Abstract
Radiation therapy (RT) is the treatment of cancer and other diseases with ionizing radiation. The ultimate goal of RT is to destroy all the disease cells while sparing healthy tissue. Towards this goal, RT has advanced significantly over the past few decades in part due to new technologies including: multileaf collimator-assisted modulation of radiation beams, improved computer-assisted inverse treatment planning, image guidance, robotics with more precision, better motion management strategies, stereotactic treatments and hypofractionation. With recent advances in nanotechnology, targeted RT with gold nanoparticles (GNPs) is actively being investigated as a means to further increase the RT therapeutic ratio. In this review, we summarize the current status of research and development towards the use of GNPs to enhance RT. We highlight the promising emerging modalities for targeted RT with GNPs and the corresponding preclinical evidence supporting such promise towards potential clinical translation. Future prospects and perspectives are discussed.
Keywords: cancer, gold nanoparticle, nanomedicine, radiotherapy, retinal disease
Background
The discovery of x-rays in 1895 by Wilhelm Conrad Röntgen and the award of a second Nobel Prize to Marie Curie for her research into radium, helped spawn the field of radiotherapy (RT), employing ionizing radiation to treat disease. Two main RT modalities emerged: external RT – where the radiation comes from a machine outside the body, and internal RT (brachytherapy) – where the radiation comes from implants or liquids placed inside the body. With either modality, RT works by damaging the DNA of disease cells directly or by creating charged particles (free radicals) within the cells that can in turn damage DNA [1]. Disease cells whose DNA is damaged beyond repair stop dividing or die via apoptosis/necrosis and the dead cells are eliminated by the body’s natural processes. The ultimate goal of RT is to destroy the disease cells with minimal damage to the normal cells, thus improving the therapeutic ratio [2].
To this end, recent technological advances have led to advanced RT modalities such as intensity-modulated radiation therapy (IMRT), image-guided RT (IGRT), stereotactic ablative body RT, proton therapy, electron beam therapy and so on, all aimed at achieving greater therapeutic efficacy, less side effects and less time under treatment. For example, IMRT at 6 MVp energy allows the creation and delivery of irregular-shaped radiation doses that conform to the target tumor whilst simultaneously avoiding significant damage to normal cells or neighboring organs at risk [3]. The increase in conformality and tighter treatment margins engendered an increased need for accuracy to circumvent the potential to miss the tumor due to organ motion and/or patient setup variations. IGRT works to assuage this need by allowing imaging of the target immediately prior to or even during treatment to guide the treatment and allow for more accurate RT [3]. The improved accuracy and greater understanding of radiation biology has in turn made dose escalation or radiation boosting feasible, and this has allowed for further improvement in the therapeutic ratio for several tumor sites [3]. Stereotactic ablative body RT is partly a consequence of this, allowing precise delivery of very high RT doses over only a few treatment fractions to ablate small, well-defined primary and oligometastatic tumors [3,4]. Meanwhile, another advanced RT approach employs high-energy proton beams to irradiate diseased tissue. The main advantage of therapy with proton beams is its ability to more precisely localize the radiation dosage compared with other types of external beam RT (EBRT) [5]. Owing to this ability, proton therapy is particularly useful in pediatric radiation oncology. With these advances, currently, over 50% of treated cancer patients receive RT [6]. In general, RT is most appropriate for patients whose disease has not spread, but can also be employed in combination with other treatment modalities such as chemotherapy, surgery or immunotherapy. It is estimated that RT contributes approximately 40% towards curative treatment [7], besides its use in palliative care.
Despite these advances in RT and associated improvements in primary treatment delivery, a significant number of cancer patients treated with RT still experience recurrence, deadly metastasis and significant toxicities to normal tissue [8]. For example, for prostate cancer, studies show that after definitive RT, biochemical failure (i.e., rise by 2 ng/ml or more above the nadir prostate-specific antigen) is seen in approximately 50% of patients after 5 years, which most often precedes clinical recurrence [9–11]. In addition, studies show that radiation boosting significantly helps prevent cancer recurrence and metastasis [12–19]. However, radiation boosting is also limited by unacceptable normal tissue toxicity [20,21], as well as other factors such as target motion [12,22–32]. There is correspondingly clear rationale to develop new treatment strategies that can overcome these limitations, allowing an increased RT dose to the tumor while sparing normal tissue [33].
Recent advances in nanotechnology have provided new opportunities for developing targeted RT modalities using gold nanoparticles (GNPs) to further increase RT therapeutic efficacy. Such targeted RT with GNPs involves first targeting the tumor cells or tumor subvolume with GNPs, and then targeting the GNPs during RT to boost RT efficacy. Following the pioneering work by Herold et al. with gold microspheres [34], Hainfeld et al. performed preclinical experiments in mouse models to establish proof-of-principle of the therapeutic benefit of intravenous injection of GNP targeting tumors during RT [35]. In the study by Hainfeld et al., the use of 7 mg/g GNPs (in tumor) with 250 kVp x-rays/photons produced 86% long-term survival compared with 20% when radiation was used alone [35], indicating major therapeutic enhancement due to the GNPs. The evident intrinsic radiosensitization potential of GNPs could be attributed to their high atomic number (Z), which endows them with the ability to readily interact with keV energy radiation via the photoelectric effect, to emit micrometer-range photo-/Auger electrons, which would boost RT dose locally. The probability of photoelectric interaction with RT photons is approximately proportional to Z3 [36]. However, beside their intrinsic radiosensitization capability, GNPs are particularly attractive for targeted RT due to their biocompatibility, and nontoxicity relative to other high Z material [37–40].
