Abstract
Background
Coronary artery disease (CAD) has been associated with HIV infection; however data are not consistent.
Objective
We performed cardiac CT to determine whether HIV-infected men have more coronary atherosclerosis than uninfected men.
Design
Cross-sectional study within the Multicenter AIDS Cohort Study(MACS).
Participants
HIV-infected (n=618) and –uninfected (n=383) men who have sex with men (MSM) had non-contrast and contrast enhanced cardiac CT if they were between 40–70 years, weighed <300 pounds, and had no history of coronary revascularization.
Measurements
Presence and extent, for those with plaque, of coronary artery calcium (CAC) on non-contrast CT, and of any plaque, non-calcified, mixed or calcified plaque and stenosis on CT angiography.
Results
1001 men underwent non-contrast CT of whom 759 had coronary CT angiography. After adjusting for age, race, center, and cohort, HIV-infected men had a greater prevalence of CAC [Prevalence ratio(PR)=1.21, 95% confidence interval (CI) 1.08–1.35, p=0.001], and any plaque [PR=1.14(1.05–1.24),p=0.001], including non-calcified plaque [PR=1.28(1.13–1.45),p<0.001) and mixed plaque [PR=1.35(1.10–1.65),p=0.004] than HIV-uninfected men. Associations between HIV-infection and any plaque and non-calcified plaque remained significant (p<0.005) after CAD risk factor adjustment. HIV-infected men also had a greater extent of non-calcified plaque after CAD risk factor adjustment (p=0.026). HIV-infected men had a greater prevalence of coronary artery stenosis>50% than HIV-uninfected men [PR=1.48(1.06–2.07),p=0.020), but not after CAD risk factor adjustment. Longer duration of highly active antiretroviral therapy [PR=1.09(1.02–1.17), p=0.007,per year] and lower nadir CD4+ T-cell count [PR=0.80(0.69–0.94),p=0.005, per 100 cells] were associated with coronary stenosis>50%.
Conclusions
Coronary artery plaque, especially non-calcified plaque, is more prevalent and extensive in HIV-infected men, independent of CAD risk factors.
Limitations
Cross-sectional observational study design and inclusion of only men.
Primary Funding Source
NHLBI and NIAID
Introduction
Advances in the treatment of human immunodeficiency virus (HIV) infection have led to dramatic decreases in AIDS-related mortality (1, 2). This extension in expected survival has led to emergence of chronic non-infectious age-related diseases, such as coronary artery disease (CAD) (3). Increased risk for CAD has been associated with HIV infection and with antiretroviral therapy (ART) (4–6). However, data are not consistent, due to differences in populations and study designs (7). For, example, the SMART study reported a lower risk for cardiovascular events among persons treated with continuous (versus intermittent) ART, associated with better suppression of HIV RNA levels and systemic inflammation (8).
The incidence of clinical CAD events among HIV-infected persons has been generally low and therefore difficult to study. Subclinical atherosclerosis can be detected non-invasively using carotid ultrasonography to measure carotid artery intima media thickness (IMT) and carotid plaque, and non-contrast cardiac computed tomography (CT) to measure coronary artery calcium (CAC). Subclinical atherosclerosis is associated with risk for events in the general population (9). Some studies have found more subclinical atherosclerosis among HIV-infected persons (10–12), but again the results are not consistent (13).
Recently, advances in technology have allowed for a more comprehensive evaluation of subclinical coronary atherosclerosis using coronary CT angiography (CTA) at lower radiation exposure (14). CTA allows for accurate assessment of the presence and extent of coronary artery plaque in addition to detection of stenosis and characterizing plaque composition. Non-calcified and mixed plaques have been associated with increased risk for plaque rupture and cardiovascular events (15, 16). We performed non-contrast cardiac CT scans and CTA to determine whether HIV-infected men have more coronary atherosclerosis than HIV-uninfected men in the Multicenter AIDS Cohort Study (MACS).
Methods
Population
The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective cohort study of the natural and treated histories of HIV-1 infection in homosexual and bisexual men, conducted in Baltimore, Chicago, Pittsburgh and Los Angeles (17). Initial enrollment in the MACS parent study occurred in 1984–85, with additional enrollment in 1987–1991 and 2001–2003. The cohort includes both HIV-infected and uninfected men who attend semiannual research visits including standardized interviews, physical examinations and blood and urine collection for laboratory measurements.
Eligibility for this MACS cardiovascular ancillary study included being an active MACS participant (with oversampling of HIV-infected men), age 40–70 years, weight< 300 lbs, and no prior history of cardiac surgery or percutaneous coronary intervention, as these procedures would interfere with the measurement of coronary atherosclerosis. All participants completed non-contrast cardiac CT scanning for coronary artery calcium (CAC) scoring between January 2010 and August 2013. Men with atrial fibrillation, chronic kidney disease [estimated glomerular filtration rate (GFR)<60 ml/min/m2 during a prior MACS study visit] or a history of IV contrast allergy were excluded from CTA studies. All eligible CTA participants had an estimated GFR>60 ml/min/m2 within one month of CTA. The study was approved by the Institutional Review Boards of all participating sites. All participants signed informed consent for this MACS ancillary study.
CT Scanning and Analysis Procedures
Details of the CT scanning procedures have been described (18). Briefly, in preparation for cardiovascular imaging, men received beta blocker or calcium channel blocker medications as needed and sublingual nitroglycerin was administered prior to IV contrast injection unless contraindicated. CT scanning equipment included 64-slice multi-detector CT at 3 centers and one center with 320-row multi-detector CT. Prospective ECG triggering protocols, which minimized radiation exposure, were used, except when the heart rate was too fast or irregular. The median (interquartile range) dose for the CTA procedure was 1.9 (1.7–2.7) mSv.
