Abstract
Objective
To evaluate the impact of antiretroviral therapy (ART) and the prognostic factors for in-intensive care unit (ICU) and 6-month mortality in human immunodeficiency virus (HIV)-infected patients.
Design
A retrospective cohort study was conducted in patients admitted to the ICU from 1996 through 2006. The follow-up period extended for 6 months after ICU admission.
Setting
The ICU of a tertiary-care teaching hospital at the Universidade de São Paulo, Brazil.
Participants
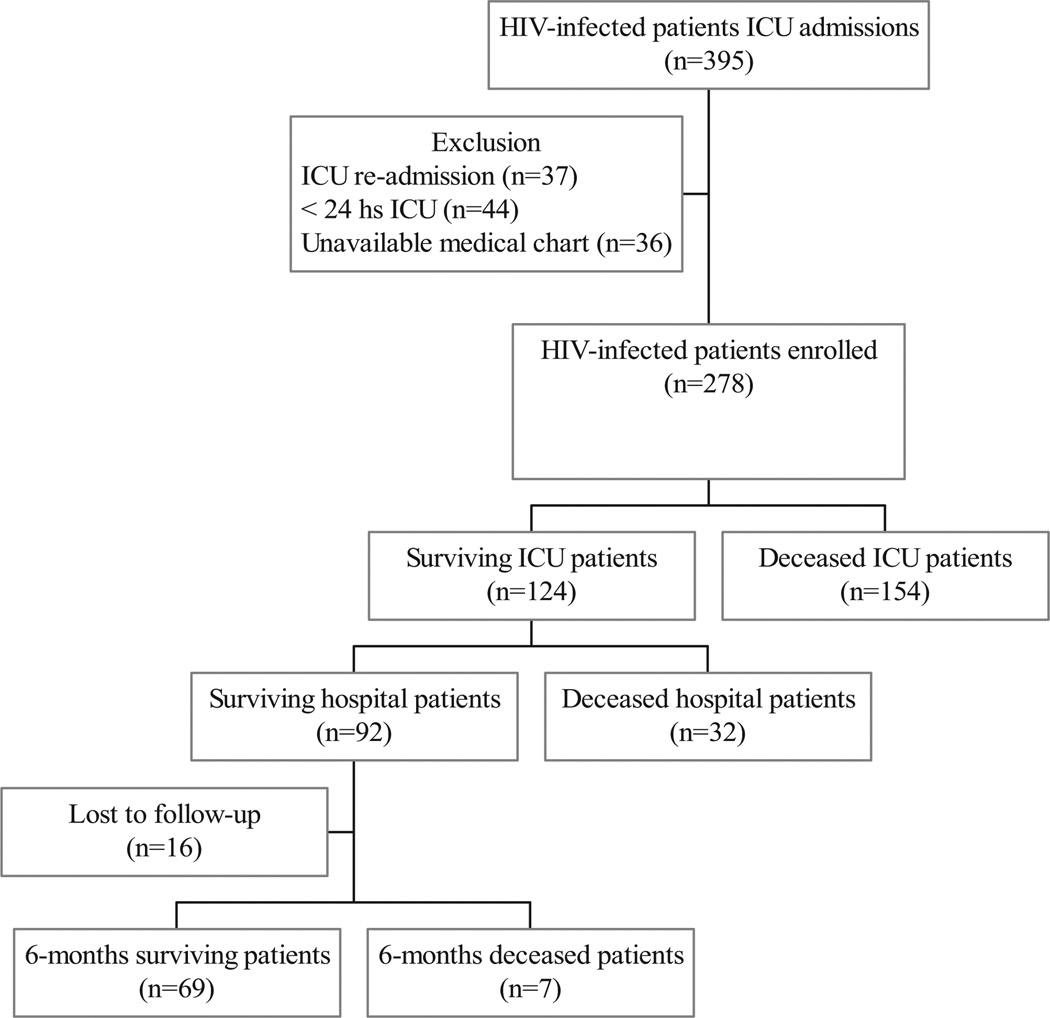
A total of 278 HIV-infected patients admitted to the ICU were selected. We excluded ICU readmissions (37), ICU admissions who stayed less than 24 hours (44), and patients with unavailable medical charts (36).
Outcome Measure
In-ICU and 6-month mortality.
Main Results
Multivariate logistic regression analysis and Cox proportional hazards models demonstrated that the variables associated with in-ICU and 6-month mortality were sepsis as the cause of admission (odds ratio [OR] = 3.16 [95% confidence interval [CI] 1.65– 6.06]); hazards ratio [HR] = 1.37 [95% CI 1.01–1.88]), an Acute Physiology and Chronic Health Evaluation II score > 19 [OR = 2.81 (95% CI 1.57–5.04); HR = 2.18 (95% CI 1.62–2.94)], mechanical ventilation during the first 24 hours [OR = 3.92 (95% CI 2.20–6.96); HR = 2.25 (95% CI 1.65–3.07)], and year of ICU admission [OR = 0.90 (95% CI 0.81– 0.99); HR = 0.92 [95% CI 0.87– 0.97)]. CD4 T-cell count <50 cells/mm3 was only associated with ICU mortality [OR = 2.10 (95% CI 1.17– 3.76)]. The use of ART in the ICU was negatively predictive of 6-month mortality in the Cox model [HR = 0.50 (95% CI 0.35– 0.71)], especially if this therapy was introduced during the first 4 days of admission to the ICU [HR = 0.58 (95% CI 0.41– 0.83)]. Regarding HIV-infected patients admitted to ICU without using ART, those who have started this treatment during ICU stay presented a better prognosis when time and potential confounding factors were adjusted for [HR 0.55 (95% CI 0.31– 0.98)].