Additional advantages of GNPs also include the fact that they can inherently provide useful imaging contrast [41–43] and are suitable for attaching other drugs, or moieties that would drive future expansion of their clinical benefits [44]. Such a multifunctional platform based on gold nanostructures with targeting ligands, therapeutic molecules and imaging contrast agents holds great promise for enhancing RT. Cognizant of all these, research on the use of GNPs to enhance RT therapeutic efficacy is burgeoning into a promising frontier in cancer research [34,45–50]. In the section entitled ‘Design considerations of GNPs for targeted RT with GNPs’ of this review, we will summarize the progress in designing GNPs for targeted RT. This will be followed in the section entitled ‘GNPs as radiosensitizers’ by a review of the hitherto theoretical and experimental evidence for targeted RT with GNPs. In the section entitled ‘Emerging GNRT modalities’, we will highlight the emerging GNP-based RT modalities with promise or potential for clinical translation. Finally, the section entitled ‘Future perspective’ will discuss future prospects and perspectives for targeted RT with GNPs.
Design considerations of GNPs for targeted RT with GNPs
Nanomedicine holds advantage over conventional medicine because nanoparticles can be designed to enable the preferential delivery of drugs to tumors owing to the enhanced permeability and retention (EPR) effect, and the fact that nanoparticles can be programmed to sustainably release therapeutic agents including in situations that could benefit from combination therapy. The EPR effect relates to the propensity of macromolecules or nanoparticles of certain sizes to preferentially accumulate at sites of increased vascular permeability such as solid tumor tissue [51]. Transportation of nanoparticles to the tumor, for example, when administered intravenously, tumor accumulation, intratumoral distribution and uptake into tumor cells are dependent on the design of the nanoparticles, specifically the size, shape and surface properties or functionalization [52].
Ferrari and colleagues showed that size can significantly affect how particles interact with tumor capillaries during transport [53,54]. A number of other studies have reinforced the importance of nanoparticle size [44,55,56], including one study reporting that small-size gold nanoparticles (<6 nm) will tend to be cleared via renal excretion within a few minutes post intravenous administration, while larger nanoparticles accumulate in reticuloendothelial system [44]. Perrault and colleagues also examined the design-dependent tumor-targeting capacity of sub- 100-nm particles in nude mice bearing subcutaneous MDA-MB-435 xenograft tumors [52]. They reported that 100-nm particles administered intravenously achieved the greatest accumulation in tumors: 4.3-times greater than that achieved by 60- and 80-nm particles; nine-times greater than the 40-nm particles; and 38-times greater than the 20-nm particles. Concerning shape, recent reports also suggest a significant role for particle shape in the in vivo performance of nanoparticles [57,58]. Specifically, shape and shape-related form factors, such as aspect ratio or edge geometry, affect particle transport characteristics, influences cell–particle interactions and alters drug release kinetics, with disk-shaped nanoparticles showing longer half-lives in circulation [59–61].
Once the nanoparticles reach the tumor, their size and shape also play a role in intratumor diffusion and intracellular uptake. In a systematic investigation of the effect of nanoparticle size (10−100 nm) on passive targeting of tumors in vivo [52], Perrault et al. found that the permeation of nanoparticles within the tumor is highly dependent on the overall size of the nanoparticle, where larger nanoparticles appear to stay near the vasculature, while smaller nanoparticles rapidly diffuse throughout the tumor matrix. The work essentially demonstrated that PEGylated GNPs must be less than 100 nm in diameter to move away from the vasculature and throughout the tumor. In consonance with this, a recent study by Wong et al. proposed a multistage system in which 100-nm nanoparticles ‘shrink’ to 10-nm nanoparticles after they extravasate from leaky regions of the tumor vasculature and are exposed to the tumor microenvironment [62]. The shrunken nanoparticles then more readily diffuse throughout the tumor’s interstitial space. It is also now generally accepted that if particle surface properties are favorable, smaller particles get internalized more efficiently by nonphagocytic cells. Huang and colleagues demonstrated that ultrasmall GNPs smaller than 10 nm display unique advantages over nanoparticles larger than 10 nm in terms of localization to, and penetration of, breast cancer cells, multicellular tumor spheroids and tumors in mice [63].
Focusing on GNPs, Chithrani et al. investigated the size and shape dependence of GNP uptake in mammalian cells [64,65]. They showed that kinetics and saturation concentrations are highly dependent upon the physical dimensions of the nanoparticles. In particular, results showed that within the size range of 2–100 nm, GNPs of diameter 50 nm demonstrate the highest uptake. With respect to shape, Chithrani et al. also reported that spherical gold nanoparticles have a higher propensity to be internalized in vitro by HeLa cells compared with rod-shaped particles of similar dimensions [66]. Spherical nanoparticles with diameters of 14 or 75 nm were taken up by cells 375–500% more compared with 74 × 14 nm rod-shaped particles. The disparity in in vitro cell uptake could be partly attributed to the difference in particle curvature, which affects the particle contact area with the cell membrane. Roy and colleagues [67] and Chithrani [68] have written excellent reviews of such studies demonstrating that particle aspect ratio, shape and volume all affect cellular internalization of nanoparticles, and describe the mechanisms of nanoparticle uptake.
