Pictures are representative of the conditions analyzed. Scale bars are 2 μm.
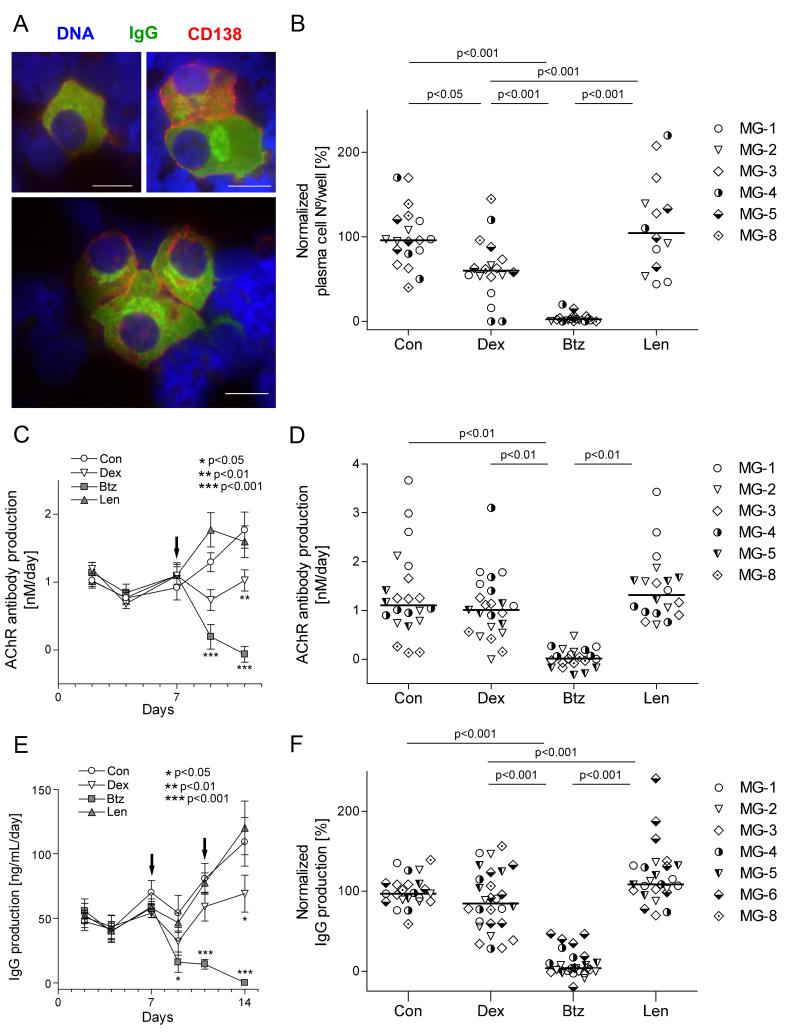
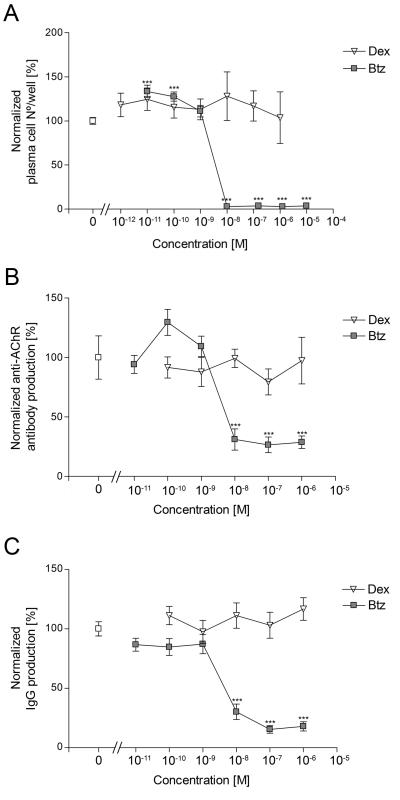
Figure 2.
Changes in plasma cell numbers and functions after addition of bortezomib (2.5 μM), lenalidomide (10 μM) or dexamethasone (10 nM) on days 7 and 11, and assessed 3 days later.
A. Representative plasma cells from cytocentrifuged thymic cells. They were readily identified by their staining for internal IgG (green) and surface CD138 (red; DNA is blue), and typical morphological features, including: relatively large size, abundant cytoplasm with positive staining for immunoglobulins and eccentric nuclei. However, many plasma cells showed dim or negative staining for CD138 despite their intense labeling for internal IgG and typical morphological features (37). They were frequently found in clumps of 2-3 or more cells together, as shown in the lower panel. Scale bars are 10 μm.
B. Normalized plasma cell numbers on day 14. Each point represents a single well (3 per condition) from the indicated patients; horizontal bars are median values. Results are expressed as percentage of the corresponding control cultures of the same patient, from which the absolute plasma cell numbers are given in Table 2.
C. Spontaneous secretion of AChR Abs in vitro, measured by radio-immunoprecipitation and expressed as nanomoles per liter per day (AChR Ab production rate). Each point represents the average of samples from patients MG-1 - MG-3 (4 replicates per patient). Samples from day 14 could not be compared to earlier time points because of inter assay-variation, and are therefore not shown.
D. AChR Ab production rate on day 11. Each point represents a measurement of one well (4 per condition) from the indicated patients; horizontal bars are median values. No AChR Ab production was detected in samples of MG-6.
E. Spontaneous secretion of total IgG in vitro. We measured IgG in supernatants by ELISA, and expressed results as nanograms of IgG per milliliter per day (IgG production).
F. Total IgG production rate on day 14. Each point represents one well (4 per condition) from the indicated patients; horizontal bars are median values. Results are normalized as for Fig 2B, and absolute IgG production rates shown in Table 2.
