Abstract
Leptospirosis is an acute, febrile disease occurring in humans and animals worldwide. Leptospira spp. are usually transmitted through direct or indirect contact with the urine of infected reservoir animals. Among wildlife species, rodents act as the most important reservoir for both human and animal infection. To gain a better understanding of the occurrence and distribution of pathogenic leptospires in rodent and shrew populations in Germany, kidney specimens of 2973 animals from 11 of the 16 federal states were examined by PCR. Rodent species captured included five murine species (family Muridae), six vole species (family Cricetidae) and six shrew species (family Soricidae). The most abundantly trapped animals were representatives of the rodent species Apodemus flavicollis, Clethrionomys glareolus and Microtus agrestis. Leptospiral DNA was amplified in 10% of all animals originating from eight of the 11 federal states. The highest carrier rate was found in Microtus spp. (13%), followed by Apodemus spp. (11%) and Clethrionomys spp. (6%). The most common Leptospira genomospecies determined by duplex PCR was L. kirschneri, followed by L. interrogans and L. borgpetersenii; all identified by single locus sequence typing (SLST). Representatives of the shrew species were also carriers of Leptospira spp. In 20% of Crocidura spp. and 6% of the Sorex spp. leptospiral DNA was detected. Here, only the pathogenic genomospecies L. kirschneri was identified.
Keywords: Leptospira spp., leptospirosis, rodents, shrews, Germany
1. Introduction
Leptospirosis is a zoonotic disease of global importance caused by spirochaetes belonging to the genus Leptospira [1]. Leptospires are usually transmitted through contact with the urine of infected animals, either directly or through exposure to contaminated water or soil. The most common portal of entry is through abrasions or cuts in the skin or via the conjunctiva [2]. The spectrum of human disease is variable and can range from subclinical infection to severe signs of multi-organ dysfunction with high case fatality rates. The severity and clinical features of the disease vary according to the leptospiral serovar as well as the age and health status of the patient [3]. To date, 20 Leptospira species with more than 300 serovars, grouped in 20 serogroups, have been described. On the molecular level, Leptospira can be divided into six environmental non-pathogenic species, nine pathogenic species and five intermediate species, for which virulence has not been demonstrated experimentally [4].
Leptospirosis is a notifiable disease in Germany, with the annual human incidence ranging from 0.06 to 0.2 (mean 0.08) per 100,000 between 2001 and 2013 [5]. Historically, leptospirosis in Germany was mainly associated with agricultural exposure risks [6]. As a result of mechanization, improvements in sanitation, and better control in animal reservoirs, epidemic leptospirosis in Germany disappeared in the early 1960s, although sporadic outbreaks associated with agricultural activities still occur [7]. Nowadays, recreational activities linked to freshwater exposures, e.g., swimming in lakes and canoeing, and residential exposures such as gardening and owning pets were identified as major risk factors for disease. Further, travelling abroad to other European and non-European countries was another important exposure risk factor identified for Germany [6]. This is consistent with a similar trend observed in other industrialized countries, especially among participants in adventure travel and water sports in exotic locations [8,9].
Among wildlife species, rodents are the most important maintenance hosts for Leptospira spp. and may transfer infection to livestock, companion animals and humans [3,10]. Maintenance hosts usually have no or mild symptoms after infection and excrete leptospires in the urine constantly and over long periods of time. They form an infection reservoir through continuous cycles of transmission from parents to offspring [11]. In contrast, accidental hosts such as humans can suffer severe acute forms of the disease. Leptospiruria in accidental hosts is intermittent and of short duration [12].
As specific rodent species may be carriers of distinct leptospiral serovars in different geographic areas, the knowledge of the prevalent serovars and the maintenance host association is essential to understand the epidemiology of the disease in a region [3].
In Germany, representatives of several genera within the order Rodentia, which include murine, vole and rat species are found. Further, shrews of different genera within the order Soricomorpha have been described [13]. Little is known about the association of the Leptospira spp. with the majority of these rodent and shrew species in Germany. Our objective therefore, was to gain a better understanding of the occurrence of pathogenic leptospires in rodent and shrew populations in Germany and to identify the most common Leptospira genomospecies. To our knowledge this is the first nationwide study in Central Europe looking at leptospiral infection in such a broad range of rodents and shrews.
2. Experimental Section
The sample collection was part of a multiannual study to investigate pathogen occurrence and distribution in rodents and other small mammals in Germany [14,15,16,17,18,19,20,21,22]. In the frame of the national network on rodent-borne pathogens [23] between 2002 and 2010 mice, voles and shrews were trapped in 60 different regions (defined by postal codes) located in 11 of the 16 federal states in Germany. The trapping was performed according to the routine protocols established at the different facilities. All animals were frozen and sent to the Friedrich-Loeffler-Institut for further analyses. Here, species, weight, size, and sex were determined and animals were necropsied according to the protocols of the network rodent-borne pathogens [23]. Further, the genus and species of all Leptospira-positive animals was confirmed by a PCR-based cytochrome b analysis [24]. If the genus and/or species could not be identified, the animals were not included in the subsequent analyses. The animals where the geographical origin was unclear were included in the analysis of the association between leptospiral status and rodent species, but not in the geographical presentation.
