Abstract
Macrophages come across active prostaglandin (PG) metabolism during inflammation, shunting early production of pro-inflammatory towards anti-inflammatory mediators terminating the process. This work for the first time provides evidence that a phytochemical may modulate the arachidonate (AA) metabolism in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages, promoting the ultimate formation of anti-inflammatory cyclopentenone 15deoxy-PGJ2. Added 1 h before LPS, indicaxanthin from Opuntia Ficus Indica prevented activation of nuclear factor-κB (NF-κB) and over-expression of PGE2 synthase-1 (mPGES-1), but up-regulated cyclo-oxygenase-2 (COX-2) and PGD2 synthase (H-PGDS), with final production of the anti-inflammatory cyclopentenone. The effects were positively related with concentration between 50 and 100 µM. Indicaxanthin did not have any effect in the absence of LPS.
A kinetic study investigating the redox status of LPS-stimulated macrophages between 0.5 and 12 h, either in the absence or in the presence of 50–100 µM indicaxanthin, revealed a differential control of ROS production, with early (0.5–3 h) modest inhibition, followed by a progressive (3–12 h) concentration-dependent enhancement over the level induced by LPS alone. In addition, indicaxanthin caused early (0.5–3 h) concentration-dependent elevation of conjugated diene lipid hydroperoxides, and production of hydroxynonenal-protein adducts, over the amount induced by LPS. In LPS-stimulated macrophages indicaxanthin did not affect PG metabolism when co-incubated with either an inhibitor of NADPH oxidase or vitamin E. It is concluded that LPS-induced pro-oxidant activity of indicaxanthin at the membrane level allows formation of signaling intermediates whose accumulation modulates PG biosynthetic pathway in inflamed macrophages.
Keywords: Indicaxanthin, Phytochemicals, Eicosanoids, Inflammation, Oxidative stress
Graphical abstract
Highlights
-
•
Phytochemical indicaxanthin promotes synthesis of anti-inflammatory prostaglandins.
-
•
Prooxidant activity of indicaxanthin causes anti-inflammatory response in macrophages.
-
•
Indicaxanthin modulates the redox status of LPS-stimulated macrophages.
-
•
Membrane lipid peroxides are signaling intermediates in inflamed macrophages.
Introduction
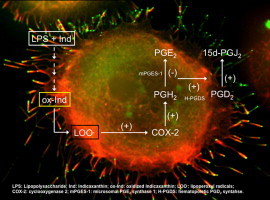
The intracellular redox status is continuously tuned to modulate signaling pathways involved in all basic functions of cell life. Sudden and timely variations, either in antioxidant or pro-oxidant direction, may be essential to permit cells to respond to various stimuli under patho-physiological conditions. In this context macrophages are an interesting paradigm. These cells are indeed crucial effectors long known to produce relatively high amounts of oxidants, a burst of reactive oxygen/nitrogen (ROS/RONS) species as a part of the molecular defense machinery against pathogens and tumor cells [1–3]. The same cells however, have to finely modulate their redox milieu to control metabolic responses during inflammation, in order that the process may switch from the acute phase to its resolution [4], thus avoiding chronicity. The metabolism of arachidonic acid (AA), with expression of inducible cyclo-oxygenase-2 (COX-2), selective activation of COX-2 downstream enzymes PGE2 synthase-1 (microsomal PGE2 synthase 1, mPGES-1) and PGD2 synthase (hematopoietic PGD2 synthase, H-PGDS), and release of mediators such as the pro-inflammatory prostaglandin E2 (PGE2), the anti-inflammatory PGD2 and its derivative 15-deoxy PGJ2 (15d-PGJ2) [5–7], are central in the inflammatory process. Both the induction of COX-2 and synthesis of either pro- or anti-inflammatory Pg have been described as redox-dependent processes [5,8]. On this basis the anti-inflammatory activity of either synthetic or natural redox-active compounds has been somewhat explained. In particular, a number of polyphenol phytochemicals has been shown to inhibit activation of transcription factors that up-regulate COX-2, thus blocking the biosynthetic cascade leading to the synthesis of PG mediators [9,10].
Indicaxanthin (4-[2-(2-Carboxy-pyrrolidine-1-yl)-vinyl]-2,3-dihydro-pyridine-2,6-dicarboxylic acid, Fig. 1) belongs to the betalain class of phytochemicals, the structure of which derives from that of betalamic acid. It is a reducing and amphipathic molecule, can interact with and partition in membranes, penetrate cells and counteract oxidative damage in various cell environments in vitro [11–16]. Moreover indicaxanthin has appeared capable of modulating specific redox-driven signaling pathways involved in the inflammatory response in cultured endothelial cells, and preventing the 7-ketocholesterol apoptotic activity in a human monocyte/macrophage cell line [17,18]. The ability to modulate the activity and/or the expression of the redox-dependent pro-inflammatory enzymes such as myeloperoxidase and NADPH oxidase (NOX) [13,17,19] may play a role in these effects.
Fig. 1.
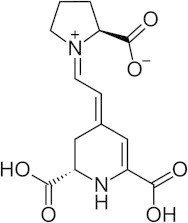
Chemical structure of Indicaxanthin.
Remarkable anti-inflammatory effects of indicaxanthin have recently been demonstrated in an animal model of acute inflammation [20]. The molecule has been shown to cause a rapid decrease of PGE2 and other inflammatory mediators at the early phase of the response, followed by resolution events. These findings prompted us to explore in vitro eventual modulatory activity of indicaxanthin on the main pathways controlling the production of eicosanoids. To this aim murine RAW 264.7 macrophages, stimulated by the bacterial lipopolysaccharide (LPS) have been used. LPS is a pro-inflammatory agent acting through a receptor-mediated signaling pathway [21] leading to the redox-dependent activation of the transcription factor NF-κB and its pro-inflammatory genes downstream [22]. Our findings for the first time show that a natural compound modulates the macrophage activation process leading to the ROS-dependent and COX-2-promoted synthesis of anti-inflammatory Pg and that early production of oxidized membrane lipids appears to be associated with the process.
Materials and methods
Reagents
Unless stated otherwise, all reagents were from Sigma (Milan, Italy) and of the highest grade commercially available.