Arrows indicate the days of drug addition. Error bars correspond to the SEM. One-way (B, D, F) or two-way (C, E) ANOVA and Bonferroni post-hoc testing were used for statistical analyses. Lenalidomide was not tested for MG-8.
In the thymus, there is normally a high rate of cell death in vivo (32, 33), primarily due to the programmed death of immature thymocytes deprived of pro-survival signals. As expected, it was also substantial in our suspension cultures of frozen/thawed cells; even from hyperplastic EOMG thymi, the majority of cells are immature thymocytes (7, 35), and no longer in contact with the rare epithelial cells on which their survival normally depends. About 20% of the input cells remained viable on day 14, and fewer in irradiated samples (~8%). Absolute values for all the parameters measured in these cultures are shown in Table 2. To maximize plasma cell recovery/activity, we used cells that had been dispersed with dispase and collagenase, and cryo-stored (7, 35); these behaved very similarly to fresh thymic cells in culture, their AChR Ab and total IgG productivity often slightly exceeding that of their fresh counterparts, probably reflecting plasma cell enrichment by depletion of thymocytes (35). In fact, cells cultured from one fresh EOMG thymus gave substantially similar results (see below, Table 2 and Supplemental Fig. S1). AutoAb production also appeared highly dependent on cell concentration and on adherent ‘feeder’ fibroblasts and macrophages (35). Although microenvironments are probably not optimal in vitro, spontaneous autoAb and total IgG production was nonetheless relatively consistent in quadruplicate wells from most of the thymi tested (Fig. 2D, F). Remarkably, both persisted for at least 2 weeks - even after irradiation (Fig. 3), although only occasional viable macrophages and fibroblasts could still be seen (not shown), again highlighting that small numbers of plasma cells are able to produce substantial amounts of IgG/ autoAb (Table 2).
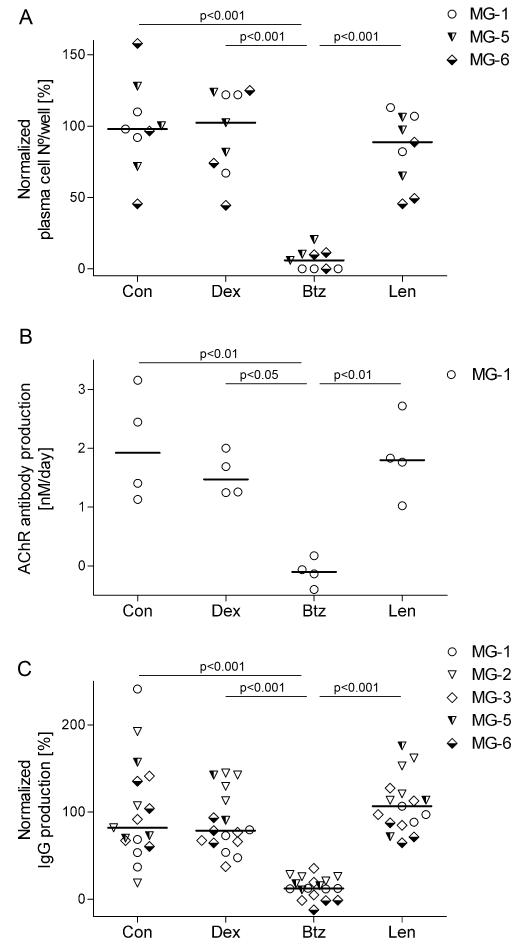
Figure 3.
Susceptibility of irradiated cells to experimental drugs. Thymic cells were irradiated and subsequently cultured for two weeks. Drugs were added on days 7 and 11 (bortezomib - 2.5 μM, lenalidomide −10 μM, dexamethasone - 10 nM) and samples were collected and analyzed as for Fig. 2.
A. Plasma cell survival from patients MG-1, 5 and 6 on day 14 shown as % of the absolute numbers in the control cultures (Table 2).
B. AChR autoAb secretion from patient MG-1 on day 11. The AChR autoAb production rate from patients MG-3, 5 and 6 was below detection limit; in MG-2, it was just above background.
C. IgG production rate from irradiated samples on day 14. Each point represents one well (3 per condition) from the indicated patients; horizontal bars are median values. One-way ANOVA and Bonferroni post-hoc testing were used for statistical analyses.
Bortezomib rapidly induces apoptosis in plasma cells from EOMG thymi
We pre-cultured thawed EOMG thymic cells for 3 days before adding 2.5 μM bortezomib. This concentration was based on previous in vitro experiments on human plasma cells (39) and the peak concentration measured in MM patients (40).
In all control samples, plasma cell ultrastructure appeared normal, with elaborate endoplasmic reticulum (ER), a well-defined Golgi complex and dense regions of (nuclear) heterochromatin in the characteristic “cart-wheel” distribution (Fig. 1A). They still appeared largely normal at 2 and 4 hours after addition of bortezomib (at 2.5 μM; Fig. 1B). However, after 8 hours, most surviving plasma cells showed signs of apoptosis (Fig. 1C), including dense condensations of chromatin around most of the nuclear membrane perimeter, and distension of the ER (41). After 24 hours, they were no longer detected in the bortezomib-treated cultures. Results were very similar with bortezomib at 0.25 μM (not shown).
Bortezomib eliminates plasma cells in cultured EOMG thymic cells
To focus on long-lived plasma cells, we next added bortezomib, lenalidomide or dexamethasone on days 7 and 11 of culture, and counted surviving plasma cells on day 14. Lenalidomide was used at 10 μM, based on previous in vitro studies (42-44) and peak levels in MM patients (45). Dexamethasone was tested at 10 nM, a level known to inhibit lymphocyte proliferation in susceptible humans (46, 47).