One kidney of each animal was used during the analysis. Cross contamination between animals was avoided through sequential dissection of single animals and stringent disinfection measures after each dissection. DNA was extracted from 30 mg of kidney tissue with the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). The DNA samples were distributed among three different laboratories for further analysis. As the laboratories have different routine protocols, these were used during the study.
Laboratory A: One thousand three hundred seventy one kidneys sent to laboratory A were analysed by a duplex PCR adapted from Gravekamp et al. [25]. The PCR reaction was set up with 12.5 µL 2× Qiagen Multiplex PCR Mastermix, 0.5 µL primer (50 pmol/µL) and 2.5 µL template DNA. Thermal cycling was carried out with the 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA). An initial denaturation step at 94 °C for 5 min was followed by template denaturation at 94 °C for 30 s, primer annealing at 55 °C for 30 s, a 30 s primer extension at 72 °C for a total of 35 cycles, with a subsequent final extension phase of 5 min at 72 °C.
Laboratory B: Four hundred forty seven samples were screened with a lipl32 PCR according to the protocol by Mayer-Scholl et al. [26]. All positive samples were then subjected to analysis with the duplex PCR used in laboratory A [25].
Laboratory C: One thousand one hundred fifty five samples were tested by lipl32 real-time PCR according to Stoddard et al. [27]. As in laboratory B, the less sensitive duplex PCR was used to further analyse all real-time PCR positive samples. The targets for the duplex PCR are the flagellin-encoding flaB gene (563 bp fragment) which only amplifies in L. kirschneri and the preprotein translocase-encoding secY gene (285 bp fragment), allowing the detection of genomospecies L. interrogans, L. borgpetersenii, L. weilii, L. noguchii, L. santarosai or L. meyeri [25].
Laboratories A, B, C: All samples yielding either the 285 bp fragment or both the 285 bp and 563 bp fragments were subsequently analysed by single locus sequence typing (SLST). The protocol was adapted from Slack et al. [28], substituting the mastermix in the protocol with the 2× Qiagen Multiplex PCR Mastermix. Due to sample size limitations only 93% of these samples could be analyzed by SLST. Samples in which the duplex PCR solely detected the 563 bp fragment were defined as genomospecies L. kirschneri. To confirm the results of the duplex PCR, a selection of 28 DNA samples was further tested by sequencing of the secY gene [29].
3. Results
3.1. Description of Examined Animals
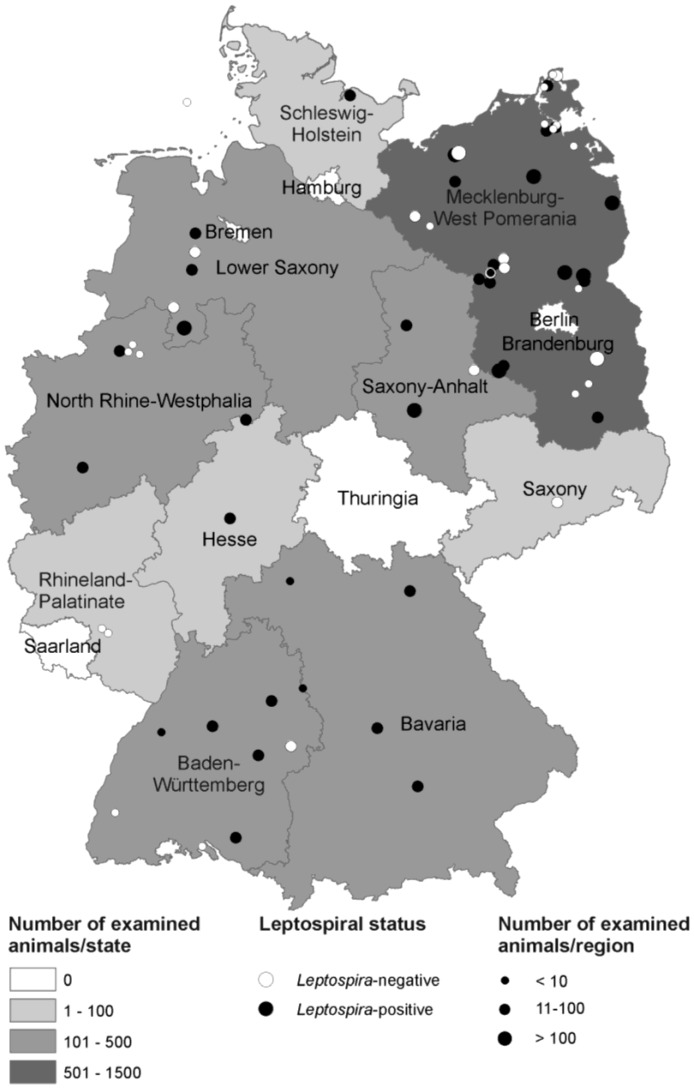
During 2002–2010 a total of 2973 rodents and other small mammals from 60 different regions in eleven German Federal states were collected. The majority of the animals originated from Brandenburg (36%), followed by Mecklenburg-West Pomerania (25%), Lower Saxony (13%) and North Rhine-Westphalia (8%) (Table 1). For the geographical distribution of the examined animals refer to Figure 1.
