Abstract
Lysosomes contribute to a multitude of cellular processes, and the pH of the lysosomal lumen plays a central mechanistic role in many of these functions. In addition to controlling the rate of enzymatic degradation for material delivered through autophagic or phagocytotic pathways, lysosomal pH regulates events such as lysosomal fusion with autophagosomes and the release of lysosomal calcium into the cytoplasm. Disruption of either the steady state lysosomal pH or of the regulated manipulations to lysosomal pH may be pathological. For example, chloroquine elevates the lysosomal pH of retinal pigmented epithelial (RPE) cells and triggers a retinopathy characterized by the accumulation of lipofuscin-like material in both humans and animals. Compensatory responses to restore lysosomal pH are observed; new data illustrate that chronic chloroquine treatment increases mRNA expression of the lysosomal/autophagy master transcription factor TFEB and of the vesicular proton pump vHATPase in the RPE/choroid of mice. An elevated lysosomal pH with upregulation of TFEB and vHATPase resembles the pathology in fibroblasts of patients with mutant presenilin 1 (PS1), suggesting a common link between age-related macular degeneration (AMD) and Alzheimer’s disease. While the absolute rise in pH is often small, elevations of only a few tenths of a pH unit can have a major impact on both lysosomal function and the accumulation of waste over decades. Accurate measurement of lysosomal pH can be complex, and imprecise measurements have clouded the field. Protocols to optimize pH measurement from fresh and cultured cells are discussed, and indirect measurements to confirm changes in lysosomal pH and degradative capacity are addressed. The ability of reacidifying treatments to restore degradative function confirms the central role of lysosomal pH in these functions and identifies potential approaches to treat diseases of accumulation like AMD and Alzheimer’s disease. In summary, various approaches to determine lysosomal pH in fresh and cultured cells, as well as the potential to restore pH levels to an optimal range, can help identify and repair pathologies associated with lysosomal defects in RPE cells and perhaps also suggest new approaches to treat lysosomal storage diseases throughout the body.
Keywords: Retinal pigmented epithelium, lysosome, autophagy, Alzheimer’s disease, lipofuscin, cathepsin D, aging
1. Chronic retinal degenerations, ion transport, autophagy and lysosomal pH
Lysosomes are best known for their acidity and their ability to degrade material. While this places them at a critical point in processing endogenous material delivered via the autophagic system in all cells, the prodigious phagocytosis of photoreceptor outer segment tips by RPE cells places an increased load on lysosomes of the RPE. In recent years it has become clear that lysosomal alkalinization may contribute to the pathologies in a number of chronic diseases and that reacidification of the lysosome is an important target for intervention (Appelqvist et al., 2013; Guha et al., 2014; Wolfe et al., 2013) (Fig. 1A). This review will present evidence for a pathological role for the chronic lysosomal alkalinization in RPE cells and will focus on different approaches to assess lysosomal pH in fresh and cultured cells.
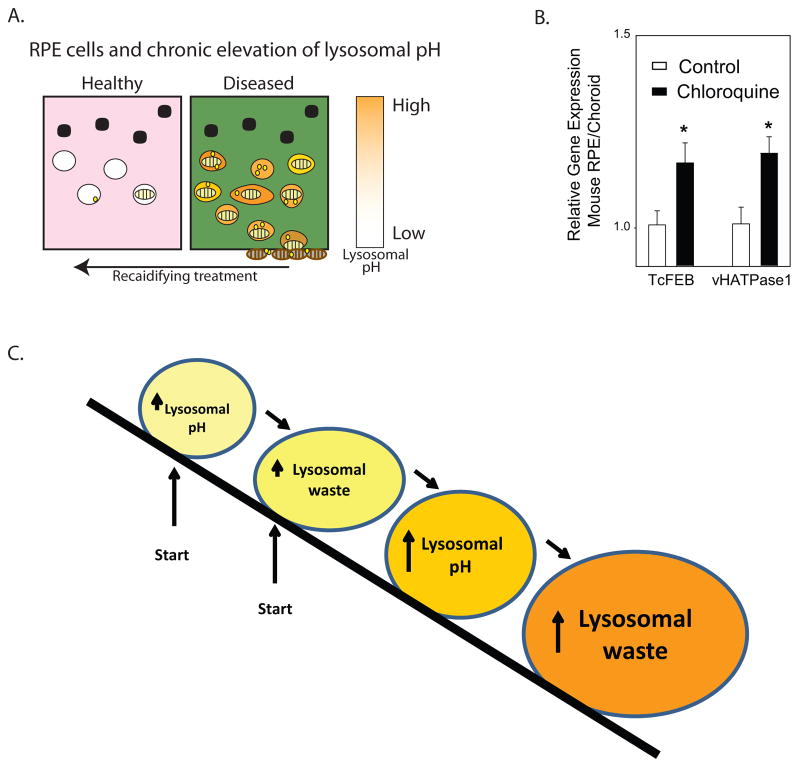
Figure 1. Lysosomal pH and accumulation of outer segment material.
A. Model of predicted effects of chronic lysosomal alkalinization on RPE cell function. In healthy RPE cells, the acid pH of the lysosomes enables efficient degradation of phagocytosed photoreceptor outer segments (striped ovals) in addition to autophagic material (yellow spheres). An elevation of lysosomal pH in diseased RPE cells impedes the enzymatic clearance of both outer segment material and autophagosomes, leading to cellular accumulation and extrusion of improperly degraded material. Treatment to reacidify lysosomes can improve clearance of outer segment material. B. Mice injected I.P. with 50 mg/kg chloroquine 3x per week for 4–6 weeks showed a compensatory increase in mRNA for the lysosomal/autohpagy transcription factor TcFEB and the lysosomal proton pump vHATPase (lysosomal V1 subunit B1, gene Atp6v1b1), as determined using the qPCR reaction. n=11–12, * p<0.05, all animals treated according to approved protocols. C. The “Snowballing” model of lysosomal alkalinization, in which an elevation in lysosomal pH leads to the accumulation of oxidized lipids which in turn leads to an elevation on lysosomal pH etc. The initial event can be either lysosomal acidification or lysosomal storage.
1.1 Chronic lysosomal disruption and chloroquine retinopathy
Healthy lysosomes are acidic; the enzymes responsible for both degrading waste material and for storing signaling molecules function optimally at a pH of 4-5-5.0 (Barrett, 1977). The regulation of lysosomal pH is complex and dynamic, with the vHATPase proton pump, anion channels and transporters, cation channels, and membrane voltage all influencing the accumulation of protons in the lysosomal lumen (Mindell, 2012). These transport mechanisms are regulated by a network of factors including cellular energetics and trafficking rates (Cang et al., 2013; Lee et al., 2010). Within RPE cells, steady state activity of the vHATPase pump is regulated by a β-crystallin anchor and by mTOR (Valapala et al., 2014), while transient fluctuations in pH levels are regulated by a variety of plasma membrane receptors and second messengers (Guha et al., 2013; Guha et al., 2012; Guha et al., 2014).
The pH of RPE cell lysosomes can be pathologically elevated by several factors. The best known of these alkalinizing agents is chloroquine. Chloroquine has been used for over 50 years to treat malaria and autoimmune disorders like rheumatoid arthritis and lupus (Goldman et al., 1953; Rinehart et al., 1957). Reports of chloroquine retinopathy have been around for nearly as long as chloroquine itself (Ben-Zvi et al., 2012; Hobbs et al., 1959; Lloyd and Hiltz, 1965; Shinjo et al., 2007; Walter, 1961). Chloroquine diffuses into acidic vesicles, becomes protonated, and gets trapped, thereby raising the pH (Homewood et al., 1972). The lysosomes of RPE cells are particularly susceptible to chloroquine because chloroquine has an affinity for pigmented cells and is retained in RPE lysosomes long after drug treatment has stopped (Bernstein et al., 1963). This affinity, combined with the high degradative load of RPE cells, leads to considerable damage to RPE cells and, secondarily, to the photoreceptors.