Indicaxanthin isolation
Indicaxanthin was separated from a methanol extract of cactus pear (Opuntia ficus-indica) fruits (yellow cultivar), by liquid chromatography, followed by semi-preparative HPLC, as previously reported [16].
Cell culture and stimulation
Murine macrophage RAW 264.7 cells (European Collection of Cell Cultures; Sigma, Milan, Italy) were cultured in D-MEM with GlutaMAX™ (Invitrogen, Milan, Italy) supplemented with 10% endotoxin-free, heat-inactivated fetal bovine serum (Invitrogen, Milan, Italy), 0.1% gentamicin and 1% non-essential amino acids at 37 °C in a humidified atmosphere with 5% CO2. Cell viability was assessed through MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] conversion assay according to manufacturer’s instructions (Invitrogen, Milan, Italy). LPS stimulation was performed as previously described [23]. Briefly, cells were seeded in 24-well plates at a density of 2.5×105 cells/mL and allowed to adhere for 12 h. Then the medium was replaced and the cells underwent a 16 h incubation with LPS (1 µg/mL, from Escherichia coli 0127: E8, 1 µg/mL), either in the absence or in the presence of suitable amounts of indicaxanthin in phosphate buffer pH 7.4. When present, indicaxanthin was added to the cells 1 h before LPS challenge. In some experiments, either diphenylene iodonium (DPI, 1 µM), or ⍺-tocopherol (⍺-T, 100 µM), in a final 0.1% ethanol concentration, were co-incubated with indicaxanthin. Control or LPS-treated cells that did not receive other additions, contained the relevant vehicle.
Measurement of PGE2, PGD2 and 15d-PGJ2
The levels of PGE2 and PGD2 in the cell-free supernatants was measured using a 96-well based EIA kit from Cayman Chemicals (Inalco, Milan, Italy), whereas 15d-PGJ2 was determined by using an EIA kit from Assay Designs (TEMA Ricerca, Bologna, Italy) according to the manufacturer’s instructions.
Western-blotting
Cell lysates were prepared as described [23]. The supernatants were collected and stored at −20 °C until tested. Proteins were determined by Bradford assay (Bio-Rad, Milan, Italy).
Immunoblotting analysis of COX-2, mPGES-1, H-PGDS and β-actin proteins was performed as follows. Total cell lysates were mixed with 6× sample buffer (50 mM Tris, 10% SDS, 10% glycerol, 100 mM DTT, 2 mg/mL bromophenol), boiled for 3 min and centrifuged at 15,000 rpm for 5 min. Samples containing 20 µg of protein were resolved on a 12% discontinuous polyacrylamide mini-gel and then electrotransferred to a polyvinylidene difluoride membrane according to the manufacturer’s instructions (Immobilon Millipore, Milan, Italy). Blots were then incubated with polyclonal antibodies against either COX-2 or mPGES-1 or H-PGDS or β-actin (Santa Cruz Biochemicals, Milan, Italy) in a blocking buffer (10% w/v non-fat dry milk in 20 mM Tris–HCl pH 7.4, 125 mM NaCl, 0.01% Tween 20 (TTBS) for 1 h at room temperature) [24]. Then membranes were washed three times with TTBS and further incubated with anti-rabbit or anti-goat IgG conjugated to horseradish peroxidase (Dako, Milan, Italy) for 1 h at room temperature. Finally, the immunoreactive bands were detected by enhanced chemiluminescence (ECL, Amersham, Milan, Italy).
Quantitative real-time reverse-transcription polymerase chain reaction
Total RNA was isolated as follows. Cells were washed twice with ice-cold PBS, harvested and resuspended in 100 µL RNAlater™. Total RNA was isolated from cell pellets using an RNeasy mini kit in accordance with the manufacturer’s instructions (QIAGEN, Milan, Italy). RNA (5–10 µg) was then reverse-transcribed to cDNA using Superscript III Reverse transcriptase following the manufacturer’s protocol (Invitrogen, Milan, Italy) and stored at −20 °C until tested.
Real-time PCR for COX-2, mPGES-1, H-PGDS and Glucose 6-Phosphate dehydrogenase (G6PDH) was carried out with an RT2 Real-Time™ SYBR Green PCR Master Mix and the RT2 PCR Primer Set in accordance with the manufacture’s instructions (SuperArray, Milan, Italy) using an ABIPRISM® 7700 Sequence Detection System (Applied Biosystems, Warrington, UK). Thermal cycling conditions included a pre-run of 2 min at 50 °C and 15 min at 95 °C followed by 40 cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s. RT-PCR data were quantified as cycle of threshold (Ct) values that represents the cycle number at which the fluorescence emission of the reporter dye passed a fixed threshold that was automatically set at 10 standard deviations (SD) above the mean baseline emission. The same threshold and baseline were set for all samples. The mean Ct value of COX-2, mPGES-1 and H-PGDS were normalized to the internal control reference gene by subtracting the mean Ct value of G3PDH from (ΔCt value). The starting gene copy number of the treated sample was determined by comparing with the copy number of the control sample following the equation: ΔΔCt=[ΔCt treated sample −ΔCt control]. The relative gene copy number was calculated by the expression 2−ΔΔCt that represents the gene induction expressed as fold increase with respect to control.
NF-κB luciferase assay
NF-κB activation was checked using a luciferase reporter plasmid, pTAL-NF-κB (Stratagene, La Jolla, CA). Transfections were carried out using Amaxa Nucleofector Technology. Briefly, RAW 264.7 cells were passaged 2 days before nucleofection, on the day of the experiment 100 µL of a cell suspension at 0.5×106 cell/mL was combined with 3 µg of highly purified DNA plasmid, transfected according to the manufacturer protocol and incubated for 12 h in a 5% CO2 atmosphere at 37 °C. Thereafter, cells were stimulated with LPS (1 µg/mL), either in the absence or in the presence of indicaxanthin (50, 75, 100 µM). After 6 h cells were washed with PBS and lysed for 5 min at 4 °C according to the manufacturer’s instructions (Promega, Madison, WI). Luciferase activity is expressed as relative luminescence units (RLU) x103 following reading in a TD/2020 luminometer (Turner Biosystems, Sunnyvale, CA).
