Abstract
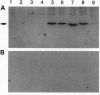
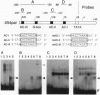
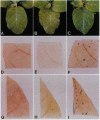
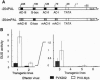
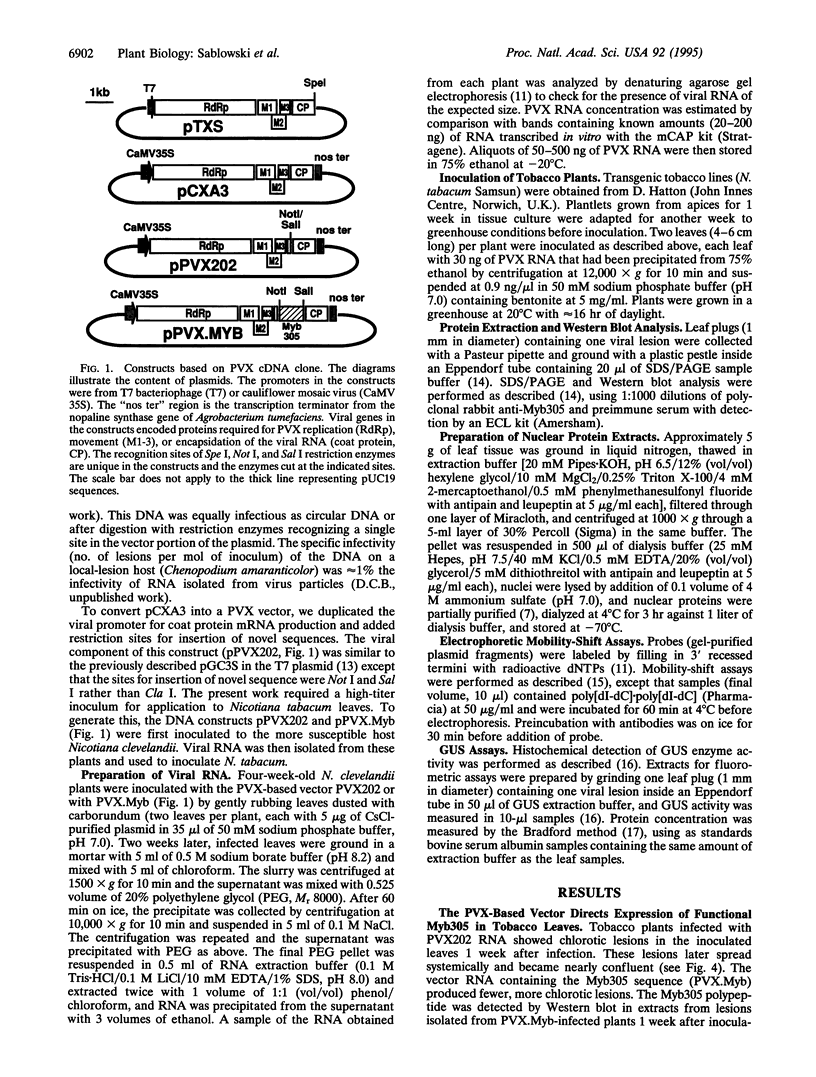
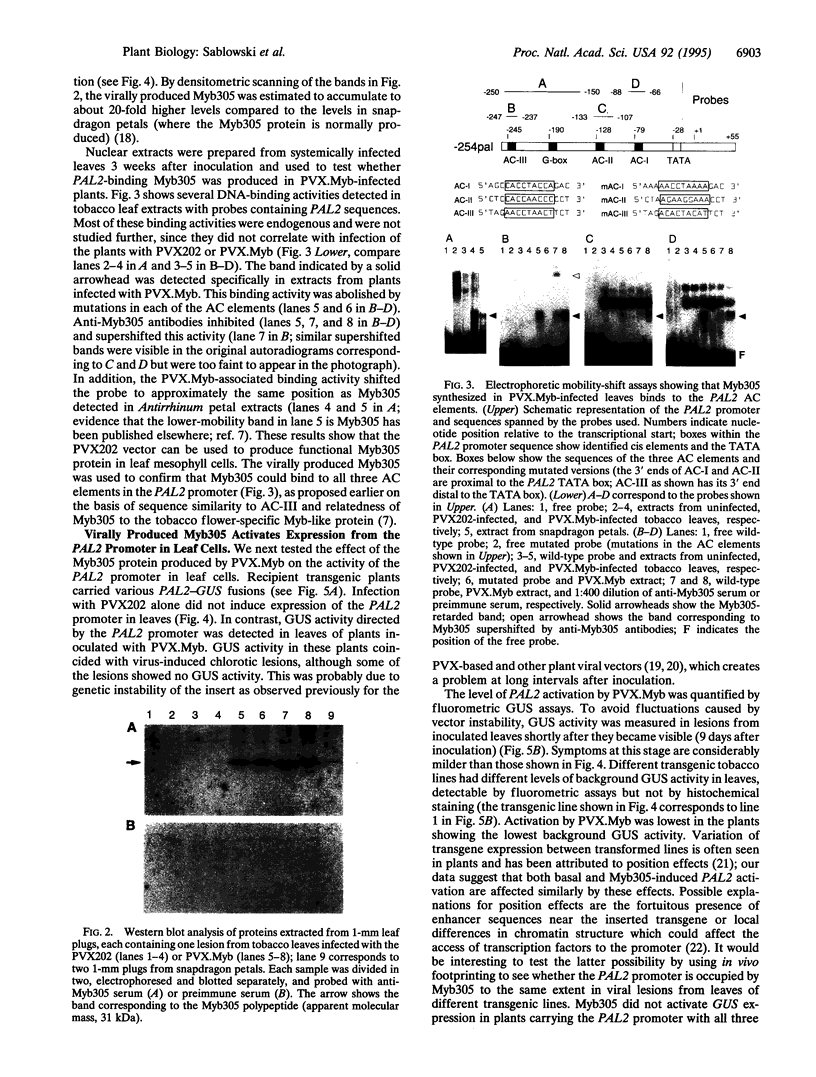
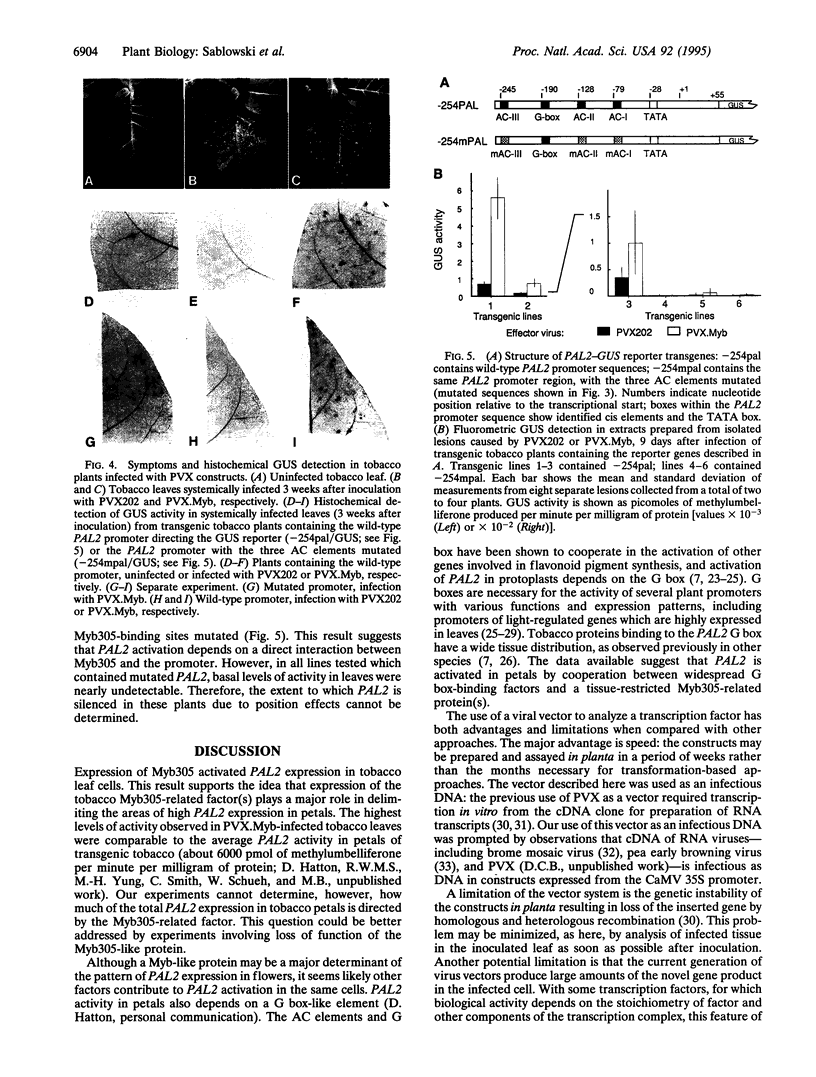
The promoter of the bean PAL2 gene (encoding phenylalanine ammonia-lyase; EC 4.3.1.5) is a model for studies of tissue-restricted gene expression in plants. Petal epidermis is one of the tissues in which this promoter is activated in tobacco. Previous work suggested that a major factor establishing the pattern of PAL2 expression in tobacco petals is the tissue distribution of a protein closely related to Myb305, which is a Myb-like transcriptional activator from snapdragon. In the present work, we show that Myb305 expression in tobacco leaves causes ectopic activation of the PAL2 promoter. To achieve Myb305 expression in planta, a viral expression vector was used. This approach combines the utility of transient assays with the possibility of direct biochemical detection of the introduced factor and may have wider application for studying the function of plant transcription factors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias J. A., Dixon R. A., Lamb C. J. Dissection of the functional architecture of a plant defense gene promoter using a homologous in vitro transcription initiation system. Plant Cell. 1993 Apr;5(4):485–496. doi: 10.1105/tpc.5.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D. C., Lloyd J., Manoussopoulos I. N., Roberts I. M., Harrison B. D. Signal for potyvirus-dependent aphid transmission of potato aucuba mosaic virus and the effect of its transfer to potato virus X. J Gen Virol. 1993 Jul;74(Pt 7):1245–1253. doi: 10.1099/0022-1317-74-7-1245. [DOI] [PubMed] [Google Scholar]
- Beck D. L., Forster R. L., Bevan M. W., Boxen K. A., Lowe S. C. Infectious transcripts and nucleotide sequence of cloned cDNA of the potexvirus white clover mosaic virus. Virology. 1990 Jul;177(1):152–158. doi: 10.1016/0042-6822(90)90469-8. [DOI] [PubMed] [Google Scholar]