Chloroquine retinopathy shares parallels with other retinal degenerations. Treatment of patients with chloroquine led to central visual loss and macular cone dysfunction, pigment changes and Bull’s eye maculopathy, in which RPE cells are lost in an expanding circle of hyperfluorescence (Kellner et al., 2006; Michaelides et al., 2011; Shinjo et al., 2007). Bull’s eye maculopathy has also been reported in patients with mutations in the retinoid flipase ABCA4, a mutation associated with the early onset retinal degeneration in Stargardt’s disease (Michaelides et al., 2007), and has some similarities with geographic atrophy, in that RPE cells are lost in an expanding ring. Interestingly, only ~7% of patients receiving chloroquine treatment display retinopathy (Scherbel et al., 1965), suggesting an additional factor, perhaps genetic makeup, predisposes some patients to an exacerbated loss of vision in response to lysosomal alkalinization.
Animal models of chloroquine retinopathy also show RPE damage and have proven useful in understanding the morphological changes induced by alkalinization of the RPE lysosomes. Chronic treatment of primates with chloroquine led to lipid accumulations in the RPE, a thickened basement membrane with collagen fibrils, and increased choroidal macrophages (Rosenthal et al., 1978). In cats, extended chloroquine treatment led to RPE hypertrophy followed by loss of photoreceptors (Meier-Ruge, 1965). In rats, chloroquine led to an accumulation of lysosomal-associated organelles in RPE cells and to lipid deposits throughout Bruch’s membrane (Ivanina et al., 1983; Peters et al., 2006). The more pronounced pathologies seen with chloroquine, as compared to analogue hydroxychloroquine, were attributed to the greater effect of chloroquine on lysosomal alkalinization (Mahon et al., 2004; Sundelin and Terman, 2002). This provides additional support for the role of lysosomal alkalinization in chloroquine retinopathy. A chronic elevation of lysosomal pH may induce both detrimental and protective changes, and compensatory changes in gene expression may occur. We report here that the RPE/choroid of mice treated with chloroquine for 3 weeks had an increased expression of mRNA for the vesicular proton pump vHATPase, and the master lysosomal/autophagy transcription factor TFEB (Fig. 1B). These rises in gene expression parallel those detected in fibroblasts from patients with a mutation in presenilin 1 (PS1) associated with elevated lysosomal pH (Coffey et al., 2014; Lee et al., 2010), and suggest that cellular attempts to compensate for chronic lysosomal alkalinization are a general phenomenon. We hypothesize that the cellular damage is more pronounced when these endogenous compensations to reacidify lysosomes are attenuated by age or secondary mutations.
1.2 Acute lysosomal disruption
Acute disruption of lysosomes can lead to more cataclysmic responses. At high concentrations, chloroquine can perturb fusion of autophagic vesicles, increase levels of LC3-II and p62, and even lead to death of ARPE-19 cells (Chen et al., 2011; Yoon et al., 2010). Direct disruption of the RPE lysosome leads to activation of the inflammasome (Tseng et al., 2013), and has been implicated in inflammasome activation by retinoid byproduct N-retinylidene-N-retinylethanolamine (A2-E) (Anderson et al., 2013). These acute responses clearly have a role to play in sudden death of RPE cells at the hands of experimentalists in vitro, and may even contribute to the end-stage death seen in geographic atrophy.
In contrast to these acute responses, most aging diseases represent moderate changes in cell physiology that build up over many years, eventually contributing to a cascade of pathological events. We believe that moderate elevations of lysosomal pH represent a common step in many disorders of aging, including age-related macular degeneration and Alzheimer’s disease. While elevation of lysosomal pH may be a primary defect in some cases, this alkalinization may also be a side effect of waste accumulation in other lysosomal storage diseases. As RPE cells filled with lipofuscin represent a type of lysosomal storage disease, any alkalinizing effects of lipofuscin on lysosomal pH would exacerbate accumulation, leading to a “snowball” effect (Fig. 1C). As such, repairing lysosomal pH is predicted to reduce the storage disorder regardless of the initial cause.
1.3 Pharmacological alkalinization of RPE lysosomes
Chloroquine is a reliable method for inducing chronic lysosomal alkalinization in RPE cells; the decades of documented chloroquine retinopathy in patients, combined with the propensity of chloroquine to accumulate in pigmented cells, makes it an ideal drug to examine the consequences of lysosomal alkalinization of RPE cells (Bernstein et al., 1963; Hobbs and Calnan, 1958). Lysosomal alkalinizing agents other than chloroquine lack the attraction to pigment and as such are less specific for RPE cells, with profoundly detrimental effects observed when given systemically. For example, systemic addition of NH4+ induces coma or metabolic acidosis (Nowik et al., 2010). Intravitreal injection may provide a compromise, however; the injection of vHATPase-inhibitor bafilomycin into the rat vitreous led to the accumulation of opsin-loaded phagolysosomes (Deguchi et al., 1994). The similar pathologies observed after chloroquine treatment and intravitreal bafilomycin injections imply that lysosomal alkalinization is itself sufficient to induce an accumulation of lipofuscin-like material.
Given the advantages of using chloroquine to alkalinize RPE lysosomes in animal models, the drug is an obvious choice for in vitro experiments. Low concentrations can stably alkalinize lysosomes for 10 days without inducing cell death (Baltazar et al., 2012). However, the central role of lysosomal alkalinization is best determined when the effects of multiple agents are compared. For example, the ability of both the vHATPase inhibitor bafilomycin and the retinoid A2-E to inhibit intra-lysosomal accumulation of methylamine was attributed to lysosomal alkalinization by A2-E following vHATPase inhibition (Bergmann et al., 2004). Likewise, the ability of both NH4+ and A2-E to reduce photoreceptor degradation in vitro was also attributed to lysosomal alkalinization (Bergmann et al., 2004). Both NH4+ and bafilomycin were used to alkalinize RPE lysosomes in vitro to examine interactions with the proteasome (Ryhanen et al., 2009). While chloroquine, NH4+, bafilomycin and tamoxifen were all capable of acute lysosomal alkalinization in our hands, tamoxifen was the most reliable and rapid, and showed the best dose-response curve (Liu et al., 2008), although tamoxifen had slightly different effects on lysosomal enzyme activity than chloroquine in pig RPE cells (Toimela et al., 1998). Of note, the alkalinizing actions of tamoxifen are unrelated to the estrogen receptor but attributed to actions specifically on the lysosomal membrane (Altan et al., 1999; Chen et al., 1999).
1.4 Genetic links between lysosomal pH and macular degeneration
It is likely that most common forms of AMD in elderly patients result from the interaction between environmental insults and genetic predisposition; age-dependent changes in the inflammatory response and chemical modulations from time-dependent accumulations are unlikely to be fully recapitulated in patients with mutations that directly elevate lysosomal pH as systemic issues would predominate. While mutations in transporters controlling lysosomal pH are not among the recognized genetic risk factors for AMD, this probably has more to do with the central role such transporters have in cellular function throughout the body than the lack of a role for lysosomal pH in the disease. The overall absence of diseases caused by mutations in transporters controlling lysosomal pH suggests that such mutations may be embryonic lethal.
