Abstract
Background
We investigated whether the free β-human chorionic gonadotropin (free β-hCG) would provide additional information to that provided by total hCG alone and thus be useful in future epidemiological studies relating hCG to maternal breast cancer risk.
Materials & methods
Cases (n = 159) and controls (n = 286) were a subset of our previous study within the Northern Sweden Maternity Cohort on total hCG during primiparous pregnancy and breast cancer risk.
Results
The associations between total hCG (hazard ratio: 0.79; 95% CI: 0.49–1.27), free β-hCG (hazard ratio: 0.85; 95% CI: 0.33–2.18) and maternal risk of breast cancer were very similar in all analyses and mutual adjustment for either one had minor effects on the risk estimates.
Conclusion
In the absence of a reliable assay on intact hCG, total hCG alone can be used in epidemiological studies investigating hCG and breast cancer risk, as free β-hCG does not appear to provide any additional information.
Keywords: breast cancer, free β-human chorionic gonadotropin, human chorionic gonadotropin, nested case–control study, pregnancy
Background
Experimental studies in rodents have demonstrated that treatment with human chorionic gonadotropin (hCG) is inversely associated with mammary tumor formation [1–4]. Initial work conducted by our group using the resources of the Northern Sweden Maternity Cohort (NSMC), relating early pregnancy hCG to subsequent risk of maternal breast cancer, provide direct human evidence in support of these observations [5,6]. HCG is composed of two distinct noncovalently associated α- and β-subunits and has six major isoforms [7] with different biological properties [8,9]. The most widely used assays for hCG determination that are practical for use in epidemiological studies are total, intact and free β-hCG (free β-hCG). The total hCG assays quantify both intact hCG and free β-hCG, which may have distinct associations with risk of breast cancer. For instance, while total hCG decreases cell proliferation and increases DNA repair in mammary cells [1,3,10], free β-hCG may posses both cellular inhibitory [11] and stimulatory activities in other cells [12,13].
In our initial study [5], concentrations of intact hCG were inversely, but not significantly associated with risk of breast cancer. At the time of extending this initial study, the intact hCG assay production was discontinued and measurements of total hCG were conducted [6]. In the extended study, we observed a significant inverse association between total hCG and breast cancer risk [6].
The aim of our present study was to determine whether assaying and relating free β-hCG separately to breast cancer risk would provide additional information to using total hCG alone. Using a subset of cases and controls included in our extended study on total hCG during a primiparous pregnancy [6], we examined the associations between free β-hCG and maternal risk of breast cancer and also explored the effect of adjustments of total hCG breast cancer models for free β-hCG.
Materials & methods
The cohort and the selection criteria for this study have been described in detail previously [6]. Briefly, the NSMC includes virtually all the pregnant women residing in one of the four northernmost counties in Sweden, who donated blood samples during the final weeks of the first trimester or early weeks of the second trimester, while attending any of the Maternity Care Units in the counties. The blood samples are stored at −20°C in a central repository at the University Hospital in Umeå (Sweden).
Eligible patients were women who were less than 40 years old at the time of blood donation and who provided a blood sample within the first 120 days of pregnancy during a singleton full-term pregnancy (≥259 days) leading to the birth of a first child (live or stillborn). Women who had used hormonal medications to become pregnant or during pregnancy, or had previously been diagnosed with any cancer (except nonmelanoma skin cancer) were excluded. Cases were women diagnosed for the first time with invasive epithelial breast cancer at least 1 year after their entry into the cohort and before 8 June 2007. Identification of eligible cases and controls involved linkages with nationwide databases (Swedish Birth Register and Swedish Cancer Registry) by using the unique ten-digit personal identification number assigned to every resident in Sweden. These databases are of high quality and over 97% complete. Information on smoking was obtained from the Swedish Birth Registry while that of BMI was obtained at the time of enrollment into the NSMC. Information regarding smoking and BMI were gathered at the same time as the blood sample was drawn, which was during the first trimester when women attended Maternity Care Units. Smoking status was obtained at the time of blood draw (during index pregnancy). Smoking during the index pregnancy was recorded for all women included in the study, when both future cases and controls were free of cancer.