Table 1.
Number of animals trapped per federal state and region.
| Federal State | No. of Regions | Total No. of Animals | Median (Range) Animals/Region |
|---|---|---|---|
| Brandenburg | 14 | 1065 | 77 (1–241) |
| Baden-Württemberg | 9 | 202 | 15 (1–76) |
| Bavaria | 6 | 107 | 13 (5–45) |
| Mecklenburg-West Pomerania | 12 | 758 | 20 (5–329) |
| Lower Saxony | 6 | 377 | 15 (1–257) |
| North Rhine-Westphalia | 6 | 226 | 8 (1–83) |
| Hesse | 1 | 32 | - |
| Rhineland-Palatinate | 1 | 6 | - |
| Saxony | 1 | 13 | - |
| Saxony-Anhalt | 2 | 150 | - |
| Schleswig-Holstein | 2 | 34 | - |
| Total | 60 | 2970 * | - |
Note: * The geographical origin of three animals could not be identified and therefore these animals were not included in the table.
Figure 1.
Number and leptospiral status of rodents and shrews trapped in the German federal states. The trapping site in the North Sea lies on the Island Helgoland. Several trapping regions defined by postal code include more than one trapping site. The map was created by ArcGIS 9.4 (ESRI, Redlands, CA, USA).
Rodent species captured included five murine species (family Muridae): harvest mouse (Micromys minutus), striped field mouse (Apodemus agrarius), yellow-necked field mouse (Apodemus flavicollis), wood mouse (Apodemus sylvaticus), house mouse (Mus musculus) and six vole species (family Cricetidae): bank vole (Clethrionomys glareolus), water vole (Arvicola amphibius), field vole (Microtus agrestis), common vole (Microtus arvalis), root vole (Microtus oeconomus) and common pine vole (Microtus subterraneus). Further, six shrew species (family Soricidae) were included in the analysis: common shrew (Sorex araneus), Millet’s shrew (Sorex coronatus), pygmy shrew (Sorex minutus), bi-coloured white-toothed shrew (Crocidura leucodon), greater white-toothed shrew (Crocidura russula) and lesser white-toothed shrew (Crocidura suaveolens) (Table 2). The genus and/or species of 12 animals could not be identified.
Table 2.
Abundance and identification of Leptospira genomospecies in rodent and shrew species investigated.
| Rodents and Shrews | Leptospira-positive Animals ** | Number of Identified Leptospira spp. *** | |||||
|---|---|---|---|---|---|---|---|
| Genus | Species | No. Trapped | Total Number | Percentage | L. kirschneri | L. interrogans | L. borgpetersenii |
| Apodemus | agrarius | 190 | 23 | 12 | 19 | 0 | 0 |
| flavicollis | 792 | 71 | 9 | 17 | 36 | 1 | |
| sylvaticus | 154 | 27 | 18 | 6 | 13 | 0 | |
| spp. total | 1136 | 121 | 11 | 42 | 49 | 1 | |
| Micromys | minutus | 6 | 0 | 0 | 0 | 0 | 0 |
| Mus | musculus | 18 | 1 | 6 | 1 | 0 | 0 |
| Arvicola | amphibius | 8 | 2 | 25 | 2 | 0 | 0 |
| Clethrionomys | glareolus | 1016 | 66 | 6 | 38 | 18 | 0 |
| Microtus | agrestis | 517 | 64 | 12 | 38 | 1 | 6 |
| arvalis | 174 | 24 | 14 | 19 | 0 | 0 | |
| oeconomus | 2 | 1 | 50 | 1 | 0 | 0 | |
| subterraneus | 4 | 0 | 0 | 0 | 0 | 0 | |
| spp. total | 697 | 89 | 13 | 58 | 1 | 6 | |
| Crocidura | leucodon | 3 | 1 | 33 | 0 | 0 | 0 |
| russula | 24 | 5 | 21 | 5 | 0 | 0 | |
| suaveolens | 3 | 0 | 0 | 0 | 0 | 0 | |
| spp. total | 30 | 6 | 20 | 5 | 0 | 0 | |
| Sorex | araneus | 36 | 2 | 6 | 0 | 0 | 0 |
| coronatus | 2 | 1 | 50 | 1 | 0 | 0 | |
| minutus | 12 | 0 | 0 | 0 | 0 | 0 | |
| spp. total | 50 | 3 | 6 | 1 | 0 | 0 | |
| Total | 2961 * | 288 | 10 | 147 | 68 | 7 | |
Notes: * The genus and/or species of 12 animals (all Leptospira-negative) could not be identified and therefore these animals were not included in the table; ** The column “Leptospira-positive animals” refers to the lipl32 and/or duplex PCR results; *** Identification of genotypes was performed by duplex PCR for L. kirschneri and SLST for L. interrogans and L. borgpetersenii. The different sensitivities of the assays must be taken into account.