The loss of certain genes associated with lysosomal ion transport is linked to retinal degenerations in mice, however. For example, the ClC-7 gene codes for a lysosomal Cl−/proton exchanger that is expressed in the RPE; mice missing this gene display retinal and neural degeneration that resembles neuronal ceroid lipidosis (Kasper et al., 2005; Kornak et al., 2001). Recent work stresses the central role of the transporter in regulating lysosomal pH (Ishida et al., 2013), although older work could not detect a pH change (Kornak et al., 2001).
Lysosomal pH may be elevated secondarily to an imbalance of lysosomal lipids. In Gaucher’s disease, the accumulation of cholesterol was associated with a secondary elevation in lysosomal pH (Sillence, 2013). Interestingly, reducing glucosylceramide synthase either chemically (Sillence, 2013) or molecularly (van der Poel et al., 2011) also alkalinized lysosomes, with the latter study suggesting glucosylceramide stimulates the vHATPase pump. This suggests that optimal levels of the protol pump require a balanced intermediate level of glucosylceramide.
The theory that activity of lysosomal transporters is modulated by levels of lysosomal lipids is supported by patch clamp studies showing sphingomyelin dramatically inhibits current through the lysosomal cation channel TRPML1 (Shen et al., 2012). In RPE cells with reduced TRPML1 levels, Fe2+ exposure leads to an increase in reactive oxygen species and to mitochondrial damage (Coblentz et al., 2014); given the accumulation of lipid-rich lipofuscin in aging RPE cells (Sparrow and Boulton, 2005) and the likelihood of Fe3+ imbalance in macular degeneration (Song and Dunaief, 2013), it is possible that lipid-mediated modulation of lysosomal transporters may alter lysosomal pH in disease.
1.5. Autophagy, proteasomes and other lysosomal functions
Lysosomes were traditionally thought of as just the “garbage disposals” of the cell, but research over the past few years has identified lysosomal involvement in a large number of cell functions, with lysosomal pH a key component to these functions (Settembre et al., 2013). In some cell types, lysosomes act as storage sites for signaling molecules like ATP and cytokines, and can influence lipid oxidation (Dou et al., 2012; Pivtoraiko et al., 2009; Stanley and Lacy, 2010; Zhang et al., 2007). Investigations to determine whether lysosomes serve similar functions in RPE cells are underway.
The actions of lysosomes and proteasomes can be interrelated in RPE cells. For example, inhibition of the proteasome by MG-132 increases the number of LAMP2-stained, and enhances autophagic clearance of protein (Ryhanen et al., 2009; Viiri et al., 2013). While proteasomes may help clear excess protein upon lysosomal alkalinization, the lack of a similar backup mechanism to clear lipids may explain the predominantly lipid composition of the waste lipofuscin retained in defunct lysosomes.
Degradation of material by lysosomal enzymes can be thought of as an end-stage step in the autophagic process. Autophagy plays a central role in the health of ocular cells and defective autophagy has been recently implicated in age-related macular degeneration, photoreceptor degradation and the visual cycle (Frost et al., 2014; Kaarniranta et al., 2013; Kim et al., 2013; Mitter et al., 2012). The elevation of lysosomal pH interferes with upstream steps in autophagy and impairs the fusion of autophagosomes with lysosomes (Kawai et al., 2007). As such, lysosomal pH is a key determinant in autophagic clearance and we propose that treatment to reacidify lysosomes may enhance autophagy in compromised cells, regardless of the cause of the initial defect.
2. Measurement of lysosomal pH
2.1 Complexities in the measurement of lysosomal pH
While it is clear that elevating the lysosomal pH of RPE cells can lead to an AMD-like damage, the accurate measurement of lysosomal pH is complex. The accumulation of H+ inside lysosomes at high concentrations requires the active pumping of an ATPase enzyme, so gradients are rapidly dissipated in dying cells, making accurate pH measurement a challenge as free protons can be hard to maintain in fixed sections. This favors the assessment of lysosomal pH in living cells, but even in live cells, the lack of an ideal reporter probe complicates measurements. For example, the LysoTracker dye readily diffuses into acidic organelles and is useful to show colocalization with compounds in lysosomes, such as the demonstration that the lipofuscin component A2-E is localized to the lysosomes of RPE cells (Sparrow et al., 1999). While the lack of LysoTracker staining can be used to demonstrate relatively large elevations in pH (Avrahami et al., 2013), LysoTracker is a single wavelength dye and therefore changes in the amount of emitted light can arise either from an elevation in pH or from a change in the number and/or area of the lysosomes. As lysosomes can change size in response to numerous signals (Bakker et al., 1997), the LysoTracker output can be misleading. We have recently found the LysoTracker signal indistinguishable in control fibroblasts and those with the PS1 mutation linked to familial Alzheimer’s disease even though lysosomes in the mutant fibroblasts were significantly alkalinized by 0.25 units (Coffey et al., 2014). Overall we feel LysoTracker is not a particularly sensitive probe for determining small changes in lysosomal pH, although it remains an excellent tool for localizing components to acidic vesicles and for detecting larger shifts in pH.
Dextran-linked rhodamine sensors are another tool used to assess pH, but come with their own set of complications. As the large dexran groups encourage endocytosis, dextran-rhodamine probes are ideal for measuring phagocytosis, but the pKa of rhodamine means that the signal is largely saturated at pH 5.0–5.5, making the probes of limited use in the accurate measurement of lysosomal pH near 4.5; small pH elevations from this baseline can consequently go undetected. The pHRodo probe is one example of such a tool that provides excellent reporting of pH in endosomes but loses sensitivity once the pH falls below 5.0 (Life Technologies; Molecular Probes handbook Figure 20.3.3); the dextran-rhodamine probes are far from ideal when it comes to detecting small differences in lysosomal pH levels.
Fluorescein isothiocyanate (FITC)-based sensors suffer from a different problem, as the dye has a pKa of 6.3–6.7. While the FITC dye has been used for pH measurements in lysosomes, a shift in pH from 5.0 to 4.5 reduced fluorescence output by only ~2%, from 5% to 3% of maximum levels (Geisow, 1984), making this dye less than ideal for the pH range found in healthy lysosomes. This is of particular concern given the number of experiments in which photoreceptor outer segments loaded with FITC were visualized as they progressed through the phagocytotic and endocytotic pathways of RPE cells. Disappearance of the FITC signal in acidic environments is a physical characteristic of the dye, and should not be confused with photoreceptor degradation. We have found that the pH-insensitive dye calcein provides a reasonable substitute (Liu et al., 2008).
One alternative to directly labeling the material to be phagocytosed is to tag pH-sensitive fluorescent reporters to dextran spheres that are themselves labeled with a fluorophore of a second color. These dual-tagged probes offer an ideal way to study the phagocytotic and endocytotic pathways. However, dextran-conjugated probes can only report from organelles actively involved in the endocytosis process. Compromised lysosomes in RPE cells with elevated pH are less likely to participate in fusion with freshly endocytosed dyes than healthy lysosomes (Chen et al., 2011). The pH readout from the dextran-conjugated reporters will thus be shifted to more acidic levels and the probe may miss the very lysosomes that are most problematic. This of course is fine for protocols in which the pH is manipulated exogenously, but in measurements to determine lysosomal pH from diseased or older cells with heterogeneous populations of lysosomes, the use of a dextran-conjugated probe can underestimate the degree of lysosomal alkalinization.