Measurement of ROS/RONS and conjugated diene (CD) hydroperoxides
Fluorescence changes that resulted from intracellular oxidation of dichlorodihydrofluorescein diacetate were monitored to reveal changes of the redox status by the endocellular content of ROS/RONS. Dichlorodihydro-fluorescein diacetate, 10 µM final concentration, was added to the cell medium 30 min before the end of treatment. The cells were collected by centrifugation for 5 min at 2000 rpm at 48 °C, washed, suspended in PBS and immediately subjected to fluorescence-activated cell sorting analysis using an EPICS XL cytofluorimeter (Beckman Coulter Inc., US). At least 1×104 cells were analyzed for each sample.
CD hydroperoxides were evaluated spectrophotometrically. The cells were precipitated (20,000 rpm, 5 min, 4 °C) and CD hydroperoxides extracted with 3 mL of a CHCl3:CH3OH mixture (2:1). The organic extract was evaporated under a nitrogen stream, re-suspended in cyclohexane and quantified by the absorbance at 234 nm, using a molar absorption coefficient of 27,000 [25] and a DU-600 spectrophotometer (Beckman Coulter Inc., US).
HNE-protein adducts determination
The levels of HNE protein adducts in cell lysates were measured using a 96-well OxiSelect™ HNE Adduct Competitive ELISA Kit from Cell Biolabs (San Diego, CA, USA).
Statistical analysis
Comparisons were made using one-way analysis of variance (ANOVA) followed by Bonferroni’s test (Instat-3 statistical software, GraphPad Software). P<0.05 was considered statistically significant.
Results and discussion
Indicaxanthin modulates PG biosynthesis in LPS-activated macrophages
LPS-stimulated RAW 264.7 cells have been used as a model to investigate on the activity of indicaxanthin in modulating PG metabolism. These cells exhibit functional characteristics of primary macrophages and this model offers the advantage of expressing COX-2 and a limited set of PG synthases downstream, namely m-PGES1 and H-PGDS [26], therefore coordinate relations among these enzymes and their products can directly be examined. It should be mentioned that, though physiological oxygen tension at tissue level is quite lower, our measurements were performed at normoxic conditions (21% O2), which makes the LPS-activated RAW 264.7 macrophages a suitable system to validate the anti-inflammatory potential of compounds in term of PG production. The time-course of the LPS-induced production of main mediators, i.e. the pro-inflammatory PGE2, anti-inflammatory PGD2 and its spontaneous dehydration product 15d-PGJ2, was analyzed either in the absence or in the presence of indicaxanthin. With respect to non-stimulated (control) cells, LPS treatment caused a release of PGE2 that accumulated during 16 h of observation (Fig. 2A), but did not have any effect on formation and release of the anti-inflammatory Pg (Fig. 2B). A 1 h pre-treatment of cells with 100 µM indicaxanthin caused a significant decrease of PGE2 (Fig. 2A) and production of PGD2 with a peak at 8 h, followed by formation of 15d-PGJ2 with a peak at 12 h (Fig. 2B). The effect positively correlated with concentration between 50 and 100 µM, whereas 5 and 25 µM indicaxanthin did not modify significantly the LPS-induced release of any mediator (Fig. 3A–C). MTT assay carried out in parallel showed that the decreased level of PGE2 did not result from loss of cell viability. Under the same conditions, exposure of cells to indicaxanthin alone (100 µM) did not affect cell viability nor have any effect on the release of PG mediators (Fig. 2A and B). In the light of these results, subsequent experiments were carried out with 50–100 µM indicaxanthin, unless specified.
Fig. 2.
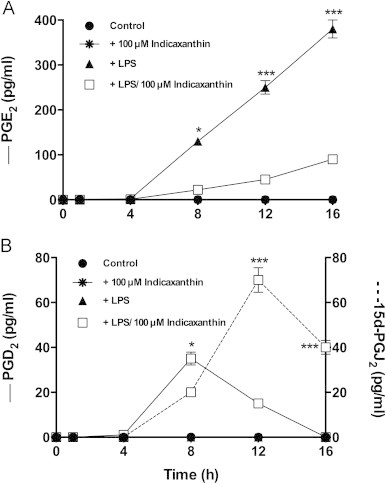
Kinetics of release of PGE2 (A) and PGD2 and 15d-PGJ2 (B) in control or LPS-activated RAW264.7 macrophages, either in the absence or in the presence of Indicaxanthin. Values are the mean±SEM of three separate experiments carried out in triplicate. Concentration differences at each time level were assessed by Bonferroni’s test with ⁎P=0.0112; ⁎⁎⁎P<0.001.
Fig. 3.
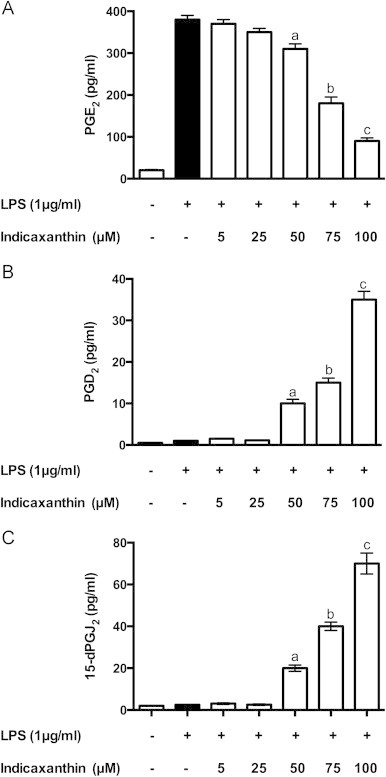
Effect of pre-treatment of LPS-activated RAW264.7 macrophages with Indicaxanthin concentrations increasing from 5 to 100 µM on release of PGE2 (A), PGD2 (B) and 15d-PGJ2 (C). Values are the mean±SEM of three separate experiments carried out in triplicate. Labeled means without a common letter differ, P<0.05.