We used thymic cells from 6 patients to test effects of these drugs on plasma cell numbers. Three days after a second dose of bortezomib, plasma cells were almost undetectable in all cases (p<0.001; Fig. 2B and Supplemental Fig. S2). Interestingly, their numbers were not changed greatly or consistently by either lenalidomide or dexamethasone (p<0.05 for the latter). In a separate experiment, we confirmed that 10 μM lenalidomide suppressed IgG production by PWM-stimulated PBMCs (not shown), as previously reported (29).
Proteasome inhibition halts spontaneous secretion of total IgG and AChR autoAbs
In thymic cell cultures which were analyzed at early time points, we could already detect significant production of both AChR Ab and total IgG at 48 hours of culture; both increased further from days 9 - 14 in the control and lenalidomide cultures (Fig. 2C, E). In striking contrast, they both consistently declined sharply after the first dose of bortezomib, and further still after the second (p<0.001). Notably, dexamethasone merely prevented their rise after day 9, which correlated with the mild reduction in plasma cell survival observed by day 14. Results were broadly similar with the one fresh thymus available to us (Table 2 and supplemental Fig. S1). At day 14, AChR Ab and total IgG production was strongly and significantly reduced by bortezomib, compared to all other conditions in all tested patients (Figure 2D, F).
Cell suspensions from five thymi were irradiated and cultured for two weeks. Plasma cells were detected in all of these cultures, though at lower frequencies than in their non-irradiated counterparts from the same patients (Table 2). There was also very substantial, persisting production of total IgG in all cases (Fig. 3C). After irradiation, AChR Ab production remained strong in one patient (Fig. 3B) and above background in a second (Table 2). Interestingly, when numbers of surviving plasma cells were sufficient (in MG1, 5, 6) they were reduced only by bortezomib (p<0.001; Fig. 3A). Notably, both autoAb and total IgG production were completely unaffected by dexamethasone, but both were again inhibited >90% by bortezomib (p<0.001; Fig. 3B, C). When all results were combined, numbers of plasma cells (whether irradiated or not) correlated strongly with spontaneous secretion of total IgG and AChR Abs (ρ=0.84; P<0.0001 and ρ =0.66; P<0.0001 respectively).
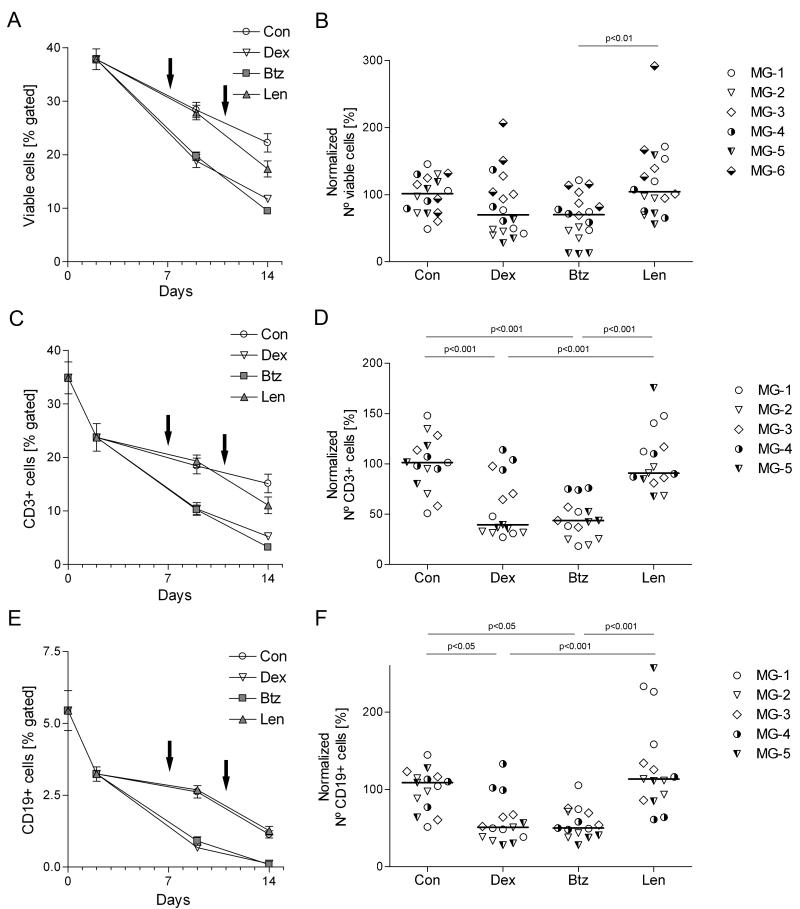
To test for effects on other cell types, we next stained unirradiated cultured cells for CD3 and CD19. Both dexamethasone (at 10 nM) and bortezomib (at 2.5 μM) reduced the numbers of T- and B-cells after the first dose (day 7; p<0.001 and p<0.05 respectively) – and to a similar extent (Fig. 4C, E), although dexamethasone had much less effect than bortezomib on Ab levels. By contrast, lenalidomide (at 10 μM) had no significant effect on either cell numbers or AChR Ab/ total IgG production (Fig. 4B, D, F). Together with the strong correlation between total IgG production and plasma cell survival, these results deeply implicate plasma cells, rather than B-cells, in the spontaneous Ab production that we observed in vitro.
Figure 4.
Quantitative overview of the drug effects on B- and T-lymphocytes. Thymic cells were cultured for 2 weeks and drugs were added on days 7 and 11 (indicated by arrows). Cells were collected and labeled for FACS analysis on days 0, 2, 9 and 14. Samples were gated to exclude cell debris, selected for viable (PI-negative) cells and for either CD3 or CD19. In, A, C and E, each point represents the average of samples from patients MG-1, MG-2 and MG-3 (3 per patient). Error bars correspond to the SEM. Each point in panels B, D and F represents one well (3 per condition) from the indicated patients for day 14; horizontal bars are median values. Results are normalized as for Fig 2B, and absolute cell numbers are shown in Table 2. At least 10,000 total events per sample were recorded; numbers of events analyzed per gate and per patient are given in Supplemental Table S1. One-way ANOVA and Bonferroni post hoc testing were used for statistical analyses.