2.2. Optimization of protocols for the use of LysoSensor Yellow/Blue DND 160
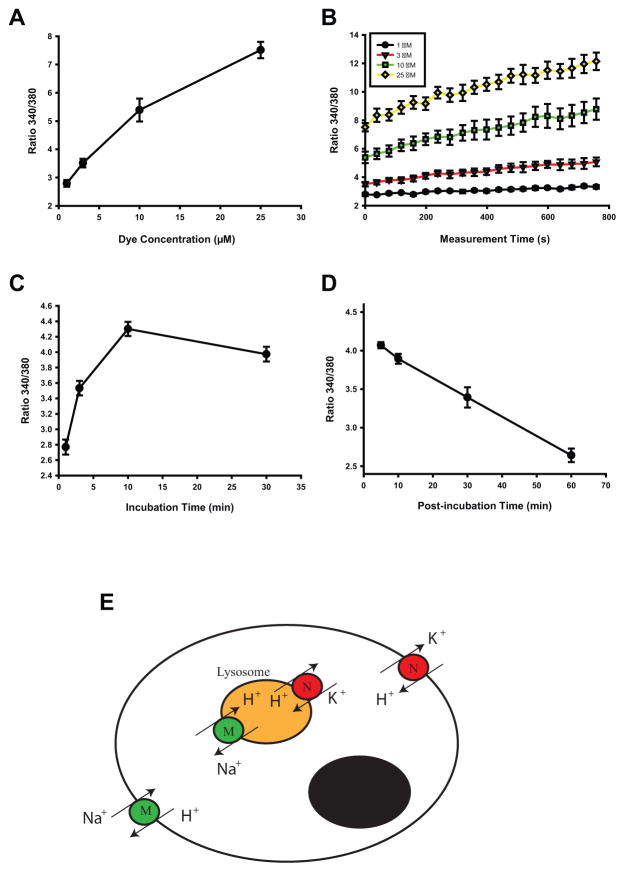
The LysoSensor Yellow/Blue DND 160 (subsequently referred to as LysoSensor Yellow/Blue) offers some advantages for pH measurement, but like the other probes, it is far from ideal. In our hands it only provides a reliable output when a complex series of controls are employed. LysoSensor Yellow/Blue is ratiometric, so the ratio of light excited at 340 nm to 380 nm and emitted at >527 nm is proportional to lysosomal pH. Like the ratiometric calcium reporter Fura-2, the ratiometric aspect of LysoSensor Yellow/Blue means that the signal is independent of dye concentration, and thus output should not greatly affected by changes in lysosomal size. Importantly, the dye is particularly sensitive over acidic pH values, making it a preferable alternative to rhodamine or FITC probes. However, LysoSensor Yellow/Blue is not particularly stable and can itself modulate pH if retained for an extended period of time. To control for this, we have performed a series of measurements to optimize parameters like loading time, concentration and post-loading interval (Fig. 2A). We have found that in cultured ARPE-19 cells, optimal results are obtained with 2–5 μM dye loaded for 3 min with measurements made 15–19 min after dye removal (Guha et al., 2012; Liu et al., 2012). For reference, best results were obtained from cultured human fibroblasts when they were incubated with 2 μM LysoSensor Yellow/Blue for 3 min and measurements made 12–14 min later (Coffey et al., 2014). Investigators are encouraged to determine optimum conditions for their own cell types.
Figure 2.
Technical challenges in optimizing the measurement of lysosomal pH.
A–D. Optimization of the parameters for measuring lysosomal pH in ARPE-19 cells with LysoSensor Yellow/Blue showing the effect of dye concentration on (A) the strength of the signal emitted at >500 nM, defined as the ratio of light excited at 340 and 380 nm, (B) the rate of change in the signal; the effect of incubation time on the signal size and (D) the decay of the signal (C) the raand emitted (A) E. Model of calibration using dual ionophores/transporters monensin (M) and nigericin (N) illustrating the complexities of calibrating across two sets of membranes.
Confidence in results from the LysoSensor Yellow/Blue probe is greatest for comparisons between simultaneous measurements; this allows any time- or dye-dependent shifts to be controlled for. While output from a microscope-based platform gives reasonable qualitative output and provides satisfying images, we have found it difficult to accurately compare the absolute responses between different preparations using a microscope. As such, we perform all quantitative measurements on a plate reader using 96- or 384-well plates. The dye is loaded and removed from all cells almost simultaneously, and the distribution of different cells and/or drug conditions is randomized across the plate to further minimize bias. Repetitive sweeps of the plate and alternating excitation at 340nm and 380nm provide measurements.
While the changes in fluorescent ratio provided by this protocol can be reliably compared to others within the plate, baseline levels for each plate can differ. To compensate for this difference, ratios across plates can be normalized to a control value in each plate, or can be calibrated. This calibration is somewhat analogous to that used for the calcium reporter Fura-2, for which the cell is bathed in solutions of various calcium concentrations and a selectively permeant ionophore enables clamping of the intracellular calcium concentration to the extracellular solution. However, calibrating the ion concentration of intracellular vesicles is topographically more complex as the concentration must be matched across two sets of membranes (Fig. 2B). To calibrate lysosomal pH, wells are perfused with a series of solutions composed of 20 mM 2-(N-morpholino)ethane sulfonic acid (MES), 110 mM KCl, and 20 mM NaCl and adjusted to pH 4.0 to 6.0 with HCl/NaOH; the intravesicular pH is then clamped using the H+/Na+ ionophore/transporter monensin and the H+/K+ ionophore/transporter nigericin, as reported (Lin et al., 2003). Calibration measurements are made simultaneously with the pH measurements in adjacent wells, ensuring that any time or dye-dependent effect is controlled for. While the relationship between the ratio of light emitted at 340/380 nm and pH is usually linear over the range examined in RPE cells (Liu et al., 2008), the calibration is subject to numerous issues involved in delivering the ionophore/transporters to both cytoplasmic and vesicular membranes and is not always reliable. In addition, the need for designated wells can be limiting when cell number is low, such as with freshly isolated RPE cells. As such, quantification is often performed on ratios with calibration performed only on an example (Coffey et al., 2014).
2.3. Modifications for lysosomal pH measurements from freshly isolated RPE cells and primary cultures
While indirect measures of pH are possible in fixed cells or in vivo, we have found it difficult to accurately measure lysosomal pH levels in RPE cells in vivo. As the most accurate comparisons of lysosomal pH are performed simultaneously on a plate reader, cultured ARPE-19 cells have been used for the majority of the experiments as they can be produced in sufficient number for screening. However, results are always confirmed in primary RPE cultures or freshly isolated cells. This is particularly true for experiments in which the status of RPE lysosomes in their endogenous state is being investigated, such as in the presence of the ABCA4−/− mutation and aging. In such cases, it is critical that lysosomal pH be measured only from freshly isolated RPE cells; cells that have divided, even once, should not be used.
When freshly isolated RPE cells are used, several adjustments to the protocol are necessary. The best results with mouse RPE cells were obtained when cells were incubated in 2–5μM LysoSensor Yellow/Blue for 5 min. Because of the reduced number of cells, and therefore reduced overall signal, available from a pair of mouse eyes, cells are distributed into the smaller volume wells of 384-well plates and drugs added ex vivo. The use of plates made of plastic with low excitation at 340 nm, such as the UV Star from Greiner Bio-One, improves the signal/noise. It should be noted that while the RPE cells from ABCA4−/− mice are themselves autofluorescent, the signal from the LysoSensor Yellow/Blue dye was ~100 fold greater (Liu et al., 2008). This dye-to background signal should be checked for each preparation, however.