Conclusions
The ICU outcome of HIV-infected patients seems to be dependent not only on acute illness severity, but also on the administration of antiretroviral treatment. (Crit Care Med 2009; 37: 000–000)
Keywords: intensive care, human immunodeficiency virus, acquired immunodeficiency syndrome, antiretroviral therapy, prognostic factors, critical care, mortality
The introduction of highly active antiretroviral therapy (HAART) and effective prophylaxis of opportunistic infections ushered in a new era in the management of human immunodeficiency virus (HIV) infection and changed the natural history of this disease (1–4). Intensive care mortality of HIV-infected patients has decreased from 37%–57.7% to 29%–37%, since the introduction of HAART (5–9). The rates of intensive care unit (ICU) admissions have also changed, especially for the spectrum of non–acquired immunodeficiency syndrome (AIDS)-associated illnesses as initial diagnoses (6, 8, 10–12). Although respiratory failure associated with Pneumocystis jirovecii pneumonia is still the most frequent reason for ICU admission, non–AIDS-associated illnesses are now becoming more common, including bacterial pneumonia and sepsis, hepatic insufficiency, cardiovascular diseases, neoplasm, and complications of chemotherapy (6, 9–11, 13–16).
Prescribing antiretroviral therapy (ART) in ICU is a subjective decision, which is most often based on the advice of the specialist in charge (9). There is no consensus, and few data are available to guide the use of ART in critically ill HIV-infected patients. Three studies conducted within the past 6 years suggest that patients who are admitted with an AIDS-defining diagnosis have the poorest prognosis and, theoretically, will receive additional benefit from ART (6, 17, 18). A retrospective study evaluating the effect of ART in 58 patients with AIDS with Pneumocystis jirovecii pneumonia found a lower mortality rate among patients with ART compared with patients without ART (25% vs. 63%, p = 0.03) (17).
Several prognostic markers of mortality for HIV-infected patients admitted to the ICU have earlier been identified in developed countries, which include Pneumocystis jirovecii pneumonia diagnosis, respiratory failure requiring mechanical ventilation, pneumothorax development, low serum albumin, and a high Acute Physiology and Chronic Health Evaluation (APACHE) II score (5, 8, 11, 19–21). However, the importance of these prognostic factors in developing countries has been poorly addressed. This study aimed at evaluating the impact of ART and prognostic factors of in-ICU and 6-month mortality in HIV-infected patients admitted to a Brazilian ICU.
METHODS
Study Population
This retrospective cohort study was conducted in a six-bed infectious disease ICU at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, a 2,200-bed teaching hospital in São Paulo, Brazil. The study enrolled HIV-infected adults admitted to the ICU from October 1, 1996 through October 30, 2006, which coincided with the implementation of universal-free access to HAART in Brazil. We included only the first ICU admission to avoid counting two outcomes for patients with multiple admissions. We excluded patients who died within 24 hours of ICU admission and patients without available medical charts. The Investigational Review Board of the Hospital das Clínicas approved the study protocol.
Data Collection
A standardized data entry form, which collected information on demographics, clinical history, and laboratory findings based on medical chart and computerized laboratory records review, was administered for each case. Variables included age, sex, risk factors for HIV infection, date of HIV diagnosis, previous opportunistic infections, coinfections, and preadmission comorbidities. The CD4 cell count and plasma HIV RNA level within 6 months of admission were obtained.
Admission diagnoses were classified as AIDS or non–AIDS-associated diseases according to the list of AIDS-defining conditions from the CDC (22). An APACHE II score was calculated for all patients. Each patient was categorized by the main reason for ICU admission: metabolic/renal, pulmonary infection, sepsis, neurologic failure, hemorrhage/hypovolemia, cardiac failure, chronic obstructive pulmonary disease exacerbation, other respiratory failures, cardiac arrest, and other conditions. Respiratory support (spontaneous breathing, invasive, or noninvasive mechanical ventilation) during the first 24 hours of ICU admission was also observed. Serum albumin and lactate dehydrogenase levels were recorded, if collected within the first 24 hours of admission. The use, regimen, and date of initiation of ART, as well as in-ICU and in-hospital adverse effects of ART, were recorded. To assess survival differences according to exposure to antiretroviral drugs, we divided the cohort into three prespecified subgroups: a) patients who were already receiving ART and continued receiving it in the ICU, b) patients who initiated ART in the ICU, and c) patients who did not use ART during their ICU stay.
We recorded survival at the time of ICU discharge and 6 months after ICU discharge. For patients without ambulatory follow-up, vital status was assessed by telephone or by the Program for Improvement of Death Information in the City of São Paulo (PRO-AIM).
Statistical Analysis
SAS 9.1 software was used for data analysis. Stepwise multivariate logistic regression analysis was performed to identify predictive factors of ICU mortality. Variables were included in the model if they reached a significance level of p < 0.20 in the univariate analysis. The Wald test was used to evaluate the significance level of the predictors in the final model. Statistical significance was determined at a p value of <0.05. Results are expressed as the odds ratio (OR) with 95% confidence interval. Cox proportional hazard models were performed to identify predictors for 6-month mortality. Variables were included in the model if they reached a significance level of p < 0.20 by the log-rank test. Statistical significance was determined at a p value of <0.05. Results are expressed as the hazards ratio (HR) with 95% confidence interval.
To assess the impact of ART, survival analyses were performed in two ways: by a proportional- hazards regression model incorporating the assigned ART utilization; and by a proportional-hazards regression model incorporating a time-dependent variable that accounts for exposure to ART. The first approach followed the intention-to-treat principle, whereas the second approach took into account the actual time intervals during which study medication was received by each patient. Potential confounding factors were included in the model, because they have been associated with mortality in the previous studies. We also included variables that reached a p value of <0.05 following comparison of the three ART-exposure groups, and with available data that fit into our model (Table 1). All possible two-factor interactions were tested by multivariate models.
Table 1.