Dose-dependence of bortezomib and dexamethasone effects on plasma cell numbers and function
We next tested broader concentration ranges of dexamethasone and bortezomib in cultures from one fresh and 6 cryo-preserved EOMG thymi. Total IgG and autoAb productivity and plasma cell numbers were all minimal in the presence of 10 nM - 10 μM bortezomib (Figs. 5A, B and C and Supplemental Fig. S2D); leukemia and MM cell lines also have IC50s of 10-20 nM (48). In sharp contrast, dexamethasone had no significant effects, even at 1 μM. Thus the minimum dose of bortezomib for eliminating plasma cells in vitro is apparently 10 nM. We also tested broader concentrations of lenalidomide, but did not observe any significant effects on plasma cell survival or function (not shown).
Figure 5.
Dose-response curves for plasma cell numbers (A), AChR Ab production (B) and total IgG secretion (C), after addition of drugs on day 4 and assay on day 7. Thymic cells from patients MG-1 and MG-3 were used in A; cells from MG-4 and MG-5 in B; and cells from MG-1, MG-3, MG-4, MG-5, MG-7 and MG-8 in C. Each point represents the average of the normalized results for all the patients analyzed (at least 3 replicates per patient) and error bars the SEM. One-way ANOVA and Bonferroni post-hoc testing were used for statistical analyses. *** p<0.001, compared with the untreated cultures (white square).
To assess their general toxicity, we sampled cultures at earlier times after addition of these drugs. At 6, 24 and 48 h, we found no significant differences in overall viability (Supplemental Fig. 3). At day 7, viabilities were reduced more by dexamethasone at 1 μM than 10 nM; still more by bortezomib at 2.5 μM, but not significantly at 10 nM, where its effects were more selective for plasma cells.
Discussion
In this study we demonstrate that bortezomib selectively eliminates long-lived autoimmune plasma cells in cultured thymus cells from 9 of 9 EOMG patients analyzed. Their spontaneous AChR autoAb and total IgG production were promptly and almost completely halted, even at 10 nM. At 0.25 and 2.5 μM – and within 8 hours – it led to ultra-structural changes in plasma cells that are characteristic not only of ER stress but also of apoptosis, as seen in vivo too (26). As far as we know, these are the first demonstrations of its efficacy directly on pathogenic autoAb-producing human plasma cells. Since bortezomib triggers apoptosis in nonneoplastic plasma cells, even after a single dose, short low-dose regimens might be sufficient to rapidly reduce their numbers and Ab levels in patients with autoimmune disorders, thus ‘buying time’ before standard immunosuppressive drugs take effect.
In our cultures, plasma cells seemed almost entirely responsible for spontaneous production of both autoAb and total IgG – which correlated strongly with their survival. Moreover, since it was maintained despite depletion of B-cells (by dexamethasone; Fig. 4E, F), these evidently contributed minimally in untreated cultures (if at all). Indeed, we have rarely found signs of mitogen-stimulable production of AChR Abs in EOMG thymi (31). These data, together with the Abs’ highly mutated heavy and light chain V region sequences, suggest that native AChR – which is continuously available in the thymus – is driving most Ab-producing cells to the terminal plasma cell stage (30). Since we have seen very similar behavior in cells from EOMG bone marrow and spleen, it is clearly not unique to the hyperplastic EOMG thymus; indeed, it was also shown by thymic remnants from thymoma/ MG patients (30, 31).
In previous studies, plasma cells from recently immunized mice died after only 4 days in suspension cultures (49). In sharp contrast, their Ig production was sustained in vitro for 2 - 4 weeks in primary cultures from human tonsils and gut-associated lymphoid tissue, and was enhanced by feeder cells and especially in whole organ cultures (50, 51). It seems unlikely that equivalent survival niches were reconstituted efficiently in our cultures of cryostored, and especially irradiated, thymic cells. We suggest that the remarkable survival of the long-lived plasma cells shown here is due to their co-clustering (Fig. 2A) and/ or to some degree of resilience or ‘self-sufficiency’. Their radio-resistance clearly shows that they are well established in many EOMG thymi and scarcely replaced in culture. Importantly, since bortezomib (but not dexamethasone) reduced their numbers and Ab production to baseline in irradiated samples, its targets must include long-lived plasma cells.
In sharp contrast with our results with bortezomib, but in agreement with previous in vivo findings (22, 52), we found only marginal effects of dexamethasone on plasma cell survival or function, even when added twice at 1 μM, and only on unirradiated cells. Evidently, most of the thymic plasma cells are dexamethasone- as well as radio-resistant; indeed, neither treatment alone – or when combined – completely eliminated them in any of our cultures. In theory, both treatments might also affect their supporting cells, and/ or damage other short-lived plasma cells or plasmablasts. In vivo, however, their precursors may be steroid-insensitive too; we noted no obvious decrease in PWM-stimulated IgG responses by (radio-sensitive) B-cells from prednisone-pretreated patients – rather, they appeared to be enriched (53).
The even smaller effects on plasma cells of the immunomodulatory drug lenalidomide may seem surprising in view of its clear benefits in MM patients (28, 29, 54). One possible explanation is that its toxicity for MM cells is mainly related to the activation of tumor suppressor genes and caspases that trigger apoptosis in transformed cells (55-57), but probably not in their non-neoplastic counterparts. Moreover, since lenalidomide also disrupts the survival niches required by MM and plasma cells (58), its effects may be under-estimated in our cultures. In addition, it is well-known for its disparate immunomodulatory properties, e.g., inhibiting Ig production by cultured PBMCs (29), but also augmenting Ab responses to vaccination (59), and enhancing proliferation and activation of T-cells (28, 58). Taken together, our in vitro results indicate that, unlike MM cells, non-neoplastic plasma cells are not directly killed by lenalidomide. However, its possible effects on their niches in vivo might valuably complement the direct actions of bortezomib in patients (42).