3.2. Identification of Leptospira spp. in Trapped Rodents and Shrews
Ten percent (288) of all examined rodents and small mammals were Leptospira-positive in the lipl32 and/or the duplex PCR. Leptospira-positive rodents belonged to the genera Arvicola (25%), Microtus (13%), and Clethrionomys (6%) within the family Cricetidae and the genera Apodemus (11%) and Mus (6%) within the family Muridae. Animals belonging to the shrew family (Soricidae) were also identified as potential vectors for the transmission of Leptospira spp. In 20% of the Crocidura spp. and 6% of the Sorex spp. leptospiral DNA was detected. The regional distribution of Leptospira-positive animals in the different murine, vole and shrew species is shown in Figure 1.
In total, 257 samples were identified as Leptospira-positive by duplex PCR. In 148 (58 %) of the duplex PCR-positive samples the flagellin-encoding flaB gene was amplified, indicating infection with the genomospecies L. kirschneri. All samples further analysed by secY sequencing were confirmed as L. kirschneri.
One hundred and nine samples where the secY gene (285 bp fragment) was amplified, were further analysed by SLST. As the sensitivity of the SLST analysis was much lower than the duplex PCR, only 69% of the samples showed an amplicon that could be sequenced. Of these, 68 samples (91%) were identified as L. interrogans and 7 (9%) as L. borgpetersenii (Table 2).
3.3. Detailed Description of Leptospira Detection in Selected Murine and Vole Species
As 96% of all trapped animals were representatives of the genera Apodemus, Clethrionomys or Microtus all subsequent data analyses focussed on these genera.
In this study Microtus spp. were most often infected by Leptospira spp. (13%), followed by Apodemus spp. (11%) and Clethrionomys glareolus with 6% positives. 72% of the Leptospira-positive Microtus spp. belonged to the species M. agrestis, 27% to M. arvalis and 1% to M. oeconomus.
Of the samples where the SLST analysis was successful, 89% of the animals of the genus Microtus were infected with L. kirschneri. The occurrence of L. kirschneri was equally distributed between M. agrestis and M. arvalis. Interestingly, six of the seven L. borgpetersenii-positive animals belonged to the species M. agrestis and were trapped at one site in Brandenburg. The other L. borgpetersenii-positive Apodemus flavicollis was caught at a neighbouring location in the same region. Microtus spp. were collected in 31 of 60 trapping regions, Leptospira-positive Microtus spp. were identified in 13 regions in seven federal states. The proportion of Leptospira-positive Microtus spp. trapped at each site ranged from 2%–100% (median 15%).
In contrast to the animals of the genus Microtus, bank voles (Clethrionomys glareolus) were less frequently infected with Leptospira spp. (6%) in comparison to all other rodent species examined (11%). Leptospira genomospecies causing infection in animals of this species were L. kirschneri (68%) and L. interrogans (32%). Bank voles were more widely distributed than Microtus spp. in the sample collection investigated and were found in 52 trapping regions, Leptospira-positive animals were trapped in 19 different regions in eight Federal States. The proportion of Leptospira-positive bank voles trapped at each site ranged from 6%–70% (median 12%).
Eleven percent of the tested animals of the genus Apodemus spp. were carriers of different Leptospira species. Leptospira-positive animals belonged to the species A. flavicollis (59%), A. sylvaticus (22%) and A. agrarius (19%). Leptospira genomospecies causing infection in this genus were L. interrogans (53%), L. kirschneri (46%) and L. borgpetersenii (1%).
A difference in the distribution of trapped male and female animals was not found in the three genera and sex was not associated with detection of leptospiral DNA (data not shown). In our study, the average size of the animals did not differ significantly between infected and non-infected animals within any of the species (data not shown).
4. Discussion and Conclusions
To date, little is known about the occurrence and distribution of pathogenic leptospires in the most common rodent and shrew species in Germany and other European countries. Most studies have described the occurrence and identity of Leptospira spp. in different rat species. In Germany, Runge et al. [30] found leptospiral DNA in 21% of the examined Norway rats (Rattus norvegicus), and in France, leptospiral renal carriage was shown in 34.7% of the studied Norway rats [31]. In Poland, renal carriage in Norway rats and black rats (Rattus rattus) ranged between 2 and 40%, depending on the region [32].
Our study indicates that, albeit with a lower abundance, pathogenic leptospires are also widely distributed in murine, vole and shrew species throughout Germany. The occurrence of Leptospira spp. in small rodents and shrews in Europe has been investigated in earlier, less extensive studies. In a serological study in southern Germany, 8% of the 266 rodents were seropositive, primarily to the serovars Grippotyphosa and Australis [33]. Europe’s largest rodent species, the beaver (Castor fiber), was recently identified as a potential reservoir host for zoonotic leptospires in Germany. L. interrogans multi locus sequence type 24 was identified as the infecting species [34].
In a Swiss study 12.6% of the 190 examined rodents and other small mammals caught in the city of Zurich carried leptospiral DNA [35]; while in a Croatian study 7% of the 227 mice and vole species were Leptospira-positive in a PCR [36]. In a recent study in rodents from Lower Austria, L. kirschneri was detected in three Apodemus mice, but genomospecies L. interrogans, L. borgpetersenii, L. weilii, L. noguchii, L. santarosai or L. meyeri were found in three voles [37].