Besides size and shape, the surface properties or functionalization of nanoparticles such as GNPs significantly determine target accumulation, intratumoral diffusion and cellular uptake. The design of the first generation of nanomaterials mainly involved investigations on surface functionalization to assess in vitro uptake and cytotoxicity without taking biological challenges of in vivo delivery into consideration [69]. The second generation of nanoparticle design was primarily focused on surface modification to confer stealth and actively target the nanoparticles to enable significant accumulation of the nanoparticles in the target volume [70–72]. In one characteristic study, Choi et al. investigated the mechanism of active targeting in solid tumors with transferrin-containing GNPs, and showed that targeted GNPs can better reach cancer cells within solid tumors than their nontargeted analogs, which depend mainly on the EPR [71]. In general, active targeting exploits the (over)expression of surface receptors on cancer cells by providing targeting ligands that can engage these receptors. Ligands that have been investigated for active targeting include proteins, aptamers and small molecules such as vitamins, peptides or carbohydrates [71,73,74]. These investigations all indicate that ligand incorporation facilitates the targeting and uptake of nanoparticles to cancer cells via receptor-mediated endocytosis. Specifically for GNPs, there is a significant body of research that has already demonstrated active targeting of tumors with GNPs associated with peptides, antibodies, oligonucleotides and liposomes [71,75–81]. Nuclear targeting of GNPs in cancer cells has also been reported [74]. It is worth mentioning that active targeting using ligands such as antibodies and peptides does not necessarily improve the dose to the tumor cells, as this may lead to more convoluted behavior and effects in vivo as discussed in a recent review by Cheng et al. [82].
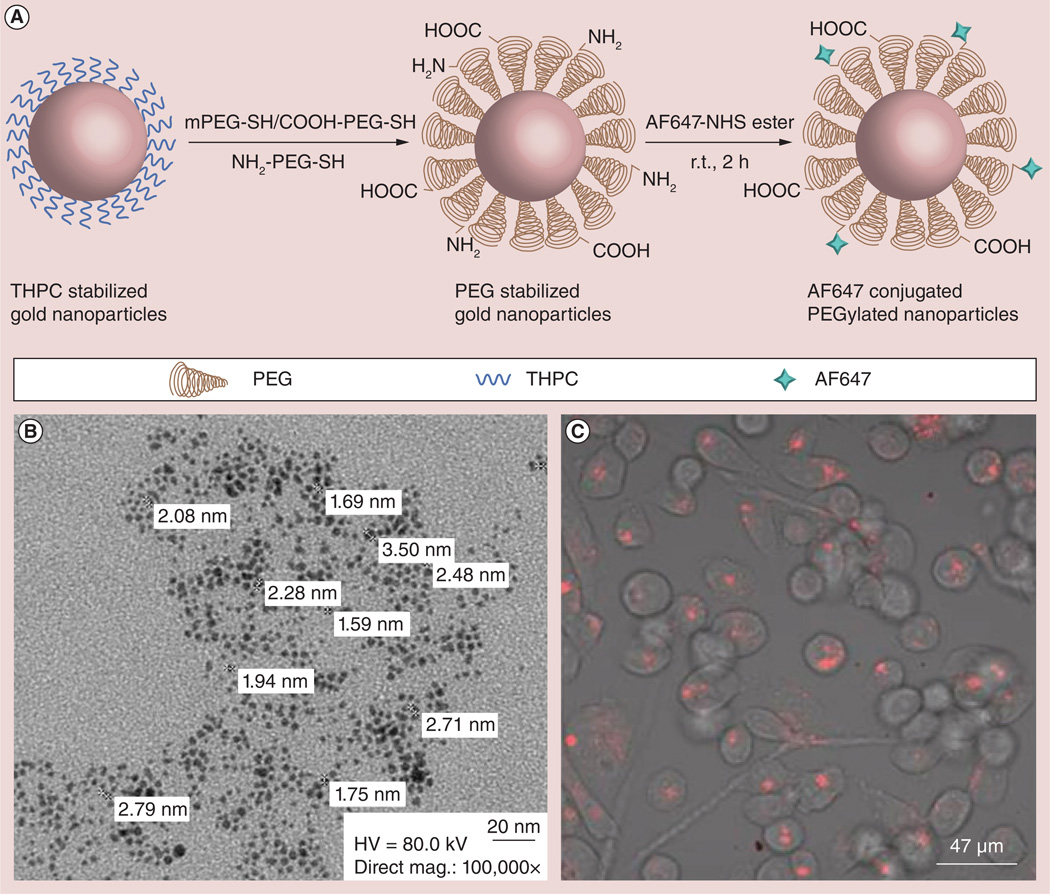
Currently, third-generation nanoparticles are being engineered for more precise control over in vivo biodistribution and disease-responsive drug release, building on the difficult lessons learned from the previous two generations [44,83]. One of our recent studies reports the development of such a third-generation platform called the AuRad™ particularly tailored for RT applications [44]. The design of the multifunctional AuRad nanoplatform for RT (Figure 1A & 1B) particularly considers size and surface functionalization as key features. The size is optimized for longer circulation, higher tumor uptake, as well as modulated clearance. Meanwhile, PEGylation allows for prolonged circulation time of the nanoparticles when administered intravenously, allowing sufficient time for potent concentration of nanoparticles to localize in the tumor. The hetero-bifunctional-polyethylene glycol with amine, carboxyl and methoxy ligands, also makes it a versatile nanoplatform to conjugate various moieties such as fluorophore, peptide, other radiosensitizers, drugs and radiolabels. This AuRad platform was tested in experiments to demonstrate their imaging capability for optical tracking in vitro and in vivo systems. Figure 1C depicts confocal fluorescence images of live cells incubated with the GNPs showing robust uptake of the fluorescent nanoparticles. Notably, no morphological damage was observed, indicating low cytotoxicity of the nanoparticles. In vivo testing of the AuRad nanoplatform is currently ongoing. Overall, based on the current literature review, the data indicate that active targeting would be a more efficacious approach for delivery of GNPs to the target for RT enhancement. Other third-generation nanoparticle platforms, which can also serve as drugdelivery agents, have been developed including biogenic GNPs [84,85], drug-loaded gold plasmonic nanoparticles for treatment of multidrug resistance in cancer [86] and other applications [87].
Figure 1. Third-generation gold nanoparticle platform for radiotherapy applications.
(A) The synthetic strategy of the PEGylated gold nanoparticles followed by conjugation with AF647. (B) Transmission electron micrograph of the THPC-coated gold nanoparticles after PEGylation and (C) the confocal fluorescence imaging of HeLA cell cultures after uptake of fluorescent gold nanoparticles.