- Bevan M., Shufflebottom D., Edwards K., Jefferson R., Schuch W. Tissue- and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. EMBO J. 1989 Jul;8(7):1899–1906. doi: 10.1002/j.1460-2075.1989.tb03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S., Kavanagh T., Baulcombe D. Potato virus X as a vector for gene expression in plants. Plant J. 1992 Jul;2(4):549–557. doi: 10.1046/j.1365-313x.1992.t01-24-00999.x. [DOI] [PubMed] [Google Scholar]
- Dawson W. O., Lewandowski D. J., Hilf M. E., Bubrick P., Raffo A. J., Shaw J. J., Grantham G. L., Desjardins P. R. A tobacco mosaic virus-hybrid expresses and loses an added gene. Virology. 1989 Sep;172(1):285–292. doi: 10.1016/0042-6822(89)90130-x. [DOI] [PubMed] [Google Scholar]
- Dillon N., Grosveld F. Chromatin domains as potential units of eukaryotic gene function. Curr Opin Genet Dev. 1994 Apr;4(2):260–264. doi: 10.1016/s0959-437x(05)80053-x. [DOI] [PubMed] [Google Scholar]
- Donald R. G., Cashmore A. R. Mutation of either G box or I box sequences profoundly affects expression from the Arabidopsis rbcS-1A promoter. EMBO J. 1990 Jun;9(6):1717–1726. doi: 10.1002/j.1460-2075.1990.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donson J., Kearney C. M., Hilf M. E., Dawson W. O. Systemic expression of a bacterial gene by a tobacco mosaic virus-based vector. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7204–7208. doi: 10.1073/pnas.88.16.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granell A., Peretó J. G., Schindler U., Cashmore A. R. Nuclear factors binding to the extensin promoter exhibit differential activity in carrot protoplasts and cells. Plant Mol Biol. 1992 Feb;18(4):739–748. doi: 10.1007/BF00020015. [DOI] [PubMed] [Google Scholar]
- Grotewold E., Drummond B. J., Bowen B., Peterson T. The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthetic gene subset. Cell. 1994 Feb 11;76(3):543–553. doi: 10.1016/0092-8674(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Hobbs S. L., Kpodar P., DeLong C. M. The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol. 1990 Dec;15(6):851–864. doi: 10.1007/BF00039425. [DOI] [PubMed] [Google Scholar]