CTA images were transferred to the core CT reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA) and were analyzed by trained, experienced readers who were blinded to participant characteristics and HIV serostatus. Each segment was analyzed using the modified 15-segment model of the American Heart Association (19). Using axial images, multi-planar reconstructions and maximum intensity projections, the reader assessed the presence, size and composition of coronary plaque and the degree of luminal narrowing stenosis in all assessable coronary segments. Plaque size was graded as 0=no plaque, 1=mild, 2=moderate, or 3=severe. Segment stenosis was defined as 0=no plaque, 1=1–29%(minimal) stenosis, 2=30–49%(mild) stenosis, 3=50–69%(moderate) stenosis, or 4 for ≥70%(severe) stenosis. The total plaque score was calculated by summing the plaque size score for all assessable coronary segments that demonstrated any plaque (either calcified, noncalcified, or mixed) up to a maximum score of 45. This measure has been shown to be highly reproducible (20). The segment involvement score was calculated as the sum of coronary artery segments with plaque, regardless of degree of stenosis.
Each coronary segment was classified as normal or containing non-calcified plaque, mixed plaque (<50% of plaque area occupied by calcium) or calcified plaque. Calcified atherosclerotic plaque was defined as any structure with attenuation >130 HU visualized separately from the intravascular lumen, identified in at least two independent planes. Non-calcified atherosclerotic plaque was defined as any discernible structure that could be clearly assignable to the vessel wall, with a CT density less than the contrast-enhanced coronary lumen but greater than the surrounding connective tissue, and identified in at least two independent planes. The non-calcified plaque score, mixed plaque score and calcified plaque score were calculated by summing the plaque scores in each non-calcified, mixed or calcified plaque segment separately. Participants and their medical care providers (if permission was obtained) received a clinical CTA report. Additional testing and therapy was determined by participants’ medical care providers.
Clinical Parameters
Participants were seen every six months as part of routine MACS research visits. Data were collected regarding CAD risk factors and HIV clinical parameters by history, physical examination and blood tests. For this analysis, data were used that had been collected at the prior MACS study visit closest to the CT scan, generally within 6 months before the CT scan. Race/ethnicity was based on self-report. Glucose, insulin, total and high-density lipoprotein cholesterol (HDL), and triglycerides were measured from fasting blood samples at the routine MACS visit. Low density lipoprotein cholesterol (LDL) was calculated using the Friedewald equation or measured directly in persons with triglycerides > 400 mg/dL or with nonfasting samples. Serum creatinine was measured at each MACS study visit and within 30 days of CT scanning for persons who underwent contrast injection. The Modification of Diet in Renal Disease (MDRD) equation was used to estimate GFR. Hypertension was defined as systolic blood pressure (BP)>140 mm Hg or diastolic BP>90 mm Hg or self-reported use of anti-hypertensive medication. Diabetes mellitus was defined as fasting serum glucose ≥ 126 mg/dL or use of medications to treat diabetes. Measures of HIV disease activity in HIV-infected men included plasma HIV RNA levels, CD4+ T-cell counts, history of an AIDS-defining malignancy or opportunistic infection, and duration of highly active anti-retroviral therapy use.
Statistics
The distributions of demographic and clinical factors in HIV-infected and -uninfected men, and completion of contrast-enhanced or non-contrast cardiac CT scans were compared using the Wilcoxon rank-sum test or chi-square test, where appropriate. Since factors associated with the initiation and progression of plaque may differ, our analytical approach treated each as separate outcomes. Analyses of associations between HIV serostatus and coronary artery plaque prevalence were performed with plaque presence defined as a score greater than zero. Poisson regression with robust variance (21) was used to evaluate associations between HIV serostatus and plaque prevalence. Separate models were performed for non-zero CAC, and for total, calcified, mixed and non-calcified plaque scores and coronary artery stenosis > 50%, adjusting for age, race, CT scanning center, and cohort status (enrolled pre- or post-2001) (minimally adjusted models). Second multivariable analyses were then performed with additional adjustment for established CAD risk factors (systolic BP, use of hypertension medications, use of diabetes medications, fasting glucose, total and HDL cholesterol, use of lipid lowering medications, body mass index, and pack-years of tobacco smoking). Linear regression was used to assess the association between HIV serostatus with plaque extent (burden) among individuals with plaque present (i.e., plaque score greater than zero) for CAC, and for total, calcified, mixed and non-calcified plaque after adjusting for age, race, CT scanning center and cohort status, and then also adjusting for CAD risk factors as described above. Since plaque scores were not normally distributed these values were natural-log transformed. To test for interaction between age and HIV serostatus, interaction terms were added to each model. Significant interactions (p <0.05) are reported in the results section. Additional analyses among HIV-infected men were performed to assess associations between HIV clinical parameters and plaque outcomes (prevalence and extent) using modified Poisson and linear regression, adjusting for age, race, CT scanning center, and cohort status (enrolled pre- or post-2001), and then additionally adjusting for CAD risk factors. To address possible unmeasured confounding, we performed simple probabilistic sensitivity analyses for selected results (22). Proportions of this “confounder”were drawn from uniform distributions defined by 0.4 and 0.7 for HIV-infected and 0.2 and 0.5 for HIV-uninfected men, and a relative risk of 5 with the outcome. To compare the prevalence of non-calcified plaque between HIV-infected and –uninfected men in coronary segments with stenosis greater than 50% we used a log-binomial model with generalized estimating equation, adjusting for age, race, CT-scan center, and cohort status. Multiple imputation was used to complete missing CAD risk factor data for multivariate models. For each outcome, the multiple imputation included all predictors and the outcome. Missing values were imputed five times based on the distribution of covariates using a Markov chain Monte Carlo (MCMC) method (23) assuming multivariate normality. Values for the following number of men were missing and imputed for multiple regression analyses-hypertension medications (11), body mass index (27), diabetes medications (11), smoking pack-years (5), lipid medications (20), total and HDL cholesterol (28), systolic blood pressure (43), fasting glucose (36). All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, NC). SAS procedures used included PROC FREQ, PROC MEANS, PROC NPAR1WAY, PROC MI, PROC MIANALYZE, PROC GENMOD, and PROC REG. Figure 1 was created by using TIBCO Spotfire S+ 8.2 (TIBCO Software Inc. Palo Alto, CA). Statistical significance was established at a p-value less than 0.05.
