Abstract
Cobalt(III) 5, 10, 15-tris(4-tert-butylphenyl) corrole with a triphenylphosphine axial ligand and rhodium(III) 5,10,15,20-tetra(p-tert-butylphenyl)porphyrin are incorporated into plasticized poly(vinyl chloride) films to fabricate nitrite-selective bulk optodes via absorbance measurements. The resulting films yield sensitive, fast and fully reversible response toward nitrite with significantly enhanced nitrite selectivity over other anions including lipophilic anions such as thiocyanate and perchlorate. The selectivity patterns differ greatly from the Hofmeister series based on anion lipophilicity and are consistent with selectivity obtained with potentiometric sensors based on the same ionophores. The optical nitrite sensors are shown to be useful for detecting rates of emission of nitric oxide (NO) from NO releasing polymers containing S-nitroso-N-acetyl-penicillamine.
Keywords: Ion-selective polymeric film, Bulk optode, Nitrite, Nitric oxide
1. Introduction
Nitrite (NO2) is an important target analyte because of its presence in food and the environment, as well as within industrial, biological/physiological samples. Physiologically, nitrite can be reduced to nitric oxide (NO) through several pathways [1–3], making nitrite therapeutically valuable, including for its cytoprotective [4] and blood-pressure lowering effects [5–6]. Nitrite itself can act as a biological signalling molecule in terms of regulation of protein expression through the formation of S-nitrosothiols [7]. Therefore, an individual’s levels of endogenous nitrite in blood plasma may convey valuable diagnostic information. Additionally, nitrite has been used as a food preservative for many years, especially in cured meats and bacon, mainly to inhibit deterioration [8–9]. Moreover, ground waters, including well water and river water, contain nitrite that is likely to be present in drinking water derived from these sources [10–11]. Reactions of nitrite with secondary amines and amides from natural breakdown products of proteins form compounds known as nitrosamines which are regarded as carcinogenic [12–13]. Consequently, monitoring the concentration of nitrite in these sources is of great importance.
Many methods have been developed for nitrite detection [14], including spectroscopic [15–18], electrochemical [19–21] and chromatographic [22–25] methods. Among these, ionophore (ion-carrier) based chemical sensors, including ion-selective polymeric membrane electrodes (ISEs) and bulk optodes, are, simple, inexpensive and fully portable nitrite sensing devices. Plasticized polymeric membranes/films containing an appropriate lipophilic ionophore and an ion-exchanger (plus a chromoionophore for optodes) are widely used to create such ion selective sensors. For anion detection, in order to achieve selectivity for the target anion and avoid response based on ion lipophilicity, ionophores (most commonly hydrophobic metal ion-ligand complexes) with specific binding affinity to the primary target anion through axial ligation reactions are often incorporated into the polymeric membrane/film. Relatively few highly selective anion ionophores have been reported in comparison to cation ion carriers. However, in the case of nitrite, a number of potentially useful ionophores have been suggested to prepare potentiometric nitrite selective membrane electrodes. These include a vitamin B12 derivative [26–27], cobalt(III) tetraphenylporphyrins [28], palladium organophosphine [29], as well as rhodium(III) porphyrins and salophens [30]. Our group has also recently studied corrole-based Co(III) complexes as nitrite ionophores to prepare polymeric membrane electrodes [31], and that such Co(III) corrole species provide greatly enhanced nitrite response and selectivity.
Even fewer ionophore-based polymeric film type optical sensors (so-called “optodes”) for nitrite have been studied. Palladium organophosphines as charged carriers have been incorporated into polymeric films to fabricate optical sensors for nitrite detection in the absorbance mode [32]. Different film formulations were studied with different types of chromoionophores in the presence and absence of lipophilic ion additives depending on the charge of the chromoinophore. The Kopelman group fabricated fluorescence sensors by applying nitrite-selective polymeric films containing a Vitamin B12 derivative ionophore (charged carrier) with optical fibers [33]. An optical flow-through cell setup was integrated with the polymeric membrane optodes for nitrite detection in fluorescence mode using a Vitamin B12 derivative ionophore [34]. All above previously reported work involved charged carrier type ionophores, and it should be noted that significant interference from thiocyanate was observed.
Herein, we report on the use of a cobalt(III) corrole species, cobalt(III) 5,10,15-tris(4-tert-butylphenyl) corrole (Co-tBC) possessing triphenylphosphine as an axial ligand to stabilize this corrole species, as a neutral carrier to fabricate nitrite-selective bulk polymeric film type optodes. Films formulated with both neutral proton chromoionophores (chromoionophores that are neutral when deprotonated) and charged proton chromoionophores (chromoionophores that are charged when deprotonated) with appropriate amounts of lipophilic ion additives are evaluated in terms of nitrite response and selectivity. It is shown that sensors made with either neutral chromoionophores or charged chromoionophores have significantly improved nitrite response and selectivity over other anions, including thiocyanate and perchlorate. For comparison purposes, rhodium(III) 5,10,15,20-tetra(p-tert-butylphenyl)porphyrin (Rh-tBTPP) is also examined as a charged carrier within polymeric films for optical nitrite sensing. Sensors made with Co-tBC doped polymeric films are used to determine NO emission rates from polymer films containing S-nitroso-N-acetyl-penicillamine. Results are in excellent agreement with those obtained from the classic colorimetric method.
2. Experimental
2.1. Materials and reagents
Cobalt(III) 5,10,15-tris(4-tert-butylphenyl) corrole triphenylphosphine (Co-tBC) (see Fig. 1A) and rhodium(III) 5,10,15,20-tetra(p-tert-butylphenyl)porphyrin (Rh-tBTPP) (see Fig. 1B) were synthesized via previously reported procedures [30–31]. The 5,10,15-tris(4-tert-butylphenyl) corrole and 5,10,15,20-tetra(p-tert-butylphenyl)porphyrin were purchased from Frontier Scientific (Logan, UT, U.S.A.). Cobalt(II) acetate tetrahydrate (Co(OAc)2•4H2O), sodium acetate anhydrous (NaOAc) and ethanol (EtOH) were products of Sigma Aldrich (Milwaukee, WI, U.S.A.). Triphenylphosphine (PPh3) was obtained from TCI America (Portland, OR, U.S.A.). For Griess assay measurement, sulphonamide, N-(1-Naphthyl)ethylenediamine and hydrochloric acid were also obtained from Sigma Aldrich.
Fig. 1.
(A) Structure of Co(III) 5, 10, 15-tris(4-tert-butylphenyl) corrole with triphenylphosphine as the axial ligand (Co-tBC) and (B) Rh(III) 5, 10, 15, 20-tetra(p-tert-butylphenyl)porphyrin chloride (Rh-tBTPP).