Subjects in this study (159 cases and 286 controls) were a subset of our previous study on hCG during primiparous pregnancy and breast cancer risk (242 cases and 450 controls) [6] who had sufficient blood samples available for free β-hCG assay. These women were comparable with those who did not have sufficient blood samples for the assay in most maternal (age at blood draw, parity, smoking and BMI) and child (gender distribution, weight and height) characteristics. However, centered mean total hCG concentrations were slightly lower among cases included in the study compared with those not included in the study (centered mean: 94,989 vs 95,184 mU/ml). Median age at breast cancer diagnosis (44.8 years; range: 25.5–60.8 years) was lower among cases included in this study than those not included (48.0 years; range: 31.7–62.7 years), while gestational day at blood draw was greater among controls included in the study (71.0 days; range: 42.0–118.0 days) compared with those controls not included (67.0 days; range: 37.0–120.0 days).
The study was approved by the Regional Ethics Committee of Umeå, Sweden.
Laboratory analyses
Total hCG and free β-hCG were quantified in the Laboratory of Clinical Chemistry, University Hospital in Umeå, Sweden using solid-phase competitive chemiluminescence assays on an Immulite 2000 Siemens analyzer (Siemens Healthcare Diagnostics Products, NY, USA). Serum specimens of individually matched case and control subjects were always included in the same laboratory run, with laboratory personnel blinded to the case–control status of the specimens. In addition to routine laboratory quality controls, a pool of serum from the cohort was created at the beginning of the study, and two aliquots, indistinguishable from the test samples, were inserted in each laboratory run. The intraand inter-batch coefficients of variation were 7.4 and 7.2% and 1.1 and 1.7% at 427 mU/ml total hCG concentration and 869 ng/ml free β-hCG concentration, respectively.
Statistical analysis
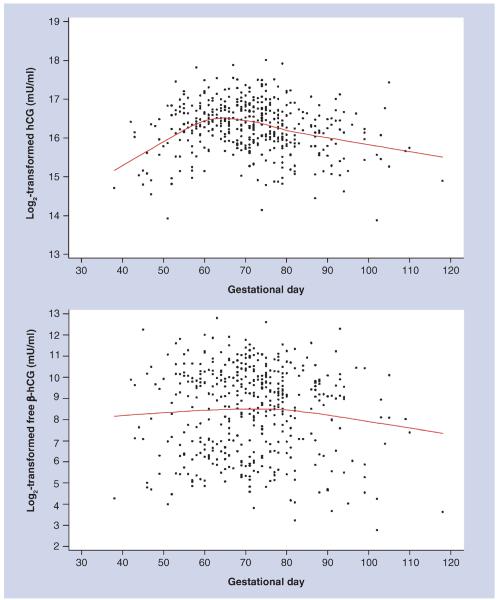
Total hCG and free β-hCG varied during pregnancy (Figure 1). We accounted for this variability in all statistical analyses using the method described by Richardson and colleagues [6,14], which we also used in our original publications [6]. Prior to analyses, original total hCG and free β-hCG values were naturally log2-transformed to limit heteroscedasticity. The mean curve of total hCG and free β-hCG variation was estimated based on all data points using local linear regression [15], a nonparametric smoothing technique that employs weighted regression and uses specific subsets of the data to estimate the curve at each point. Hormonal concentrations for each woman were computed as the difference (residual) between the assay value and the estimated mean determined for the day of gestation when the sample was drawn. Henceforth, total hCG and free β-hCG concentrations refer to their residuals.
Figure 1. Maternal human chorionic gonadotropin and free β-human chorionic gonadotropin concentrations (log2-transformed) by gestational day at blood draw.
hCG: Human chorionic gonadotropin.