The measurement of lysosomal pH from RPE cells of the ABCA4−/− mouse highlights several key issues in the measurement of lysosomal pH from fresh cells. In this model, the primary defect is found in the photoreceptors, and for the consequences of the mutation to impact the RPE cells, the two cell types must be adjacent (Weng et al., 1999). The delivery of excess A2-PE from photoreceptors to RPE cells is required for the production of A2-E by RPE cells and, presumably, the subsequent damage to the lysosomes. As such, even a single cell division by primary RPE cells in culture will result in the production of new lysosomes, untainted by the A2-E as they have been removed from their association with the mutant photoreceptors. In these new lysosomes from daughter cells, the altered phenotype is no longer present, or at best the magnitude of the defect is diluted out. In experiments comparing lysosomal pH in RPE cells in ABCA4−/− versus control mice (Liu et al., 2008), measurements are always performed from freshly isolated RPE cells. Likewise, in trials demonstrating that activators of the lysosomal chloride channel CFTR (Liu et al., 2012) or agonists for the D5 dopamine receptor (Guha et al., 2012) were capable of restoring lysosomal pH, measurements were always performed on RPE cells that had been removed from the eye of ABCA4−/− mice less than 3 hrs previously. Preliminary findings suggesting an age-dependent elevation in lysosomal pH were likewise performed on freshly isolated RPE cells that had not yet divided (Mitchell and Laties, 2012).
With regards to the age of the cultures, it may be relevant that lysosomal pH measurement of control and mutant PS1 fibroblasts was most consistent on cells grown for at least 6 days on their substrate (Coffey et al., 2014). While a detailed analysis of culture duration and response has yet to be performed for RPE cells, the “snowballing” hypothesis illustrated in Figure 1C implies that some time is needed to accumulate waste material before the lysosomal pH is altered. This requirement for some delay is also consistent with the findings that 24hr exposure of RPE cells to the retinoid A2-E had no immediate effect on lysosomal pH (Lakkaraju et al., 2007; Liu et al., 2008) but that three weeks of exposure to A2-E did elevate the lysosomal pH (Holz et al., 1999; Liu et al., 2008). The alkalinizing effect of a three-week nextended exposure to A2-E has recently been confirmed (Poliakov et al., 2014). These observations are also consistent with the age-dependent lysosomal alkalinization found in ABCA4−/− mice (Liu et al., 2008). The mechanisms by which A2-E associated accumulations lead to lysosomal alkalinization are currently being investigated.
3. Indirect approaches to support the detection of lysosomal alkalinization
3.1. Assessment of cathepsin D activity
Given that the direct measurement of lysosomal pH is complex and is best performed in live cells, additional support for pH disruption is both necessary and informative. While degradation, detailed in the section below, is perhaps the most relevant assay, additional protocols can provide support for the detection of defective lysosomes and some can be translated to the in vivo condition more readily than direct measurement of lysosomal pH.
The assays used most effectively in our laboratory involve the lysosomal protease cathepsin D. The maturation of cathepsin D is pH-sensitive, as catalytic enzymes require an acidic milieu for effective cleavage of pro forms into active forms (Richo and Conner, 1994). Western blotting has confirmed that the ratio of mature to pro-cathepsin isoforms to immature pro forms is greater in cells with an acidic lysosome than in those in which the lysosomal pH is chronically alkalinized (Coffey et al., 2014). As this approach uses standard immunoblots, it has the advantage that it can be performed from preserved tissue and does not require live cells.
The BODIPY FL-pepstatin A assay provides a similar output from live cells. Not only is the production of mature cathepsin D dependent upon an acidic lumen, but the protease activity is also optimal at an acidic pH, with degradative activity decreasing by 80% when the pH rises from 4.5 to 5.3 (Barrett, 1977). Access to the binding site can be measured with fluorescent BODIPY FL-pepstatin A; the fluorescent signal is greatly increased when pH falls to 4.5 (Chen et al., 2000). In ARPE-19 cells, the fluorescent signal of BODIPY FL-pepstatin A is greater under control conditions than in cells treated with chloroquine to raise lysosomal pH (Baltazar et al., 2012). Likewise, stimulation of the P2X7 receptor increased lysosomal pH, and reduced the BODIPY FL-pepstatin A signal (Guha et al., 2013). Again, human cells with mutant PS1 show decreased BODIPY FL-pepstatin A staining compared to control, consistent with their elevated lysosomal pH (Coffey et al., 2014)., It should be kept in mind that under chronically pH elevation, a loss of Bodipy pepstatin A fluorescence can result from either a decrease in the amount of mature cathepsin D or a decrease in the pH-dependent access to the binding site; both factors will sum.
Standard biochemical measures of lysosomal enzyme activity should be approached with caution, as most of these kits and assays measure enzyme activity in a pre-made solution of fixed pH. This will prevent the detection of any change in enzyme activity caused solely by a shift in lysosomal pH. This may explain why addition of A2-E had no direct effect on the activity of lysosomal enzymes when tested in lysed suspensions (Bermann et al., 2001); indirect effects on enzyme activity arising from its ability to raise lysosomal pH would be missed by this approach. Of course, for enzymes like cathepsin D where acidity is needed for enzyme maturation in addition to direct activity, such measurements may detect evidence for chronic alkalinization. A fluorometric assay was recently used to demonstrate a decline in cathepsin D activity in mice missing the Cryba1 gene, a defect that led to lysosomal alkalinization (Valapala et al., 2014); presumably levels of active enzyme were reduced by the chronic rise in lysosomal pH.
3.2. Lysosomal alkalinization and degradation
Lysosomal alkalinization can damage RPE cells by impairing degradation; the lysosomal enzymes responsible for degrading ingested outer segments and autophagic material are impaired by alkalinization. As mentioned, cathepsin D activity decreases dramatically with a modest rise in lysosomal pH(Barrett, 1977). Because cathepsin D is the enzyme primarily responsible for the breakdown of opsin (Feeney-Burns et al., 1987), a moderate lysosomal alkalinization can slow outer segment clearance. RPE cells also have a large load of photoreceptor-derived lipids to degrade, and the activity of lysosomal lipases needed to handle this lipid burden is also very pH-dependent, with a 75% drop in the ability to degrade triglycerides observed when pH goes from 4.8 to 5.4 (Ameis et al., 1994).
Graphic evidence that lysosomal alkalinization interferes with outer segment clearance is shown by electron microscopy showing the accumulation of photoreceptor outer segment debris in RPE cells of rats treated with chloroquine (Peters et al., 2006). However, in vitro assays can be more quantitative while also isolating the effect of lysosomal pH changes more directly. Monitoring the disappearance of fluorescently labeled outer segments has been performed for many years. However, the pH dependence of FITC makes the classic techniques of labeling outer segments with FITC problematic, as discussed above. Labeling outer segments with the pH-insensitive dye calcein avoids this issue; elevating lysosomal pH led to an increased retention of calcein fluorescence, while treatment to restore acidity reduced the calcein fluorescence (Liu et al., 2012; Liu et al., 2008).
The lipofuscin-like autofluorescence of incorrectly processed photoreceptor outer segments also provide a reasonable measure by which clearance can be quantified. Detection is improved by using a flow cytometer and repeated feeding of cultured cells for at least a week with isolated outer segments. This outer segment-derived autofluorescence co-localizes with lysosomes, consistent with accumulation of partially digested remains in lysosome-like organelles (Guha et al., 2012). The autofluorescence of cultured RPE cells showed a small increase after treatment with chloroquine alone, suggesting that lysosomal alkalinization increased an autofluorescent signal arising from the autophagic degradation of endogenous RPE material (Baltazar et al., 2012; Guha et al., 2013). The addition of outer segments and chloroquine together to cultured RPE cells increased autofluorescence substantially. Critically, this autofluorescence decreases when cells are treated with agents to reacidify lysosomal pH, such as D5 dopamine receptor agonists or acid nanoparticles (Baltazar et al., 2012; Guha et al., 2012). This implies that the impaired degradation was due to a rise in lysosomal pH. A similar reduction in autofluorescence was observed when lysosomal pH was reacidified with the P2X7 receptor antagonist BBG (Guha et al., 2013). Together these experiments demonstrate that increased clearance of lipofuscin-like autofluorescence is a general response to lysosomal acidification and that drugs to reacidify lysosomes have promise as treatment for diseases of accumulation like AMD.