For optical sensor film preparation, poly(vinyl chloride) (PVC), o-nitrophenyloctyl ether (o-NPOE), tridodecylmethylammonium chloride (TDMACl), chromoionophore I (9-(diethylamino)-5-(octadecanoylimino)-5H-benzo[a]phenoxazine (ETH 5294)), chromoionophore II (9-dimethylamino-5-[4-(16-butyl-2,14-dioxo-3,15-dioxaeicosyl)phenylimino]benzo[a]phenoxazine (ETH 2439)), chromoionophore III (9-(diethylamino)-5-[(2-octyldecyl)imino]benzo[a]phenoxazine (ETH 5350)), chromoionophore IV (5-octadecanoyloxy-2-(4-nitrophenylazo)phenol (ETH 2412)), chromoionophore V (9-(diethylamino)-5-(2-naphthoylimino)-5H-benzo[a]phenoxazine), chromoionophore VI (4′,5′-dibromofluorescein octadecyl ester (ETH 7075)), chromoionophore VII (9-dimethylamino-5-[4-(15-butyl-1,13-dioxo-2,14-dioxanonadecyl)phenylimino]benzo[a]phenoxazine (ETH 5418)) and anhydrous tetrahydrofuran (THF) were purchased from Fluka and used without further purification. For NO releasing film preparation, S-nitroso-N-acetyl-penicillamine (SNAP) was synthesized using a previously reported method [35]. N-Acetyl-DL-penicillamine (NAP), and methanol were purchased from Sigma Aldrich. The CarboSil polymer was purchased from the Polymer Technology Group (Berkeley, CA).
All salts for standard solutions were purchased from Sigma Aldrich and were used as received. Measurements were performed in 50 mM NaH2PO4/Na2HPO4 buffer solutions with pH 4.5 or 5.0. All solutions were prepared with 18.2 MΩ water (Milli-Q, Millipore Corporation, Billerica, MA, USA). Activity coefficients were calculated according to the two-parameter Debye-Hückel formalism of Meier [36].
2.2. Film preparation for bulk optodes
For optode thin film cocktail preparations, a total of 100 mg of components containing 10 or 20 mmol/kg of the ionophore, 10 mmol/kg of the neutral chromoionophore or 10 mmol/kg of the charged chromoionophore with 10 mmol/kg of TDMACl plus o-NPOE and PVC with a mass ratio of 2:1 were dissolved in 1 mL THF. To obtain the optical sensing thin films, 50 μL aliquots of the membrane cocktail were coated with a pipette onto glass slides (51 mm × 9 mm), and the remaining THF was allowed to evaporate completely in fume hoods before testing. The thickness of the formed thin film was determined to be ~ 5 μm [37–38]. The resulted film is transparent with no visible aggregation.
2.3. Absorbance measurements
All absorbance measurements were made using a PerkinElmer Lambda35 UV/Vis spectrophotometer. Before testing, film-coated glass slides were inserted into 1 cm cuvettes containing sample solutions to condition for 30 min. For each sample, a ratiometric method was used for calculation of α value (fraction of unprotonated chromoionophore) by recording the absorbance ratio of two peaks at two given wavelengths corresponding to the deprotonation peak and protonation peak of each chromoionophore. More specifically, for films prepared with chromoionophore I, ratios of absorbance values at 666 nm and 545 nm were measured for different sample solutions. The ratios of absorbance values at 668 nm and 526 nm, 648 nm and 512 nm, 428 nm and 568 nm, 665 nm and 566 nm, 472 nm and 536 nm and 677 nm and 550 nm were recorded for films formulated with chromoionophore II, III, IV, V, VI and VII, respectively. For calculation of α values, absorbance spectra of fully protonated and deprotonated chromoionophores were recorded by conditioning the optode membranes in 0.1 M HCl and 0.1 M NaOH solutions. All calculated curves were fitted to experimental points by adjusting Kcoex.
The sensor reversibility experiments were carried out by using one optical sensing film and changing the sample solution only. Noise due to opening the instrument chamber was removed from the absorbance tracings.
2.4. Preparation of SNAP-doped NO release film
The casting solution was prepared by dissolving 400 mg CarboSil in 3 mL THF. Ten wt% SNAP (44.5 mg) was then added into the polymer solution, and the mixture was stirred for 10 min. The film solution was cast into a Teflon ring (d=2.5cm) on a Teflon plate and dried overnight under ambient conditions, followed by 48 h of drying under vacuum to remove any additional solvent. The disk formed was evenly cut into 8 pieces and those pieces were divided into 2 portions, one portion containing 3 pieces and the other portion containing the remaining 5 pieces (to provide higher rate of NO production compared to 1st portion in given volume of solution phase). Since in the presence of oxygen within aqueous test solutions, nitric oxide is converted to nitrite with a stoichiometry of 1:1 [39], the concentration of nitrite after a given time can be used to calculate the total NO released from the film. To convert NO released from the polymer film to nitrite, the portions with 3 film pieces and that with 5 film pieces were incubated in 50 mM phosphate buffer solution (4 mL, pH 4.5) for 12 h under 37 °C in two separate amber vials and the resulting solutions were labeled as sample # 1 and sample # 2, respectively.
2.5. Determination of NO
To determine the NO level in the samples by using the conventional colorimetric Griess method [40–41], the Griess assay reagent solutions were prepared as follows: 21.52 mg sulphonamide was dissolved in 10 mL water to yield a 12.5 mM solution; concentrated HCl was diluted to 6 M; 32.4 mg N-(1-Naphthyl)ethylenediamine was dissolved in 10 mL water to give a solution with the concentration of 12.5 mM. Both the sulphonamide and N-(1-Naphthyl)ethylenediamine solutions were stored at 4 °C and the latter was kept away from light. For sample measurements, 100 μL of a sample solution was added into a cuvette and diluted with 700 μL phosphate buffer (50 mM, pH 4.5), followed by addition of the Griess reagent (100 μL sulphonamide, 100 μL HCl and 100 μL N-(1-Naphthyl)ethylenediamine). After color development for 15 min at room temperature, the absorbance at 540 nm was measured. Results for each sample solution were compared to absorbance values obtained using nitrite standard solutions treated in the same manner (to obtain the calibration curve). For measurements via the new Co-tBC based polymeric optical sensors, the same sample solution dilution procedure was performed and the diluted sample was detected by the o-NPOE plasticized PVC membrane optodes doped with 20 mmol/kg Co-tBC and 10 mmol/kg chromoionophore I in the absorbance mode. All tests were conducted in triplicate.