- Jackson D., Culianez-Macia F., Prescott A. G., Roberts K., Martin C. Expression patterns of myb genes from Antirrhinum flowers. Plant Cell. 1991 Feb;3(2):115–125. doi: 10.1105/tpc.3.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh T., Goulden M., Santa Cruz S., Chapman S., Barker I., Baulcombe D. Molecular analysis of a resistance-breaking strain of potato virus X. Virology. 1992 Aug;189(2):609–617. doi: 10.1016/0042-6822(92)90584-c. [DOI] [PubMed] [Google Scholar]
- Kearney C. M., Donson J., Jones G. E., Dawson W. O. Low level of genetic drift in foreign sequences replicating in an RNA virus in plants. Virology. 1993 Jan;192(1):11–17. doi: 10.1006/viro.1993.1002. [DOI] [PubMed] [Google Scholar]
- Kumagai M. H., Turpen T. H., Weinzettl N., della-Cioppa G., Turpen A. M., Donson J., Hilf M. E., Grantham G. L., Dawson W. O., Chow T. P. Rapid, high-level expression of biologically active alpha-trichosanthin in transfected plants by an RNA viral vector. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):427–430. doi: 10.1073/pnas.90.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. W., Dron M., Schmid J., Dixon R. A., Lamb C. J. Developmental and environmental regulation of a phenylalanine ammonia-lyase-beta-glucuronidase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9284–9288. doi: 10.1073/pnas.86.23.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loake G. J., Faktor O., Lamb C. J., Dixon R. A. Combination of H-box [CCTACC(N)7CT] and G-box (CACGTG) cis elements is necessary for feed-forward stimulation of a chalcone synthase promoter by the phenylpropanoid-pathway intermediate p-coumaric acid. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9230–9234. doi: 10.1073/pnas.89.19.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois R., Dietrich A., Hahlbrock K., Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989 Jun;8(6):1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane S. A., Gilmer D., Davies J. W. Efficient inoculation with CaMV 35 S promoter-driven DNA clones of the tobravirus PEBV. Virology. 1992 Apr;187(2):829–831. doi: 10.1016/0042-6822(92)90488-b. [DOI] [PubMed] [Google Scholar]