AF647: Alexa Fluor 647; HV: High voltage; mag.: Magnification; mPEG: Monofunctional polyethylene gylcol; PEG: Polyethylene glycol; r.t.: Room temperature; THPC: Tetrakis(hydroxymethyl)phosphonium chloride. Reproduced with permission from [44] Translational Cancer Research.
It is worth noting that delivery of the GNPs to target tumor cells could also benefit from approaches being considered to facilitate the delivery of drugs administered intravenously. Such recently reviewed approaches [88], include modifying the tumor microenvironment to facilitate desired distribution of the nanoparticles. Such modification includes normalization of the tumor components such as the extracellular matrix using matrix-modifying agents in order to enable the delivery of sufficiently potent concentrations of targeted GNPs to the whole tumor. Although, it should be noted that normalization is transient, and thus, RT would have to be administered during the window of normalization [89,90]. Other studies have also shown that normalizing the extracellular matrix by degradation of its components, particularly the collagen and glycosaminoglycan content, lead to a major improvement (2–10-fold) in drug penetration [91,92]. Such degradation would thus increase the accessible volume of the diffusing GNPs. More studies would be needed to determine optimal combination of these approaches appropriate for enhancing RT using GNPs.
Overall, in order to optimize therapeutic ratio due to GNP radiosensitization, the optimal nanoparticle design should enable maximization of GNP localization in the tumor, with cellular uptake of GNPs close to the nucleus, while ensuring minimal distribution of GNPs to neighboring healthy tissue during RT. It is not yet completely clear, based on the current literature, what the optimal GNP size or shape is to achieve this. This is probably a combination of these factors and functionalization. In addition, while the EPR and associated GNP design considerations are important in determining optimal delivery strategy, studies show that tumor complexity (tumor type, position, heterogeneity and so on) has to be taken into consideration and the fact that preclinical models may not accurately represent human disease conditions [93–95].
GNPs as radiosensitizers
Theoretical studies
There is now ample theoretical evidence establishing that GNPs can be employed as radiosensitizers to enhance RT, due to photon-induced emission of photo-/Auger electrons from the GNPs [1,2,47,48,50,96,100]. The often-cited initial theoretical studies are those of Cho and coworkers [47,50], who carried out Monte Carlo simulations to investigate the dosimetric feasibility of GNP-aided radiation therapy (GNRT) via brachytherapy using low-energy γ-/x-ray sources. The main conclusion from these studies was that significant radiosensitization via photoelectric mechanism may be achieved for lower (kV) energy RT photons such as those used in brachytherapy, while clinical MV x-rays will yield minimal radiosensitization. This conclusion was also reached by other authors [101,102] who typically assumed a uniform distribution of intravenously administered GNPs.
In contrast to calculations assuming a uniform distribution throughout the target volume, Berbeco and coworkers [1,97–99] employed Monte Carlo-generated spectra for analytic microdosimetry calculations, which predicted major dose enhancement to the tumor vasculature for both brachytherapy sources and EBRT with MV x-rays. The authors recognized that because intravenously administered targeted GNPs preferentially accumulate around the tumor vasculature [52,88], this could be harnessed for therapeutic gain to deliver ablative RT dose enhancement to the sensitive tumor endothelial cells while maintaining established dose constraints for organs at risk. Further work by the same group showed that for such RT beams, greater dose enhancement or radiosensitization could be achieved for split-IMRT fields and for out-of-field areas of an RT linear accelerator machine with no filter to flatten the beam [2]. An ensuing recommendation was that appropriate mapping of GNP location in tumor and normal tissue for individual patients at the time of RT is essential for efficient and safe delivery of GNRT.
In 2011, Lechtman et al. carried out more Monte Carlo investigations assessing implications on clinical scenario of GNP radiosensitization with regards to photon energy, nanoparticle size, concentration and location [101]. The investigations indicated that the range of photon-induced electrons escaping a single GNP depends on the nanoparticle’s size. This could be explained by self-absorption of emitted electrons by larger nanoparticles, with the effect apparently minimal for ultrasmall GNPs. In a more recent theoretical study using Monte Carlo simulations, Zygmanski et al. studied the dose enhancement of GNPs at nanometer-to-micrometer distances with high spatial resolution, while also assessing the dependence of predicted levels of dose enhancement on spatial geometry [103]. The results revealed strong dependence of dose enhancement or predicted levels of radiosensitization on theoretical simulation microgeometry. A main conclusion was that careful simulation of the irradiation geometry is required to avoid artifacts that tend to exaggerate the computed dose enhancement factor as in some previous studies.
Experimental studies
Meanwhile, a significant number of corroborative in vitro experimental studies have demonstrated that GNPs can significantly enhance RT at kV energies [55,104–108]. These include a study by Chithrani et al. focusing on the radiosensitization properties of nanoparticles in the size range from 14 to 74 nm [55]. They found that radiosensitization was dependent on the number of GNPs internalized within the cells. The 50-nm GNPs with the highest internalization also showed the highest radiosensitization enhancement factor (1.43 at 220 kVp) compared with GNPs of 14 and 74 nm (enhancement factors of 1.20 and 1.26, respectively).