3. Results and discussion
3.1. Anion carrier optode response mechanism
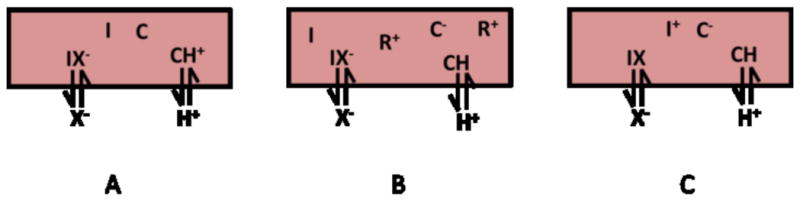
Nitrite-selective bulk optode responses are based on the coextraction of nitrite and protons into the polymer film containing both the nitrite selective ionophore, which may be neutral or charged, and a proton-sensitive chromoionophore in the presence or absence of lipophilic cationic sites (R+) depending on the neutrality of the membrane. When a neutral ionophore is employed, either a neutral chromoionophore (see Fig. 2A) or a charged chromoionophore with a proper amount of lipophilic cationic sites (see Fig. 2B) can be incorporated into the polymeric film. When a charged ionophore is present, a charged chromoionophore is required to maintain membrane neutrality in the organic polymeric phase (see Fig. 2C). The coextraction processes can be summarized as simple net ionic equations (1):
| (1) |
| (2) |
| (3) |
where X− is the analyte anion, I is the neutral carrier, I+ is the charged carrier ionophore, C is a neutral chromoionophore and C− is a charged chromoionophore. Taking mass balance and charge balance into account, the above three net ionic equations can be rewritten in an analytically more useful form to relate proton (aH+) and ion activities (aX−) to optical responses and membrane parameters as follows [32, 42–43]:
| (4) |
| (5) |
| (6) |
where Kcoex is the coextraction constant, CT is the total amount of chromoionophore, IT is the total amount of ionophore, and RT is the total amount of lipophilic ionic sites. The value of 1−α is the protonation degree of the chromoionophore, which is defined as the ratio of the chromoionophore concentration in its protonated form to its total concentration and can be connected to the apparent absorbance values (see equation 7), where A is the absorbance of the chromoionophore for a given equilibrium condition, and Aprot and Adeprot are the absorbance values at fully protonated and deprotonated forms respectively. In a buffered system, 1−α is only dependent on the analyte ion activity since the proton activity is constant.
Fig. 2.
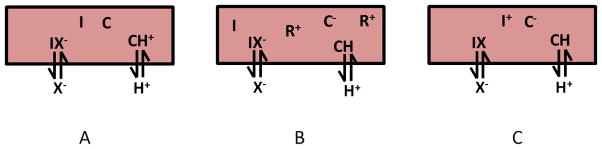
Optical sensing schemes for nitrite ion with a neutral carrier coupled with (A) a neutral chromoionophore, (B) a charged chromoionophore and lipophilic ionic sites and (C) a charged carrier coupled with a charged chromoionophore. Note: C=chromoionophore, I = ionophore, X− = nitrite or other anion, R+ = lipophilic cationic site additive.
| (7) |
3.2. Cobalt(III) corrole as a neutral ionophore optical nitrite sensing
3.2.1. Optodes response
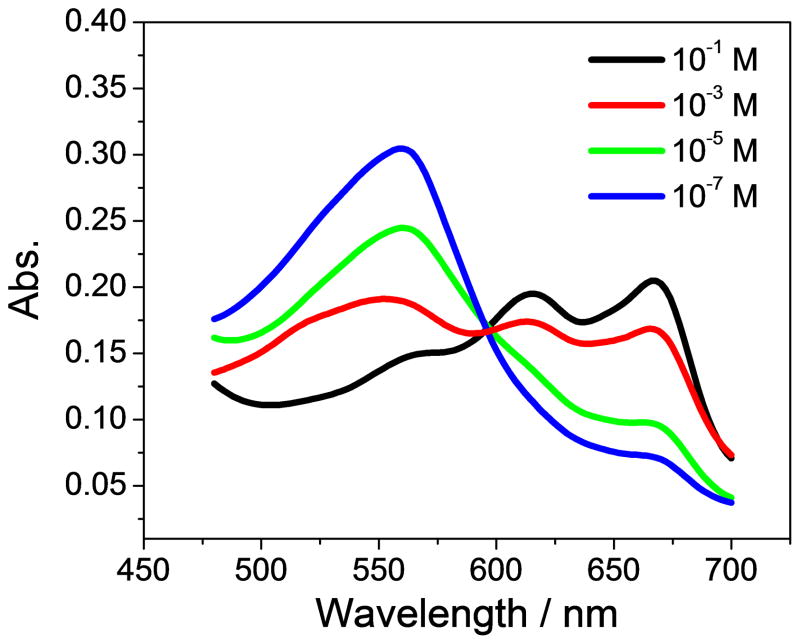
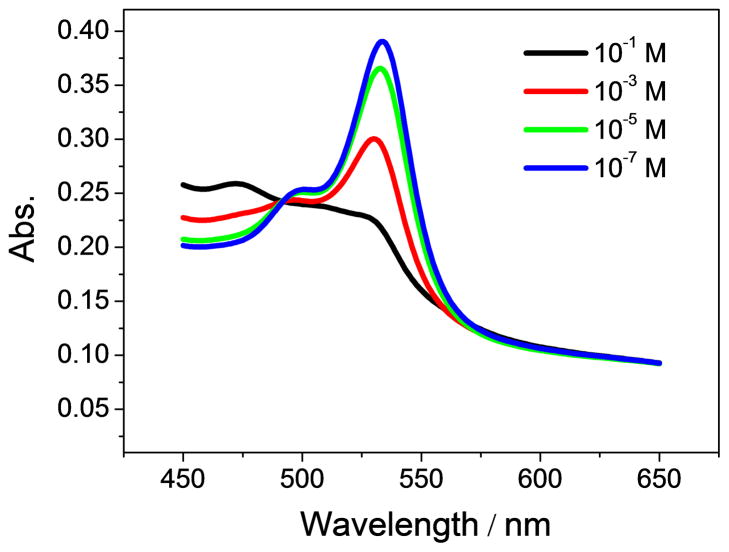
The response mechanism of the anion carrier largely determines the composition of the optical sensing films. It has been reported and determined by potentiometry that Co-tBC is a neutral carrier within polymeric membrane electrodes [31]. Therefore, to maintain membrane neutrality, a neutral chromoionophore or a charged chromoionophore with the appropriate amount of lipophilic ionic sites should be employed together with the neutral ionophore in polymeric film optodes. In the presence of a neutral chromoionophore and neutral carrier, the binding of nitrite and Co-tBC is accompanied by a concomitant protonation of the chromoionophore to maintain the charge neutrality within the film. As shown in Figure 3A, as the nitrite concentration increases and more nitrite enters the film to bind with the ionophore, more protons are coextracted to protonate chromoionophore I, resulting in the absorbance change (increase of protonation peak at 666 nm and decrease of deprotonation peak at 545 nm). In contrast, if a charged chromoionophore is incorporated into the polymeric membrane, a proper amount of lipophilic ionic sites should also be introduced to act as the initial counterion to the chromoionophore. Similarly, an increase in nitrite level in the sample phase is accompanied by an increased degree of protonation of the chromoionophore, as indicated by a spectra change (increase of protonation peak at 472 nm and decrease of deprotonation peak at 536 nm). As the binding of nitrite and the ionophore occurs, the ion-ionophore complex is charged while the protonated chromoionophore becomes neutral. In the presence of lipophilic ionic additives, the electroneutrality of the sensing film is still ensured. The spectra changes observed in Fig. 3 indicate Co-tBC can successfully function as a nitrite-selective ionophore when incorporated in a polymeric membrane to fabricate sensitive bulk optodes for nitrite detection.