Spearman correlation coefficients were used to analyze the associations between total hCG and free β-hCG. Variations in hormone concentrations by median age at blood draw, child's gender, smoking status and parity at diagnosis among control women were investigated by SAS Proc Mixed. Conditional logistic regression analysis was used to calculate the odds ratios (OR) and corresponding 95% CIs of breast cancer according to tertiles of hormone concentrations (based on the distribution among control women) and on a continuous scale. We adjusted for potential confounders (smoking and child gender), but these made no differences on the risk estimates, thus are not presented. The regression models for total hCG were adjusted for free β-hCG and vice versa. We also examined the risks of breast cancer using the ratio of total hCG to free β-hCG. Trend tests were conducted using hormone variables as ordered categories and likelihood ratio tests were used to assess statistical significance. Stratified analyses were conducted based on median age at blood draw, median age at breast cancer diagnosis, median lag-time to cancer diagnosis, smoking (yes vs no), child's gender (male vs female) and hormone receptor status (estrogen receptor [ER] and progesterone receptor [PR]). Tests of heterogeneity between the ORs in different strata were based on χ2 statistics calculated as the deviations of logistic regression coefficients observed in each stratum relative to the overall regression coefficient. Our power calculations indicated that we had an 80% power to detect an OR of 0.75 on a continuous scale of any of the hormone variables.
Statistical significance was defined as p-values <0.05 and all statistical tests were two-sided. Analyses were conducted using SAS (V9.2, SAS Institute, Inc., NC, USA).
Results
Cases and controls were highly comparable in all the study characteristics (Table 1). Median age at breast cancer diagnosis was 44.8 years (range: 25.5–60.8 years) and the median lag time to cancer diagnosis was 17.8 years (range: 2.7–28.6 years). Data on ER and PR status were available for 140 (88% of total) and 144 (91% of total) cases, respectively. Of these, 110 (79%) and 30 (21%) were ER+ and ER−, respectively, while 103 (72%) and 41 (28%) were PR+ and PR−, respectively. Mean levels corresponding to total hCG (97,355 vs 94,989 mU/ml; p = 0.52) and free β-hCG (803 vs 726 ng/ml; p = 0.28) concentrations centered at the median gestational day were slightly higher among controls than cases, but the differences were not statistically significant. There was moderate correlation between total hCG and free β-hCG (r = 0.42; p < 0.001). As expected, total hCG and free β-hCG concentrations changed with increasing gestational age and their concentrations were within the range expected for that period of pregnancy (Figure 1).
Table 1.
Selected study characteristics of study subjects (Umeå, Sweden, 1975–2008).
| Characteristic | Cases (n = 159) | Controls (n = 286) | p-value* |
|---|---|---|---|
| Age at blood draw, years (range) | 26.8 (18.3–39.0) | 26.6 (18.3–39.2) | – |
| Gestational day (range) | 72.0 (38.0–105.0) | 71.0 (42.0–118.0) | 0.68 |
| Parity at diagnosis | – | – | 0.18 |
| − 1, n (%) | 38 (24%) | 56 (20%) | – |
| − 2, n (%) | 82 (52%) | 145 (50%) | – |
| − ≥2, n (%) | 39 (24%) | 85 (30%) | – |
| Child's gender | – | – | 0.63 |
| − Male, n (%) | 90 (57%) | 155 (54%) | – |
| − Female, n (%) | 69 (43%) | 131 (46%) | – |
| Child's weight, g (range) | 3470 (2520–4680) | 3485 (2115–4770) | 0.13 |
| Child's length, cm (range) | 50.0 (37.0–56.0) | 50.0 (45.5–57.0) | 0.61 |
| Smoking | – | – | 0.41 |
| − No, n (%) | 107 (67%) | 204 (71%) | – |
| − Yes, n (%) | 52 (33%) | 81 (28%) | – |
| BMI, kg/m2 (range) | 22.0 (17.7–30.5) | 22.2 (16.8–45.0) | 0.36 |
| Age at diagnosis, years (range) | 44.8 (25.5–60.8) | – | – |
| Lag-time, years (range) | 17.8 (2.7–28.6) | – | – |
| Hormones† | – | – | – |
| − hCG(mU/ml) | 94,989 (57,757–140,430) | 97,355 (55,016–141,279) | 0.52 |
| − Free β-hCG (ng/ml) | 726 (68–1741) | 803 (62–1942) | 0.28 |
p-values calculated by conditional logistic regression.