The inducible COX-2 is central in the eicosanoid cascade during inflammation, since its PGH2 product may serve as a substrate for either mPGES-1 or H-PGDS, forming PGE2 or PGD2, respectively. A regulated balance between these enzyme activities and their products differentiates the acute inflammatory response vs the subsequent resolution phase. Indicaxanthin pre-treatment reverted the LPS-induced pro-/anti-inflammatory eicosanoid ratio, then the mechanism through which the COX-2/PGE2-PGD2 biosynthetic axes had been altered was investigated by measuring the level of the enzymes concerned. The analysis of both RNA and protein levels showed that, in accordance with the formation of PGE2, LPS-stimulation caused a remarkable increase of mRNA and protein level of COX-2 (Fig. 4A and D) and its downstream m-PGES1enzyme (Fig. 4B and E), accompanied by only a modest elevation of H-PGDS (Fig. 4C and F). The same analysis revealed remarkable effects of indicaxanthin, on the COX-2/PG biosynthesis pathway: the phytochemical up-regulated COX-2 (Fig. 4A and D), whose level appeared up to three times higher than observed in the presence of LPS alone; down-regulated mPGES-1 (Fig. 4B and E) and up-regulated H-PGDS (Fig. 4C and F), consistently with the observed PG production. All these effects were positively related with concentration. Indicaxanthin alone did not bring about any significant alteration of the expression of COX-2, PGES-1 and H-PGDS (not shown).
Fig. 4.
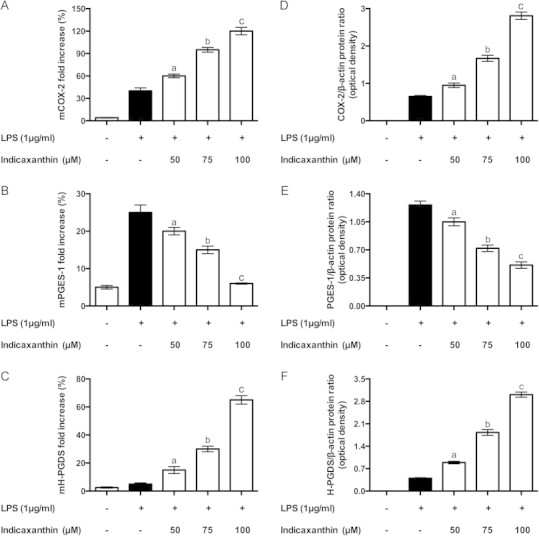
COX-2, PGES-1, H-PGDS mRNA (A, C, and E) and COX-2, PGES-1, H-PGDS protein levels (B, D, and F) in control or LPS-activated RAW264.7 macrophages, either in the absence or in the presence of Indicaxanthin. Immunoblots are representative images of three experiments with comparable results. Values are the mean±SEM of three separate experiments carried out in triplicate. Labeled means without a common letter differ, P<0.05.
Indicaxanthin modulates LPS-induced variations of the cell redox environment
Activation of various isoforms of NADPH oxidase (NOX) including the macrophage NOX-4 [3,27] and ROS production are associated with LPS activity, and are essential to determine a well-timed evolution of the macrophage response with early formation of pro- and final of anti-inflammatory mediators. ROS elevation has indeed known to be involved in the AA release from membrane, COX-2 expression and PG production [28,29], and may differentially regulate LPS-induced PGD2 and PGE2 production in macrophages, activating the former with no effect on the latter [24]. The cell redox tone was investigated by monitoring the ROS level in macrophages stimulated with LPS, that were pre-treated or not with 50–100 µM indicaxanthin, between 30 min and 12 h. Data reported in Fig. 5A show elevation of ROS by LPS within 30 min, with no further significant modification until the end of observation. Pre-treatment with indicaxanthin had a time-dependent bimodal effect, i.e. an early (30 min) decrease of ROS level was observed, followed by a slow increase over 3 h, evolving in a sharp rise between 6 h and 12 h (Fig. 5A). The pro-oxidant effect was positively related with concentration and final level of ROS in the presence of 100 µM indicaxanthin was four-fold higher than observed under the mere LPS stimulation (Fig. 5). Indicaxanthin alone (100 µM) was ineffective (Fig. 5A and B). It deserves to be mentioned that data not reported provided evidence that pre-treatment with low indicaxanthin concentrations (5 and 25 µM) that did not show any effect on PG synthesis (Fig. 3), prevented the LPS-dependent ROS production at any time-point. While confirming that redox-dependent mechanisms that control AA metabolic pathways in the macrophages require an increased oxidative tone [28,29], all these findings suggested that the pro-oxidant activity of indicaxanthin had a main role in modulating the COX-2/PG biosynthetic axes. In accordance with the literature data [24], through over-production of ROS, indicaxanthin could bring about a timely expression and activity of both COX-2 and H-PGDS, this resulting in shifting PGH2 towards the terminal production of 15d-PGJ2.
Fig. 5.
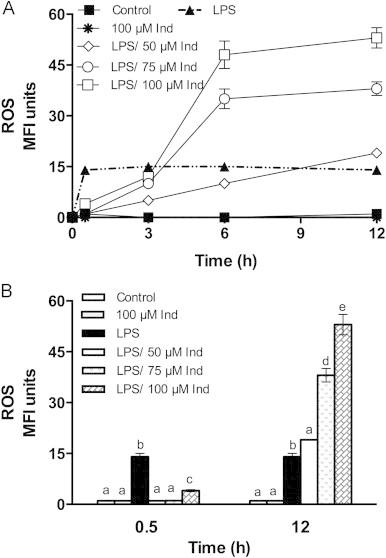
Kinetics of LPS-induced ROS production in RAW264.7 macrophages, either in the absence (full symbols) or in the presence (open symbols) of Indicaxanthin. Values are the mean±SEM of three separate experiments carried out in triplicate (A). Statistical significance of the data at 0.5 and 12 h is reported in (B). Labeled means without a common letter differ, P<0.05.
LPS-induced ROS production in macrophages precedes several signaling events leading to early activation of NF-κB [8,30], that controls a rapid and coordinated transcription of a number of genes including COX-2 [31], and mPGES-1 [32]. Because indicaxanthin pre-treatment resulted in early decrease of ROS levels, we examined whether NF-κB activation was affected. RAW 264.7 cells were transfected with an NF-κB reporter construct prior to stimulation with LPS. Basal luciferase activity of un-stimulated cells increased four-fold in LPS-activated macrophages (Fig. 6). Pre-treatment with indicaxanthin caused a clear concentration-dependent inhibition of NF-κB activation (Fig. 6). Indicaxanthin alone did not affect the activation status of NF-κB (not shown).