Fig. 3.
Absorption spectra of nitrite optical sensing films prepared with a PVC-NPOE cocktail containing (A) 20 mmol/kg ionophore, 10 mmol/kg chromoionophore I; and (B) 20 mmol/kg ionophore, 10 mmol/kg chromoionophore VI and 10 mmol/kg TDMACl, measured at varying nitrite levels, ranging from 0.1 μM to 0.1 M, in 50 mM phosphate buffer solutions (pH 4.5).
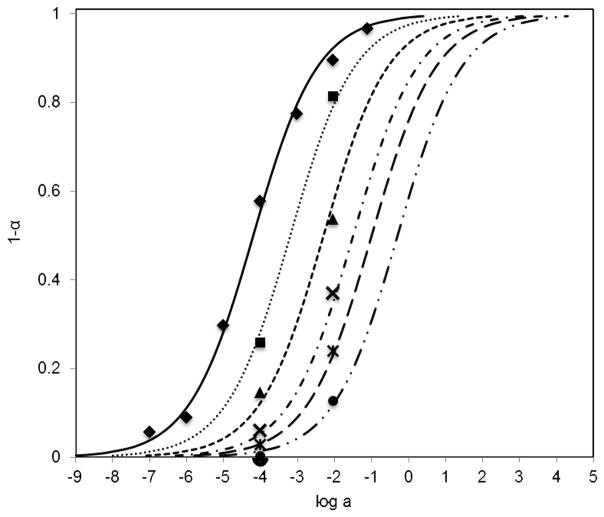
The response calibration curves toward nitrite are shown in Figure 4. The solid lines are the theoretical curves according to equation 4 and the points (□, ■ and ●) are from experimental data, which fit well with the theoretical curves. Since the film composition and buffer pH can substantially affect membrane equilibrium and sensor response characteristics, optodes made with two different film compositions were initially studied as well as two different pH buffered test solutions. Figure 4 illustrates that when the ionophore amount is doubled (□), almost all the chromoionophores are protonated in the presence of a high concentration of nitrite, while only ~ 90% of the chromoionophores are protonated when there is less ionophore in the membrane (●) with the response range shifted to a higher concentration range. Comparing the two buffer solutions, the pH 4.5 yields higher α values than pH 5.0, indicating a better sensor response with increased proton activity. However, if the buffer pH value is < pH 4.5, nitrite will start to be protonated in the sample phase, resulting in lower nitrite activity.
Fig. 4.
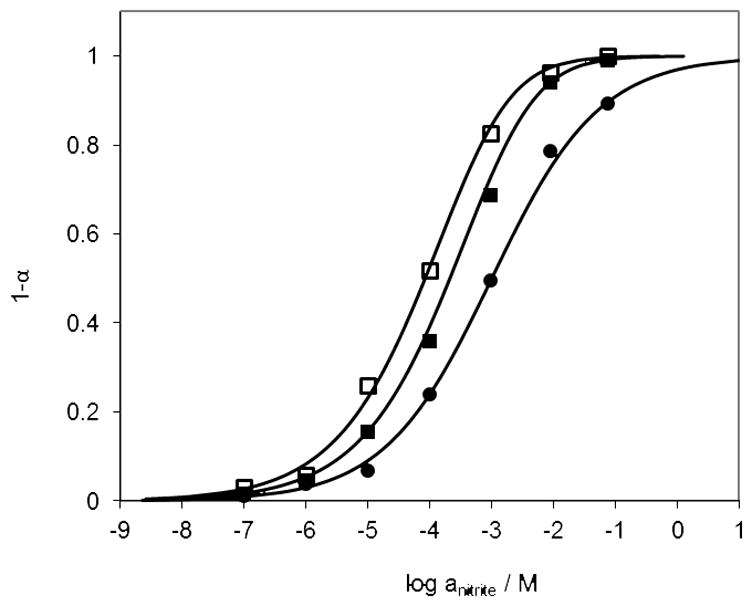
Response of optical nitrite sensing polymeric films to solutions prepared in 50 mM phosphate buffer solutions with different nitrite concentrations as function of sample pH and different amount of ionophore in film. Films were o-NPOE plasticized PVC doped with (□) 20 mmol/kg ionophore and 10 mmol/kg chromoionophore I (buffer pH 4.5); (■) 20 mmol/kg ionophore and 10 mmol/kg chromoionophore I (buffer pH 5.0); and (●) 10 mmol/kg ionophore and 10 mmol/kg chromoionophore I (buffer, pH 4.5).