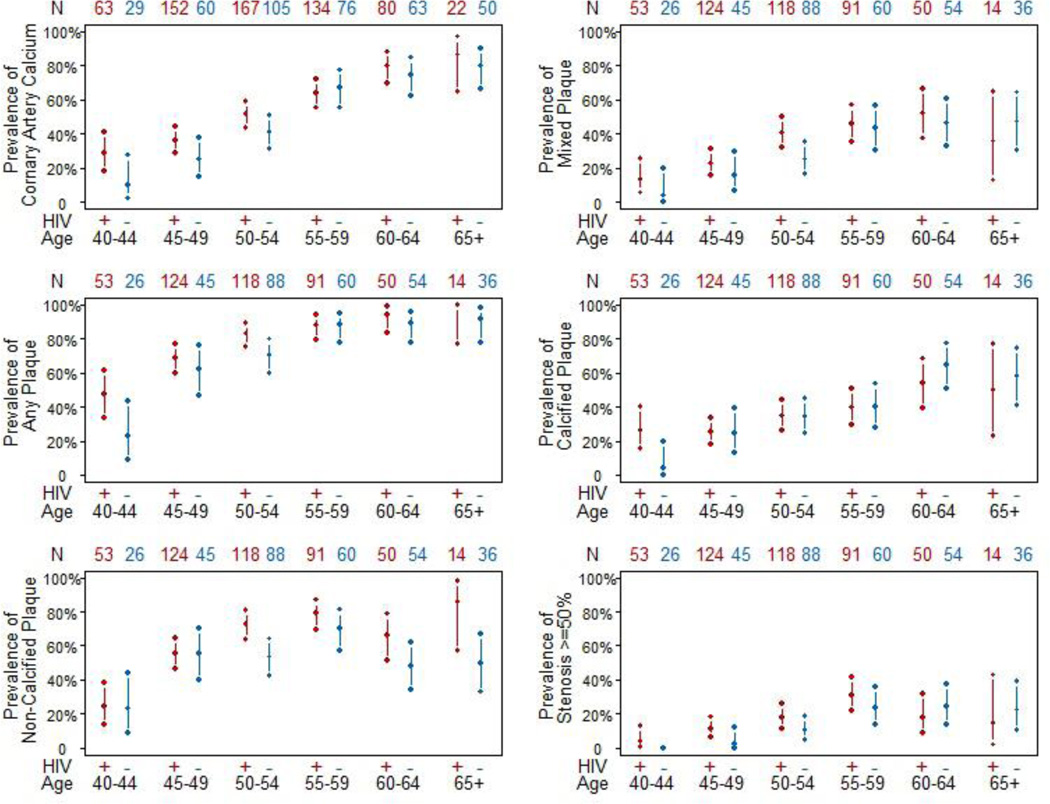
Figure 1.
Unadjusted prevalences with 95% confidence intervals of plaque stratified by HIV serostatus in 5 year age increments. N on the top of each panel shows the number of men in each group. Interaction for age by HIV serostatus for the presence of non-calcified plaque was significant (p= 0.006).
The funding source did not have any role in the design, conduct, or analysis of this study or in the decision to submit the manuscript for publication.
Results
There were 1001 men with non-contrast CT scan results available, of whom 759 had coronary CT angiography results, after excluding one man with a technically limited CAC scan. The median time between the MACS visit where covariates were assessed and the CT scan date was 2 months (interquartile range: 1 – 4 months). The study population characteristics are presented in Table 1. The 618 HIV-infected men were slightly younger and more likely to be African-American than the 383 HIV-uninfected. More HIV-infected men were current smokers, had greater cumulative pack-years of smoking, a lower body mass index (BMI), and higher serum creatinine. LDL and HDL cholesterol were lower in HIV-infected men, and triglyceride levels were higher. Lipid lowering therapy was used by approximately one-third of all men. Men who underwent only a non-contrast CT scan had higher serum creatinine, were slightly older, had a higher prevalence of diabetes and hypertension, and lower LDL cholesterol. No discernible differences in the results described below were observed when restricting the CAC sample to those who also received contrast scans.
Table I.