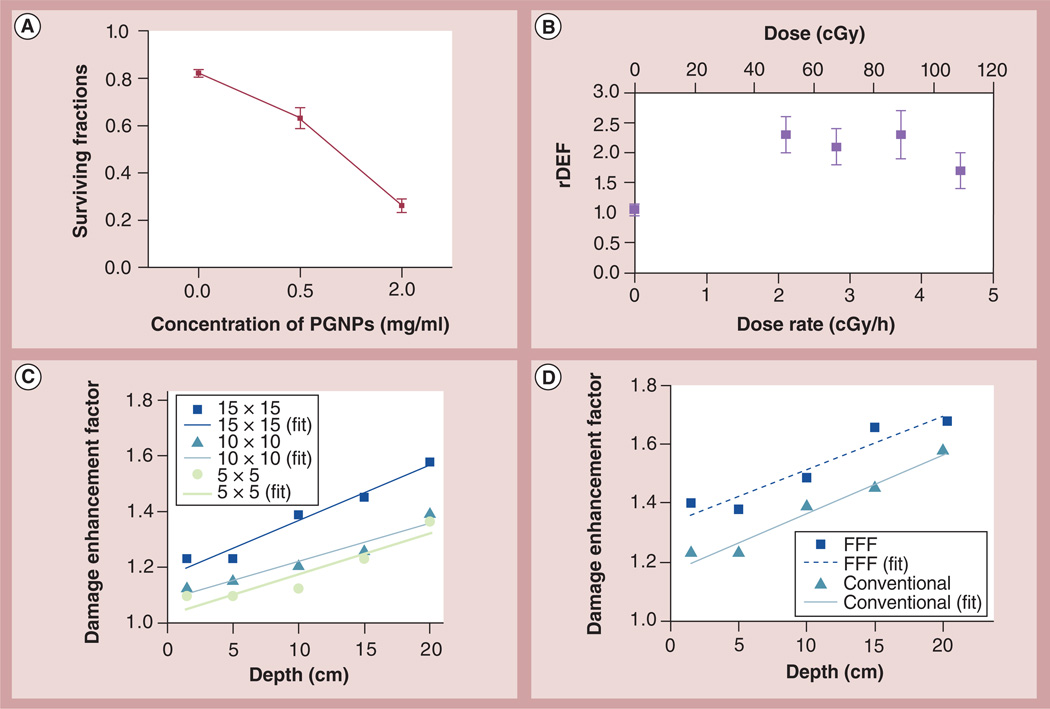
More recently published in vitro results using the third-generation ultrasmall GNP formulation described above are shown in Figure 2A for HeLA cells after uptake of the fluorescent GNPs [44]. The authors reported that nonirradiated (control) cells incubated with or without the GNPs formed approximately 174 colonies. After irradiation with a dose of 2 Gy, the number of colonies for control cells without GNPs decreased to 151, compared with 121 and 42 for 0.5 and 2 mg/ml GNPs, respectively. Overall the survival results obtained indicated major therapeutic enhancement with increasing concentrations of GNPs. A number of other in vitro studies [4,55,104–108] have also shown significant therapeutic enhancement at kV energies, including one study employing GNPs as adjuvants to clinically applicable brachytherapy sources, 125I [4]. The data (Figure 2B) indicate that the biologic effect (residual or unrepaired DNA damage) when irradiating cancer cells in the presence of 0.2 mg/ml concentration of GNPs is approximately 70–130% greater than without GNPs. Meanwhile, without radiation, the GNPs showed minimal effect on the cancer cells, indicating relative nontoxicity of GNPs. As Figure 2A indicates, such enhancement can be increased with an increase in GNP concentration.
Figure 2. In vitro DNA damage enhancement by gold nanoparticle during irradiation.
(A) Surviving fraction of HeLA cells irradiated with 220 kVp x-rays (2 Gy) as a function of GNP concentration. (B) Comparison of unrepaired (residual) radiation damage for HeLA cells incubated with and without GNPs. The rDEF represents the ratio of the residual DNA damage with and without GNPs. The rDEF is shown for four sets of irradiation experiments (2.1–4.5 cGy/h), as well as for no irradiation (0 cGy/h). The figure is from Ngwa et al. [4]. (C) Evidence of dose enhancement during 6 MV external RT; damage enhancement as a function of depth in tissue-equivalent material for conventional (open-field) photon-field delivery with square apertures of sizes 5, 10 and 15 cm. (D) Damage enhancement as a function of depth in tissue-equivalent material for conventional (15 × 15 cm) and FFF (15 × 15 cm) photon-field delivery.
FFF: Flattening filter free; PGNP: PEGylated gold nanoparticle; rDEF: Residual DNA damage enhancement factor. (A) Reproduced with permission from [44] Translational Cancer Research. (B) Reproduced with permission from [4]. (C & D) Reproduced with permission from [3].
Most cancer patients are treated with external RT using MV-energy photons produced by clinical linear accelerators. However, few experimental studies have been performed with MV beams, partly due to the early theoretical studies that predict insignificant radiosensitization at MV energies. Following the original prediction that significant dose enhancement in the immediate vicinity of GNPs can be achieved even for MV beams, Berbeco and coworkers also carried out in vitro studies to verify their predictions [3]. Figure 2C & 2D highlight some published results from this MV x-ray study using 0.05 mg/ml added to HeLa cells and incubated for 24 h [3]. The results indicated that GNPs increase DNA damage in clinical MV beams, and that the damage to tumor cells cultured with GNPs increases when the flattening filter of the RT linear accelerator is removed. This flattening filter-free treatment modality is now commercially available and is currently becoming more clinically accepted. In addition, as the figure shows, damage enhancement was observed to increase as a function of increasing depth. Altogether, the measured relative DNA damage enhancement results validated the theoretically predicted trends [1,99] as a function of depth and delivery mode for clinical MV photon beams. However, the specific concentrations of the GNPs in the HeLa cells were not determined in the in vitro study and hence the dose enhancement cannot be directly compared with theoretical studies. The additional depths and modalities studied by Berbeco and coworkers also demonstrate that damage enhancement with GNPs depends on treatment factors that affect the proportion of low-energy photons within the beam [3].