Demographic and baseline clinical characteristics of 278 HIV-infected patients admitted to the ICU, stratified according to the antiretroviral therapy used
| Characteristics | Overall (n = 278) |
Patients With ART During ICU Staya (n = 143) |
Patients Beginning ART During ICU Stay (n = 61) |
Patients Without ART During ICU Stay (n = 74) |
pb |
|---|---|---|---|---|---|
| Age—mean years ± SD | 39.9 ± 10.4c | 40.4 ± 9.6 | 40.3 ± 9.3 | 38.5 ± 12.2 | 0.43 |
| Sex—male, n (%) | 199 (71.6) | 103 (72.0) | 45 (73.8) | 51 (68.9) | 0.81 |
| HIV transmission category | |||||
| Heterosexual, n (%) | 163 (58.6) | 83 (58.0) | 37 (60.7) | 43 (58.1) | 0.94 |
| Homosexual, n (%) | 43 (15.5) | 26 (18.2) | 8 (13.1) | 9 (12.2) | 0.43 |
| Injection drug user, n (%) | 37 (13.3) | 17 (11.9) | 6 (9.8) | 14 (18.9) | 0.23 |
| Other/unknown, n (%) | 35 (12.6) | 17 (11.9) | 10 (16.4) | 8 (10.1) | 0.58 |
| Medical history | |||||
| Time since HIV diagnosis median days (IR) | 92 (10–1445)d | 370 (40–1608) | 17 (5–605) | 14 (2–1445) | <0.01 |
| History of ART use previous to hospital admission, n (%) | 125 (45) | 92 (64) | 12 (20) | 21 (28) | <0.01 |
| CD4 T-cell count, median cells/mL (IR) | 39 (16–92) | 41.5 (17–94) | 26.5 (12–58) | 42 (14–117) | 0.13 |
| HIV RNA load, median log10 copies/mL (IR) | 5.39 (4.70–5.79) | 5.23 (4.28–5.77) | 5.48 (4.96–5.85) | 5.41 (5.08–5.81) | 0.11 |
| Antihepatitis C virus positive, n (%) | 43 (15.5) | 22/128 (17.2) | 3/51 (5.9) | 18/66 (27.3) | 0.01 |
| HbsAg positive, n (%) | 29 (10.4) | 13/125 (10.4) | 5/50 (10.0) | 11/66 (16.7) | 0.40 |
| Comorbidity,e n (%) | 73 (26.3) | 40 (28.0) | 10 (16.4) | 23 (31.1) | 0.12 |
| Main reason for ICU admission | |||||
| Respiratory, n (%) | 92 (33.1) | 42 (29.4) | 28 (45.9) | 22 (29.7) | 0.05 |
| Sepsis, n (%) | 87 (31.3) | 48 (33.6) | 17 (27.9) | 22 (29.7) | 0.68 |
| Neurologic, n (%) | 54 (19.4) | 25 (17.5) | 13 (21.3) | 16 (21.6) | 0.70 |
| Others | 45 (16.2) | ||||
| Opportunistic infection | |||||
| Tuberculosis, n (%) | 95 (34.3) | 43 (30.1) | 24 (39.3) | 28 (37.8) | 0.30 |
| Pneumocystis jirovecii pneumonia, n (%) | 64 (23.2) | 29 (20.3) | 17 (27.9) | 18 (25.0) | 0.48 |
| Cytomegalovirus, n (%) | 51 (18.4) | 25 (17.5) | 15 (24.6) | 11 (15.1) | 0.36 |
| Toxoplasmosis, n (%) | 49 (17.7) | 22 (15.4) | 13 (21.3) | 14 (19.2) | 0.56 |
| Cryptococcosis, n (%) | 29 (10.5) | 20 (14.0) | 4 (6.6) | 5 (6.8) | 0.14 |
| Clinical characteristics | |||||
| APACHE II score median (IR) | 19 (15–23) | 20 (16–24) | 18 (14–12) | 18.5 (14–23) | 0.05 |
| Albumin, median g/dL (IR) | 21 (16–28) | 22 (18–29) | 21 (17–27) | 19 (15–27) | 0.03 |
| DHL, median mg/dL (IR) | 743 (459–1143) | 689 (456–1042) | 743 (443–1143) | 828 (493–1515) | 0.30 |
| Mechanical ventilation in the first day, n (%) | 153 (55.0) | 73 (51.0) | 42 (68.9) | 38 (51.3) | 0.05 |
| Survival | |||||
| In-ICU mortality, n (%) | 154 (55.4) | 76/143 (53.1) | 35/61 (57.4) | 43/74 (58.1) | 0.74 |
| Six-mo mortality, n (%) | 193 (69.4) | 98/143 (68.5) | 41/61 (67.2) | 54/74 (73.0) | 0.73 |
AIDS, acquired immunodeficiency syndrome; RNA, ribonucleic acid; APACHE, Acute Physiology and Chronic Health Evaluation; ART, antiretroviral therapy; HIV, human immunodeficiency virus; HCV, hepatitis C virus; HbsAg, Hepatitis B virus surface antigen; ICU, intensive care unit; LDH, lactate dehydrogenase; SD, standard deviation; IR, interquartile range.
Patients already receiving antiretroviral therapy and continuing to receive it in the ICU;
chi-square or Kruskall-Wallis test;
mean and standard deviation were used for continuous variables with normal distribution;
median and interquartile range were used for continuous variables with non-normal distribution;
diabetes mellitus, liver, or chronic renal failure, chronic cardiovascular and pulmonary diseases, and non–AIDS-related malignancies.
To better evaluate the opportune moment to initiate ART, we defined the time to treatment as the interval between admission and the administration of ART. Cox regression analysis was used to estimate the hazard ratio of 6-month mortality at different periods of time to treatment.
RESULTS
Demographic and Clinical Characteristics
A total of 395 HIV-infected patients admitted to the ICU were reviewed. We excluded ICU readmissions (37), ICU admissions that stayed <24 hours (44), and patients with unavailable medical charts (36). The remaining 278 admissions were included in this study (Fig. 1). A total of 124 HIV-infected patients survived while in the ICU. Among the 92 hospital survivors, 70 patients (76.1%) have received ART.
Figure 1.