In our study Microtus spp. were most often carriers of leptospires (13%), followed by representatives of the genus Apodemus (11%). This is in contrast to a study from Croatia where house mice from an urban area showed the highest carrier rate and confirmed its role as a major reservoir in this area [36]. This difference could be due to the much lower number of house mice investigated in our study compared to the other species.
Leptospirosis is usually perceived as a zoonotic disease mainly restricted to rural areas. Yet, in a recent German study, at least 12% of the reported human leptospirosis cases were contracted in urban areas and were primarily related to residential activities, such as gardening and working on private ponds [6]. In two studies in England, Apodemus spp. were nearly exclusively found in residential gardens [38,39]. Also in rural areas, the wood mouse (Apodemus sylvaticus) is often associated with habitats close to humans [40]. As this rodent species and humans share their environment, the wood mouse may be important in the epidemiology of human leptospirosis.
In this study, L. kirschneri was the most prevalent Leptospira genomospecies. L. kirschneri was most common in the vole species Microtus arvalis and the murine species Apodemus agrarius. Previously published data indicate that the serovar Grippothyphosa, which belongs to the genomospecies L. kirschneri, is most often associated with mice of the genus Apodemus in Europe [3]. Interestingly, in our study Apodemus flavicollis and A. sylvaticus were more often infected with L. interrogans.
The public health relevance of the serovar Grippothyphosa is well documented [41]. At the beginning of the previous century, infections with the serovar Grippotyphosa were known as “mudfever” in Germany and associated with a variety of fieldwork activities [42]. In an outbreak of leptospirosis in strawberry harvesters in Germany in 2007, the common vole (M. arvalis) was identified as the most likely reservoir for the infection of the field workers. Leptospires isolated from the animal kidneys were identified by DNA sequencing and by monoclonal antibodies as Leptospira kirschneri serovar Grippotyphosa and serovar Vanderhoedeni [7].
In contrast to another study [43], the average body size, which is used in some rodents as an approximation of age and sexual maturity [44] did not differ significantly between infected and non-infected animals. This could be due to differing rodent species examined in the two studies.
A limitation to the current study is that the serovars of the infecting leptospires were not determined. Serological classification by microagglutination is the gold standard method performed to confirm human leptospirosis in clinical cases. As a multitude of serovars are associated with a single genomospecies [3], it is difficult to infer from the molecular identification of the genomospecies to the serologically detected infecting serovar. This makes the comparison of clinical and epidemiological studies based solely on molecular methods difficult. An additional limitation was that the data origin and quality were heterogeneous, as trapping and laboratory analyses were performed according to the protocols established at the different facilities. The screening for Leptospira spp. in 46% of the kidney samples was performed with the duplex PCR, which is less sensitive than the lipl32 conventional PCR [26]. The analytical sensitivity of the lipl32 conventional PCR for rodent kidney samples is 102 leptospires/mL [26]. The sensitivity of the real-time PCR is expected to be comparable, as the analytical sensitivity in spiked blood and urine samples ranged from 101–102 leptospires/mL [27]. The specificity of all three assays was 100% [26,27]. Therefore, the proportion of Leptospira-positive animals is most likely underestimated.
In conclusion, this study was based on a very large panel of rodent species, a wide geographical coverage of Germany and a high sample size. Leptospiral DNA was detected not only in different murine and vole species, but also in some shrew species. SLST analysis revealed first insights into a host association of Leptospira genomospecies.
Future studies should aim to investigate the leptospiral status of rodent species by molecular and serological assays in association with their natural habitat, environmental conditions and population dynamics in order to better understand the epidemiology of Leptospira in these animal populations.
Acknowledgments
We thank the following persons for trapping rodents and small mammals, performing the necropsies and other support: Rainer Allgöwer, Daniel Balkema, Margrit Bemmann, Jana Blumhardt, Peter Borkenhagen, Thomas Büchner, Lena Buschke, Anne Balkema-Buschmann, Claudia Dettmer, Paul Dremsek, Ulrike Duve, Jona Freise, Susanne Freyse, Robert Friedrich, Dieter Fuchs, Andreas Gehrke, Anja Globig, Bärbel Hammerschmidt, Torsten Heidecke, Martin Hornung, Ommo Hüppop, Jens Jacob, Martin Kaatz, Robert Klopfleisch, Christian Kretzschmar, Heike Kubitza, Johannes Lang, Jens Lewitzki, Paul-Walter Löhr, Christina Maresch, Daniel Masur, Roswitha Mattis, Marc Mertens, Ralph-Udo Mühle, Michael Noack, Lutz Ohlmeyer, Brita Oltmann, Hans-Joachim Pelz, Carsten Pötzsch, Ulrike M. Rosenfeld, Mathias Schlegel, Jonas Schmidt-Chanasit, Christina Schnick, Josephine Schröter, Kati Sevke, Hanan Sheikh Ali, Ramona Spließ, Martina Steffen, Ingolf Stodian, Kirsten Tackmann, Jens P. Teifke, Cornelia Triebenbacher, Konrad Wanka, Daniel Windolph, Wolfgang Wegener, Matthias Wenk, and Hinrich Zoller.