The in vitro studies by Chitrani et al. did synchronously investigate GNP radiosensitization for EBRT as a function of size in the range 14–74 nm; GNPs in the size of 50 nm achieved the highest radiosensitization for this size range for 6 MVp photon treatment with a radiosensitization enhancement factor of approximately 1.17 when administering a concentration of 7 × 109 particles/ml [55]. Another study employed CT26 murine cancer cells treated with 500 µM of ultrasmall (6.1 nm) PEGylated GNPs for 48 h [107]. A dose enhancement factor of approximately 1.32 was reported. Meanwhile Zhang et al. also reported results showing that all sizes of the polyethylene glycol-coated GNPs can cause a significant decrease in cancer cell survival after irradiation [56]. Overall, the outcomes of in vitro studies at MV energies are consistent with theoretical predictions that the radiosensitization with GNPs at MV energies would be smaller relative to radiosensitization at KV energies [42,109].
A report by Butterworth et al. reviewing some of this in vitro evidence highlighted the disparity between observed in vitro experimental findings and the theoretical predictions, where the predicted dose enhancement or radiosensitization is often different from the results observed from in vitro findings [109]. The authors highlighted emerging evidence pointing to more than just a physical radiosensitization effect via photoelectric mechanism, and suggest oxidative stress as one of the central mechanisms of radiobiological response. Some of this evidence came from a study evaluating cytotoxicity and radiation enhancement by GNPs, which found that irradiation of cells with 1.9 nm GNPs induce a range of cell line-specific responses including decreased clonogenic survival, increased apoptosis and induction of DNA damage, which may be mediated through the production of reactive oxygen species (ROS) [110]. Work by others has also concluded that the mechanism of radiosensitization may involve more than just via the photoelectric effect [42,111]. Misawa et al. concluded that smaller diameter GNPs with a larger surface area lead to a greater yield of ROS probably due to catalytic effects [111]. Higher ROS levels overtime for smaller GNPs with the same functionalization have also been reported by other authors [56,112]. Besides size, ROS may also depend on the functionalization or surface coating of the GNPs, with one report showing that more hydrophobic coatings produce greater ROS levels [113]. However, as the most recent theoretical work by Zygmanski et al. points out, careful simulation of the irradiation geometry in the theoretical studies could potentially reconcile some of the discrepancies highlighted [103].
Beyond in vitro experimental evidence, the existing evidence for GNP radiosensitization in vivo is mainly at kV energies [35,45,46,114,115]. This includes the study by Hainfeld et al. showing that the 1-year survival increased from 20 to 86% for animals irradiated in combination with GNPs. More recently Joh et al. have also shown in vivo evidence of GNP radiosensitization at kV energies for mice with brain tumors [116]. The use of GNPs as adjuvants to 175 kVp x-ray irradiations led to significantly increased survival of mice with orthotopic brain tumors. Another study showed that tumor-bearing mice exhibited a 1.7-fold improvement in the median survival time compared with mice receiving radiation alone [114].
Emerging GNRT modalities
A review of currently published studies points to the following emerging approaches or directions toward development and potential translation of targeted RT with GNPs.
Brachytherapy or superficial RT
Given the demonstrated high levels of GNP radiosensitization at low energies, the conclusions from a number of studies is that GNRT using brachytherapy is the clinically feasible approach [47,48,101,102,117]. However, as one of these studies indicates, the clinical challenge when using low dose rate (LDR) brachytherapy seeds is that the prescribed dose is delivered continuously over the span of weeks [101]. In which case, it would be necessary to understand the full pharmacokinetic dynamics of different-sized GNPs with various conjugations to ensure they remain in the cancer cells for extended periods. Some of these authors see an easier pathway forward if higher dose rate brachytherapy is considered including with miniature electronic 50 kVp x-ray sources [47,50,101].
A study by Raman et al. also indicates that GNPs could be used to enhance RT for cancer patients with superficial tumors [104]. Owing to the low penetration of lower energy x-rays, tumors near the surface could potentially be targeted with GNPs and treated with x-rays at orthovoltage x-ray energies. This direction is consistent with the in vivo findings reported in the work by Hainfeld et al. with orthovoltage energy x-rays, which showed major survival advantage could be achieved for superficial tumors represented in this case by the tumor xenografts targeted with GNPs intravenously [35].