3.2.2. Selectivity
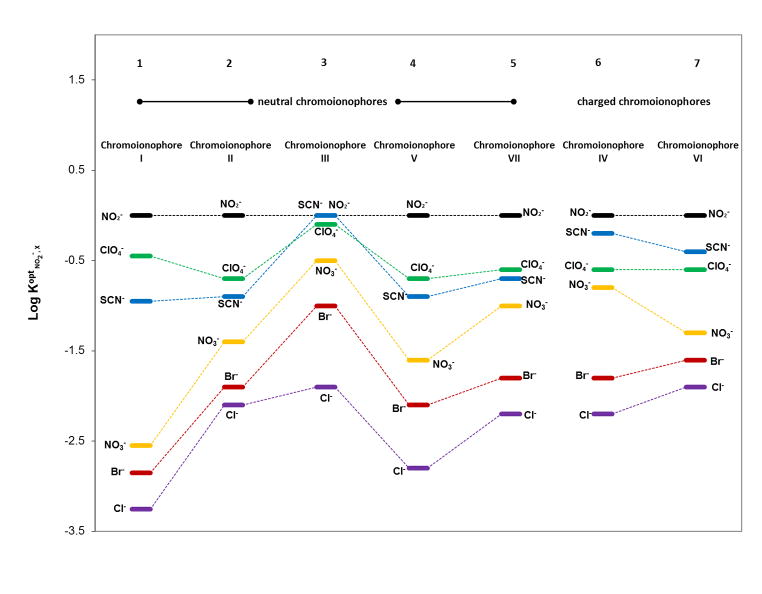
Experimental selectivity coefficients for different films can be estimated from the horizontal distance of two optical response calibration curves at a given degree of protonation, 1−α, which was 0.5 in this study. Since the response of ionophore-based ion-selective sensors (both optical and potentiometric) is highly dependent on the ionophore, selectivity patterns obtained from bulk optodes formulated with Co-tBC are similar to the selectivity patterns observed previously with potentiometric anion sensors employing the same ionophore, and completely different than the classical Hofmeister series. For example, optical sensor 1 (20 mmol/kg ionophore and 10 mmol/kg chromoionophore I) yields the same selectivity pattern as potentiometric polymer membrane electrodes formulated with 1 wt% ionophore and 10 mol% TDMACl (NO2−> ClO4− > SCN− > NO3− > Br− > Cl−), but is more selective to nitrite (with more negative log selectivity coefficient values ( )) over SCN−, ClO4− and NO3− [31]. With the proper amount of ionophore, type and amount of chromoionophore, lipophilic ion additives, and sample phase pH, the sensors also exhibit good nitrite selectivity over the most lipophilic ones (ClO4− and SCN−). Generally, polymer film optodes (1–5, Fig. 5) doped with neutral chromoionophores have better selectivity towards nitrite than those with charged chromoionophores (6–7, Fig. 5), especially regarding interference from lipophilic anions. A possible reason for this is that the incorporation of lipophilic ionic sites increases the ionic strength of the film, which favors other anions that can compete with nitrite, leading to worse selectivity for nitrite over other anions. Since the response of anion-selective optodes is based on a carrier-mediated coextraction equilibrium, it relates to both the complex formation constant of the ionophore and the proton-binding affinity of the chromoionophore in the film (pKa) [37]. Membrane optodes containing chromoionophores with higher pKa values (order of pKa values: chromoionophore I > V > II > VII > III [44–45]) generally have better selectivity, and this can be seen in Figure 5.
Fig. 5.
Logarithm of optical selectivity coefficients (relative to nitrite) of Co-tBC (20 mmol/kg)-based polymer film optodes doped with different chromoionophores in the presence or absence of TDMACl. Polymeric films were doped with (1) 10 mmol/kg chromoionophore I; (2) 10 mmol/kg chromoionophore II; (3) 10 mmol/kg chromoionophore III; (4) 10 mmol/kg chromoionophore V; (5) 10 mmol/kg chromoionophore VII; (6) 10 mmol/kg chromoionophore IV and 10 mmol/kg TDMACl; (7) chromoionophore VI and 10 mmol/kg TDMACl. Measurements were carried out in 50 mM phosphate buffer, pH 4.5.
3.2.3. Response time and reversibility
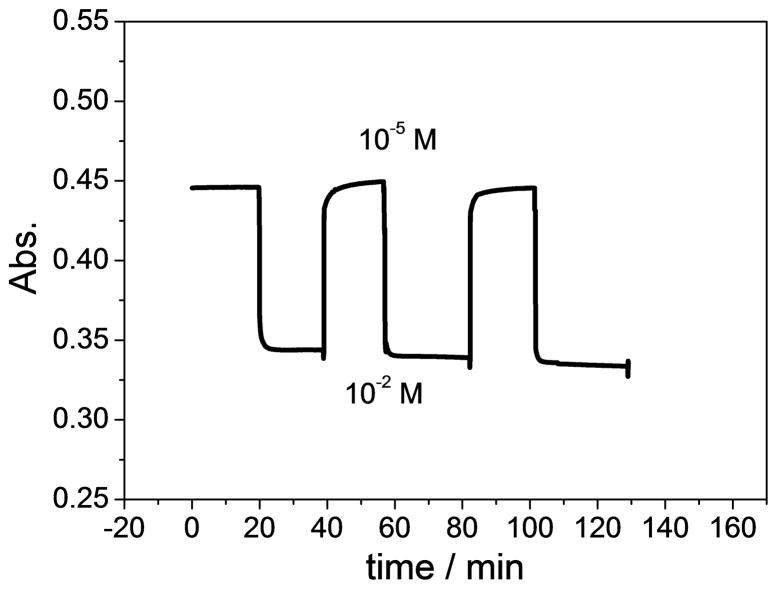
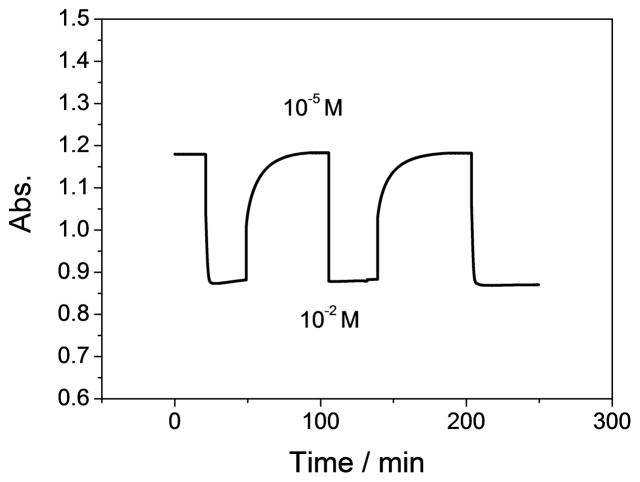
The response time of the optical sensor is measured as the time require for the films to reach 95% of the equilibrium response. The dynamic response time is shown in Figure 6 and is found to be less than 1 min, with excellent signal reproducibility of the nitrite optode film when switching between sample solutions containing 10−5 M and 10−2 M sodium nitrite. The response time observed is comparable to the previously reported organopalladium-based membrane optodes tested in the absorbance mode [32] and is much shorter than optodes based on the same ionophore tested in the fluorescence mode [46] and optodes based on electropolymerized cobalt(II) porphyrin [47].
Fig. 6.
Reversibility of optical nitrite response in phosphate buffer solution (pH 4.5) for polymer film optodes formulated with 20 mmol/kg Co-tBC and 10 mmol/kg chromoionophore I in o-NPOE plasticized PVC, when changing nitrite concentration back and forth between 10-2 M and 10-5 M. Absorbance values were measured at 545 nm.