Our special thanks to Enno Luge, Tanja Skladnikiewicz-Ziemer and Cornelia Göllner for the analysis of the samples and Franz J. Conraths and Martin H. Groschup for continuous support.
The work of RGU was supported by the Federal Ministry of Education and Research (BMBF) through the National Research Platform for Zoonoses (Network “Rodent-borne pathogens”; project numbers 01KI1018 and 01KI1303).
Author Contributions
Anne Mayer-Scholl, Rainer G. Ulrich, Martin Pfeffer, Holger C. Scholz and Karsten Nöckler conceptualize the paper objective, conducted the analyses and wrote the manuscript. Jens Andre Hammerl, Sabrina Schmidt, Dietlinde Woll and Astrid Thomas carried out the laboratory work, performed the sequence analyses and collaborated on the interpretation of findings and writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bharti A.R., Nally J.E., Ricaldi J.N., Matthias M.A., Diaz M.M., Lovett M.A., Levett P.N., Gilman R.H., Willig M.R., Gotuzzo E., Vinetz J.M. Peru—United States Leptospirosis Consortium. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003;3:757–771. doi: 10.1016/S1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 2.Levett P.N. Leptospirosis. Clin. Microbiol. Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faine S., Adler B., Bolin C., Perolat P. Leptospira and Leptospirosis. MediSci; Melbourne, Australia: 1999. pp. 83–86. [Google Scholar]
- 4.Picardeau M. Diagnosis and epidemiology of leptospirosis. Med. Mal. Infec. 2013;43:1–9. doi: 10.1016/j.medmal.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Robert Koch Institute [(accessed on 15 July 2014)]. Available online: https://www3.rki.de/survstat/
- 6.Jansen A., Schöneberg I., Frank C., Alpers K., Schneider T., Stark K. Leptospirosis in Germany 1962–2003. Emerg. Infect. Dis. 2005;11:1048–1054. doi: 10.3201/eid1107.041172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai S., van Treeck U., Lierz M., Espelage W., Zota L., Sarbu A., Czerwinski M., Sadkowska-Todys M., Avdicová M., Reetz J., Luge E., Guerra B., Nöckler K., Jansen A. Resurgence of field fever in a temperate country: An epidemic of leptospirosis among seasonal strawberry harvesters in Germany in 2007. Clin. Infect. Dis. 2009;48:691–697. doi: 10.1086/597036. [DOI] [PubMed] [Google Scholar]
- 8.van Crevel R., Speelman P., Gravekamp C., Terpstra W.J. Leptospirosis in travellers. Clin. Infect. Dis. 1994;19:132–134. doi: 10.1093/clinids/19.1.132. [DOI] [PubMed] [Google Scholar]
- 9.Sejvar J., Bancroft E., Winthrop K., Bettinger J., Bajani M., Bragg S., Shutt K., Kaiser R., Marano N., Popovic T., Tappero J., Ashford D., Mascola L., Vugia D., Perkins B., Rosenstein N. Eco-challenge investigation team. Leptospirosis in “eco-challenge” athletes, Malaysian Borneo, 2000. Emerg. Infect. Dis. 2003;9:702–707. doi: 10.3201/eid0906.020751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meerburg B.G., Singleton G.R., Kijlstra A. Rodent-borne diseases and their risks for public health. Crit. Rev. Microbiol. 2009;35:221–270. doi: 10.1080/10408410902989837. [DOI] [PubMed] [Google Scholar]
- 11.Hartskeerl R.A., Terpstra W.J. Leptospirosis in wild animals. Vet. Quart. 1996;18:S149–S150. doi: 10.1080/01652176.1996.9694722. [DOI] [PubMed] [Google Scholar]
- 12.Monahan A.M., Callanan J.J., Nally J.E. Host-pathogen interactions in the kidney during chronic leptospirosis. Vet. Pathol. 2009;46:792–799. doi: 10.1354/vp.08-VP-0265-N-REV. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell-Jones J., Amori G., Bogdanowicz W., Krystufek B., Reijnders P.J.H., Spitzenberger F., Stubbe M., Thissen J.B.M., Vohralik V., Zima J. The Atlas of European Mammals. T & AD Poyser, Academic Press; London, UK: 1999. pp. 32–311. [Google Scholar]
- 14.Essbauer S.S., Schmidt-Chanasit J., Madeja E.L., Wegener W., Friedrich R., Petraityte R., Sasnauskas K., Jacob J., Koch J., Dobler G., Conraths F.J., Pfeffer M., Pitra C., Ulrich R.G. Nephropathia epidemica outbreak in a metropolitan area, Germany. Emerg. Infect. Dis. 2007;13:1271–1273. doi: 10.3201/eid1308.061425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehlers B., Kuchler J., Yasmum N., Dural G., Schmidt-Chanasit J., Jäkel T., Matuschka F.-R., Richter D., Essbauer S., Hughes D.J., Summers C., Bennett M., Stewart J.P., Ulrich R.G. Identification of novel rodent herpesviruses, including the first gammaherpesvirus of Mus musculus. J. Virol. 