Mean levels (10th–90th percentile) corresponding to hCG and free β-hCG concentrations centred at the median gestational day (71).
hCG: Human chorionic gonadotropin.
As expected, total hCG concentrations were higher among women carrying a female child than among women carrying a male child (104,253 vs 91,524 mU/ml; p-value = 0.004) as was free β-hCG (915 vs 708 ng/ml; p-value = 0.061) (Table 2). Total hCG concentrations were also higher among nonsmokers than in smokers (103,477 vs 82,515 mU/ml; p < 0.0001), but no significant differences in free β-hCG concentrations were observed by smoking status (smokers: 785 ng/ml and nonsmokers: 807 ng/ml; p = 0.86). Concentrations of both hormones were similar by categories of maternal age.
Table 2.
Geometric means of maternal human chorionic gonadotropin and free β-human chorionic gonadotropin concentrations by maternal characteristics at enrollment and child gender.
| Characteristics | n | hCG (mU/ml) | Free β-hCG (ng/ml) |
|---|---|---|---|
| Median age at blood draw | |||
| <26.6 | 141 | 96,552 | 792 |
| ≥26.6 | 145 | 98,135 | 813 |
| p-value | 0.72 | 0.85 | |
| Child's gender | |||
| Male | 155 | 91,524 | 708 |
| Female | 131 | 104,253 | 915 |
| p-value | 0.004 | 0.061 | |
| Smoking | |||
| No | 204 | 103,477 | 807 |
| Yes | 81 | 82,515 | 785 |
| p-value | <0.0001 | 0.86 | |
| Parity at diagnosis | |||
| 1 | 56 | 94,219 | 911 |
| ≥2 | 230 | 98,118 | 777 |
| p-value | 0.49 | 0.33 | |
hCG: Human chorionic gonadotropin
In the analyses including all subjects, the risk estimates for breast cancer for concentrations of total hCG and free β-hCG were very similar (Table 3). Adjusting total hCG models for free β-hCG attenuated only slightly risk estimates (<10%) in analyses across tertiles or on a continuous scale of the hormone. The ORs for breast cancer among women in the highest tertiles of total hCG concentrations were 0.79 (95% CI: 0.49–1.27; p-trend = 0.31) and 0.85 (95% CI 0.49–1.47; p-trend = 0.56) after adjustment for free β-hCG. Adjustment of the free β-hCG analyses for total hCG concentrations had minimal effect on the risk estimates. For free β-hCG, the corresponding ORs were 0.85 (95% CI: 0.33–2.18; p-trend = 0.34) and 0.89 (95% CI: 0.34–2.33; p-trend = 0.43) across tertiles and 0.92 on continuous scales, respectively. Largely similar results were obtained using the total hCG/free β-hCG ratio. The associations were similar in virtually all the subgroup analyses and were not materially different in analyses stratified by receptor status (data not shown). For total hCG, the ORs appeared to diverge when stratified by median age at breast cancer diagnosis (44.8 years); the OR was 1.05 (95% CI: 0.68–1.62) among younger women and 0.72 (95% CI: 0.43–1.19) among older women, but the test for heterogeneity was not statistically significant (phet = 0.27). No such divergence was, however observed for free β-hCG; OR was 0.91 (95% CI: 0.73–1.13) among younger women compared with 0.93 (95% CI: 0.71–1.21) among older women (data not shown).
Table 3.