Fig. 6.
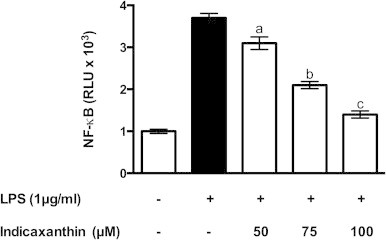
NF-κB activation in control or LPS-activated RAW264.7 macrophages, either in the absence or in the presence of Indicaxanthin. Values are the mean±SEM of three separate experiments carried out in triplicate. Labeled means without a common letter differ, P<0.05.
As a global picture, the effects of indicaxanthin in LPS-activated macrophages appeared first associated with an inhibition of NF-κB activation, which may account for mPGES-1 down-regulation; subsequently, through activation of ROS generating systems, indicaxanthin may allow COX-2 and H-PGDS over-expression.
Modulatory activity of indicaxanthin on PG biosynthesis in LPS-stimulated RAW 234.7 macrophages is associated with lipid oxidation
Activity of indicaxanthin on ROS production appeared to depend on (i) the presence of LPS, (ii) concentration and (iii) time. These observations suggested that indicaxanthin had to be transformed in an active compound in an LPS-dependent reaction and possibly other mediators accumulated. Oxidized phospholipids have appeared to possess a number of biological activities and induce signaling and transcriptional pathways that may affect various aspects of the inflammatory process [33]. Endogenously oxidized lipids have been suggested as part of a defense strategy, serving as negative feedback mechanisms in promoting resolution of inflammation [34]. Similar roles have been considered for reactive aldehydes such as hydroxyl-nonenal (HNE) and related end-products of lipid oxidation, whose time-dependent accumulation can be regarded as a hormetic and adaptative response [35,36] starting signaling pathways [37–39].
Membrane phospholipids in particular containing polyunsaturated acyl chains such as 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine, are very prone to oxidation by NOX-derived reactive species [34]. Lipid oxidation in LPS-stimulated RAW 264.7 macrophages, that had been or not pre-treated with 50–100 µM indicaxanthin, was monitored at time-intervals by the formation of CD hydroperoxides. Data reported in Fig. 7A show that, under the conditions applied, LPS stimulation brought about formation of CD hydroperoxides with a peak at 6 h, followed by a decline. Indicaxanthin pre-treatment caused an early and concentration-dependent increase of CD hydroperoxides (Fig. 7A and B), well over the amount measured in the presence of LPS alone. The level of CD hydroperoxides peaked at 30 min, followed by a decline as faster as higher the indicaxanthin concentration (Fig. 7A). Production of HNE, as protein adducts, was evaluated in the same experiments, either in the absence or in the presence of indicaxanthin. Appearance of HNE adducts was consistent with the CD hydroperoxide production in LPS-stimulated macrophages (Fig. 7C). Likewise, the increased production of CD hydroperoxides by pre-treatment with indicaxanthin was paralleled by HNE-protein levels higher than observed with LPS alone (Fig. 7C), that increased at the increase of indicaxanthin (Fig. 7D). Indicaxanthin alone (100 µM) did not cause formation of CD peroxides nor HNE in the absence of LPS, during the entire observation time (Fig. 5A–D). Then, while showing that in LPS-stimulated macrophages indicaxanthin promoted a concentration-dependent lipid oxidation, our data indicated that the activity of LPS was a necessary requirement for its pro-oxidant activity. Indicaxanthin is amphipathic and partitions in phospholipid bilayers at a level between the polar heads and hydrocarbon chains [40–42]. Previous studies showed that while behaving as a lipoperoxyl radical-scavenger, indicaxanthin exhibited some pro-oxidant activity in liposomal membranes of unsaturated lipids in the presence of a lipid-soluble azo-initiator [15]. This was ascribed to the capacity of the oxidized molecule of self-regenerating at the expenses of unsaturated lipids, and was more evident at the increasing of its concentration over 20 µM [15]. On this basis we tentatively maintain that indicaxanthin, early oxidized as a consequence of LPS-induced NOX activity and lipoperoxide formation, may promote further lipid oxidation at the macrophage membrane. In the LPS-stimulated macrophage system, however, oxidized lipids did not exert cytotoxicity, rather their formation appeared coherent with cell responses, suggesting a role as functional mediators.
Fig. 7.
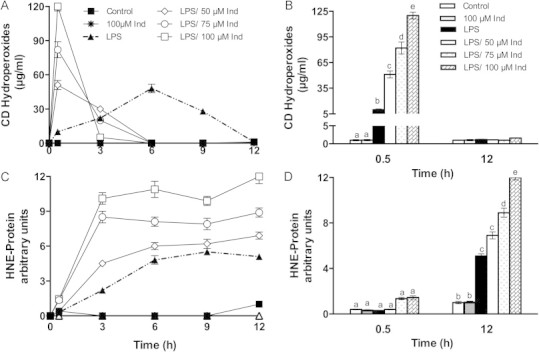
Kinetics of LPS-induced CD lipid hydroperoxide (A), and HNE-protein adducts (C) production in RAW264.7 macrophages, either in the absence (solid symbols) or in the presence (open symbols) of Indicaxanthin. Values are the mean±SEM of three separate experiments carried out in triplicate (A). Statistical significance of the data at 0.5 and 12 h is reported in (B). Labeled means without a common letter differ, P<0.05.