While levels of an added fluorescent label like calcein to outer segments, or detection of autofluorescence levels provides an index of turnover, it is important to confirm that the accumulated material being measured is of outer segment origin. In this regard, measuring the protein opsin with immunoblots provides a specific assay of outer segment degradation, as the protein is derived only from added outer segments. Treatment of ARPE-19 cells with outer segments over 7 days dramatically increased the 40 kDa band of opsin; the ability of acid nanoparticles to reduce this opsin band by over 90% provided a clear support for the theory that lowering lysosomal pH improves the degradation of photoreceptor outer segments by RPE cells and reduces lipofuscin (Baltazar et al., 2012).
4. Summary
In summary, increasing evidence implicates lysosomal alkalinization in diseases of accumulation such as AMD and Alzheimer’s disease. For example, raising lysosomal pH increases markers associated with retinal degeneration, stimuli associated with RPE damage elevate lysosomal pH, and lysosomal alkalinization alone leads to a macular degeneration. Perhaps most importantly, reacidifying lysosomes with drugs or nanoparticles can reverse signs of retinal degeneration. The complexities in accurately measuring lysosomal pH and degradation may explain some of the controversy over the relative contribution of defective lysosomal pH to AMD and Alzheimer’s disease. These complexities also stress the need for numerous complementary approaches when evaluating the presence of a perturbed lysosomal pH or of potential treatments. The preference for live cells when performing accurate measurements highlights a complementary role for both cultured and freshly isolated cells. Analysis of enzymes like cathepsin D can provide an indirect support that can be performed on protein samples from fixed tissue. The ability of chloroquine to induce maculopathy provides in vivo support for a role for lysosomal alkalinization, while analysis of mRNA from mice treated with chloroquine assigns modern markers to this classic model. The analysis of lysosomal degradation from RPE cells is facilitated by their uptake of exogenous photoreceptor outer segments; the effect of lysosomal pH on the processing and turnover of photoreceptor outer segments can be monitored by using a pH-insensitive label such as calcein-AM, by quantifying the autofluorescence present after prolonged exposure to outer segments, or by probing immunoblots for a specific marker like opsin. The convergence of all these approaches from cultured, fresh and in situ material provides compelling evidence that chronic alkalinization of the lysosomal lumen is a major issue for RPE cells. Given the emerging role for lysosomes in the storage and release of cellular signals, additional pathways impacted by lysosomal alkalinization are sure to emerge. Our ability to restore an acidic lysosomal pH with a number of treatments may help repair many of these functions.
Highlights.
Evidence for contribution of RPE lysosomal alkalinization in retinal degenerations
Elevated expression of TcFEB and vHATPase in RPE cells in vivo after chronic lysosomal alkalinization with chloroquine
Advantages and optimization of different techniques for measuring lysosomal pH
Detection of secondary markers of lysosomal alkalinization in fresh and fixed tissue
Linkage between lysosomal alkalinization, impaired degradation and lipofuscin-like autofluorescence
Acknowledgments
The authors would like to thank Ji Liu for help with the early studies that went into this work. This work is supported by grants from the NIH R01EY013434 (CHM), R01EY015537 (CHM), R01EY10420 (KBB), P30EY001583 (CHM & KBB), T32GM075770 (JMB), Research to Prevent Blindness (AML), the Paul and Evanina Bell Mackall Foundation Trust (AML) and the Jody Sack Fund (WL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altan N, Chen Y, Schindler M, Simon SM. Tamoxifen inhibits acidification in cells independent of the estrogen receptor. Proc Natl Acad Sci U S A. 1999;96:4432–4437. doi: 10.1073/pnas.96.8.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameis D, Merkel M, Eckerskorn C, Greten H. Purification, characterization and molecular cloning of human hepatic lysosomal acid lipase. Eur J Biochem. 1994;219:905–914. doi: 10.1111/j.1432-1033.1994.tb18572.x. [DOI] [PubMed] [Google Scholar]
- Anderson OA, Finkelstein A, Shima DT. A2E induces IL-1β production in retinal pigment epithelial cells via the NLRP3 inflammasome. PLoS One. 2013;8:e67263. doi: 10.1371/journal.pone.0067263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelqvist H, Waster P, Kagedal K, Ollinger K. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5:214–226. doi: 10.1093/jmcb/mjt022. [DOI] [PubMed] [Google Scholar]
- Avrahami L, Farfara D, Shaham-Kol M, Vassar R, Frenkel D, Eldar-Finkelman H. Inhibition of glycogen synthase kinase-3 ameliorates beta-amyloid pathology and restores lysosomal acidification and mammalian target of rapamycin activity in the Alzheimer disease mouse model: in vivo and in vitro studies. J Biol Chem. 2013;288:1295–1306. doi: 10.1074/jbc.M112.409250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker AC, Webster P, Jacob WA, Andrews NW. Homotypic fusion between aggregated lysosomes triggered by elevated [Ca2+](i) in fibroblasts. J Cell Sci. 1997;110:2227–2238. doi: 10.1242/jcs.110.18.2227. [DOI] [PubMed] [Google Scholar]
- Baltazar GC, Guha S, Boesze-Battaglia K, Laties AM, Tyagi P, Kompella UB, Mitchell CH. Acidic nanoparticles restore lysosomal pH and degradative function in compromised RPE cells. PloS One. 2012b;7:e49635. doi: 10.1371/journal.pone.0049635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. Protinases in mammalian cells and tissues. Elsiver/North-Hollard Biomedical Press; New York: 1977. [Google Scholar]
- Ben-Zvi I, Kivity S, Langevitz P, Shoenfeld Y. Hydroxychloroquine: from malaria to autoimmunity. Clin Rev Allergy Immunol. 2012;42:145–153. doi: 10.1007/s12016-010-8243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann M, Schutt F, Holz FG, Kopitz J. Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration. Faseb J. 2004;18:562–564. doi: 10.1096/fj.03-0289fje. [DOI] [PubMed] [Google Scholar]
- Bermann M, Schutt F, Holz FG, Kopitz J. Does A2E, a retinoid component of lipofuscin and inhibitor of lysosomal degradative functions, directly affect the activity of lysosomal hydrolases? Exp Eye Res. 2001;72:191–195. doi: 10.1006/exer.2000.0949. [DOI] [PubMed] [Google Scholar]
- Bernstein H, Zvaifler N, Rubin M, Mansour AM. The ocular deposition of chloroquine. Invest Ophthalmol. 1963;2:384–392. [PubMed] [Google Scholar]