Odds ratios† and 95% CIs of breast cancer within tertiles of human chorionic gonadotropin and free β-human chorionic gonadotropin concentrations and on a continuous scale.
| Tertiles | Continuous | |||||
|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | p-trend | Per unit increase | p-value | |
| Total hCG | ||||||
| Cases/controls (n) | 66/96 | 40/95 | 53/95 | – | 159/286 | – |
| Crude total hCG | 1.0 (ref) | 0.60 (0.37–0.99) | 0.79 (0.49–1.27) | 0.31 | 0.89 (0.64–1.24) | 0.49 |
| Adjusted for free β-hCG | 1.0 (ref) | 0.63 (0.37–1.06) | 0.85 (0.49–1.47) | 0.56 | 0.97 (0.65–1.43) | 0.86 |
| Free β-hCG | ||||||
| Cases/controls (n) | 53/96 | 61/95 | 45/95 | – | 159/286 | – |
| Crude free β-hCG | 1.0 (ref) | 1.16 (0.47–2.85) | 0.85 (0.33–2.18) | 0.34 | 0.92 (0.77–1.09) | 0.31 |
| Adjusted for total hCG | 1.0 (ref) | 1.17 (0.47–2.91) | 0.89 (0.34–2.33) | 0.43 | 0.92 (0.75–1.13) | 0.44 |
| Ratio of total hCG to free β-hCG | ||||||
| Cases/controls (n) | 67/96 | 43/95 | 49/95 | – | 159/286 | – |
| Total hCG/free β-hCG | 1.0 (ref) | 0.64 (0.40–1.02) | 0.74 (0.46–1.19) | 0.18 | 1.01 (0.97–1.06) | 0.58 |
Crude odds ratios presented. Adjustments for potential confounders (smoking and child gender) did not alter risk estimates by up 5%.
hCG: Human chorionic gonadotropin; ref: Reference.
Discussion
Elevated total hCG and free β-hCG concentrations during early pregnancy were associated with nonstatistically significant reductions in maternal risk of breast cancer, with the risk estimates being identical for the two isoforms. Adjusting the total hCG analyses for free β-hCG did not result in strengthening the association with breast cancer, while risk estimates of the free β-hCG models were not altered by adjustment for total hCG. Our study suggests that in exploring the associations between hCG during pregnancy and breast cancer risk, the use of free β-hCG may not provide additional information to that obtained using total hCG alone. Based on reports from the literature and the results from our study, we believe that epidemiological studies relating hCG to risks could utilize total hCG alone.
HCG, together with its free subunits (α- and β-subunits) is produced by placental trophoblastic cells, as well as other nontrophoblastic cells such as pituitary and neoplastic cells [8,16]. The six major isoforms of hCG are intact hCG (hCG), nicked hCG (hCGn), free β-subunit (free β-hCG), nicked hCG β-subunit (hCGβn), hCG β-core fragment (hCGβcf) and hCG α-subunit (hCGα) [7]. HCG is also glycosylated in various tissues resulting in hypoglycosylated and hyperglycosylated hCG [17,18].
The free α- and β-subunits have no gonadotrophic activities because they cannot stimulate the luteinizing hormone/hCG receptor by themselves [19]. Thus, only the intact α–β complex is hormonally active [12]. Nevertheless, experimental studies have shown that free β-hCG may have both cell growth stimulating [12,13] and inhibitory activities [11], acting via pathways unrelated to its gonadotrophic activities [12]. Differences in the biological activity between hCG and free β-hCG has been demonstrated in mammary and other cell types [1,3,11–13]. In an in vitro study investigating the effects of hCG and its subunits on bladder cancer cell growth, hCG and hCGα had no effect on cell growth, whereas free β-hCG stimulated cell growth [13], which suggests an independent mechanism of action unrelated to its hormone functions. One such mechanism of action is an antiapoptotic effect, which may occur via possible interactions with the TGFβ receptor complex [12]. This is because free β-hCG and TGFβ are structurally similar [12,20], both having an exposed cystine knot making free β-hCG a TGFβ antagonist, while hCG has no exposed cystine knot [21].
The associations between maternal and child characteristics and hormone concentrations are similar to results reported in previous studies. Smokers had lower hCG and free β-hCG concentrations than nonsmokers [22–25], although the difference was not statistically significant for free β-hCG. Studies have consistently shown that the impact of smoking is stronger on hCG than free β-hCG [22–25]. While smoking may be associated with up to a 20% reduction in hGG levels, its impact on free β-hCG levels might be no greater than a 6% reduction [24]. The mechanisms through which smoking impacts hCG levels are not fully understood. It has been suggested that smoking might reduce hCG concentrations by causing morphological changes in the villus barrier and placental trophoblasts, thereby affecting their synthetic capabilities [23,26–27]. Similarly, previous studies have shown that women carrying a female child have higher hCG and free β-hCG concentrations [23,28]. HCG levels decline with parity with an average of 3.1% decrease per previous birth[29]. Nevertheless, the effect of previous pregnancy on hCG levels appears to manifest only in pregnancies that have reached the third trimester [29].