Characteristics of Study Population
| HIV Seropositive |
HIV Seronegative | p-value* | CT with contrast | Non-contrast CT only |
p-value† | |
|---|---|---|---|---|---|---|
| N | 618 | 383 | 759 | 242 | ||
| Age (years) | 53.2(6.5) | 55.8(7.4) | <0.001 | 53.8(7.0) | 55.3(6.7) | 0.002 |
| Race | ||||||
| Caucasian (%) | 52.6 | 66.8 | <0.001 | 57.4 | 59.9 | 0.40 |
| African-American (%) | 34.0 | 24.8 | 30.3 | 31.0 | ||
| Hispanic/Other (%) | 13.4 | 8.4 | 12.3 | 9.1 | ||
| Hypertension (%) | 50.5 | 44.3 | 0.062 | 45.4 | 56.8 | 0.003 |
| Systolic blood pressure (mm Hg) | 126.7(15.0) | 127.8(14.5) | 0.34 | 126.8(14.2) | 128.4(16.5) | 0.188 |
| Hypertension medications (%) | 35.9 | 31.2 | 0.127 | 31.1 | 43.7 | <0.001 |
| Diabetes (%) | 13.0 | 8.9 | 0.053 | 9.3 | 18.1 | <0.001 |
| Diabetes medications (%) | 8.8 | 6.6 | 0.21 | 6.5 | 12.6 | 0.003 |
| Tobacco use | ||||||
| Never smoker (%) | 25.2 | 25.2 | 0.009 | 25.2 | 25.3 | 0.63 |
| Current smoker (%) | 30.9 | 22.6 | 27.0 | 30.0 | ||
| Former smoker (%) | 43.9 | 52.2 | 47.8 | 44.7 | ||
| Smoking pack-years** | 5.6(0–22.8) | 1.8(0–21.4) | 0.024 | 4.4(0–21.4) | 4.5(0–26.9) | 0.47 |
| Body Mass Index (kg/m2) | 26.1(4.5) | 27.3(4.8) | <0.001 | 26.6(4.5) | 26.6(5.2) | 0.49 |
| Glucose (mg/dL) | 102.6(25.7) | 100.7(31.2) | 0.016 | 100.1(23.7) | 107.4(38.2) | 0.002 |
| Total Cholesterol (mg/dL) | 188.0(42.3) | 191.1(36.3) | 0.128 | 188.9(38.8) | 190.1(44.1) | 0.67 |
| LDL Cholesterol (mg/dL) | 105.3(35.2) | 112.4(32.4) | <0.001 | 109.1(34.1) | 104.4(34.7) | 0.027 |
| HDL Cholesterol (mg/dL) | 48.7(16.4) | 53.2(16.0) | <0.001 | 50.3(16.1) | 50.7(17.4) | 0.88 |
| Triglycerides (mg/dL) | 174.1(130.6) | 125.7(76.3) | <0.001 | 148.8(101.4) | 177.3(150.3) | 0.010 |
| Lipid lowering medications (%) | 35.0 | 28.5 | 0.032 | 31.8 | 34.9 | 0.37 |
| Serum Creatinine (mg/dL) | 1.1(0.5) | 1.0(0.2) | 0.034 | 1.0(0.2) | 1.3(0.8) | <0.001 |
| HIV clinical factors | 618 | 450 | 168 | |||
| Current HIV RNA undetectable, < 50 copies/mL (%) | 82.4 | 80.4 | 87.7 | 0.037 | ||
| Current HIV RNA (copies/mL)& | 723(139–24000) | 1121(138–34,100) | 602(197–7675) | 0.68 | ||
| Current CD4+ T-cell count (cells/mm3) | 601(427–766) | 602(428–773) | 599(417–757) | 0.74 | ||
| CD4+ T-cell count nadir (cells/mm3) | 244(134–332) | 254(155–339) | 216(98–318) | 0.009 | ||
| HAART experienced (%) | 96.0 | 95.3 | 97.6 | 0.199 | ||
| Protease Inhibitor use (%) | 48.7 | 47.3 | 52.4 | 0.26 | ||
| NNRTI use (%) | 47.1 | 48.2 | 44.0 | 0.36 | ||
| HAART Duration (years) | 12.5(8.7–14.1) | 12.2(8.5–14.0) | 13.1(9.6–14.4) | 0.031 | ||
| Protease Inhibitor Duration (years) | 5.1(0.4–9.6) | 4.8(0.3–8.9) | 6.0(1.4–11.0) | 0.017 | ||
| NNRTI Duration (years) | 3.8(0.6–7.9) | 3.9(0.6–7.8) | 3.5(0.6–8.0) | 0.76 | ||
| History of AIDS (%) | 14.2 | 11.1 | 22.6 | <0.001 |
Data are presented stratified by HIV serostatus for the entire cohort and then stratified by whether or not a CT with contrast was performed. Laboratory Glucose and Triglycerides results represent fasting levels. Data are reported as mean (standard deviation) or percentage. P values are unadjusted.
P value comparing seropositive and seronegative values.
P value comparing CT with contrast versus non-contrast only values.
Median (interquartile range: 25%– 75%) for non-normally distributed variables and men (SD) for normally distributed variables.
Among 107 HIV+ men (87 with contrast CT scan) with detectable current HIV RNA (>50 copies/mL) levels. HAART= highly active retroviral therapy; NNRTI= non-nucleoside reverse transcriptase inhibitors
The prevalences of having any CAC (Agatston score>0) on non-contrast CT scans were 53.1% among HIV-infected and 52.0 % in HIV-uninfected men (Table 2). After adjusting for age, race, study center, and cohort, there was a greater prevalence of CAC in HIV-infected men [prevalence ratio (PR)=1.21 (95% confidence interval (CI): 1.08, 1.35)] (Table 3). After additional adjustment for CAD risk factors, this association became borderline significant [PR=1.12(0.99, 1.26)]. Among persons with CAC present by CT scan, no association existed between HIV serostatus and CAC extent.