Both bortezomib (at higher concentrations) and dexamethasone reduced CD19+ and CD3+ lymphocytes in our cultures. This is in line with the reported effects of bortezomib on activated human B-cells (60) and total circulating B-cells in EAMG rats (26). Moreover, bortezomib influences T-cell subset distributions, inducing apoptosis in activated CD4+ T-cells, preventing the activation of memory T-cells (61), but preserving resting and regulatory T-cells (62-64), and promoting their de novo generation (64). Additional effects of bortezomib on activated B- and T-cells, or on antigen-presenting B-cells, could be an advantage in treating MG patients, e.g., in preventing the generation of new autoreactive plasma cells while also eliminating the existing long-lived subset.
The susceptibility of plasma cells that we observed here, even to 10 nM bortezomib, is striking. Treating autoimmune patients with lower doses/ shorter courses of bortezomib may offer valuable therapeutic benefits while minimizing side-effects, since even partial elimination of pathogenic plasma cells might be adequate – especially if combined with plasma exchange (65, 66). At doses commonly used to treat MM, SLE, TTP and acute Ab-mediated transplant rejection (1.3 mg/m2; resulting in plasma levels of Cmax = 600 nM (40)), bortezomib can cause serious thrombocytopenia or peripheral neuropathy, particularly in MM patients given other chemo-therapeutics to eliminate as many neoplastic cells as possible. In contrast, adverse effects were significantly fewer with ‘light touch’ regimens that maintained therapeutic effects in patients with hyper-acute Ab-mediated transplant rejection (24, 65-67) and also in MM patients (40). Finally, some second generation proteasome inhibitors have equal or greater potency but lower neurotoxicity than bortezomib, and are already being tested in clinical trials (68).
In conclusion, our study using EOMG thymic cells, in combination with our previous results in the EAMG model (26), gives proof-of-principle for using proteasome inhibitors for the elimination of nonneoplastic plasma cells in autoAb-mediated disorders. This therapeutic strategy could have the important advantage of to rapidly reducing autoAb titers during the lag period before the standard immunosuppressants have taken full effect. However, this potential benefit needs to be balanced very carefully against the possibility that side-effects still persist at very low doses of bortezomib.
Supplementary Material
Acknowledgements
We are very grateful to Dr. Henry Kaminski and Dr Marguerite E. Hill for helpful discussions, to the patients for their samples and to Sr E Goodger and the late Prof John Newsom-Davis for access to them. We are also very grateful to Dr. Jan Damoiseaux and Jozien Jaspers-Spits for granting access to their laboratory and for their assistance with the FACS analysis. We would also like to thank Miro Vysocansky and Judith Hounjet for their help with plasma cell counting.
Grant support: This work was supported by a Marie-Curie fellowship from the European Union to A.M.G; grants from the UK Medical Research Council to N.W.; a Sara Borrell contract of ISCIII Spain to G.N.G. (CD10/00027); grants from the Prinses Beatrix Fonds (Project WAR08-12) and the Association Française contre les Myopathies to P.M.-M; a Veni fellowship of the Netherlands Organization for Scientific Research and a fellowship of the Brain Foundation of the Netherlands to M.L.
The School for Mental Health and Neuroscience of Maastricht University receives financial support from Takeda for testing proteasome inhibitors.
Abbreviations
- AChR
acetylcholine receptor
- α-BT
α-bungarotoxin
- ANCA
anti-neutrophil cytoplasmic antibodies
- EOMG
early-onset myasthenia gravis
- ER
endoplasmic reticulum
- EAMG
experimental autoimmune MG
- GC
germinal center
- MM
multiple myeloma
- PI
propidium iodide
- SLE
systemic lupus erythematosus
- TTP
thrombotic thrombocytopenic purpura
References
- 1.Irani S, Lang B. Autoantibody-mediated disorders of the central nervous system. Autoimmunity. 2008;41:55–65. doi: 10.1080/08916930701619490. [DOI] [PubMed] [Google Scholar]
- 2.Sahashi K, Engel AG, Lambert EH, Howard FM., Jr. Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J Neuropathol Exp Neurol. 1980;39:160–172. doi: 10.1097/00005072-198003000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Heinemann S, Merlie J, Lindstrom J. Modulation of acetylcholine receptor in rat diaphragm by anti-receptor sera. Nature. 1978;274:65–68. doi: 10.1038/274065a0. [DOI] [PubMed] [Google Scholar]
- 4.Gregersen PK, Kosoy R, Lee AT, Lamb J, Sussman J, McKee D, Simpfendorfer KR, Pirskanen-Matell R, Piehl F, Pan-Hammarstrom Q, Verschuuren JJ, Titulaer MJ, Niks EH, Marx A, Strobel P, Tackenberg B, Putz M, Maniaol A, Elsais A, Tallaksen C, Harbo HF, Lie BA, Raychaudhuri S, de Bakker PI, Melms A, Garchon HJ, Willcox N, Hammarstrom L, Seldin MF. Risk for myasthenia gravis maps to a (151) Pro-->Ala change in TNIP1 and to human leukocyte antigen-B*08. Annals of Neurology. 2012;72:927–935. doi: 10.1002/ana.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Panse R, Bismuth J, Cizeron-Clairac G, Weiss JM, Cufi P, Dartevelle P, De Rosbo NK, Berrih-Aknin S. Thymic remodeling associated with hyperplasia in myasthenia gravis. Autoimmunity. 2010;43:401–412. doi: 10.3109/08916930903563491. [DOI] [PubMed] [Google Scholar]