The effect of pregnancy hormones on maternal breast cancer risk is complex. In addition to hCG, other pregnancy hormones are believed to plan an important role in the protection conferred by pregnancy on maternal breast cancer risk. Studies have shown that α-fetoprotein (AFP) may also have a protective effect on maternal breast cancer risk [30]. AFP binds to estradiol and prevents estrogen-dependent growth of breast cancer cells [31]. Elevated AFP levels during later part of pregnancy [14,32], but not during the first trimester have been associated with reduced risk of breast cancer [32].
A limitation of this study is its smaller sample size compared with the parent study [6], because we could only analyze free β-hCG among women who had sufficient serum samples available. Although the association between total hCG and breast cancer risk was not statistically significant, it was in the same direction as in the parent study. From our power calculations, we had an 80% power to detect an OR of 0.75 on continuous scale of any of the hormone variables. Nevertheless, larger studies with adequate power for subgroup analyses, particularly with regards to receptor status, are needed.
In conclusion, despite the small size of our study, our data suggests that in the absence of a reliable assay on intact hCG, which represents the best way to measure hCG concentrations, total hCG alone could be used in epidemiological research relating hCG during pregnancy to cancer risk.
Future perspective
HCG has six important isoforms of which total, intact and free-β isoforms are the most widely used in epidemiological studies. As efforts to standardize hCG assays continue, and in the absence of a reliable assay on intact hCG, future epidemiological studies evaluating the associations of hCG with maternal risk of breast cancer can relate total hCG alone to risk.
EXECUTiVE SUMMARY.
Both free β-human chorionic gonadotropin (hCG) and total hCG may be associated with an inverse risk of breast cancer.
• The associations between free β-hCG and total hCG in relation to maternal breast cancer risk are similar.
In the absence of a reliable assay on intact hCG, total hCG alone can be used in epidemiological studies relating hCG to cancer risk, since free β-hCG does not appear to provide added information.
Acknowledgments
Ethical conduct of research The study was approved by the Regional Ethics Committee of Umeå, Sweden. No animals were used in this study.
USPHS grants CA 114329 (P Toniolo), CA120061 (A Lukanova) and Cancer Center grant CA16087 from the National Cancer Institute, Department of Health and Human Services and Lions Cancer Research Foundation, Umeå University. AT Toriola is supported by the Washington University School of Medicine, Barnes-Jewish Hospital Foundation and Siteman Cancer Center.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Srivastava P, Russo J, Russo IH. Chorionic gonadotropin inhibits rat mammary carcinogenesis through activation of programmed cell death. Carcinogenesis. 1997;18(9):1799–1808. doi: 10.1093/carcin/18.9.1799. [DOI] [PubMed] [Google Scholar]; •• Experimental study revealed that human chorionic gonadotropin (hCG)-induced programmed cell death in rats is p53-dependent and modulatd by c-myc expression.
- 2.Lopez D, Sekharam M, Coppola D, Carter WB. Purified human chorionic gonadotropin induces apoptosis in breast cancer. Mol. Cancer Ther. 2008;7(9):2837–2844. doi: 10.1158/1535-7163.MCT-08-0339. [DOI] [PubMed] [Google Scholar]; • Intramural injection of purified hCG increased the apoptotic index in breast cancer xenografts.
- 3.Russo IH, Russo J. Hormonal approach to breast cancer prevention. J. Cell Biochem. Suppl. 2000;34:1–6. [PubMed] [Google Scholar]; • Details the molecular mechanisms through which hCG might prevent breast cancer and the possible therapeutic applications.