2007;81:8091–8100. doi: 10.1128/JVI.00255-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guenther S., Grobbel M., Heidemanns K., Schlegel M., Ulrich R.G., Ewers C., Wieler L.H. First insights into antimicrobial resistance among faecal Escherichia coli isolates from small wild mammals in rural areas. Sci. Total Envir. 2010;408:3519–3522. doi: 10.1016/j.scitotenv.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt-Chanasit J., Essbauer S., Petraityte R., Yoshimatsu K., Tackmann K., Conraths F.J., Sasnauskas K., Arikawa J., Thomas A., Pfeffer M., Scharninghausen J.J., Splettstoesser W., Wenk M., Heckel G., Ulrich R.G. Extensive host sharing of central European Tula virus. J. Virol. 2010;84:459–474. doi: 10.1128/JVI.01226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ettinger J., Hofmann J., Enders M., Tewes F., Oehme R.M., Rosenfeld U.M., Ali H.S., Schlegel M., Essbauer S., Osterberg A., Jacob J., Reil D., Klempa B., Ulrich R.G., Krüger D.H. Multiple synchronous Puumala virus outbreaks, Germany, 2010. Emerg. Infect. Dis. 2012;18:1461–1464. doi: 10.3201/eid1809.111447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlegel M., Radosa L., Rosenfeld U.M., Schmidt S., Triebenbacher C., Löhr P.-W., Fuchs D., Heroldová M., Jánová E., Stanko M., Mošanský L., Fričová J., Pejčoch M., Suchomel J., Purchart L., Groschup M.H., Krüger D.H., Klempa B., Ulrich R.G. Broad geographical distribution and high genetic diversity of shrew-borne Seewis hantavirus in Central Europe. Virus Genes. 2012;45:48–55. doi: 10.1007/s11262-012-0736-7. [DOI] [PubMed] [Google Scholar]
- 20.Schlegel M., Klempa B., Auste B., Bemmann M., Schmidt-Chanasit J., Büchner T., Groschup M.H., Meier M., Buschmann A., Zoller H., Krüger D.H., Ulrich R.G. Multiple dobrava-belgrade virus spillover infections, Germany. Emerg. Infect. Dis. 2009;15:2017–2020. doi: 10.3201/eid1512.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann J., Meisel H., Klempa B., Vesenbeckh S.M., Beck R., Michel D., Schmidt-Chanasit J., Ulrich R.G., Grund S., Enders G., Krüger D.H. Hantavirus outbreak, Germany, 2007. Emerg. Infect. Dis. 2008;14:850–852. doi: 10.3201/eid1405.071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Melo V.W., Ali H.S., Freise J., Essbauer S., Mertens M., Wanka K., Drewes S., Ulrich R.G., Heckel G. Spatiotemporal dynamics of Puumala hantavirus associated with its rodent host, myodes glareolus. Mol. Ecol. 2014 doi: 10.1111/eva.12263. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulrich R.G., Schmidt-Chanasit J., Schlegel M., Jacob J., Pelz H.J., Mertens M., Wenk M., Büchner T., Masur D., Sevke K., Groschup M.H., Gerstengarbe F.W., Pfeffer M., Oehme R., Wegener W., Bemmann M., Ohlmeyer L., Wolf R., Zoller H., Koch J., Brockmann S., Heckel G., Essbauer S.S. Network “rodent-borne pathogens” in Germany: Longitudinal studies on the geographical distribution and prevalence of hantavirus infections. Parasitol. Res. 2008;103:S121–S129. doi: 10.1007/s00436-008-1054-9. [DOI] [PubMed] [Google Scholar]
- 24.Schlegel M., Ali H.S., Stieger N., Groschup M.H., Wolf R., Ulrich R.G. Molecular identification of small mammal species using novel cytochrome b gene-derived degenerated primers. Biochem. Genet. 2012;50:440–447. doi: 10.1007/s10528-011-9487-8. [DOI] [PubMed] [Google Scholar]
- 25.Gravekamp C., van de Kemp H., Franzen M., Carrington D., Schoone G.J., van Eys G.J., Everard C.O., Hartskeerl R.A., Terpstra W.J. Detection of seven species of pathogenic leptospires by PCR using two sets of primers. J. Gen. Microbiol. 1993;139:1691–1700. doi: 10.1099/00221287-139-8-1691. [DOI] [PubMed] [Google Scholar]
- 26.Mayer-Scholl A., Draeger A., Luge E., Ulrich R., Nöckler K. Comparison of two PCR systems for the rapid detection of Leptospira. spp. from kidney tissue. Curr. Microbiol. 2011;62:1104–1106. doi: 10.1007/s00284-010-9829-5. [DOI] [PubMed] [Google Scholar]
- 27.Stoddard R.A., Gee J.E., Wilkins P.P., McCaustland K., Hoffmaster A.R. Detection of pathogenic Leptospira. spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn. Microbiol. Infect. Dis. 2009;64:247–255. doi: 10.1016/j.diagmicrobio.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Slack A.T., Symonds M.L., Dohnt M.F., Smythe L.D. Identification of pathogenic Leptospira. species by conventional or real-time PCR and sequencing of the DNA gyrase subunit B encoding gene. BMC Microbiol. 2006;6 doi: 10.1186/1471-2180-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Victoria B., Ahmed A., Zuerner R.L., Ahmed N., Bulach D.M., Quinteiro J., Hartskeerl R.A. Conservation of the S10-spc α locus within otherwise highly plastic genomes provides phylogenetic insight into the genus Leptospira. PLoS One. 2008 doi: 10.1371/journal.pone.0002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Runge M., von Keyserlingk M., Braune S., Becker D., Plenge-Bönig A., Freise J.F., Pelz H.J., Esther A. Distribution of rodenticide resistance and zoonotic pathogens in Norway rats in Lower Saxony and Hamburg, Germany. Pest. Manag. Sci. 2012;69:403–408. doi: 10.1002/ps.3369. [DOI] [PubMed] [Google Scholar]