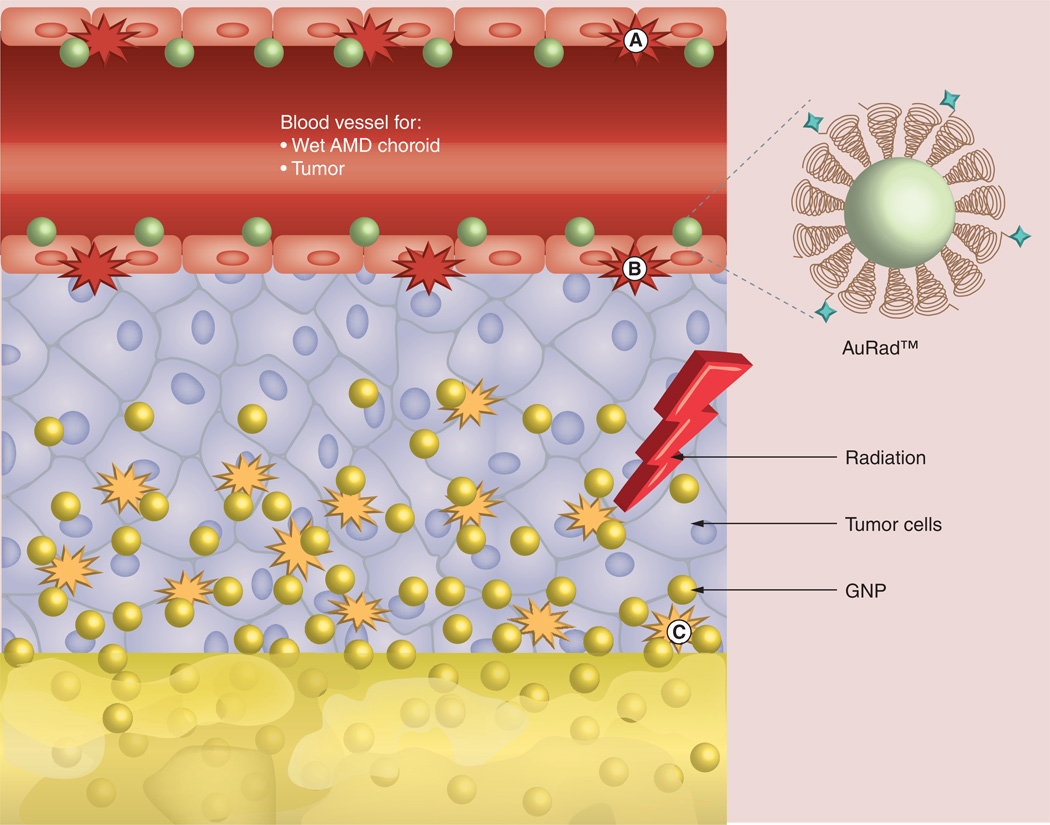
GNPs as tumor vascular-disrupting agents
Another emerging approach supported by theoretical [1,2,97,99,118–120] and in vitro experimental evidence [3,4] is the one proposed by Berbeco and coworkers to employ GNPs as tumor vascular-disrupting agents (VDAs) during either brachytherapy or EBRT with 6 MV x-rays. VDAs, as originally proposed by Denekamp [121,122], are designed to selectively target the established tumor blood vessels. This approach thus assumes the feasibility of specifically targeting the endothelial cells with GNPs at sufficiently potent concentrations, enough to elicit ablative localized dose enhancement. This assumption is justifiable given that preclinical work has already demonstrated the feasibility for 7 and 18 mg/g concentrations through passive targeting [35]. In addition, given the greater accessibility to the endothelium, and its unique features compared with healthy tissue endothelium, it may be possible to adapt the above active targeting strategies to realize sufficiently potent concentrations around the tumor vasculature. VDAs are designed to be administered acutely to secure more rapid effects [123]. It is expected that the preferential accumulation of potent GNP concentration near the vasculature resulting in major endothelial RT boosting or vasculature dose painting [98,116] could rapidly disrupt the tumor vasculature. This could, in turn, result in massive ‘downstream’ tumor cell killing similar to VDAs. Therefore, the preferential accumulation of GNPs near the tumor vasculature, when administered intravenously [52], could be harnessed in this approach with additional active targeting, for example, with the third generation GNP platforms. It may be worth noting that in the pioneering study by Hainfeld et al. [35], such accumulation of GNPs near the vasculature was also reported, and could have contributed to the major survival benefits by mice treated with GNPs. Recent in vivo work on brain tumors also reported vascular dose-painting experiments supporting the notion that GNPs could be used to enhance radiation damage to tumor-associated vasculature, which would precipitate vascular shutdown and extensive tumor cell death [116].
In proposing the VDA approach, Berbeco et al. [99] cited experimental evidence from Garcia-Barros et al. whose studies indicated that damage to tumor microvasculature during RT may be a more important mechanism for tumor eradication than clonogenic cell death itself [124]. They also cited preclinical studies by Boerman et al. [125] indicating that a restricted or concentrated distribution of radiopharmaceuticals around blood vessels is more effective than distributing them uniformly within the tumor. Besides the work in these citations, there is growing consensus that VDAs are more effective as adjuvants to RT [126,127]. This is partly because in VDA applications, the rapid induction of central necrosis may still leave a rim of viable oxygenated and possibly malignant cells persisting at the tumor–normal tissue interface, which could make good target for RT [128]. In the approach considered by Berbeco et al., the GNPs would essentially serve as such adjuvants – to brachytherapy or EBRT [99]. A major advantage of the approach by Berbeco and coworkers [1–3,97,99] is the fact that EBRT could also be employed, not only brachytherapy. The approach also recognizes that for EBRT, the proportion of low energy photons in a clinical MV photon beam depends on the depth in material and that beam ‘softening’ will occur at depth in a patient increasing the potential for photon interaction with GNPs for vasculature dose painting.
When using currently available VDAs, the concern is that more than just the vasculature may be targeted by systemic exposure to the VDAs, with potential damage to vascular compartments outside the tumor. This may contribute to acute coronary syndromes and thrombo-embolic events [129]. In fact, close monitoring of patients receiving VDAs for any cardiovascular toxicity is usually imperative [127]. In this regard, GNPs may actually have an advantage in potential applications as VDAs during RT since GNPs are relatively nontoxic [38] by themselves. Currently, there are ongoing studies to further corroborate the in vitro findings by Berbeco and coworkers in vivo towards potential clinical translation of this novel modality, namely, employing GNPs as VDAs during RT.