- 4.Rao Ch V, Li X, Manna SK, Lei ZM, Aggarwal BB. Human chorionic gonadotropin decreases proliferation and invasion of breast cancer MCF-7 cells by inhibiting NF-kappaB and AP-1 activation. J. Biol. Chem. 2004;279(24):25503–25510. doi: 10.1074/jbc.M400683200. [DOI] [PubMed] [Google Scholar]
- 5.Lukanova A, Andersson R, Wulff M, et al. Human chorionic gonadotropin and alpha-fetoprotein concentrations in pregnancy and maternal risk of breast cancer: a nested case–control study. Am. J. Epidemiol. 2008;168(11):1284–1291. doi: 10.1093/aje/kwn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toniolo P, Grankvist K, Wulff M, et al. Human chorionic gonadotropin in pregnancy and maternal risk of breast cancer. Cancer Res. 2010;70(17):6779–6786. doi: 10.1158/0008-5472.CAN-09-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Study demonstrated that maternal breast cancer risk decreased with increasing concentrations of total hCG and the association might be modified by age at diagnosis.
- 7.Sturgeon CM, Berger P, Bidart J-M, et al. Differences in recognition of the 1st WHO International Reference Reagents for hCG-related isoforms by diagnostic immunoassays for human chorionic gonadotropin. Clin. Chem. 2009;55(8):1484–1491. doi: 10.1373/clinchem.2009.124578. [DOI] [PubMed] [Google Scholar]; •• Demonstrated large interassay differences in the recognition of hCG isoforms.
- 8.Medeiros SF, Norman RJ. Human choriogonadotrophin protein core and sugar branches heterogeneity: basic and clinical insights. Hum. Reprod. Update. 2009;15(1):69–95. doi: 10.1093/humupd/dmn036. [DOI] [PubMed] [Google Scholar]
- 9.Iles RK, Delves PJ, Butler SA. Does hCG or hCGbeta play a role in cancer cell biology? Mol. Cell Endocrinol. 2010;329(1–2):62–70. doi: 10.1016/j.mce.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Yuri T, Lai YC, Yoshizawa K, Tsubura A. Human chorionic gonadotropin inhibits N-methyl-N-nitrosourea-induced mammary carcinoma growth in female Lewis rats. In Vivo. 2012;26(3):361–367. [PubMed] [Google Scholar]
- 11.Lunardi-Lskandar Y, Bryant JL, Zeman RA, et al. Tumorigenesis and metastasis of neoplastic Kaposi's sarcoma cell line in immunodeficient mice blocked by a human pregnancy hormone. Nature. 1995;375(6526):64–68. doi: 10.1038/375064a0. [DOI] [PubMed] [Google Scholar]
- 12.Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br. J. Cancer. 2000;82(9):1553–1556. doi: 10.1054/bjoc.2000.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillott DJ, Iles RK, Chard T. The effects of beta-human chorionic gonadotrophin on the in vitro growth of bladder cancer cell lines. Br. J. Cancer. 1996;73(3):323–326. doi: 10.1038/bjc.1996.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson BE, Hulka BS, Peck JL, et al. Levels of maternal serum alpha-fetoprotein (AFP) in pregnant women and subsequent breast cancer risk. Am. J. Epidemiol. 1998;148(8):719–727. doi: 10.1093/oxfordjournals.aje.a009691. [DOI] [PubMed] [Google Scholar]
- 15.Cleveland WS. Smoothing by local regression: principles and methods. In: Härdle W, Schimek M, editors. Statistical Theory and Computational Aspects of Smoothing. 1st Edition Springer; NY, USA: 1996. pp. 113–120. [Google Scholar]
- 16.Stenman UH, Tiitinen A, Alfthan H, Valmu L. The classification, functions and clinical use of different isoforms of HCG. Hum. Reprod. Update. 2006;12(6):769–784. doi: 10.1093/humupd/dml029. [DOI] [PubMed] [Google Scholar]