Table 2.
CT Scan Results
| CT Scan Parameters | HIV Seropositive | HIV Seronegative | Total |
|---|---|---|---|
| Non-contrast CT Scans (N) | 618 | 383 | 1001 |
| Coronary Artery Calcium Present: Agatston Score > 0 (%) | 53.1(49.1,57) | 52(46.9,57.0) | 52.6(49.5,55.7) |
| Coronary Artery Calcium Score among those with calcium present (N=527) | 70(22–190) | 77(23–226) | 71(22–208) |
| Contrast-enhanced CT Scans (N) | 450 | 309 | 759 |
| Prevalence of any coronary plaque (%) | 77.6(73.7,81.4) | 74.4(69.5,79.3) | 76.3(73.3,79.3) |
| Prevalence of non-calcified plaque (%) | 63.3(58.9,67.8) | 53.1(47.5,58.7) | 59.2(55.7,62.7) |
| Prevalence of mixed plaque (%) | 34.7(30.3,39.1) | 31.7(26.5,36.9) | 33.5(30.1,36.8) |
| Prevalence of calcified plaque (%) | 34.7(30.3,39.1) | 39.5(34.0,45.0) | 36.6(33.2,40.1) |
| Prevalence of any coronary stenosis > 50% (%) | 16.9(13.4,20.4) | 14.6(10.6,18.5) | 15.9(13.3,18.6) |
| Prevalence of any coronary stenosis > 70% (%) | 6.9(4.5,9.2) | 7.1(4.2,10.0) | 7(5.2,8.8) |
| Segment involvement score | 2(1–4) | 2(1–4) | 2(1–4) |
| Total Plaque Score | 2(1–5) | 2(0–5) | 2(1–5) |
| Non-calcified Plaque Score | 1(0–3) | 1(0–2) | 1(0–2) |
| Mixed Plaque Score | 0(0–1) | 0(0–1) | 0(0–1) |
| Calcified Plaque Score | 0(0–1) | 0(0–2) | 0(0–1) |
Plaque variables are reported as median and interquartile range (IQR) or percentage (95% confidence interval) of men with plaque.
Table 3.
Associations between HIV Serostatus and Coronary Artery Plaque
| Minimally adjusted model | Adjusted for CAD Risk Factors | |||
|---|---|---|---|---|
| Prevalence of Plaque | PR (95% CI)* | P value | PR (95% CI)† | P value |
| Non-contrast CT Scans (N=1001) | ||||
| Coronary artery calcium present | 1.21(1.08,1.35) | 0.001 | 1.12(0.99,1.26) | 0.076 |
| Contrast-enhanced CT Scans (N=759) | ||||
| Any plaque present | 1.14(1.05,1.24) | 0.001 | 1.13(1.04,1.23) | 0.004 |
| Non-calcified plaque present | 1.28(1.13,1.45) | 0.000 | 1.25(1.10,1.43) | 0.001 |
| Mixed plaque present | 1.35(1.10,1.65) | 0.004 | 1.22(0.98,1.52) | 0.070 |
| Calcified plaque present | 1.05(0.88,1.27) | 0.58 | 1.02(0.84,1.23) | 0.88 |
| Coronary artery stenosis > 50% | 1.48(1.06,2.07) | 0.020 | 1.23(0.86,1.75) | 0.26 |
| Coronary artery stenosis > 70% | 1.20(0.70,2.05) | 0.51 | 0.76(0.44,1.30) | 0.31 |
| Extent of Plaque |
Mean Difference (95% CI)* |
P value |
Mean Difference (95% CI)† |
P value |
| Non-contrast CT Scans | ||||
| Coronary Artery Calcium (N=527)(Agatston Score) | 0.07(−0.23,0.38) | 0.65 | −0.03(−0.34,0.29) | 0.88 |
| Contrast-enhanced CT Scans | ||||
| Segment Involvement Score | 0.14(0.02,0.25) | 0.023 | 0.11(−0.01,0.22) | 0.075 |
| Total Coronary Plaque Score (N=579) | 0.19(0.05,0.33) | 0.009 | 0.13(−0.01,0.27) | 0.062 |
| Non-calcified Plaque Score (N=449) | 0.16(0.03,0.29) | 0.015 | 0.15(0.02,0.29) | 0.026 |
| Mixed Plaque Score (N=254) | 0.15(−0.05,0.35) | 0.133 | 0.16(−0.04,0.36) | 0.109 |
| Calcified Plaque Score (N=278) | −0.02(−0.21,0.17) | 0.83 | −0.07(−0.27,0.12) | 0.46 |
Adjusted for age, race, center, and cohort (pre- vs. post-2001);
Adjusted for age, race, center, cohort, and CAD risk factors; PR= prevalence ratio (HIV seropositive compared to HIV seronegative); CI= confidence interval. CAD risk factors include systolic blood pressure, antihypertensive medication use, diabetes medication use, fasting glucose, total cholesterol, HDL cholesterol, use of lipid-lowering medications, body mass index and smoking (pack-years). Analyses of extent of plaque (in natural log scale) include men with plaque present (plaque score > 0). Mean differences represent HIV seropositive- HIV seronegative.