In the light that both ROS generated and oxidized lipids appeared associated with the indicaxanthin capacity of modulating progression of PG biosynthesis in LPS-stimulated macrophages, we researched whether and to what extent indicaxanthin stimulated the formation of the mediator PGD2 in the presence of either DPI, as an inhibitor of NOX, or ⍺-T, as a competing lipoperoxyl radical-scavenger [15]. As expected, formation of CD lipid hydroperoxides was prevented by DPI, either without or with indicaxanthin pre-treatment (Fig. 8). On the other hand, the indicaxanthin-promoted production of PGD2 was also prevented by DPI. Co-incubation with ⍺-T inhibited the indicaxanthin-stimulated formation of lipid hydroperoxides, the level of which was comparable with that observed with LPS alone, as was the accumulated PGD2 (Fig. 8). The data above may suggest that promoting lipid oxidation indicaxanthin provides macrophages with active intermediates starting signaling pathways and driving metabolic responses. Among other effects, lipoperoxides and reactive aldehydes may up-regulate PG biosynthesis in RAW 264.7 macrophages [43], have appeared capable of inhibiting LPS signaling [44,45], may prevent activation of the NF-κB pathway [46,47], block NADPH oxidase at low [48], whereas activate ROS generating systems at elevated concentrations [48–50], induce COX-2 expression [43,47]. The non-conventional effect of indicaxanthin exerted through pro-oxidant activity of relatively high concentrations may offer new perspectives to shed light on yet poorly known mechanisms that switch macrophage PG metabolism leading to production of anti-inflammatory mediators, and appears to mimic and accelerate a patho-physiological activity of the macrophage [51,52] more than simply arresting its pro-inflammatory response. On the basis of our present knowledge it is not easy to assess whether, or to what extent, this activity has a role in the potent anti-inflammatory effect of indicaxanthin administered to rats at the small concentrations consistent with dietary intake [20].
Fig. 8.
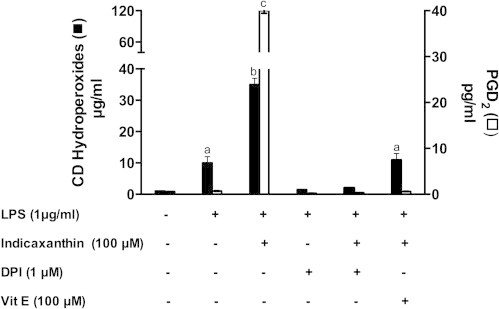
Effect of DPI and vit E on the production of CD hydroperoxides and PGD2 in LPS-activated RAW264.7 macrophages, either in the absence or in the presence of Indicaxanthin. Amounts of CD hydroperoxides at 0.5 h, and of PGD2 at 8 h, are represented. Values are the mean±SEM of three separate experiments carried out in triplicate. Labeled means without a common letter differ, P<0.05.
Appendix A. Supplementary material
Supplementary Data
Supplementary Data
References
- 1.Johnston R.B., Jr. Oxygen metabolism and the microbicidal activity of macrophages. Federation Proceedings. 1978;37:2759–2764. 213318 [PubMed] [Google Scholar]
- 2.Qin L., Liu Y., Wang T., Wei S.J., Block M.L., Wilson B., Liu B., Hong J.S. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. Journal of Biological Chemistry. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. 14578353 [DOI] [PubMed] [Google Scholar]
- 3.Rada B., Leto T.L. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contributions to Microbiology. 2008;15:164–187. doi: 10.1159/000136357. 18511861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan C.N., Brain S.D., Buckley C.D., Gilroy D.W., Haslett C., O’Neill L.A.J., Perretti M., Rossi A.G., Wallace J.L. Resolution of inflammation: state of the art, definitions and terms. FASEB Journal. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi U.H., Kaushal N., Ravindra K.C., Hegde S., Nelson S.M., Narayan V., Vunta H., Paulson R.F., Prabhu K.S. Selenoprotein-dependent up-regulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) gamma. Journal of Biological Chemistry. 2011;286:27471–27482. doi: 10.1074/jbc.M111.260547. 21669866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vunta H., Davis F., Palempalli U.D., Bhat D., Arner R.J., Thompson J.T., Peterson D.G., Reddy C.C., Prabhu K.S. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. Journal of Biological Chemistry. 2007;282:17964–17973. doi: 10.1074/jbc.M703075200. 17439952 [DOI] [PubMed] [Google Scholar]
- 7.Surh Y., Na H., Park J., Lee H., Kim W., Yoon I., Kim D. 15-Deoxy-Δ12,14-prostaglandin J2, an electrophilic lipid mediator of anti-inflammatory and pro-resolving signaling. Biochemical Pharmacology. 2011;82:1335–1351. doi: 10.1016/j.bcp.2011.07.100. [DOI] [PubMed] [Google Scholar]
- 8.Zamamiri-Davis F., Lu Y., Thompson J.T., Prabhu K.S., Reddy P.V., Sordillo L.M., Reddy C.C. Nuclear factor-kappaB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency. Free Radical Biology & Medicine. 2002;32:890–897. doi: 10.1016/s0891-5849(02)00775-x. 11978490 [DOI] [PubMed] [Google Scholar]
- 9.Surh Y.J., Kundu J.K., Na H.K., Lee J.S. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. Journal of Nutrition. 2005;135:2993S–3001S. doi: 10.1093/jn/135.12.2993S. 16317160 [DOI] [PubMed] [Google Scholar]
- 10.Cerella C., Sobolewski C., Dicato M., Diederich M. Targeting COX-2 expression by natural compounds: a promising alternative strategy to synthetic COX-2 inhibitors for cancer chemoprevention and therapy. Biochemical Pharmacology. 2010;80:1801–1815. doi: 10.1016/j.bcp.2010.06.050. 20615394 [DOI] [PubMed] [Google Scholar]
- 11.Tesoriere L., Allegra M., Butera D., Gentile C., Livrea M.A. Cytoprotective effects of the antioxidant phytochemical indicaxanthin in beta-thalassemia red blood cells. Free Radical Research. 2006;40:753–761. doi: 10.1080/10715760600554228. 16984002 [DOI] [PubMed] [Google Scholar]
- 12.Tesoriere L., Butera D., D’Arpa D., Di Gaudio F., Allegra M., Gentile C., Livrea M.A. Increased resistance to oxidation of betalain-enriched human low density lipoproteins. Free Radical Research. 2003;37:689–696. doi: 10.1080/1071576031000097490. 12868496 [DOI] [PubMed] [Google Scholar]