Nitrite optode thin films show no visible change within two weeks if stored in dark without contacting any aqueous solution. A few crystals are formed after two weeks indicating a change in film composition. If films are in contact with aqueous solution for a certain amount of time (usually ~ 2 days) instead of being stored in dark and dry condition, it was found that the thin film can be easily peeled off from the glass slide.
3.3. Rhodium(III) porphyrin as the charged ionophore
Rhodium(III) porphyrins have recently been discovered to exhibit excellent performance in terms of nitrite selectivity over other common anions [30]. Rhodium is from the same group as cobalt and exhibits similar axial ligation chemistry, although not exactly the same. Unlike corroles, when complexed to metal(III) ions, porphyrins are −2 charged and the resulting metal complex functions as a charged carrier when incorporated into polymeric films. Fabricating polymeric optodes by employing Rh(III) porphyrins as ionophores requires that a charged chromoionophore be present in the film to maintain charge neutrality (see Fig. 2C). As shown in Figure 7, sensors formulated with 10 mmol/kg Rh-tBTPP and 10 mmol/kg chromoionophore VI show very sensitive and selective response to nitrite over the range of 10−7 to 10−2 M. As shown in Figure 7, The selectivity pattern deviates substantially from the Hofmeister selectivity pattern.. Sensors prepared with the Rh(III) porphyrin exhibit selectivity to nitrite over other anions including more lipophilic perchlorate and thiocyanate ( : SCN−, −1; ClO4−, −1.9; NO3−, −2.7; Br−, −3.2; Cl−, −3.9), which is consistent with the selectivity coefficients data observed via potentiometry using an ion-selective membrane electrode. The signal reproducibility is shown in Figure 8, with a very reversible optical response when nitrite concentration is switched between 10−5 and 10−2 M. The response time of the optode, t95, is found to be within 1 min with a longer recovery time of ~ 8min, indicating strong binding affinity between nitrite and Rh-tBTPP, indicating a larger on-rate constant and a much smaller off-rate constant. The Rh-tBTPP-based bulk optodes provide better selectivity to nitrite over ClO4− compared to Co-tBC-based optical sensors (see above, Fig. 5), while Co-tBC-based sensors are more selective to nitrite over NO3− than Rh-tBTPP-based sensors. Comparable selectivity over other anions (SCN−, Br−, Cl−) is observed by sensors prepared with both ionophores.
Fig. 7.
Optical response of o-NPOE-PVC sensing film doped with 10 mmol/kg Rh-tBTPP and 10 mmol/kg chromoionophore VI toward various anions measured in 50 mM phosphate buffer, pH 4.5: (●) chloride, (*) bromide, (×) nitrate, (▲) perchlorate, (■) thiocyanate, (◆) nitrite.
Fig. 8.
Reversibility of potentiometric response to nitrite in phosphate buffer solution (pH 4.5) for optical sensing films formulated with 10 mmol/kg Rh-tBTPP and 10 mmol/kg chromoionophore VI in o-NPOE plasticized PVC, when changing nitrite concentration back and forth between 10-2 M and 10-5 M. Absorbance measured at 536 nm.
3.4. Nitric oxide detection
To establish the usefulness of optical sensors made with polymeric membranes prepared with Co-tBC (20 mmol/kg) and chromoionophore I (10 mmol/kg), the determination of nitrite converted from nitric oxide released by S-nitroso-N-acetyl-penicillamine-doped CarboSil films were carried out and compared with the conventional Griess colorimetric assay method. In oxygen-containing aqueous solution, NO can be oxidized to nitrite with a stoichiometry of 1:1 [39]. Thus, the concentration of nitrite in the soaking solution can provide information about the amounts of NO released from such polymeric films. Such NO release polymers are very useful biomedically to coat various devices to prevent clotting and infection [35, 48–50]. As shown in Table 1, the results obtained for NO released from SNAP-doped films with the polymeric membrane nitrite optode are in excellent agreement with the results obtained using the colorimetric Griess method for the two samples examined. The biggest advantage of the optical sensor over the Griess assay method is that one sensor can be used multiple times (owing to the reversibility), and there are no reagents that need to be made fresh each day for analytical measurements. For real sample applications in the case of detecting NO gas production, to prevent possible interfering ions present, the sample can be purged with nitrogen gas to sweep the NO to a recipient buffer solution where it would form nitrite and the optical sensor would be used to determine nitrite levels in that recipient solution. Another option to correct for any interference from lipophilic anion species such as perchlorate or thiocyanate that might be present in a sample along with nitrite, would be to utilize a second optical film sensor composed of the a lipophilic quaternary ammonium salt anion-exchanger (e.g., TDMAC) along with a pH indicator that would be negatively charged in deprotonated state. Such films will exhibit the greatest optical response to lipophilic anions and hence the presence of interferences could be accounted for when using the new nitrite selective optical sensing films described here.
Table 1.
Results of NO release rates from SNAP-doped CarboSil film over 12 h period by using both conventional Griess assay and polymeric film-based nitrite selective bulk optodes based the on Co-tBCionophore (n=3).
| Sample | Optical Sensors | Griess Assay | ||
|---|---|---|---|---|
|
| ||||
| [NO2−] (mol•L−1) | NO emission rate (mol•min−1) | [NO2−] (mol•L−1) | NO emission rate (mol•min−1) | |
| #1 | 1.91±0.09×10−4 | 1.06±0.05×10−9 | 1.96±0.06×10−4 | 1.09±0.02×10−9 |
| #2 | 3.37±0.1×10−4 | 1.88±0.08×10−9 | 3.29±0.03×10−4 | 1.82±0.08×10−9 |
4. Conclusions
In summary, both the cobalt(III) 5,10,15-tris(4-tert-butylphenyl) corrole triphenylphosphine and rhodium(III) 5,10,15,20-tetra(p-tert-butylphenyl)porphyrin ionophores can function as nitrite-selective ionophores in polymeric film type optical sensors and provide similar anion selectivity patterns with previously reported polymeric membrane electrodes [30–31]. For the Co-tBC (neutral carrier)-based optical sensors, films formulated with neutral chromoionophores show better selectivity to nitrite than charged chromoionophores due to the presence of lipophilic ion additives. Sensors doped with chromoionophores I and V (see Fig. 5) provide greatly enhanced nitrite selectivity to other anions including the most lipophilic anions, perchlorate and thiocyanate. Chromoionophores with different pKa values also influence sensor performance. The higher the pKa, of the chomoionophore the better the response and selectivity observed for nitrite. It is also observed that the lower the pH value of the buffer, the more sensitive the sensor is. Sensor response ranges and detection limits can be tuned by changing film formulations and test buffer pH values. For Rh-tBTPP (charged carrier)-based optical sensors, a charged chromoionophore must be employed in the sensing membrane to maintain charge neutrality. Selectivity patterns and coefficients obtained from the Rh-tBTPP-based optical sensors are in excellent agreement with those obtained when using same ionophore to prepare potentiometric membrane electrodes[30]. Sensors based on both of the two ionophores have fast response and recovery times in response to changes in nitrite concentration. Results also demonstrate the potential for immediate application of sensors in quantitating the rates of nitric oxide release from polymeric films doped with various NO donors, with data for the new optical sensors correlating well with the classical Griess assay for determining nitrite levels derived from the liberated NO.