Among men who underwent cardiac CT scans with contrast enhancement, the prevalence of plaque in any coronary segment was 77.6% for HIV-infected men and 74.4% for HIV-uninfected men. After adjustment for age, race, center, and cohort, there was a greater prevalence of plaque in HIV-infected men than uninfected men [PR=1.14(1.05, 1.24)], which persisted after adjustment for CAD risk factors [PR=1.13(1.04, 1.23)]. Among men with plaque, plaque extent, measured as total plaque score, and segment involvement score, were both greater among HIV-infected men (p=0.009 and p=0.023, respectively), but differences were attenuated and only borderline significant after adjustment for CAD risk factors (p=0.07 and p=0.06, respectively). There was some suggestion that the HIV association with segment involvement score and total plaque score may differ by race, whereby the scores were higher among HIV-infected, but not among African-Americans (data not shown).
Additional analyses were performed to determine the association between HIV serostatus and plaque composition on CT angiography (non-calcified, mixed or calcified plaque). More HIV-infected than HIV-uninfected men (63.3% versus 53.1%) had non-calcified plaque. This higher prevalence of non-calcified plaque in HIV-infected men persisted after adjusting for age, race, center and cohort [PR=1.28(1.13,1.45)], and remained significant after adjusting for additional CAD risk factors [PR=1.25(1.10,1.43)]. To address possible unmeasured confounding, we performed simple probabilistic sensitivity analyses (22). Even in the presence of very strong confounding, the significant association between non-calcified plaque and HIV serostatus remained. Among men with non-calcified plaque, there was also a significant association between extent of non-calcified plaque and HIV infection, (mean difference in log plaque score= 0.15(0.02,0.29) for HIV-infected compared to uninfected men upon full adjustment).
In both HIV-infected and –uninfected men, mixed plaque and calcified plaque were less prevalent than non-calcified plaque (table 2). There was a greater prevalence of mixed plaque in HIV-infected than uninfected men [PR=1.35(1.10,1.65)]. This association was attenuated and borderline significant after adjusting for CAD risk factors [PR=1.22(0.98,1.52)]. Among men with mixed plaque present, there was no association between HIV serostatus and the extent of mixed plaque. There was also no association between HIV serostatus and the presence or extent of calcified plaque on CT angiography (Table 3).
The prevalence of a coronary artery stenosis >50% in any coronary segment was 16.9% among HIV-infected men and 14.6% among uninfected men, [PR=1.48(1.06, 2.07)] after adjusting for age, race, center, and cohort. This association was no longer statistically significant after adjusting for CAD risk factors [PR=1.23(0.86, 1.75)]. There were 192 coronary segments among 121 men that demonstrated ≥ 50% stenosis on CT angiography. Among these 192 coronary segments, the proportion of non-calcified plaque in coronary segments among HIV-infected men was 45% compared with 35% of segments among uninfected men (p=0.23, adjusted for age, race, center and cohort, and controlling for within-person correlation). We repeated the analyses in table 3 including only the men with suppressed HIV RNA and the results were nearly identical to the results with the entire cohort.
The unadjusted prevalences (and 95% confidence intervals) of each plaque type stratified by HIV serostatus and by age are shown in Figure 1. Advancing age was associated with an increase in prevalence of coronary artery plaque and stenosis; however, we noted a significant interaction between age and HIV serostatus for the presence of non-calcified plaque; advancing age was associated with non-calcified plaque in HIV-infected men [PR=1.17 (95% CI 1.11, 1.23) per 5 years increase in age], but not in HIV-uninfected men [PR=1.03 (95% CI 0.96, 1.11) per 5 years increase in age] (p for interaction=0.006).
We examined HIV-related clinical stage-of-disease parameters to evaluate associations with subclinical atherosclerosis that might contribute to a greater prevalence and extent of some types of coronary artery plaque among HIV-infected men. There were no significant associations of the presence of CAC, any plaque on CT angiography, mixed or calcified plaque, with any of the following: detectable plasma HIV RNA levels, duration of HAART use, history of AIDS, or current or nadir CD4+ T-cell count (all p >0.10) either in minimally or fully adjusted models. Higher nadir CD4 cell count was associated with lower prevalence of non-calcified plaque [PR=0.95(0.91,1.00)] in the fully adjusted model. There were significant associations seen with extent of plaque and HIV clinical parameters. CAC extent was positively associated with the presence of a detectable HIV RNA level [estimate=0.56(0.05,1.06)] and mixed plaque extent was positively associated with history of AIDS [estimate= 0.38(0.03,0.73)] in fully adjusted models. There were no other significant (p<0.05) associations between HIV clinical parameters and extent of plaque.
In contrast, multiple HIV clinical parameters were associated with the presence of coronary artery stenosis >50% (Table 4), which were not attenuated with the inclusion of traditional CAD risk factors. Longer duration of HAART use and lower nadir CD4+ T-cell count were both associated with the presence of coronary artery stenosis >50% in separate regression models. When both of these factors were placed in the same model, these associations all remained significantly associated with stenosis >50% [duration of HAART use (per year)- PR=1.08(1.01,1.16); nadir CD4+ T-cell count (per 100 cell increase)-PR=0.82(0.70,0.96)]. Similar results were seen when limiting the analyses to only those men with suppressed HIV RNA. Although the prevalence of ≥ 50% stenosis was much higher (PR=1.4) among those with detectable viral load and among those with a history of AIDS, they were not statistically significant. There was no association with current CD4+ T-cell count.
Table 4.