- 13.Allegra M., Furtmüller P.G., Jantschko W., Zederbauer M., Tesoriere L., Livrea M.A., Obinger C. Mechanism of interaction of betanin and indicaxanthin with human myeloperoxidase and hypochlorous acid. Biochemical and Biophysical Research Communications. 2005;332:837–844. doi: 10.1016/j.bbrc.2005.05.031. 15913556 [DOI] [PubMed] [Google Scholar]
- 14.Tesoriere L., Butera D., Allegra M., Fazzari M., Livrea M.A. Distribution of betalain pigments in red blood cells after consumption of cactus pear fruits and increased resistance of the cells to ex vivo induced oxidative hemolysis in humans. Journal of Agricultural and Food Chemistry. 2005;53:1266–1270. doi: 10.1021/jf048134+. 15713051. [DOI] [PubMed] [Google Scholar]
- 15.Tesoriere L., Allegra M., Butera D., Gentile C., Livrea M.A. Kinetics of the lipoperoxyl radical-scavenging activity of indicaxanthin in solution and unilamellar liposomes. Free Radical Research. 2007;41:226–233. doi: 10.1080/10715760601026614. 17364949 [DOI] [PubMed] [Google Scholar]
- 16.Butera D., Tesoriere L., Di Gaudio F., Bongiorno A., Allegra M., Pintaudi A.M., Kohen R., Livrea M.A. Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: betanin and indicaxanthin. Journal of Agricultural and Food Chemistry. 2002;50:6895–6901. doi: 10.1021/jf025696p. 12405794 [DOI] [PubMed] [Google Scholar]
- 17.Tesoriere L., Attanzio A., Allegra M., Gentile C., Livrea M.A. Phytochemical indicaxanthin suppresses 7-ketocholesterol-induced THP-1 cell apoptosis by preventing cytosolic Ca(2+) increase and oxidative stress. British Journal of Nutrition. 2013;110:230–240. doi: 10.1017/S000711451200493X. 23228674 [DOI] [PubMed] [Google Scholar]
- 18.Gentile C., Tesoriere L., Allegra M., Livrea M.A., D’Alessio P. Antioxidant betalains from cactus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1 expression. Annals of the New York Academy of Sciences. 2004;1028:481–486. doi: 10.1196/annals.1322.057. 15650274 [DOI] [PubMed] [Google Scholar]
- 19.Tesoriere L., Attanzio A., Allegra M., Gentile C., Livrea M.A. Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-κB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1β-exposed Caco-2 cells. British Journal of Nutrition. 2014;111:415–423. doi: 10.1017/S0007114513002663. [DOI] [PubMed] [Google Scholar]
- 20.Allegra M., Ianaro A., Tersigni M., Panza E., Tesoriere L., Livrea M.A. Indicaxanthin from cactus pear fruit exerts anti-inflammatory effects in carrageenin-induced rat pleurisy. Journal of Nutrition. 2014;144:185–192. doi: 10.3945/jn.113.183657. 24306215 [DOI] [PubMed] [Google Scholar]
- 21.Pålsson-McDermott E.M., O’Neill L.A. Signal transduction by the lipopolysaccharide receptor, toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. 15379975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akira S., Takeda K. Toll-like receptor signalling. Nature Reviews Immunology. 2004;4:499–511. doi: 10.1038/nri1391. 15229469 [DOI] [PubMed] [Google Scholar]
- 23.Yi P.F., Bi W.Y., Shen H.Q., Wei Q., Zhang L.Y., Dong H.B., Bai H.L., Zhang C., Song Z., Qin Q.Q., Lv S., Wu S.C., Fu B.D., Wei X.B. Inhibitory effects of sulfated 20(S)-ginsenoside Rh2 on the release of pro-inflammatory mediators in LPS-induced RAW 264.7 cells. European Journal of Pharmacology. 2013;712:60–66. doi: 10.1016/j.ejphar.2013.04.036. 23665488 [DOI] [PubMed] [Google Scholar]
- 24.Zhao G., Yu R., Deng J., Zhao Q., Li Y., Joo M., van Breemen R.B., Christman J.W., Xiao L. Pivotal role of reactive oxygen species in differential regulation of lipopolysaccharide-induced prostaglandins production in macrophages. Molecular Pharmacology. 2013;83:167–178. doi: 10.1124/mol.112.080762. 23071105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pryor W.A., Castle L. Chemical methods for the detection of lipid hydroperoxides. Methods in Enzymology. 1984;105:293–299. doi: 10.1016/s0076-6879(84)05037-0. 6727667 [DOI] [PubMed] [Google Scholar]
- 26.Norris P.C., Reichart D., Dumlao D.S., Glass C.K., Dennis E.A. Specificity of eicosanoid production depends on the TLR-4-stimulated macrophage phenotype. Journal of Leukocyte Biology. 2011;90:563–574. doi: 10.1189/jlb.0311153. 21653236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ngkelo A., Meja K., Yeadon M., Adcock I., Kirkham P.A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Gialpha dependent PI-3kinase signalling. Journal of Inflammation. 2012;9:1. doi: 10.1186/1476-9255-9-1. 22239975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez J., Moreno J.J. Role of Ca2+-independent phospholipase A2 on arachidonic acid release induced by reactive oxygen species. Archives of Biochemistry and Biophysics. 2001;392:257–262. doi: 10.1006/abbi.2001.2439. 11488600 [DOI] [PubMed] [Google Scholar]
- 29.Martínez J., Moreno J.J. Influence of superoxide radical and hydrogen peroxide on arachidonic acid mobilization. Archives of Biochemistry and Biophysics. 1996;336:191–198. doi: 10.1006/abbi.1996.0549. 8954566 [DOI] [PubMed] [Google Scholar]
- 30.Hayden M.S., West A.P., Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. 17072327 [DOI] [PubMed] [Google Scholar]
- 31.Tanabe T., Tohnai N. Cyclooxygenase isozymes and their gene structures and expression. Prostaglandins & Other Lipid Mediators. 2002;68–69:95–114. doi: 10.1016/s0090-6980(02)00024-2. 12432912 [DOI] [PubMed] [Google Scholar]
- 32.Díaz-Muñoz M.D., Osma-García I.C., Cacheiro-Llaguno C., Fresno M., Iñiguez M.A. Coordinated up-regulation of cyclooxygenase-2 and microsomal prostaglandin E synthase 1 transcription by nuclear factor kappa B and early growth response-1 in macrophages. Cellular Signalling. 2010;22:1427–1436. doi: 10.1016/j.cellsig.2010.05.011. 20546888 [DOI] [PubMed] [Google Scholar]