Flow diagram of human immunodeficiency virus (HIV)-infected patients enrolled in the study at the Infectious Diseases Intensive Care Unit of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo from 1996 to 2006. ICU, intensive care unit.
The majority of the ICU cohort consisted of men (71.6%) and heterosexuals (58.6%). The mean age was 39.9 years, and the median time since HIV diagnosis was 92 days. The ICU admission represented the initial HIV diagnosis for 38% of the patients. Most ICU admissions were because of AIDS-defining conditions (80.6%) and low CD4 count (median 39 cells/mL). More than one third of the subjects had tuberculosis (34.3%). Respiratory failure and sepsis were the most common reasons for ICU admission (33.1% and 31.3%, respectively). Most patients required mechanical ventilation (75.2%) and vasoactive drugs (61.1%). Patients presented with a median APACHE II score of 19, serum albumin of 21 g/L, and a serum lactate dehydrogenase level of 743 mg/dl (Table 1). The median lengths of ICU and hospital stays were 7 days (interquartile range 4–15 days) and 26 days (interquartile range 13–52 days), respectively.
Prognostic Factors
By univariate analysis, the factors associated with ICU mortality (p < 0.05) were year of ICU admission, injecting drug use risk for HIV infection, CD4 T-cell count <50 cells/mm3, sepsis as the main reason for ICU admission, an APACHE II score >19, a serum albumin level lower than 20 g/L, an lactate dehydrogenase serum level higher than 1,000 mg/dL, and use of mechanical ventilation during the first 24 hours of ICU admission. After multivariate logistic regression analysis, the only independent predictors of ICU mortality were year of ICU admission, CD4 T cell count <50 cells/mm3, sepsis as the main reason for ICU admission, an APACHE II score >19, and use of mechanical ventilation during the first 24 hours of ICU admission (Table 2).
Table 2.
Univariate and multivariate analyses of variables associated with in-ICU and 6-month mortality among 278 HIV-infected patients admitted to the Infectious Diseases ICU of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo from 1996 to 2006
| ICU Mortality | Six-mo Mortality | |||
|---|---|---|---|---|
| Variables | Crude Odds Ratio (95% CI) |
Adjusted Odds Ratio (95% CI) |
Crude Hazard Ratio (95% CI) |
Adjusted Hazard Ratio (95% CI) |
| Age (per 10-year increase) | 1.04 (0.83–1.30) | 1.10 (0.97–1.24)b | ||
| Year of ICU admission (per 1-year increase) | 0.84 (0.71–0.99)a | 0.90 (0.81–0.99) | 0.95 (0.90–0.99)b | 0.92 (0.87–0.97) |
| HIV transmission category | ||||
| Heterosexual | 1.000a | 1.000b | 1.000 | |
| Homosexual | 0.41 (0.19–0.86)a | 0.64 (0.44–0.94)b | 0.69 (0.42–1.12) | |
| Injecting drug use | 1.40 (0.68–2.86)a | 1.40 (0.86–2.27)b | 1.69 (1.15–2.50) | |
| Others/unknown | 0.55 (0.26–1.19)a | 0.69 (0.46–1.04)b | 1.74 (1.21–2.71) | |
| Medical history | ||||
| CD4 T-cell count <50 cells/mm3 | 1.93 (1.17–3.19)a | 2.10 (1.17–3.76) | 1.29 (0.95–1.74)b | |
| History of ART use previous to hospital admission | 0.75 (0.47–1.21)a | 0.96 (0.72–1.28) | ||
| Use of ART during ICU stayd | 0.86 (0.50–1.47) | 0.77 (0.56–1.06)b | 0.50 (0.35–0.71) | |
| Antihepatitis C virus positive | 1.73 (0.89–3.36)a | 1.47 (0.95–2.29)b | ||
| HbsAg positive | 1.79 (0.80–4.04)a | 1.36 (0.87–2.13) | ||
| Main reason for ICU admission | ||||
| Respiratory failure | 0.68 (0.41–1.12)a | 0.65 (0.48–0.90)b | ||
| Sepsis | 4.01 (2.25–7.12)a | 3.16 (1.65–6.06) | 1.84 (1.37–2.48)b | 1.37 (1.01–1.88) |
| Opportunistic infection | ||||
| Tuberculosis | 0.76 (0.46–1.26) | 0.71 (0.52–0.96)b | ||
| Cryptococcosis | 1.94 (0.85–4.42)a | 1.52 (1.00–2.32)b | ||
| Clinical characteristics | ||||
| APACHE II score >19 | 3.79 (2.28–6.30)a | 2.81 (1.57–5.04) | 2.45 (1.84–3.27)b | 2.18 (1.62–2.94) |
| Albumin <20 g/L | 2.48 (1.34–4.58)c | 1.59 (1.13–2.22)c | ||
| LDH higher than 1,000 mg/dL | 2.03 (1.12–3.67)c | 1.35 (0.95–1.93)c | ||
| Mechanical ventilation in the first 24 hours | 4.40 (2.65–7.30)a | 3.92 (2.20–6.96) | 2.29 (1.70–3.10)b | 2.25(1.65–3.07) |
APACHE, Acute Physiology and Chronic Health Evaluation; ART, antiretroviral therapy; HIV, human immunodeficiency virus; ICU, intensive care unit; LDH, lactate dehydrogenase; CI, confidence interval.
Variables with p < 0.2 included in the logistic regression stepwise;
variables with p < 0.2 included in the Cox proportional hazards model stepwise;
albumin and LDH were excluded because of high missing rates;
patients who were already receiving antiretroviral therapy and continued to receive it in the ICU or those who initiated antiretroviral therapy in the ICU.
By univariate analysis, the factors associated with 6-month mortality (p < 0.05) were year of ICU admission, injecting drug use and others/unknown risks for HIV infection, respiratory failure or sepsis as the main reason for ICU admission, diagnosis of cryptococcosis or tuberculosis, an APACHE II score >19, a serum albumin level lower than 20 g/L, and use of mechanical ventilation during the first 24 hours of ICU admission. Using Cox proportional hazard models, the only independent predictors for 6-month mortality were year of ICU admission, injection drug user and others/unknown risk factors for HIV infection, use of ART during ICU stay, sepsis as the main reason of admission, an APACHE II score >19, and use of mechanical ventilation during the first 24 hours of ICU admission (Table 2).