- 6.Castleman B, Norris EH. The pathology of the thymus in myasthenia gravis; a study of 35 cases. Medicine. 1949;28:27–58. doi: 10.1097/00005792-194902000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Willcox HN, Newsom-Davis J, Calder LR. Cell types required for anti-acetylcholine receptor antibody synthesis by cultured thymocytes and blood lymphocytes in myasthenia gravis. Clin Exp Immunol. 1984;58:97–106. [PMC free article] [PubMed] [Google Scholar]
- 8.Gold R, Hohlfeld R, Toyka KV. Progress in the treatment of myasthenia gravis. Ther Adv Neurol Disord. 2008;1:36–51. doi: 10.1177/1756285608093888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hehir MK, Burns TM, Alpers J, Conaway MR, Sawa M, Sanders DB. Mycophenolate mofetil in AChR-antibody-positive myasthenia gravis: outcomes in 102 patients. Muscle Nerve. 2010;41:593–598. doi: 10.1002/mus.21640. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Manera J, Martinez-Hernandez E, Querol L, Klooster R, Rojas-Garcia R, Suarez-Calvet X, Munoz-Blanco JL, Mazia C, Straasheijm KR, Gallardo E, Juarez C, Verschuuren JJ, Illa I. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78:189–193. doi: 10.1212/WNL.0b013e3182407982. [DOI] [PubMed] [Google Scholar]
- 11.Cupps TR, Gerrard TL, Falkoff RJ, Whalen G, Fauci AS. Effects of in vitro corticosteroids on B cell activation, proliferation, and differentiation. J Clin Invest. 1985;75:754–761. doi: 10.1172/JCI111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function*. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 13.Brown TE, Ahmed A, Filo RS, Knudsen RC, Sell KW. The immunosuppressive mechanism of azathioprine. I. In vitro effect on lymphocyte function in the baboon. Transplantation. 1976;21:27–35. doi: 10.1097/00007890-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Helmreich E, Kern M, Eisen HN. The secretion of antibody by isolated lymph node cells. J Biol Chem. 1961;236:464–473. [PubMed] [Google Scholar]
- 15.Hibi T, Dosch HM. Limiting dilution analysis of the B cell compartment in human bone marrow. Eur J Immunol. 1986;16:139–145. doi: 10.1002/eji.1830160206. [DOI] [PubMed] [Google Scholar]
- 16.Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 17.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–386. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 18.Hiepe F, Dorner T, Hauser AE, Hoyer BF, Mei H, Radbruch A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011;7:170–178. doi: 10.1038/nrrheum.2011.1. [DOI] [PubMed] [Google Scholar]
- 19.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr., Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meister S, Schubert U, Neubert K, Herrmann K, Burger R, Gramatzki M, Hahn S, Schreiber S, Wilhelm S, Herrmann M, Jack HM, Voll RE. Extensive immunoglobulin production sensitizes myeloma cells for proteasome inhibition. Cancer Res. 2007;67:1783–1792. doi: 10.1158/0008-5472.CAN-06-2258. [DOI] [PubMed] [Google Scholar]
- 21.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 22.Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, Wiethe C, Winkler TH, Kalden JR, Manz RA, Voll RE. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 23.Gomez AM, Willcox N, Molenaar PC, Buurman W, Martinez-Martinez P, De Baets MH, Losen M. Targeting plasma cells with proteasome inhibitors: possible roles in treating myasthenia gravis? Ann N Y Acad Sci. 2012;1274:48–59. doi: 10.1111/j.1749-6632.2012.06824.x. [DOI] [PubMed] [Google Scholar]
- 24.Woodle ES, Alloway RR, Girnita A. Proteasome inhibitor treatment of antibody-mediated allograft rejection. Curr Opin Organ Transplant. 2011;16:434–438. doi: 10.1097/MOT.0b013e328348c0e5. [DOI] [PubMed] [Google Scholar]
- 25.Shortt J, Oh DH, Opat SS. ADAMTS13 antibody depletion by bortezomib in thrombotic thrombocytopenic purpura. N Engl J Med. 2013;368:90–92. doi: 10.1056/NEJMc1213206. [DOI] [PubMed] [Google Scholar]
- 26.Gomez AM, Vrolix K, Martinez-Martinez P, Molenaar PC, Phernambucq M, van der Esch E, Duimel H, Verheyen F, Voll RE, Manz RA, De Baets MH, Losen M. Proteasome inhibition with bortezomib depletes plasma cells and autoantibodies in experimental autoimmune myasthenia gravis. J Immunol. 2011;186:2503–2513. doi: 10.4049/jimmunol.1002539. [DOI] [PubMed] [Google Scholar]
- 27.Bontscho J, Schreiber A, Manz RA, Schneider W, Luft FC, Kettritz R. Myeloperoxidase-specific plasma cell depletion by bortezomib protects from anti-neutrophil cytoplasmic autoantibodies-induced glomerulonephritis. J Am Soc Nephrol. 2011;22:336–348. doi: 10.1681/ASN.2010010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies F, Baz R. Lenalidomide mode of action: linking bench and clinical findings. Blood Rev. 2010;24(Suppl 1):S13–19. doi: 10.1016/S0268-960X(10)70004-7. [DOI] [PubMed] [Google Scholar]
- 29.Shannon E, Sandoval F, Greig N, Stagg P. Lenalidomide alone or lenalidomide plus dexamethasone significantly inhibit IgG and IgM in vitro... A possible explanation for their mechanism of action in treating multiple myeloma. Int Immunopharmacol. 2012;12:441–446. doi: 10.1016/j.intimp.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiono H, Roxanis I, Zhang W, Sims GP, Meager A, Jacobson LW, Liu JL, Matthews I, Wong YL, Bonifati M, Micklem K, Stott DI, Todd JA, Beeson D, Vincent A, Willcox N. Scenarios for autoimmunization of T and B cells in myasthenia gravis. Ann N Y Acad Sci. 2003;998:237–256. doi: 10.1196/annals.1254.026. [DOI] [PubMed] [Google Scholar]