- 17.Gronowski AM, Grenache DG. Characterization of the hCG variants recognized by different hCG immunoassays: an important step toward standardization of hCG measurements. Clin. Chem. 2009;55(8):1447–1449. doi: 10.1373/clinchem.2009.129205. [DOI] [PubMed] [Google Scholar]
- 18.Cole L. New discoveries on the biology and detection of human chorionic gonadotropin. Reprod. Biol. Endocrinol. 2009;7(1):8. doi: 10.1186/1477-7827-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 1981;50(1):465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- 20.Lapthorn AJ, Harris DC, Littlejohn A, et al. Crystal structure of human chorionic gonadotropin. Nature. 1994;369(6480):455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- 21.Cole LA. HCG variants, the growth factors which drive human malignancies. Am. J. Cancer Res. 2012;2(1):22–35. [PMC free article] [PubMed] [Google Scholar]
- 22.Wald NJ, Kennard A, Hackshaw A, Mcguire A. Antenatal screening for Down's syndrome. Health Technol. Assess. 1998;2(1):i–iv. 1–112. [PubMed] [Google Scholar]
- 23.De Graaf IM, Cuckle HS, Pajkrt E, Leschot NJ, Bleker OP, Van Lith JMM. Co-variables in first trimester maternal serum screening. Prenat. Diagn. 2000;20(3):186–189. [PubMed] [Google Scholar]
- 24.Bestwick JP, Huttly WJ, Wald NJ. First trimester Down's syndrome screening marker values and cigarette smoking: new data and a meta-analysis on free β human chorionic gonadotophin, pregnancy-associated plasma protein-A and nuchal translucency. J. Med. Screen. 2008;15(4):204–206. doi: 10.1258/jms.2008.008049. [DOI] [PubMed] [Google Scholar]
- 25.Lambert-Messerlian G, Palomaki GE, Canick JA. Adjustment of serum markers in first trimester screening. J. Med. Screen. 2009;16(2):102–103. doi: 10.1258/jms.2009.009028. [DOI] [PubMed] [Google Scholar]
- 26.Tislaric D, Brajenovic-Milic B, Ristic S, et al. The influence of smoking and parity on serum markers for Down's syndrome screening. Fetal Diag. Ther. 2002;17(1):17–21. doi: 10.1159/000047999. [DOI] [PubMed] [Google Scholar]
- 27.Demir R, Demir AY, Yinanc M. Structural changes in placental barrier of smoking mother. A quantitative and ultrastructural study. Pathol. Res. Pract. 1994;190(7):656–667. doi: 10.1016/s0344-0338(11)80744-2. [DOI] [PubMed] [Google Scholar]
- 28.Yaron Y, Lehavi O, Orr-Urtreger A, et al. Maternal serum HCG is higher in the presence of a female fetus as early as week 3 post-fertilization. Hum. Reprod. 2002;17(2):485–489. doi: 10.1093/humrep/17.2.485. [DOI] [PubMed] [Google Scholar]
- 29.Wald NJ, Watt HC. Serum markers for Down's syndrome in relation to number of previous births and maternal age. Prenat. Diagn. 1996;16(8):699–703. doi: 10.1002/(SICI)1097-0223(199608)16:8<699::AID-PD919>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson HI, Lemanski N, Agarwal A, et al. A proposed unified mechanism for the reduction of human breast cancer risk by the hormones of pregnancy. Cancer Prev. Res. (Phila.) 2010;3(2):212–220. doi: 10.1158/1940-6207.CAPR-09-0050. [DOI] [PubMed] [Google Scholar]
- 31.Bennett JA, Semeniuk DJ, Jacobson HI, Murgita RA. Similarity between natural and recombinant human alpha-fetoprotein as inhibitors of estrogen-dependent breast cancer growth. Breast Cancer Res. Treat. 1997;45(2):169–179. doi: 10.1023/a:1005841032371. [DOI] [PubMed] [Google Scholar]
- 32.Melbye M, Wohlfahrt J, Lei U, et al. Alpha-fetoprotein levels in maternal serum during pregnancy and maternal breast cancer incidence. J. Natl Cancer Inst. 2000;92(12):1001–1005. doi: 10.1093/jnci/92.12.1001. [DOI] [PubMed] [Google Scholar]