Predictors of Coronary Artery Stenosis ≥ 50% among HIV Seropositive Men
| Minimally adjusted model | Adjusted for CAD Risk Factors | |||
|---|---|---|---|---|
| PR (95% CI)* | P value | PR (95% CI)† | P value | |
| Detectable current HIV RNA > 50 copies/mL | 1.26(0.76,2.1) | 0.37 | 1.43(0.84,2.43) | 0.187 |
| Duration of HAART (yrs) | 1.09(1.02,1.17) | 0.007 | 1.09(1.02,1.17) | 0.007 |
| History of AIDS | 1.49(0.92,2.41) | 0.103 | 1.40(0.87,2.26) | 0.170 |
| Current CD4+ T cell count (per 100 cell increase) | 1.01(0.93,1.09) | 0.87 | 1.00(0.92,1.08) | 0.97 |
| Nadir CD4+ T cell count (per 100 cell increase) | 0.79(0.68,0.92) | 0.002 | 0.80(0.69,0.94) | 0.005 |
Adjusted for age, race, center, and cohort;
Adjusted for age, race, center, cohort, and CAD risk factors; Separate Poisson regression models were performed for each predictor variable; PR= prevalence ratio (HIV seropositive compared to HIV seronegative); CI= confidence interval. CAD risk factors include systolic blood pressure, antihypertensive medication use, diabetes medication use, fasting glucose, total cholesterol, HDL cholesterol, use of lipid-lowering medications, body mass index and smoking (pack-years). HAART= highly active antiretroviral therapy.
Discussion
In the MACS, we found a greater prevalence and extent of coronary atherosclerosis on coronary CT angiography among HIV-infected men, than among HIV-uninfected men. Coronary CT angiography allows identification of specific plaque composition. Non-calcified plaque prevalence and extent were each independently and positively associated with HIV positive serostatus even after adjusting for CAD risk factors. Non-calcified plaques may be more prone to rupture, leading to acute coronary syndromes (24, 25) though in this cohort there have been relatively few coronary clinical events identified to date. Among HIV-infected men, coronary artery stenosis greater than 50% was associated with lower nadir CD4+ T-cell count and longer treatment with HAART.
It is important to note that the MACS includes an HIV-uninfected control group drawn from the same reference population with a similar lifestyle, namely men who have sex with men (MSM), and this helps to minimize unmeasured potential confounding and selection bias. A smaller study of 102 HIV-infected men and women and 41 HIV-uninfected controls also found a greater number of coronary segments containing non-calcified plaque among HIV-infected patients (26). Our results confirm in a larger study a greater prevalence and extent of non-calcified plaque among HIV-infected men. The larger sample size allowed us to have enough power to also identify a significant interaction by age for non-calcified plaque. The prevalence of non-calcified plaque increased with advancing age in HIV-infected men, but not in uninfected men, which is also consistent with the higher prevalence in the infected men, observed as early as 50 years (Figure 1). Additional studies that include repeat, longitudinal, assessments of plaque in this cohort are needed to determine if non-calcified plaques calcify at a slower rate in HIV-infected men or if the incidence of new non-calcified plaques is increased.
We found positive associations between the presence of coronary artery stenosis >50% and both a lower nadir CD4+ T-cell count and greater years of highly active antiretroviral therapy (HAART) use. Low CD4+ T-cell count has been associated with carotid intima media thickness(IMT) (10) and carotid artery plaque (27), though these associations were not seen in some other studies (28). The SMART study demonstrated that continuous ART use reduced CAD events compared to interrupted ART (8). Coronary artery stenosis > 50% is an indication of advanced atherosclerosis. Lower nadir CD4+ T-cell count is likely a marker of longer duration of uncontrolled viremia in years prior to the initiation of effective anti-retroviral therapy. In addition, both lower nadir CD4+ T cell count and longer duration of HAART use are markers of longer duration of HIV infection and exposure to older formulations of ART, with greater potential adverse metabolic effects, although controlling for traditional CAD risk factors accounts for some of these metabolic effects. Our future studies will investigate associations between coronary atherosclerosis with specific ART regimens and with measures of inflammation and immune activation.
Our study has important strengths, including the use of coronary CT angiography, which allows detailed assessment of subclinical coronary atherosclerosis, and a large ethnically diverse participant population from a well characterized cohort that includes an appropriate HIV-uninfected control group. Limitations include the cross-sectional study design, inclusion of men and not women, and limitations inherent to observational studies. There were differences in some CVD risk factors between HIV-infected and uninfected men, for example more HIV-infected men reported smoking compared to HIV-uninfected men. We performed detailed assessment of potential confounders and performed analyses with and without adjustment for CAD risk factors. Therefore, the differences in plaque by HIV infection that persisted are not attributable to these population confounders. Some risk factor data were missing for a small number of men and were therefore imputed. We also performed risk factor adjusted models excluding these individuals and the results were essentially unchanged. Lastly for the outcome for which we found pronounced differences (non-calcified plaque), we performed sensitivity analyses and determined that unmeasured confounding would have to be extremely strong to attenuate the observed association.
The assessment of an association with non-calcified plaque was possible due to the use of contrast enhanced CT scans. Non-calcified plaque cannot be identified on non-contrast cardiac CT scans which are used to measure CAC. CAC is known to be a potent predictor of CAD events in the general population, and a CAC score of zero is generally associated with a very low risk for a CAD event (9). Future studies are needed to assess this risk in patients with HIV who may have more non-calcified plaque. CTA can also differentiate mixed plaque (plaque that has <50% calcification) from calcified plaque. Calcified plaque may reflect more advanced stable atherosclerosis, whereas mixed plaque, like non-calcified plaque may be more prone to rupture. Since this study is cross-sectional, future studies will be needed to determine whether there are differences in the progression of the atherosclerotic process in HIV patients, which might lead to a greater prevalence of non-calcified plaque.