- 31.Hill ME, Shiono H, Newsom-Davis J, Willcox N. The myasthenia gravis thymus: a rare source of human autoantibody-secreting plasma cells for testing potential therapeutics. J Neuroimmunol. 2008;201-202:50–56. doi: 10.1016/j.jneuroim.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 32.Bai M, Doukas M, Papoudou-Bai A, Barbouti A, Stefanaki K, Galani V, Kanavaros P. Immunohistological analysis of cell cycle and apoptosis regulators in thymus. Ann Anat. 2013;195:159–165. doi: 10.1016/j.aanat.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Le PT, Maecker HT, Cook JE. In situ detection and characterization of apoptotic thymocytes in human thymus. Expression of bcl-2 in vivo does not prevent apoptosis. J Immunol. 1995;154:4371–4378. [PubMed] [Google Scholar]
- 34.Purton JF, Boyd RL, Cole TJ, Godfrey DI. Intrathymic T cell development and selection proceeds normally in the absence of glucocorticoid receptor signaling. Immunity. 2000;13:179–186. doi: 10.1016/s1074-7613(00)00018-2. [DOI] [PubMed] [Google Scholar]
- 35.Willcox HN, Newsom-Davis J, Calder LR. Greatly increased autoantibody production in myasthenia gravis by thymocyte suspensions prepared with proteolytic enzymes. Clin Exp Immunol. 1983;54:378–386. [PMC free article] [PubMed] [Google Scholar]
- 36.van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, De Baets MH, van de Winkel JG, Aalberse RC, Parren PW. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 37.Frigyesi I, Adolfsson J, Ali M, Christophersen MK, Johnsson E, Turesson I, Gullberg U, Hansson M, Nilsson B. Robust isolation of malignant plasma cells in multiple myeloma. Blood. 2014;123:1336–1340. doi: 10.1182/blood-2013-09-529800. [DOI] [PubMed] [Google Scholar]
- 38.Tokoyoda K, Hauser AE, Nakayama T, Radbruch A. Organization of immunological memory by bone marrow stroma. Nat Rev Immunol. 2010;10:193–200. doi: 10.1038/nri2727. [DOI] [PubMed] [Google Scholar]
- 39.Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, Stegall MD. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9:201–209. doi: 10.1111/j.1600-6143.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 40.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, Arnulf B, Kropff M, Cavet J, Esseltine DL, Feng H, Girgis S, van de Velde H, Deraedt W, Harousseau JL. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 41.Hacker G. The morphology of apoptosis. Cell Tissue Res. 2000;301:5–17. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- 42.Chauhan D, Singh AV, Ciccarelli B, Richardson PG, Palladino MA, Anderson KC. Combination of novel proteasome inhibitor NPI-0052 and lenalidomide trigger in vitro and in vivo synergistic cytotoxicity in multiple myeloma. Blood. 2010;115:834–845. doi: 10.1182/blood-2009-03-213009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian Z, Zhang L, Cai Z, Sun L, Wang H, Yi Q, Wang M. Lenalidomide synergizes with dexamethasone to induce growth arrest and apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leuk Res. 2011;35:380–386. doi: 10.1016/j.leukres.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 44.Matsuoka A, Tochigi A, Kishimoto M, Nakahara T, Kondo T, Tsujioka T, Tasaka T, Tohyama Y, Tohyama K. Lenalidomide induces cell death in an MDS-derived cell line with deletion of chromosome 5q by inhibition of cytokinesis. Leukemia. 2010;24:748–755. doi: 10.1038/leu.2009.296. [DOI] [PubMed] [Google Scholar]
- 45.Hofmeister CC, Yang X, Pichiorri F, Chen P, Rozewski DM, Johnson AJ, Lee S, Liu Z, Garr CL, Hade EM, Ji J, Schaaf LJ, Benson DM, Jr., Kraut EH, Hicks WJ, Chan KK, Chen CS, Farag SS, Grever MR, Byrd JC, Phelps MA. Phase I trial of lenalidomide and CCI-779 in patients with relapsed multiple myeloma: evidence for lenalidomide-CCI-779 interaction via P-glycoprotein. J Clin Oncol. 2011;29:3427–3434. doi: 10.1200/JCO.2010.32.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De A, Blotta HM, Mamoni RL, Louzada P, Bertolo MB, Foss NT, Moreira AC, Castro M. Effects of dexamethasone on lymphocyte proliferation and cytokine production in rheumatoid arthritis. J Rheumatol. 2002;29:46–51. [PubMed] [Google Scholar]
- 47.Hearing SD, Norman M, Smyth C, Foy C, Dayan CM. Wide variation in lymphocyte steroid sensitivity among healthy human volunteers. J Clin Endocrinol Metab. 1999;84:4149–4154. doi: 10.1210/jcem.84.11.6156. [DOI] [PubMed] [Google Scholar]
- 48.Horton TM, Gannavarapu A, Blaney SM, D’Argenio DZ, Plon SE, Berg SL. Bortezomib interactions with chemotherapy agents in acute leukemia in vitro. Cancer Chemother Pharmacol. 2006;58:13–23. doi: 10.1007/s00280-005-0135-z. [DOI] [PubMed] [Google Scholar]