Highlights.
We examine cobalt(III) corroles and rhodium(III) porphyrins as ionophores in polymeric films for optical sensors to detect nitrite.
Different types of proton chromoionophores are evaluated to optimize nitrite response.
Selectivity over lipophilic anions such as perchlorate and thiocyanate is observed.
Both ionophores yield optical sensors that are fully reversible.
The cobalt(III) corrole based sensor is employed to determine nitric oxide emission rates from NO donor doped polymers with good accuracy.
Acknowledgments
We greatly appreciate the support from National Institutes of Health for this work (Grant EB-000784).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zweier JL, Wang PH, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric-oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg JON, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans - measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin N, Odriscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502–502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 4.Duranski MR, Greer JJM, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang XD, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Investig. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro D, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive NO-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 6.Tsuchiya K, Takiguchi Y, Okamoto M, Izawa Y, Kanematsu Y, Yoshizumi M, Tamaki T. Malfunction of vascular control in lifestyle-related diseases: Formation of systemic hemoglobin-nitric oxide complex (HbNO) from dietary nitrite. J Pharmacol Sci. 2004;96:395–400. doi: 10.1254/jphs.fmj04006x3. [DOI] [PubMed] [Google Scholar]
- 7.Bryan N, Fernandez B, Bauer S, Garcia-Saura M, Milsom A, Rassaf T, Maloney R, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 8.Santos WJR, Lima PR, Tanaka AA, Tanaka SMCN, Kubota LT. Determination of nitrite in food samples by anodic voltammetry using a modified electrode. Food Chem. 2009;113:1206–1211. [Google Scholar]
- 9.Honikel K-O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008;78:68–76. doi: 10.1016/j.meatsci.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Ward MH, Cerhan JR, Colt JS, Hartge P. Risk of non-hodgkin lymphoma and nitrate and nitrite from drinking water and diet. Epidemiology. 2006;17:375–382. doi: 10.1097/01.ede.0000219675.79395.9f. [DOI] [PubMed] [Google Scholar]
- 11.Binghui Z, Zhixiong Z, Jing Y. Ion chromatographic determination of trace iodate, chlorite, chlorate, bromide, bromate and nitrite in drinking water using suppressed conductivity detection and visible detection. J Chromatogr A. 2006;1118:106–110. doi: 10.1016/j.chroma.2006.01.139. [DOI] [PubMed] [Google Scholar]
- 12.Fiddler W, Pensabene JW, Piotrowski EG, Phillips JG, Keating J, Mergens WJ, Newmark HL. Inhibition of formation of volatile nitrosamines in fried bacon by the use of cure-solubilized α-tocopherol. J Agric Food Chem. 1978;26:653–656. doi: 10.1021/jf60217a046. [DOI] [PubMed] [Google Scholar]
- 13.Tricker A. N-nitroso compounds and man: sources of exposure, endogenous formation and occurrence in body fluids. Eur J Cancer Prev. 1997;6:226–268. [PubMed] [Google Scholar]
- 14.Moorcroft MJ, Davis J, Compton RG. Detection and determination of nitrate and nitrite: a review. Talanta. 2001;54:785–803. doi: 10.1016/s0039-9140(01)00323-x. [DOI] [PubMed] [Google Scholar]
- 15.Senra-Ferreiro S, Pena-Pereira F, Lavilla I, Bendicho C. Griess micro-assay for the determination of nitrite by combining fibre optics-based cuvetteless UV-Vis micro-spectrophotometry with liquid-phase microextraction. Anal Chim Acta. 2010;668:195–200. doi: 10.1016/j.aca.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Tomé MJ, Esquembre R, Mallavia R, Mateo CR. Development of a dual-analyte fluorescent sensor for the determination of bioactive nitrite and selenite in water samples. J Pharm Biomed Anal. 2010;51:484–489. doi: 10.1016/j.jpba.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Helaleh MIH, Korenaga T. Fluorometric determination of nitrite with acetaminophen. Microchem J. 2000;64:241–246. [Google Scholar]
- 18.Lapat A, Székelyhidi L, Hornyák I. Spectrofluorimetric determination of 1,3,5-trinitro-1,3,5-triazacyclohexane (Hexogen, RDX) as a nitramine type explosive. Biomed Chromatogr. 1997;11:102–104. doi: 10.1002/(SICI)1099-0801(199703)11:2<102::AID-BMC660>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Shariar SM, Hinoue T. Simultaneous voltammetric determination of nitrate and nitrite ions using a copper electrode pretreated by dissolution/redeposition. Anal Sci. 2010;26:1173–1179. doi: 10.2116/analsci.26.1173. [DOI] [PubMed] [Google Scholar]
- 20.Muchindu M, Waryo T, Arotiba O, Kazimierska E, Morrin A, Killard AJ, Smyth MR, Jahed N, Kgarebe B, Baker PGL, Iwuoha EI. Electrochemical nitrite nanosensor developed with amine- and sulphate-functionalised polystyrene latex beads self-assembled on polyaniline. Electrochim Acta. 2010;55:4274–4280. [Google Scholar]
- 21.Doherty AP, Stanley MA, Leech D, Vos JG. Oxidative detection of nitrite at an electrocatalytic [Ru(bipy)2poly-(4-vinylpyridine)10Cl]Cl electrochemical sensor applied for the flow injection determination of nitrate using a Cu/Cd reductor column. Anal Chim Acta. 1996;319:111–120. [Google Scholar]
- 22.Kodamatani H, Yamazaki S, Saito K, Tomiyasu T, Komatsu Y. Selective determination method for measurement of nitrite and nitrate in water samples using high-performance liquid chromatography with post-column photochemical reaction and chemiluminescence detection. J Chromatogr A. 2009;1216:3163–3167. doi: 10.1016/j.chroma.2009.01.096. [DOI] [PubMed] [Google Scholar]