ART and Outcome
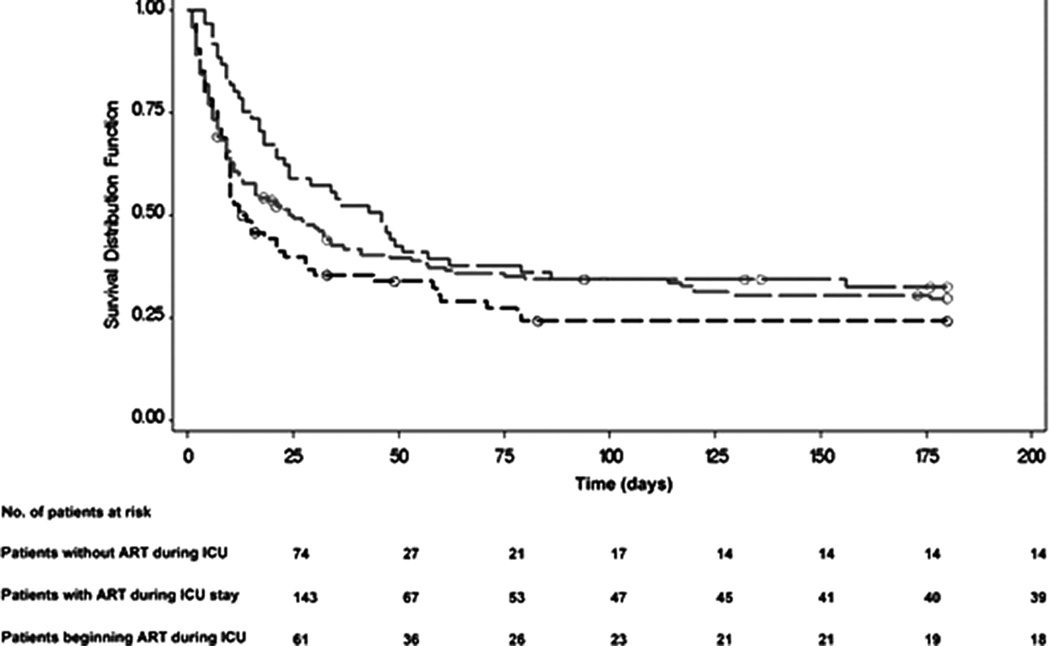
Kaplan-Meier survival curves for all patients stratified according to the category of ART exposition were significantly different among the groups (Wilcoxon, p = 0.03). This difference was especially prominent when comparing patients who underwent ART in the ICU to those who did not (Wilcoxon, p < 0.01). The survival of patients with previous and continuous use of ART in the ICU was not different from patients without ART, according to this category approach (Wilcoxon, p = 0.39) (Fig. 2).
Figure 2.
Kaplan-Meier survival curves for all intensive care unit (ICU) human immunodeficiency virus (HIV)-infected patients stratified according to the use of antiretroviral therapy. The survival of patients who began antiretroviral therapy (ART) during ICU stay (green) was significantly different from patients without ART during ICU stay (p = 0.004) (black). The survival of patients with previous and continuous use of ART at the ICU (red) was not different from HIV-infected patients without antiretroviral therapy during ICU stay (p = 0.39).
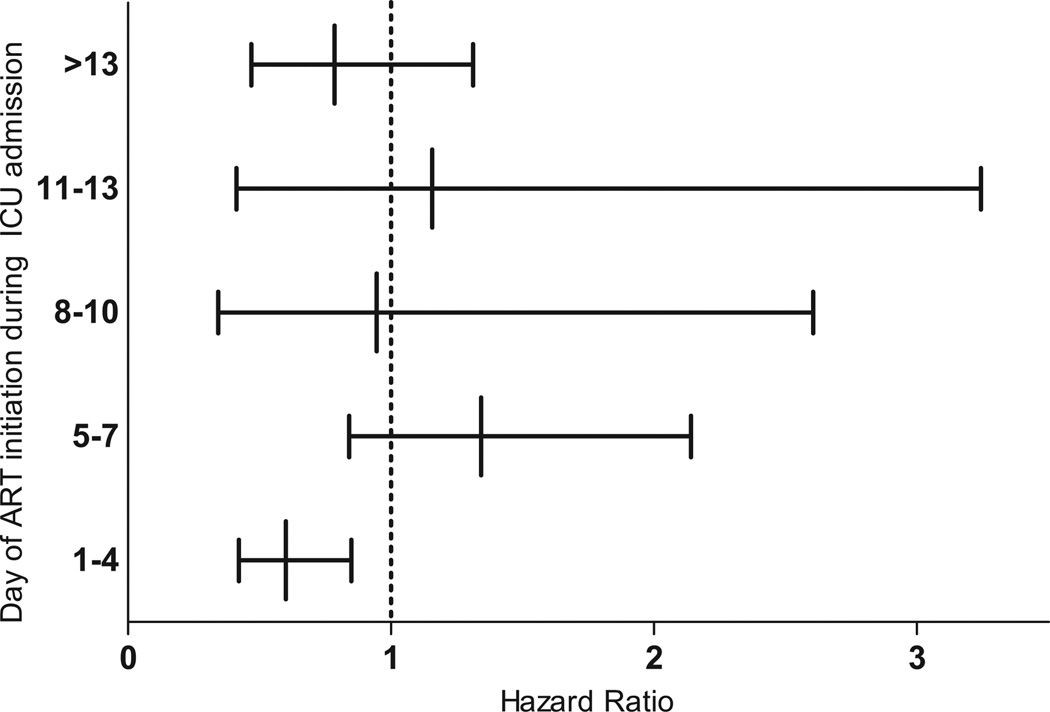
Cox regression analysis identified a protective effect of ART (HR = 0.50, 95% CI 0.35– 0.71) after adjusting for potential confounding factors, such as age, APACHE II score, year of admission, presence or absence of sepsis, Pneumocystis jirovecii pneumonia, comorbidities, hepatitis C, previous use of ART, time since HIV diagnosis, and mechanical ventilation at ICU admission (Table 2), particularly in patients who used this therapy during the first 4 days of ICU admission (HR = 0.58, 95% CI 0.41– 0.83) (Fig. 3).