- 49.Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, Muehlinghaus G, Szyska M, Radbruch A, Manz RA. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 50.van Laar JM, Melchers M, Teng YK, van der Zouwen B, Mohammadi R, Fischer R, Margolis L, Fitzgerald W, Grivel JC, Breedveld FC, Lipsky PE, Grammer AC. Sustained secretion of immunoglobulin by long-lived human tonsil plasma cells. Am J Pathol. 2007;171:917–927. doi: 10.2353/ajpath.2007.070005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mesin L, Di Niro R, Thompson KM, Lundin KE, Sollid LM. Long-lived plasma cells from human small intestine biopsies secrete immunoglobulins for many weeks in vitro. J Immunol. 2011;187:2867–2874. doi: 10.4049/jimmunol.1003181. [DOI] [PubMed] [Google Scholar]
- 52.Miller JJ, 3rd, Cole LJ. Resistance of long-lived lymphocytes and plasma cells in rat lymph nodes to treatment with prednisone, cyclophosphamide, 6-mercaptopurine, and actinomycin D. J Exp Med. 1967;126:109–125. doi: 10.1084/jem.126.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willcox N, Schluep M, Sommer N, Campana D, Janossy G, Brown AN, Newsom-Davis J. Variable corticosteroid sensitivity of thymic cortex and medullary peripheral-type lymphoid tissue in myasthenia gravis patients: structural and functional effects. Q J Med. 1989;73:1071–1087. [PubMed] [Google Scholar]
- 54.Dimopoulos MA, Richardson PG, Brandenburg N, Yu Z, Weber DM, Niesvizky R, Morgan GJ. A review of second primary malignancy in patients with relapsed or refractory multiple myeloma treated with lenalidomide. Blood. 2012;119:2764–2767. doi: 10.1182/blood-2011-08-373514. [DOI] [PubMed] [Google Scholar]
- 55.Gandhi AK, Kang J, Capone L, Parton A, Wu L, Zhang LH, Mendy D, Lopez-Girona A, Tran T, Sapinoso L, Fang W, Xu S, Hampton G, Bartlett JB, Schafer P. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10:155–167. doi: 10.2174/156800910791054239. [DOI] [PubMed] [Google Scholar]
- 56.Verhelle D, Corral LG, Wong K, Mueller JH, Moutouh-de Parseval L, Jensen-Pergakes K, Schafer PH, Chen R, Glezer E, Ferguson GD, Lopez-Girona A, Muller GW, Brady HA, Chan KW. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007;67:746–755. doi: 10.1158/0008-5472.CAN-06-2317. [DOI] [PubMed] [Google Scholar]
- 57.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP, Anderson KC. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 58.Gorgun G, Calabrese E, Soydan E, Hideshima T, Perrone G, Bandi M, Cirstea D, Santo L, Hu Y, Tai YT, Nahar S, Mimura N, Fabre C, Raje N, Munshi N, Richardson P, Anderson KC. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood. 2010;116:3227–3237. doi: 10.1182/blood-2010-04-279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noonan K, Rudraraju L, Ferguson A, Emerling A, Pasetti MF, Huff CA, Borrello I. Lenalidomide-induced immunomodulation in multiple myeloma: impact on vaccines and antitumor responses. Clin Cancer Res. 2012;18:1426–1434. doi: 10.1158/1078-0432.CCR-11-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heidt S, Roelen DL, Vergunst M, Doxiadis, Claas FH, Mulder A. Bortezomib affects the function of human B cells: possible implications for desensitization protocols. Clin Transpl. 2009:387–392. [PubMed] [Google Scholar]
- 61.Kim JS, Lee JI, Shin JY, Kim SY, Shin JS, Lim JH, Cho HS, Yoon IH, Kim KH, Kim SJ, Park CG. Bortezomib can suppress activation of rapamycin-resistant memory T cells without affecting regulatory T-cell viability in non-human primates. Transplantation. 2009;88:1349–1359. doi: 10.1097/TP.0b013e3181bd7b3a. [DOI] [PubMed] [Google Scholar]
- 62.Berges C, Haberstock H, Fuchs D, Miltz M, Sadeghi M, Opelz G, Daniel V, Naujokat C. Proteasome inhibition suppresses essential immune functions of human CD4+ T cells. Immunology. 2008;124:234–246. doi: 10.1111/j.1365-2567.2007.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heider U, Rademacher J, Kaiser M, Kleeberg L, von Metzler I, Sezer O. Decrease in CD4+ T-cell counts in patients with multiple myeloma treated with bortezomib. Clin Lymphoma Myeloma Leuk. 2010;10:134–137. doi: 10.3816/CLML.2010.n.019. [DOI] [PubMed] [Google Scholar]
- 64.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, Caballero-Velazquez T, Gutierrez-Cossio S, Hernandez-Campo P, Diez-Campelo M, Herrero-Sanchez C, Rodriguez-Serrano C, Santamaria C, Sanchez-Guijo FM, Del Canizo C, San Miguel JF. Treatment with bortezomib of human CD4+ T cells preserves natural regulatory T cells and allows the emergence of a distinct suppressor T-cell population. Haematologica. 2009;94:975–983. doi: 10.3324/haematol.2008.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, Gentili S, Patriarca F, Nozzoli C, Levi A, Guglielmelli T, Benevolo G, Callea V, Rizzo V, Cangialosi C, Musto P, De Rosa L, Liberati AM, Grasso M, Falcone AP, Evangelista A, Cavo M, Gaidano G, Boccadoro M, Palumbo A. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–4753. doi: 10.1182/blood-2010-07-294983. [DOI] [PubMed] [Google Scholar]
- 66.Everly MJ. A summary of bortezomib use in transplantation across 29 centers. Clin Transpl. 2009:323–337. [PubMed] [Google Scholar]
- 67.Diwan TS, Raghavaiah S, Burns JM, Kremers WK, Gloor JM, Stegall MD. The impact of proteasome inhibition on alloantibody-producing plasma cells in vivo. Transplantation. 2011;91:536–541. doi: 10.1097/TP.0b013e3182081333. [DOI] [PubMed] [Google Scholar]
- 68.Kirk CJ. Discovery and development of second-generation proteasome inhibitors. Semin Hematol. 2012;49:207–214. doi: 10.1053/j.seminhematol.2012.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.