- 23.Malik AK, Faubel W. Capillary electrophoretic determination of tetramethylthiuram disulphide (thiram) Anal Lett. 2000;33:2055–2064. [Google Scholar]
- 24.Greenway GM, Haswell SJ, Petsul PH. Characterisation of a micro-total analytical system for the determination of nitrite with spectrophotometric detection. Anal Chim Acta. 1999;387:1–10. [Google Scholar]
- 25.Liu Z, Xi X, Dong S, Wang E. Liquid chromatography-amperometric detection of nitrite using a polypyrrole modified glassy carbon electrode doped with tungstodiphosphate anion. Anal Chim Acta. 1997;345:147–153. [Google Scholar]
- 26.Schulthess P, Ammann D, Krautler B, Caderas C, Stepanek R, Simon W. Nitrite-selective liquid membrane-electrode. Anal Chem. 1985;57:1397–1401. [Google Scholar]
- 27.Stepanek R, Krautler B, Schulthess P, Lindemann B, Ammann D, Simon W. Aquocyanocobalt(III)-hepta(2-phenylethyl)-cobyrinate as a cationic carrier for nitrite-selective liquid-membrane electrodes. Anal Chim Acta. 1986;182:83–90. [Google Scholar]
- 28.Malinowska E, Meyerhoff ME. Role of axial ligation on potentiometric response of Co(III) tetraphenylporphyrin-doped polymeric membranes to nitrite ions. Anal Chim Acta. 1995;300:33–43. [Google Scholar]
- 29.Badr IHA, Meyerhoff ME, Hassan SSM. Potentiometric anion selectivity of polymer membranes doped with palladium organophosphine complex. Anal Chem. 1995;67:2613–2618. doi: 10.1021/ac00111a019. [DOI] [PubMed] [Google Scholar]
- 30.Pietrzak M, Meyerhoff ME. Polymeric membrane electrodes with high nitrite selectivity based on rhodium(III) porphyrins and salophens as ionophores. Anal Chem. 2009;81:3637–3644. doi: 10.1021/ac900092f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S, Meyerhoff ME. Study of Cobalt(III) corrole as the neutral ionophore for nitrite and nitrate detection via polymeric membrane electrodes. Electroanalysis. 2013;25:2579–2585. doi: 10.1002/elan.201300400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badr IHA. Nitrite-selective optical sensors based on organopalladium ionophores. Anal Lett. 2001;34:2019–2034. [Google Scholar]
- 33.Barker SLR, Shortreed MR, Kopelman R. Anion selective optodes: development of a fluorescent fiber optic sensor for the determination of nitrite activity. Proc SPIE. 1996;2836:304–310. [Google Scholar]
- 34.Demuth C, Spichiger UE. Response function and analytical parameters of nitrite-selective optode membranes in absorbance and fluorescence mode. Anal Chim Acta. 1997;355:259–268. [Google Scholar]
- 35.Brisbois EJ, Handa H, Major TC, Bartlett RH, Meyerhoff ME. Long-term nitric oxide release and elevated temperature stability with S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As polymer. Biomaterials. 2013;34:6957–6966. doi: 10.1016/j.biomaterials.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meier PC. Two-parameter debye-hückel approximation for the evaluation of mean activity coefficients of 109 electrolytes. Anal Chim Acta. 1982;136:363–368. [Google Scholar]
- 37.Bakker E, Bühlmann P, Pretsch E. Carrier-based ion-selective electrodes and bulk optodes. 1. general characteristics. Chem Rev. 1997;97:3083–3132. doi: 10.1021/cr940394a. [DOI] [PubMed] [Google Scholar]
- 38.Xie L, Qin Y, Chen H-Y. Polymeric optodes based on upconverting nanorods for fluorescent measurements of pH and metal ions in blood samples. Anal Chem. 2012;84:1969–1974. doi: 10.1021/ac203003w. [DOI] [PubMed] [Google Scholar]
- 39.Ignarro LJ, Fukuto JM, Griscavage JM, Rogers NE, Byrns RE. Oxidation of nitric oxide in aqueous solution to nitrite but not nitrate: comparison with enzymatically formed nitric oxide from L-arginine. Proc Natl Acad Sci USA. 1993;90:8103–8107. doi: 10.1073/pnas.90.17.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griess P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt „Ueber einige Azoverbindungen. Ber Dtsch Chem Ges. 1879;12:426–428. [Google Scholar]
- 41.Shinn MB. Colorimetric method for determination of nitrate. Ind Eng Chem, Anal Ed. 1941;13:33–35. [Google Scholar]
- 42.Seiler K, Simon W. Theoretical aspects of bulk optode membranes. Anal Chim Acta. 1992;266:73–87. [Google Scholar]
- 43.Bakker E, Simon W. Selectivity of ion-sensitive bulk optodes. Anal Chem. 1992;64:1805–1812. [Google Scholar]
- 44.Qin Y, Bakker E. Quantitive binding constants of H+-selective chromoionophores and anion ionophores in solvent polymeric sensing membranes. Talanta. 2002;58:909–918. doi: 10.1016/s0039-9140(02)00405-8. [DOI] [PubMed] [Google Scholar]
- 45.Liu X, Qin Y. Ion-exchange reaction of silver(I) and copper(II) in optical sensors based on thiaglutaric diamide. Anal Sci. 2008;24:1151–1156. doi: 10.2116/analsci.24.1151. [DOI] [PubMed] [Google Scholar]
- 46.Mohr GJ, Wolfbeis OS. Optical nitrite sensor based on a potential-sensitive dye and a nitrite-selective carrier. Analyst. 1996;121:1489–1494. [Google Scholar]
- 47.Yang S-T, Bachas LG. Fiber optic chemical sensor for nitrite based on an electropolymerized cobaltporphyrin film. Talanta. 1994;41:963–968. doi: 10.1016/0039-9140(94)e005s-j. [DOI] [PubMed] [Google Scholar]
- 48.Cai W, Wu J, Xi C, Ashe AJ, III, Meyerhoff ME. Carboxyl-ebselen-based layer-by-layer films as potential antithrombotic and antimicrobial coatings. Biomaterials. 2011;32:7774–7784. doi: 10.1016/j.biomaterials.2011.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai W, Wu J, Xi C, Meyerhoff ME. Diazeniumdiolate-doped poly(lactic-co-glycolic acid)-based nitric oxide releasing films as antibiofilm coatings. Biomaterials. 2012;33:7933–7944. doi: 10.1016/j.biomaterials.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Y, Meyerhoff ME. Nitric oxide-releasing/generating polymers for the development of implantable chemical sensors with enhanced biocompatibility. Talanta. 2008;75:642–650. doi: 10.1016/j.talanta.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]