Figure 3.
Hazard ratio and confidence intervals according to the time of initiation of antiretroviral therapy in 278 HIV-infected patients admitted to the intensive care unit (ICU). Hazard ratios are adjusted on the basis of age, Acute Physiology and Chronic Health Evaluation II, year of admission, presence or absence of sepsis, Pneumocystis jirovecii pneumonia, comorbidities, hepatitis C, lactate dehydrogenase, previous use of antiretroviral therapy, time of human immunodeficiency virus diagnosis, and mechanical ventilation during the first day of ICU admission. ART, antiretroviral therapy.
Although the use of ART previous to ICU admission was not an independent predictor of mortality, the interaction term with exposure to ART during ICU admission as a time-dependent covariate identified this variable as a possible effect modifier in multivariate analyses (p = 0.059). The use of ART was more beneficial for those patients with a history of ART use previous to hospital admission (HR = 0.46 [95% CI 0.25– 0.84]) than in patients without a history of ART (HR = 0.92 [95% CI 0.58 –1.46]). Furthermore, patients who have used ART previously to hospital admission and discontinued this therapy during ICU admission have higher mortality risk (HR = 2.00; 95% CI 0.971– 4.13) compared with patients who have continued this therapy in the ICU (HR = 1.00; 95% CI 0.30 –1.53).
In the subgroup analysis using exposure to ART as a time-dependent covariate to compare only patients who started ART during the ICU stay (n = 61) with patients who did not (n = 74), ART remained a significant negative risk factor for 6-month mortality (HR = 0.55; 95% CI 0.31– 0.98).
We were unable to obtain reliable information on ART adherence after hospital discharge and CD4 response, which could change survival. To access this issue, we performed a uniform interval-censored survival analysis using follow-up periods of 30 days. From 60 days on, use of ART in the ICU began to be associated with survival (HR = 0.48; 95% CI 0.34–69).
The most common prescription for beginning of treatment during ICU stay was two nucleoside/nucleotide transcriptase inhibitors and one non-nucleoside/nucleotide transcriptase inhibitor (33.7%). The most common regimen prescribed for beginning of treatment before ICU admission was two nucleotide transcriptase inhibitors and one protease inhibitor (31.4%). Adverse effects of ART were found in 18.1% of patients and mainly included hematologic disorders (8.8%), pancreatitis (2%), and lactic acidosis (2.9%) (Table 3). Anemia was observed in patients treated with zidovudine and pancreatitis in patients using stavudine. All adverse drug reactions demanded either a switch in or discontinuation of ART. Three patients with tuberculosis developed immune reconstitution syndrome during their ICU stay, but it was not necessary to discontinue ART. Mortality was not different among patients with continuous ART and patients who interrupted or modified ART during the hospital stay (p = 0.14, log-rank).
Table 3.
Antiretroviral therapy used by HIV-infected patients admitted to the ICU and adverse effects during hospitalization
| Antiretroviral Regimen | Overall (n = 204) |
Patients With ART Beginning Previous ICU Admission (n = 143) |
Patients With ART Beginning During ICU Stay (n = 61)a |
|---|---|---|---|
| 2 NRTI + 1 PI, n (%) | 64 (31.4) | 50 (35.0) | 14 (23.0) |
| 2 NRTI + 1 NNRTI, n (%) | 61 (29.9) | 39 (27.3) | 23 (37.7) |
| 2 NRTI + boosted PI, n (%) | 36 (17.7) | 21 (15.4) | 14 (23.0) |
| 2 NRTI, n (%) | 24 (11.8) | 17 (11.9) | 7 (11.5) |
| Others, n (%) | 19 (9.3) | 16 (11.2) | 3 (4.9) |
| Discontinue/modify ART, n (%) | 43 (21.1) | 33 (23.1) | 10 (16.4) |
| Adverse effects, n (%) | 37 (18.1) | 30 (20.1) | 7 (11.4) |
| Hematologic disorders,b n (%) | 18 (8.8) | 16 (11.9) | 2 (3.3) |
| Acute pancreatitis, n (%) | 4 (2) | 2 (1.4) | 2 (3.3) |
| Lactic acidosis, n (%) | 6 (2.9) | 5 (3.5) | 1 (1.6) |
| Others, n (%) | 6 (2.9) | 4 (2.8) | 2 (3.3) |
NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitors, ART, antiretroviral therapy; ICU, intensive care unit.
Patients who were already receiving antiretroviral therapy and continued to receive it in the ICU or those who initiated antiretroviral therapy in the ICU;
anemia, bicytopenia, and pancytopenia.
DISCUSSION
Despite the well-known benefits of ART, questions related to when ART should be continued or started during the critical care of HIV-infected patients remain unanswered. Although ART in the ICU setting presents distinct challenges related to drug delivery, doses, drug interactions, and antiretroviral-associated toxic effects (23–28), the findings of our study demonstrate benefit of this therapy in HIV-infected critically ill patients at 6 months after ICU admission. Furthermore, we identified several risk factors associated with in-ICU and 6-month mortality in developing countries.
The advantage of ART found in our population reflected the impact of early introduction of therapy in the context of extremely low CD4 counts (median of 39 cells/mm3) and high frequency of AIDS- defining conditions (80.6%). Recently, Zolopa et al (29) conducted a trial in which 282 patients with acute AIDS-related opportunistic infection were randomly assigned to receive either immediate ART on study entry or deferred ART after opportunistic infection treatment was completed (at least 4 weeks after study entry). They verified a reduced frequency of AIDS progression and death in the immediate ART arm, particularly during the first 6 months, with a more rapid increase in CD4 cell count probably reducing the period of vulnerability to disease progression and death.