- 31.Aviat F., Blanchard B., Michel V., Blanchet B., Branger C., Hars J., Mansotte F., Brasme L., de Champs C., Bolut P., Mondot P., Faliu J., Rochereau S., Kodjo A., Andre-Fontaine G. Leptospira. exposure in the human environment in France: A survey in feral rodents and in fresh water. Comp. Immunol. Microbiol. Infect. 2009;32:463–476. doi: 10.1016/j.cimid.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Theuerkauf J., Perez J., Taugamoa A., Niutoua I., Labrousse D., Gula R., Bogdanowicz W., Jourdan H., Goarant C. Leptospirosis risk increases with changes in species composition of rat populations. Naturwissenschaften. 2013;100:385–388. doi: 10.1007/s00114-013-1033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kocianová E., Kozuch O., Bakoss P., Rehácek J., Kovácová E. The prevalence of small terrestrial mammals infected with tick-borne encephalitis virus and leptospirae in the foothills of the southern Bavarian forest, Germany. Appl. Parasitol. 1993;34:283–290. [PubMed] [Google Scholar]
- 34.Woll D., Karnath C., Pfeffer M., Allgöwer R. Genetic characterization of Leptospira spp. from beavers found dead in south-west Germany. Vet. Microbiol. 2012;158:232–234. doi: 10.1016/j.vetmic.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 35.Adler H., Vonstein S., Deplazes P., Stieger C., Frei R. Prevalence of Leptospira. spp. in various species of small mammals caught in an inner-city area in Switzerland. Epidemiol. Infect. 2002;128:107–109. doi: 10.1017/s0950268801006380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turk N., Milas Z., Margaletic J., Staresina V., Slavica A., Riquelme-Sertour N., Bellenger E., Baranton G., Postic D. Molecular characterization of Leptospira. spp. strains isolated from small rodents in Croatia. Epidemiol. Infect. 2003;130:159–166. doi: 10.1017/S0950268802008026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt S., Essbauer S.S., Mayer-Scholl A., Poppert S., Schmidt-Chanasit J., Klempa B., Henning K., Schares G., Groschup M.H., Spitzenberger F., Heckel G., Richter D., Ulrich R.G. Multiple infections of rodents with zoonotic pathogens in Austria. Vector-Borne Zoonotic Dis. 2014;14:467–475. doi: 10.1089/vbz.2013.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker P.J., Ansell R.J., Dodds P.A.A., Webber C.E., Harris S. Factors affecting the distribution of small mammals in an urban area. Mammal Rev. 2003;33:95–100. doi: 10.1046/j.1365-2907.2003.00003.x. [DOI] [Google Scholar]
- 39.Dickman C.R., Doncaster C.P. The ecology of small mammals in urban habitats. I. Populations in a patchy environment. J. Anim. Ecol. 1987;56:629–640. doi: 10.2307/5073. [DOI] [Google Scholar]
- 40.Tattersall F.H. House mice and wood mice in and around an agricultural building. J. Zool. Lond. 1999;249:469–472. doi: 10.1111/j.1469-7998.1999.tb01217.x. [DOI] [Google Scholar]
- 41.Dupouey J., Faucher B., Edouard S., Richet H., Kodjo A., Drancourt M., Davoust B. Human leptospirosis: An emerging risk in Europe? Comp. Immunol. Microbiol. Infect. 2014;37:77–83. doi: 10.1016/j.cimid.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Mochmann H. The role of the field hamster (Cricetus. cricetus) as of a source of infection of the swamp-field fever. Z. Hyg. Infektionskr. 1957;143:327–333. doi: 10.1007/BF02156820. [DOI] [PubMed] [Google Scholar]
- 43.Sauer J.R., Slade N.A. Size-based demography of vertebrates. Annu. Rev. Ecol. Syst. 1987;18:71–90. [Google Scholar]
- 44.Paiva-Cardoso M.N., Collares-Pereira M., Gilmore C., Dunbar K., Frizzel C., Santos-Reis M., Vale-Goncalves H., Rodrigues J., Mackie D., Ellis W.A. Sexual Maturity State as Risk Factor to Leptospira. Infection in Small Mammals in Tras-os-Montes e Alto Douro Region, Northern Portugal; Proceedings of the European Meeting of Leptospirosis, Eurolepto 2012; Dubrovnik, Croatia. 31 May–2 June 2012. [Google Scholar]