- Marcotte W. R., Jr, Russell S. H., Quatrano R. S. Abscisic acid-responsive sequences from the em gene of wheat. Plant Cell. 1989 Oct;1(10):969–976. doi: 10.1105/tpc.1.10.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendree W. L., Jr, Ferl R. J. Functional elements of the Arabidopsis Adh promoter include the G-box. Plant Mol Biol. 1992 Aug;19(5):859–862. doi: 10.1007/BF00027081. [DOI] [PubMed] [Google Scholar]
- Mori M., Mise K., Kobayashi K., Okuno T., Furusawa I. Infectivity of plasmids containing brome mosaic virus cDNA linked to the cauliflower mosaic virus 35S RNA promoter. J Gen Virol. 1991 Feb;72(Pt 2):243–246. doi: 10.1099/0022-1317-72-2-243. [DOI] [PubMed] [Google Scholar]
- Neuhaus G., Neuhaus-Url G., Katagiri F., Seipel K., Chua N. H. Tissue-Specific Expression of as-1 in Transgenic Tobacco. Plant Cell. 1994 Jun;6(6):827–834. doi: 10.1105/tpc.6.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablowski R. W., Moyano E., Culianez-Macia F. A., Schuch W., Martin C., Bevan M. A flower-specific Myb protein activates transcription of phenylpropanoid biosynthetic genes. EMBO J. 1994 Jan 1;13(1):128–137. doi: 10.1002/j.1460-2075.1994.tb06242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lefert P., Dangl J. L., Becker-André M., Hahlbrock K., Schulz W. Inducible in vivo DNA footprints define sequences necessary for UV light activation of the parsley chalcone synthase gene. EMBO J. 1989 Mar;8(3):651–656. doi: 10.1002/j.1460-2075.1989.tb03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staiger D., Kaulen H., Schell J. A CACGTG motif of the Antirrhinum majus chalcone synthase promoter is recognized by an evolutionarily conserved nuclear protein. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6930–6934. doi: 10.1073/pnas.86.18.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer I. M., Brouwer M., Spelt C. E., Mol J. N., Stuitje A. R. The TACPyAT repeats in the chalcone synthase promoter of Petunia hybrida act as a dominant negative cis-acting module in the control of organ-specific expression. Plant J. 1992 Jul;2(4):525–535. doi: 10.1111/j.1365-313x.1992.00525.x. [DOI] [PubMed] [Google Scholar]