Our high ICU and 6-month mortality rates (55.4% and 69.4%, respectively) suggest that the survival of HIV-infected patients who require intensive care in Brazil is still poor when compared with industrialized countries (5–8). This discrepancy can be explained in part by differences in healthcare assistance, with relatively few ICU beds present in Brazilian public hospitals, and by the clinical characteristics of our population. In our study, up to 35% of the patients were unaware of their status at the time of ICU admission or had a recent HIV diagnosis. Furthermore, most patients had low albumin levels (median of 21 g/L), high APACHE II scores (median of 19), and respiratory failure requiring mechanical ventilation (5–8).
In this investigation, sepsis as a cause of admission, an APACHE II score >19, and mechanical ventilation during the first 24 hours of ICU admission were independent predictors of in-ICU and 6-month mortality in HIV-infected patients. Similar findings have been reported in industrialized countries (5–8, 11, 14, 20). HIV-infected patients with sepsis had significantly higher mortality rates than those with respiratory failure (76.9% and 57.8%, respectively, p = 0.01). Previous studies have highlighted the impact of sepsis in critically ill HIV-infected patients (16, 30–34), especially in developing countries (18). Our results reinforce the importance of sepsis diagnosis in this population. Efforts to ensure early diagnosis and treatment of severe sepsis would probably reduce the mortality of HIV-infected patients admitted to ICU.
Regardless of the study’s retrospective nature, we were unable to obtain reliable information on ART adherence after ICU discharge and CD4 response, both of which can change the short-term survival (i.e., 6 months after ICU discharge). To access this issue, we performed sensitive analyses with different censoring times. Even with 60 days of censure time, exposure to ART in the ICU remained associated with survival, probably because most events occurred soon after ICU discharge.
Another difficulty of studying ICU outcomes for HIV-infected patients is the changing attitudes toward aggressive care for patients with AIDS and the variation in levels of care through the years (Fig. 1, supplemental material). For this reason, the data obtained in our analysis were adjusted by the year of admission. Furthermore, the ability to generalize the results may be limited. They reflect the late diagnosis and treatment of HIV infection in Brazil (35), hospital admission and discharge policies, and the practice patterns of our staff. In addition, the patient population in different institutions and countries probably has unique characteristics and treatment preferences that influence the spectrum of the disease.
Despite the limitations, our findings provide crucial information to support future prospective randomized clinical trials to address the benefit of ART on survival of critically ill HIV-infected patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank the clinical, laboratory, and administrative staff of Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, especially Ho Yeh Li and Luciana Gusti, for their help in collecting data and suggestions in the questionnaire. We are grateful to Edméa Costa Pereira from the Program for Improvement of Death Information in the City of São Paulo (PRO-AIM). Furthermore, we thank Albert I. Ko and Guilherme S. Ribeiro for their advice during study implementation and Marshall Glesby and Albert I. Ko for comments on the manuscript. Finally, we thank Angela T. Paes for statistical revision.
Supported, in part, by the Fogarty International Center (grant D43 TW00919) (to JC and MGC).
Footnotes
The authors have no potential conflicts of interest to disclose.
REFERENCES
- 1.Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30(Suppl 1):S5–S14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 2.Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. Aids. 1999;13:1933–1942. doi: 10.1097/00002030-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 3.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 4.Moore RD, Chaisson RE. Natural history of opportunistic disease in an HIV-infected urban clinical cohort. Ann Intern Med. 1996;124:633–642. doi: 10.7326/0003-4819-124-7-199604010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Narasimhan M, Posner AJ, DePalo VA, et al. Intensive care in patients with HIV infection in the era of highly active antiretroviral therapy. Chest. 2004;125:1800–1804. doi: 10.1378/chest.125.5.1800. [DOI] [PubMed] [Google Scholar]
- 6.Morris A, Creasman J, Turner J, et al. Intensive care of human immunodeficiency virus-infected patients during the era of highly active antiretroviral therapy. Am J Respir Crit Care Med. 2002;166:262–267. doi: 10.1164/rccm.2111025. [DOI] [PubMed] [Google Scholar]
- 7.Afessa B, Green B. Clinical course, prognostic factors, and outcome prediction for HIV patients in the ICU. The PIP (Pulmonary complications, ICU support, and prognostic factors in hospitalized patients with HIV) study. Chest. 2000;118:138–145. doi: 10.1378/chest.118.1.138. [DOI] [PubMed] [Google Scholar]
- 8.Casalino E, Wolff M, Ravaud P, et al. Impact of HAART advent on admission patterns and survival in HIV-infected patients admitted to an intensive care unit. Aids. 2004;18:1429–1433. doi: 10.1097/01.aids.0000131301.55204.a7. [DOI] [PubMed] [Google Scholar]
- 9.Huang L, Quartin A, Jones D, et al. Intensive care of patients with HIV infection. N Engl J Med. 2006;355:173–181. doi: 10.1056/NEJMra050836. [DOI] [PubMed] [Google Scholar]
- 10.Nuesch R, Geigy N, Schaedler E, et al. Effect of highly active antiretroviral therapy on hospitalization characteristics of HIV-infected patients. Eur J Clin Microbiol Infect Dis. 2002;21:684–687. doi: 10.1007/s10096-002-0792-3. [DOI] [PubMed] [Google Scholar]