- Cang C, Zhou Y, Navarro B, Seo YJ, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell. 2013;152:778–790. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Chen WNU, Zhou MJ, Arttamangkul S, Haugland RP. Probing the cathepsin D using a BODIPY EL-pepstatin A: applications in fluorescence polarization and microscopy. J Biochem Biophys Meth. 2000;42:137–151. doi: 10.1016/s0165-022x(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Chen PM, Gombart ZJ, Chen JW. Chloroquine treatment of ARPE-19 cells leads to lysosome dilation and intracellular lipid accumulation: possible implications of lysosomal dysfunction in macular degeneration. Cell Biosci. 2011;1:10. doi: 10.1186/2045-3701-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Schindler M, Simon SM. A mechanism for tamoxifen-mediated inhibition of acidification. J Biol Chem. 1999;274:18364–18373. doi: 10.1074/jbc.274.26.18364. [DOI] [PubMed] [Google Scholar]
- Coblentz J, St Croix C, Kiselyov K. Loss of TRPML1 promotes production of reactive oxygen species: is oxidative damage a factor in mucolipidosis type IV? Biochem J. 2014;457:361–368. doi: 10.1042/BJ20130647. [DOI] [PubMed] [Google Scholar]
- Coffey EE, Beckel JM, Laties AM, Mitchell CH. Lysosomal alkalization and dysfunctional autphagy in fibroblasts with the Alzheimer’s disease-linked presenilin 1 A246E mutation can be reversed with cAMP. Neuroscience. 2014;263:111–124. doi: 10.1016/j.neuroscience.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi J, Yamamoto A, Yoshimori T, Sugasawa K, Moriyama Y, Futai M, Suzuki T, Kato K, Uyama M, Tashiro Y. Acidification of phagosomes and degradation of rod outer segments in rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1994;35:568–579. [PubMed] [Google Scholar]
- Dou Y, Wu HJ, Li HQ, Qin S, Wang YE, Li J, Lou HF, Chen Z, Li XM, Luo QM, Duan S. Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res. 2012;22:1022–1033. doi: 10.1038/cr.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney-Burns L, Gao CL, Tidwell M. Lysosomal enzyme cytochemistry of human RPE, Bruch’s membrane and drusen. Invest Ophthalmol Vis Sci. 1987;28:1138–1147. [PubMed] [Google Scholar]
- Frost LS, Mitchell CH, Boesze-Battaglia K. Autophagy in the eye: significance for ocular cell health. Exp Eye Res. 2014 doi: 10.1016/j.exer.2014.04.010. http://dx.doi.org/10.1016/j.exer.2014.04.010. [DOI] [PMC free article] [PubMed]
- Geisow MJ. Fluorescein conjugates as indicators of subcellular pH - a critical-evaluation. Exp Cell Res. 1984;150:29–35. doi: 10.1016/0014-4827(84)90698-0. [DOI] [PubMed] [Google Scholar]
- Goldman L, Cole DP, Preston RH. Chloroquine diphosphate in treatment of discoid lupus erythematosus. J Am Med Assoc. 1953;152:1428–1429. doi: 10.1001/jama.1953.63690150002009a. [DOI] [PubMed] [Google Scholar]
- Guha S, Baltazar GC, Coffey EE, Tu L-A, Lim JC, Beckel JM, Eysteinsson T, Lu W, O’Brien-Jenkins A, Patel S, Laties AM, Mitchell CH. Lysosomal alkalinization, lipid oxidation, impaired autophagy and reduced phagosome clearance triggered by P2X7 receptor activation in retinal pigmented epithelial cells. Faseb J. 2013;27:4500–4509. doi: 10.1096/fj.13-236166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha S, Baltazar GC, Tu LA, Liu J, Lim JC, Lu W, Argall A, Boesze-Battaglia K, Laties AM, Mitchell CH. Stimulation of the D5 dopamine receptor acidifies the lysosomal pH of retinal pigmented epithelial cells and decreases accumulation of autofluorescent photoreceptor debris. J Neurochem. 2012;122:823–833. doi: 10.1111/j.1471-4159.2012.07804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha S, Liu J, Baltazar GC, Laties AM, Mitchell CH. Rescue of compromised lysosomes enhances degradation of photoreceptor outer segments and reduce lipofuscin-like autofluorescence. Adv Exp Med Biol. 2014;801:105–111. doi: 10.1007/978-1-4614-3209-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs HE, Calnan CD. The ocular complications of chloroquine therapy. Lancet. 1958;1:1207–1209. doi: 10.1016/s0140-6736(58)91911-1. [DOI] [PubMed] [Google Scholar]
- Hobbs HE, Sorsby A, Freedman A. Retinopathy following chloroquine therapy. Lancet. 1959;2:478–480. doi: 10.1016/s0140-6736(59)90604-x. [DOI] [PubMed] [Google Scholar]
- Holz FG, Schutt F, Kopitz J, Eldred GE, Kruse FE, Volcker HE, Cantz M. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 1999;40:737–743. [PubMed] [Google Scholar]
- Homewood CA, Warhurst DC, Peters W, Baggaley VC. Lysosomes, pH and the anti-malarial action of chloroquine. Nature. 1972;235:50–52. doi: 10.1038/235050a0. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Nayak S, Mindell JA, Grabe M. A model of lysosomal pH regulation. J Gen Physiol. 2013;141:705–720. doi: 10.1085/jgp.201210930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanina TA, Zueva MV, Lebedeva MN, Bogoslovsky AI, Bunin AJ. Ultrastructural alterations in rat and cat retina and pigment epithelium induced by chloroquine. Graefes Arch Clin Exp Ophthalmol. 1983;220:32–38. doi: 10.1007/BF02307013. [DOI] [PubMed] [Google Scholar]
- Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Vereb Z, Salminen A, Boulton ME, Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D, Planells-Cases R, Fuhrmann JC, Scheel O, Zeitz O, Ruether K, Schmitt A, Poet M, Steinfeld R, Schweizer M, Kornak U, Jentsch TJ. Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J. 2005;24:1079–1091. doi: 10.1038/sj.emboj.7600576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A, Uchiyama H, Takano S, Nakamura N, Ohkuma S. Autophagosome-lysosome fusion depends on the pH in acidic compartments in CHO cells. Autophagy. 2007;3:154–157. doi: 10.4161/auto.3634. [DOI] [PubMed] [Google Scholar]
- Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006;47:3531–3538. doi: 10.1167/iovs.05-1290. [DOI] [PubMed] [Google Scholar]
- Kim JY, Zhao H, Martinez J, Doggett TA, Kolesnikov AV, Tang PH, Ablonczy Z, Chan CC, Zhou Z, Green DR, Ferguson TA. Noncanonical autophagy promotes the visual cycle. Cell. 2013;154:365–376. doi: 10.1016/j.cell.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell. 2001;104:205–215. doi: 10.1016/s0092-8674(01)00206-9. [DOI] [PubMed] [Google Scholar]
- Lakkaraju A, Finnemann SC, Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc Natl Acad Sci U S A. 2007;104:11026–11031. doi: 10.1073/pnas.0702504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HJ, Herman P, Lakowicz JR. Fluorescence lifetime-resolved pH imaging of living cells. Cytometry A. 2003;52:77–89. doi: 10.1002/cyto.a.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lu W, Guha S, Baltazar GC, Coffey EE, Laties AM, RCR, Reenstra WW, Mitchell CH. Cystic fibrosis transmembrane conductance regulator (CFTR) contributes to reacidification of alkalinized lysosomes in RPE cells. Am J Physiol Cell Physiol. 2012;303:C160–169. doi: 10.1152/ajpcell.00278.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lu W, Reigada D, Nguyen J, Laties AM, Mitchell CH. Restoration of lysosomal pH in RPE cells from cultured human and ABCA4(−/−) mice: pharmacologic approaches and functional recovery. Invest Ophthalmol Vis Sci. 2008;49:772–780. doi: 10.1167/iovs.07-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd LA, Hiltz JW. Ocular complications of chloroquine therapy. Can Med Assoc J. 1965;92:508–513. [PMC free article] [PubMed] [Google Scholar]