In conclusion, non-calcified coronary artery plaque was more prevalent and extensive in HIV-infected men, suggesting increased risk for cardiovascular events. Men with more advanced HIV infection, as demonstrated by low nadir CD4+ T cell count and a greater number of years on HAART have a higher prevalence of clinically significant coronary stenosis > 50%. Additional studies are needed to identify how best to prevent progression of atherosclerosis in this unique population and correlation with future events. Although coronary CT angiography is not indicated as a screening test in asymptomatic individuals, these results emphasize the importance of assessing and modifying traditional cardiovascular risk factors in this population, especially in men with a history of a low nadir CD4+ T cell count.
Acknowledgements
Funding
The MACS CVD 2 study is funded by National Heart Lung and Blood Institute (NHLBI), RO1 HL095129-01 (Post), with additional support from UL1 RR 025005 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers (Principal Investigators) at The Johns Hopkins Bloomberg School of Public Health (Joseph B. Margolick), Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services (John P. Phair, Steven M. Wolinsky), University of California, Los Angeles (Roger Detels), and University of Pittsburgh (Charles R. Rinaldo), and the Data Center located at the Johns Hopkins Bloomberg School of Public Health (Lisa P. Jacobson). The MACS is funded by the National Institute of Allergy and Infectious Diseases (NIAID), with additional supplemental funding from the National Cancer Institute (NCI). UO1-AI-35042, UL1-RR025005, UO1-AI-35043, UO1-AI-35039, UO1-AI-35040, UO1-AI-35041. Website located at http://www.statepi.jhsph.edu/macs/macs.html.
We would like to thank Andrea Stronski for administrative support for this study and Adrian Dobs MD, MHS, Joseph Margolick, MD, and A. Richey Sharrett MD, PhD for their reviews of the manuscript draft and contributions to the MACS CVD study. Dr. Post had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.”
Data collection forms used as part of the core MACS visit are available on the MACS website at http://www.statepi.jhsph.edu/macs/macs.html. The protocol for this cardiovascular study and the computer code used to generate results reported in the published article are available upon request. Individual level MACS data are available upon request after review of a concept sheet by the MACS executive committee. Please contact Dr. Post at wpost@jhmi.edu.
References
- 1.May MT, Sterne JA, Costagliola D, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451. doi: 10.1016/S0140-6736(06)69152-6. [DOI] [PubMed] [Google Scholar]
- 2.Wada N, Jacobson LP, Cohen M, et al. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984–2008. Am J Epidemiol. 2013;177(2):116–125. doi: 10.1093/aje/kws321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 4.Mary-Krause M, Cotte L, Simon A, et al. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17:2479. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 5.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 6.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007 Jul;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozzette SA, Ake CF, Tam HK, et al. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 8.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 9.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 10.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 11.Currier JS, Kendall MA, Henry WK, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS. 2007;21(9):1137–1145. doi: 10.1097/QAD.0b013e32811ebf79. [DOI] [PubMed] [Google Scholar]
- 12.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingsley LA, Cuervo-Rojas J, Munoz A, et al. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS. 2008;22(13):1589–1599. doi: 10.1097/QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging. 2011;4(5):537–548. doi: 10.1016/j.jcmg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadi N, Nabavi V, Hajsadeghi F, et al. Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol. 2011;107(1):10–16. doi: 10.1016/j.amjcard.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 16.Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Kaslow RA, Ostrow DG, Detels R, et al. The multicenter AIDS cohort study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 18.Hacioglu Y, Gupta M, Choi T-Y, George RT, Deible CR, Jacobson L, et al. Use of Cardiact CT Angiography Imaging in an Epidemiology Study - the Methodology of the Multicenter AIDS Cohort Study Cardiovascular Disease Substudy. Anadolu Kardiyol Derg. 2013;13(3):207–214. doi: 10.5152/akd.2013.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, American Heart Association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 20.Pagali SR, Madaj P, Gupta M, et al. Interobserver variations of plaque severity score and segment stenosis score in coronary arteries using 64 slice multidetector computed tomography: a substudy of the ACCURACY trial. J Cardiovasc Comput Tomogr. 2010;4(5):312–318. doi: 10.1016/j.jcct.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004 Apr 1;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 22.Greenland S. Basic methods for sensitivity analysis of biases. Int J Epi. 1996;25(6):1107–1116. [PubMed] [Google Scholar]
- 23.Schafer JL. Analysis of Incomplete Multivariate Data. New York: Chapman & Hill; 1997. [Google Scholar]
- 24.Ahmadi N, Nabavi V, Hajsadeghi F, et al. Mortality incidence of patients with non-obstructive coronary artery disease diagnosed by computed tomography angiography. Am J Cardiol. 2011;107(1):10–16. doi: 10.1016/j.amjcard.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Motoyama S, Kondo T, Sarai M, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 26.Lo J, Abbara S, Shturman L, Soni A, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22(13):1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delaney JA, Scherzer R, Biggs ML, et al. Associations of antiretroviral drug use and HIV-specific risk factors with carotid intima-media thickness. AIDS. 2010;24(14):2201–2209. doi: 10.1097/QAD.0b013e32833d2132. [DOI] [PMC free article] [PubMed] [Google Scholar]