- 11.Palacios R, Hidalgo A, Reina C, et al. Effect of antiretroviral therapy on admissions of HIV-infected patients to an intensive care unit. HIV Med. 2006;7:193–196. doi: 10.1111/j.1468-1293.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- 12.Boumis E, Petrosillo N, Girardi E, et al. Changing patterns in the etiology of HIV-associated bacterial pneumonia in the era of highly active antiretroviral therapy. Eur J Clin Microbiol Infect Dis. 2001;20:71–73. doi: 10.1007/s100960000420. [DOI] [PubMed] [Google Scholar]
- 13.Alves C, Nicolas JM, Miro JM, et al. Reappraisal of the aetiology and prognostic factors of severe acute respiratory failure in HIV patients. Eur Respir J. 2001;17:87–93. doi: 10.1183/09031936.01.17100870. [DOI] [PubMed] [Google Scholar]
- 14.Vincent B, Timsit JF, Auburtin M, et al. Characteristics and outcomes of HIV-infected patients in the ICU: Impact of the highly active antiretroviral treatment era. Intensive Care Med. 2004;30:859–866. doi: 10.1007/s00134-004-2158-z. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg AL, Seneff MG, Atiyeh L, et al. The importance of bacterial sepsis in intensive care unit patients with acquired immunodeficiency syndrome: Implications for future care in the age of increasing antiretroviral resistance. Crit Care Med. 2001;29:548–556. doi: 10.1097/00003246-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Mrus JM, Braun L, Yi MS, et al. Impact of HIV/AIDS on care and outcomes of severe sepsis. Crit Care. 2005;9:R623–R630. doi: 10.1186/cc3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris A, Wachter RM, Luce J, et al. Improved survival with highly active antiretroviral therapy in HIV-infected patients with severe Pneumocystis carinii pneumonia. Aids. 2003;17:73–80. doi: 10.1097/00002030-200301030-00010. [DOI] [PubMed] [Google Scholar]
- 18.Vargas-Infante YA, Guerrero ML, Ruiz-Palacios GM, et al. Improving outcome of human immunodeficiency virus-infected patients in a Mexican intensive care unit. Arch Med Res. 2007;38:827–833. doi: 10.1016/j.arcmed.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Casalino E, Mendoza-Sassi G, Wolff M, et al. Predictors of short- and long-term survival in HIV-infected patients admitted to the ICU. Chest. 1998;113:421–429. doi: 10.1378/chest.113.2.421. [DOI] [PubMed] [Google Scholar]
- 20.Nickas G, Wachter RM. Outcomes of intensive care for patients with human immunodeficiency virus infection. Arch Intern Med. 2000;160:541–547. doi: 10.1001/archinte.160.4.541. [DOI] [PubMed] [Google Scholar]
- 21.Bedos JP, Dumoulin JL, Gachot B, et al. Pneumocystis carinii pneumonia requiring intensive care management: Survival and prognostic study in 110 patients with human immunodeficiency virus. Crit Care Med. 1999;27:1109–1115. doi: 10.1097/00003246-199906000-00030. [DOI] [PubMed] [Google Scholar]
- 22.Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists; AIDS Program, Center for Infectious Diseases. MMWR Morb Mortal Wkly Rep. 1987;36(Suppl 1):1S–15S. [PubMed] [Google Scholar]
- 23.Heyland DK, Tougas G, King D, et al. Impaired gastric emptying in mechanically ventilated, critically ill patients. Intensive Care Med. 1996;22:1339–1344. doi: 10.1007/BF01709548. [DOI] [PubMed] [Google Scholar]
- 24.Tarling MM, Toner CC, Withington PS, et al. A model of gastric emptying using paracetamol absorption in intensive care patients. Intensive Care Med. 1997;23:256–260. doi: 10.1007/s001340050325. [DOI] [PubMed] [Google Scholar]
- 25.Battegay M, Nuesch R, Hirschel B, et al. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6:280–287. doi: 10.1016/S1473-3099(06)70463-7. [DOI] [PubMed] [Google Scholar]
- 26.Wit FW, Weverling GJ, Weel J, et al. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis. 2002;186:23–31. doi: 10.1086/341084. [DOI] [PubMed] [Google Scholar]
- 27.Sulkowski MS, Mehta SH, Chaisson RE, et al. Hepatotoxicity associated with protease inhibitor-based antiretroviral regimens with or without concurrent ritonavir. Aids. 2004;18:2277–2284. doi: 10.1097/00002030-200411190-00008. [DOI] [PubMed] [Google Scholar]
- 28.Bonnet F, Bonarek M, Abridj A, et al. [Severe lactic acidosis in HIV-infected patients treated with nucleosidic reverse transcriptase analogs: A report of 9 cases] Rev Med Interne. 2003;24:11–16. doi: 10.1016/s0248-8663(02)00702-6. [DOI] [PubMed] [Google Scholar]
- 29.Zolopa A, Andersen J, Komarow L, et al. Immediate vs deferred ART in the setting of acute AIDS-related opportunistic infection: Final results of a randomized strategy trial, ACTG A5164. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2008. [Google Scholar]
- 30.Thyrault M, Gachot B, Chastang C, et al. Septic shock in patients with the acquired immunodeficiency syndrome. Intensive Care Med. 1997;23:1018–1023. doi: 10.1007/s001340050451. [DOI] [PubMed] [Google Scholar]
- 31.De Palo VA, Millstein BH, Mayo PH, et al. Outcome of intensive care in patients with HIV infection. Chest. 1995;107:506–510. doi: 10.1378/chest.107.2.506. [DOI] [PubMed] [Google Scholar]
- 32.Afessa B, Morales I, Weaver B. Bacteremia in hospitalized patients with human immunodeficiency virus: A prospective, cohort study. BMC Infect Dis. 2001;1:13. doi: 10.1186/1471-2334-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afessa B, Green B. Bacterial pneumonia in hospitalized patients with HIV infection: The Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest. 2000;117:1017–1022. doi: 10.1378/chest.117.4.1017. [DOI] [PubMed] [Google Scholar]
- 34.Timsit JF. Open the intensive care unit doors to HIV-infected patients with sepsis. Crit Care. 2005;9:629–630. doi: 10.1186/cc3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aids MdS-SdVeS-PNdDe, editor. 2005. CN-DST/AIDS: Boletim Epidemiológico—Aids e DST; p. 48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.