- 33.Bochkov V.N. Inflammatory profile of oxidized phospholipids. Thrombosis and Haemostasis. 2007;97:348–354. 17334500 [PubMed] [Google Scholar]
- 34.Bochkov V.N., Oskolkova O.V., Birukov K.G., Levonen A.L., Binder C.J., Stöckl J. Generation and biological activities of oxidized phospholipids. Antioxidants & Redox Signaling. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. 19686040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vatsyayan R., Lelsani P.C., Chaudhary P., Kumar S., Awasthi S., Awasthi Y.C. The expression and function of vascular endothelial growth factor in retinal pigment epithelial (RPE) cells is regulated by 4-hydroxynonenal (HNE) and glutathione S-transferaseA4-4. Biochemical and Biophysical Research Communications. 2012;417:346–351. doi: 10.1016/j.bbrc.2011.11.113. 22155253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radical Biology & Medicine. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. 23747930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harry R.S., Hiatt L.A., Kimmel D.W., Carney C.K., Halfpenny K.C., Cliffel D.E., Wright D.W. Metabolic impact of 4-hydroxynonenal on macrophage-like RAW 264.7 function and activation. Chemical Research in Toxicology. 2012;25:1643–1651. doi: 10.1021/tx3001048. 22799741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poli G., Leonarduzzi G., Biasi F., Chiarpotto E. Oxidative stress and cell signalling. Current Medicinal Chemistry. 2004;11:1163–1182. doi: 10.2174/0929867043365323. 15134513 [DOI] [PubMed] [Google Scholar]
- 39.Forman H.J., Fukuto J.M., Miller T., Zhang H., Rinna A., Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Archives of Biochemistry and Biophysics. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. 18602883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tesoriere L., Gentile C., Angileri F., Attanzio A., Tutone M., Allegra M., Livrea M.A. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. European Journal of Nutrition. 2013;52:1077–1087. doi: 10.1007/s00394-012-0414-5. 22806766 [DOI] [PubMed] [Google Scholar]
- 41.Liveri M.L., Sciascia L., Lombardo R., Tesoriere L., Passante E., Livrea M.A. Spectrophotometric evidence for the solubilization site of betalain pigments in membrane biomimetic systems. Journal of Agricultural and Food Chemistry. 2007;55:2836–2840. doi: 10.1021/jf0632536. 17381111 [DOI] [PubMed] [Google Scholar]
- 42.Turco Liveri M.L., Sciascia L., Allegra M., Tesoriere L., Livrea M.A. Partition of indicaxanthin in membrane biomimetic systems. A kinetic and modeling approach. Journal of Agricultural and Food Chemistry. 2009;57:10959–10963. doi: 10.1021/jf902266m. 19919126 [DOI] [PubMed] [Google Scholar]
- 43.Kumagai T., Matsukawa N., Kaneko Y., Kusumi Y., Mitsumata M., Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. Journal of Biological Chemistry. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. 15355999 [DOI] [PubMed] [Google Scholar]
- 44.Erridge C., Kennedy S., Spickett C.M., Webb D.J. Oxidized phospholipid inhibition of Toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. Journal of Biological Chemistry. 2008;283:24748–24759. doi: 10.1074/jbc.M800352200. 18559343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marantos C., Mukaro V., Ferrante J., Hii C., Ferrante A. Inhibition of the lipopolysaccharide-induced stimulation of the members of the MAPK family in human monocytes/macrophages by 4-hydroxynonenal, a product of oxidized omega-6 fatty acids. American Journal of Pathology. 2008;173:1057–1066. doi: 10.2353/ajpath.2008.071150. 18772336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Page S., Fischer C., Baumgartner B., Haas M., Kreusel U., Loidl G., Hayn M., Ziegler-Heitbrock H.W., Neumeier D., Brand K. 4-hydroxynonenal prevents NF-kappaB activation and tumor necrosis factor expression by inhibiting IkappaB phosphorylation and subsequent proteolysis. Journal of Biological Chemistry. 1999;274:11611–11618. doi: 10.1074/jbc.274.17.11611. 10206970 [DOI] [PubMed] [Google Scholar]
- 47.Bochkov V.N., Leitinger N. Anti-inflammatory properties of lipid oxidation products. Journal of Molecular Medicine. 2003;81:613–626. doi: 10.1007/s00109-003-0467-2. 13679995 [DOI] [PubMed] [Google Scholar]
- 48.Starosta V., Wu T., Zimman A., Pham D., Tian X., Oskolkova O., Bochkov V., Berliner J.A., Birukova A.A., Birukov K.G. Differential regulation of endothelial cell permeability by high and low doses of oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine. American Journal of Respiratory Cell and Molecular Biology. 2012;46:331–341. doi: 10.1165/rcmb.2011-0153OC. 21997484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S., Gharavi N.M., Honda H., Chang I., Kim B., Jen N., Li R., Zimman A., Berliner J.A. A role for NADPH oxidase 4 in the activation of vascular endothelial cells by oxidized phospholipids. Free Radical Biology and Medicine. 2009;47:145–151. doi: 10.1016/j.freeradbiomed.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouhanizadeh M., Hwang J., Clempus R.E., Marcu L., Lassègue B., Sevanian A., Hsiai T.K. Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: implication of NADPH oxidase. Free Radical Biology & Medicine. 2005;39:1512–1522. doi: 10.1016/j.freeradbiomed.2005.07.013. 16274886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Covarrubias A., Byles V., Horng T. ROS sets the stage for macrophage differentiation. Cell Research. 2013;23:984–985. doi: 10.1038/cr.2013.88. 23835480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Choksi S., Chen K., Pobezinskaya Y., Linnoila I., Liu Z.G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Research. 2013;23:898–914. doi: 10.1038/cr.2013.75. 23752925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data
Supplementary Data