- Mahon GJ, Anderson HR, Gardiner TA, McFarlane S, Archer DB, Stitt AW. Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr Eye Res. 2004;28:277–284. doi: 10.1076/ceyr.28.4.277.27835. [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W. Experimental investigation of the morphogenesis of chloroquine retinopathy. Arch Ophthalmol. 1965;73:540–544. doi: 10.1001/archopht.1965.00970030542017. [DOI] [PubMed] [Google Scholar]
- Michaelides M, Chen LL, Brantley MA, Jr, Andorf JL, Isaak EM, Jenkins SA, Holder GE, Bird AC, Stone EM, Webster AR. ABCA4 mutations and discordant ABCA4 alleles in patients and siblings with bull’s-eye maculopathy. Br J Ophthalmol. 2007;91:1650–1655. doi: 10.1136/bjo.2007.118356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelides M, Stover NB, Francis PJ, Weleber RG. Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapy. Arch Ophthalmol. 2011;129:30–39. doi: 10.1001/archophthalmol.2010.321. [DOI] [PubMed] [Google Scholar]
- Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- Mitchell CH, Laties AM. Retinal Degeneration 2012 Abstract. 2012. Age-dependent defects in the lysosomes of RPE cells are similar to those of ABCA4−/− mice. [Google Scholar]
- Mitter SK, Rao HV, Qi X, Cai J, Sugrue A, Dunn WA, Jr, Grant MB, Boulton ME. Autophagy in the retina: a potential role in age-related macular degeneration. Adv Exp Med Biol. 2012;723:83–90. doi: 10.1007/978-1-4614-0631-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowik M, Kampik NB, Mihailova M, Eladari D, Wagner CA. Induction of metabolic acidosis with ammonium chloride (NH4Cl) in mice and rats - species differences and technical considerations. Cell Physiol Biochem. 2010;26:1059–1072. doi: 10.1159/000323984. [DOI] [PubMed] [Google Scholar]
- Peters S, Reinthal E, Blitgen-Heinecke P, Bartz-Schmidt KU, Schraermeyer U. Inhibition of lysosomal degradation in retinal pigment epithelium cells induces exocytosis of phagocytic residual material at the basolateral plasma membrane. Ophthalmic Research. 2006;38:83–88. doi: 10.1159/000090268. [DOI] [PubMed] [Google Scholar]
- Pivtoraiko VN, Stone SL, Roth KA, Shacka JJ. Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death. Antioxid Redox Signal. 2009;11:481–496. doi: 10.1089/ars.2008.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliakov E, Strunnikova NV, Jiang JK, Martinez B, Parikh T, Lakkaraju A, Thomas C, Brooks BP, Redmond TM. Multiple A2E treatments lead to melanization of rod outer segment-challenged ARPE-19 cells. Mol Vis. 2014;20:285–300. [PMC free article] [PubMed] [Google Scholar]
- Richo GR, Conner GE. Structural requirements of procathepsin D activation and maturation. J Biol Chem. 1994;269:14806–14812. [PubMed] [Google Scholar]
- Rinehart RE, Rosenbaum EE, Hopkins CE. Chloroquine therapy in rheumatoid arthritis. Northwest Med. 1957;56:703–705. [PubMed] [Google Scholar]
- Rosenthal AR, Kolb H, Bergsma D, Huxsoll D, Hopkins JL. Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci. 1978;17:1158–1175. [PubMed] [Google Scholar]
- Ryhanen T, Hyttinen JM, Kopitz J, Rilla K, Kuusisto E, Mannermaa E, Viiri J, Holmberg CI, Immonen I, Meri S, Parkkinen J, Eskelinen EL, Uusitalo H, Salminen A, Kaarniranta K. Crosstalk between Hsp70 molecular chaperone, lysosomes and proteasomes in autophagy-mediated proteolysis in human retinal pigment epithelial cells. J Cell Mol Med. 2009;13:3616–3631. doi: 10.1111/j.1582-4934.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbel AL, Mackenzi Ah, Nousek JE, Atdjian M. Ocular lesions in rheumatoid arthritis and related disorders with particular reference to retinopathy - a study of 741 patients treated with and without chloroquine drugs. New Eng J Med. 1965;273:360–366. doi: 10.1056/nejm196508122730704. [DOI] [PubMed] [Google Scholar]
- Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, Xu H. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinjo SK, Maia OO, Jr, Tizziani VA, Morita C, Kochen JA, Takahashi WY, Laurindo IM. Chloroquine-induced bull’s eye maculopathy in rheumatoid arthritis: related to disease duration? Clin Rheumatol. 2007;26:1248–1253. doi: 10.1007/s10067-006-0478-9. [DOI] [PubMed] [Google Scholar]
- Sillence DJ. Glucosylceramide modulates endolysosomal pH in Gaucher disease. Mol Genet Metab. 2013;109:194–200. doi: 10.1016/j.ymgme.2013.03.015. [DOI] [PubMed] [Google Scholar]
- Song D, Dunaief JL. Retinal iron homeostasis in health and disease. Front Aging Neurosci. 2013;5:24. doi: 10.3389/fnagi.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci. 1999;40:2988–2995. [PubMed] [Google Scholar]
- Stanley AC, Lacy P. Pathways for cytokine secretion. Physiology. 2010;25:218–229. doi: 10.1152/physiol.00017.2010. [DOI] [PubMed] [Google Scholar]
- Sundelin SP, Terman A. Different effects of chloroquine and hydroxychloroquine on lysosomal function in cultured retinal pigment epithelial cells. APMIS. 2002;110:481–489. doi: 10.1034/j.1600-0463.2002.100606.x. [DOI] [PubMed] [Google Scholar]
- Toimela T, Salminen L, Tahti H. Effects of tamoxifen, toremifene and chloroquine on the lysosomal enzymes in cultured retinal pigment epithelial cells. Pharmacol Toxicol. 1998;83:246–251. doi: 10.1111/j.1600-0773.1998.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Tseng WA, Thein T, Kinnunen K, Lashkari K, Gregory MS, D’Amore PA, Ksander BR. NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:110–120. doi: 10.1167/iovs.12-10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valapala M, Wilson C, Hose S, Bhutto IA, Grebe R, Dong A, Greenbaum S, Gu L, Sengupta S, Cano M, Hackett S, Xu G, Lutty GA, Dong L, Sergeev Y, Handa JT, Campochiaro P, Wawrousek E, Zigler JS, Sinha D. Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/betaA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy. 2014;10:480–496. doi: 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poel S, Wolthoorn J, van den Heuvel D, Egmond M, Groux-Degroote S, Neumann S, Gerritsen H, van Meer G, Sprong H. Hyperacidification of trans-Golgi network and endo/lysosomes in melanocytes by glucosylceramide-dependent V-ATPase activity. Traffic. 2011;12:1634–1647. doi: 10.1111/j.1600-0854.2011.01263.x. [DOI] [PubMed] [Google Scholar]
- Viiri J, Amadio M, Marchesi N, Hyttinen JM, Kivinen N, Sironen R, Rilla K, Akhtar S, Provenzani A, D’Agostino VG, Govoni S, Pascale A, Agostini H, Petrovski G, Salminen A, Kaarniranta K. Autophagy activation clears ELAVL1/HuR-mediated accumulation of SQSTM1/p62 during proteasomal inhibition in human retinal pigment epithelial cells. PLoS One. 2013;8:e69563. doi: 10.1371/journal.pone.0069563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter CJ. Retinopathy following chloroquine therapy. Med J Aust. 1961;48:741–742. doi: 10.5694/j.1326-5377.1961.tb69111.x. [DOI] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Wolfe DM, Lee JH, Kumar A, Lee S, Orenstein SJ, Nixon RA. Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur J Neurosci. 2013;37:1949–1961. doi: 10.1111/ejn.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YH, Cho KS, Hwang JJ, Lee SJ, Choi JA, Koh JY. Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Invest Ophthalmol Vis Sci. 2010;51:6030–6037. doi: 10.1167/iovs.10-5278. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]