RT application with in situ dose painting using GNPs
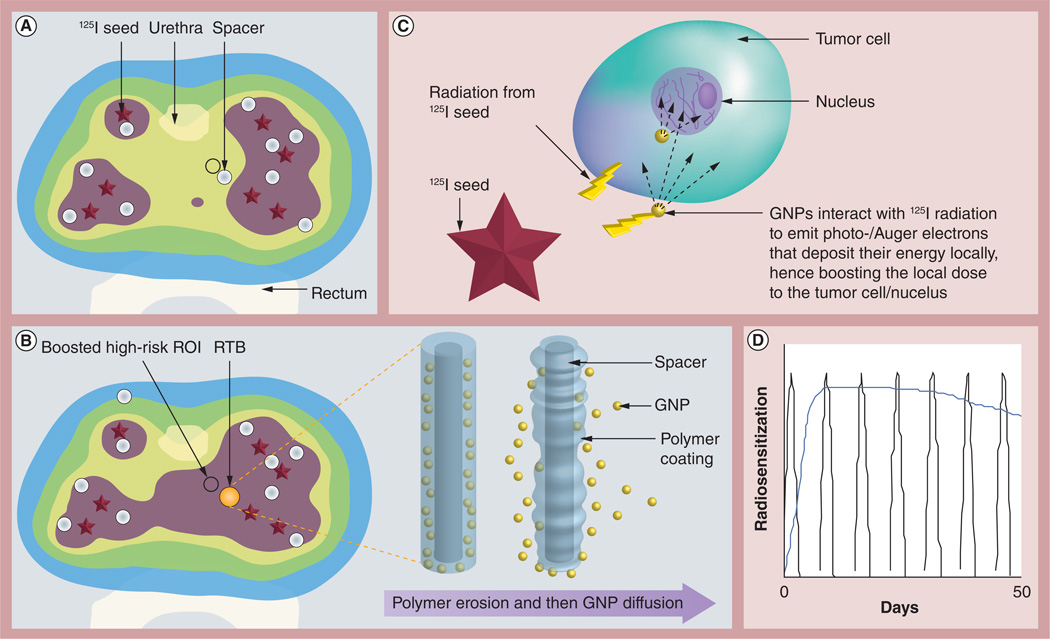
In a 2010 study, Cormack et al. proposed that current inert IGRT biomaterials (brachytherapy spacers and fiducials) routinely used in the clinic to ensure spatial accuracy during RT could be upgraded to smarter ones by loading them with radiosensitizing drugs which can be released in situ after implantation [130]. Cognizant of the existing evidence that GNPs can be employed as radiosensitizers during RT, the RT application with in situ dose painting (RAID) with GNPs approach involves specifically loading these RT biomaterials with GNPs, which can be released in situ after implantation, to provide subvolume radiation boosting or dose painting without an increase in normal tissue toxicity. The modus operandi for the RAID approach is illustrated in Figure 3 for prostate brachytherapy. In current brachytherapy practice, inert spacers (shown in white in Figure 3A) are used to achieve a desired placement of the radioactive LDR seeds specified by the physician’s treatment plan. In the RAID approach, the inert brachytherapy spacers will be upgraded to smarter ones loaded with radiosensitizing GNPs. Such a gold-loaded RT biomaterial (RTB) is shown in gold color in Figure 3B. The RTBs are produced by coating the spacers with polymer films containing GNPs similar to procedures for coating fiducials with polymer films loaded with poly(d,l-lactide-co-glycolide) nanoparticles [131]. Once implanted, the polymer coating on the RTB begins to degrade, releasing the GNPs in situ, which then diffuse into the tumor subvolume (as illustrated on the right of Figure 3B). The sustained release of GNPs, in situ, from the RTB and consequent 3D intratumor biodistribution over time can be customized by varying GNP size, initial concentration, functionalization, location of smart biomaterial and so on. This would address the concern raised by Lechtmann et al. on finding ways to continuously radiosensitize the tumor with the GNPs during continuous LDR brachytherapy [101].
Figure 3. Radiotherapy application with in situ dose painting.
(A) 125I radiation-only dose distribution. (B) Dose distribution using the radiotherapy application with in situ dose painting approach where a gold-loaded RTB is used instead of usual inert spacer. (C) Not to scale. Tumor cell in high-risk ROI receives a dose boost due to 125I-induced emission of photo-/Auger electrons. (D) GNP release kinetics can be customized for sustained in situ radiosensitization (blue curve) compared with the current approach of weekly systemic administration of radiosensitizer (multiple peaks).
GNP: Gold nanoparticle; ROI: Region of interest; RTB: Radiotherapy biomaterial.
Figure 3C illustrates photon-induced emission of micrometer-range photo-/Auger electrons from the GNPs to boost dose to tumor cells [47,97,118]. Because the dose enhancement is more highly localized than even brachytherapy radiation, this subvolume boost may be achieved without any significant increase in dose to the rectum and other organs at risk not containing GNPs. Figure 3D illustrates that the GNP release kinetics from RTB can be customized for sustained radiosensitization (blue curve) compared with current approaches of weekly systemic administration of radiosensitizer (multiple peaks) [131].
The RAID approach recognizes that the tumor is heterogenous with high-risk tumor subvolumes (see Figure 3A & 3B), and that the GNPs may not distribute uniformly throughout the tumor. Nevertheless, the RTB could be targeted to high-risk tumor subvolumes identified, for example, by MRI or PET imaging, in which case a high concentration of released GNPs would be around the area where boosting is most needed. To illustrate how this would work clinically, the color wash in Figure 3A represents the dose distribution prescribed by a physician’s treatment plan for a patient using 125I seed irradiation only. The small circle is a hypothetical high-risk tumor region of interest (identified e.g., via functional imaging) needing a dose boost, which cannot be achieved without increased dose (toxicity) to the urethra/rectum. In comparison, Figure 3B shows dose distribution using the RAID approach when the RTB is used instead of the usual inert spacer. In this RAID approach (Figure 3B), the 125I radiation plus additional dose from GNPs (see Figure 3C) leads to a subvolume boost to the high-risk region of interest without increased toxicity to the urethra/rectum.
Other major advantages of the RAID approach include the fact that in situ administration of GNPs circumvents a central problem in the use of nanoparticles – that is, delivering a substantial fraction of GNPs to the intended treatment site, hence bypassing the barriers, restricting the delivery of the nanoparticles. In addition, the RAID approach proffers minimal additional inconvenience to patients, because implantation of these RTBs such as spacers or fiducials is already part of routine clinical RT practice. Furthermore, even though Figure 3 is for prostate brachytherapy, this can also be extended to other sites that routinely employ these RTBs, for example, in the lung in the case of external RT.
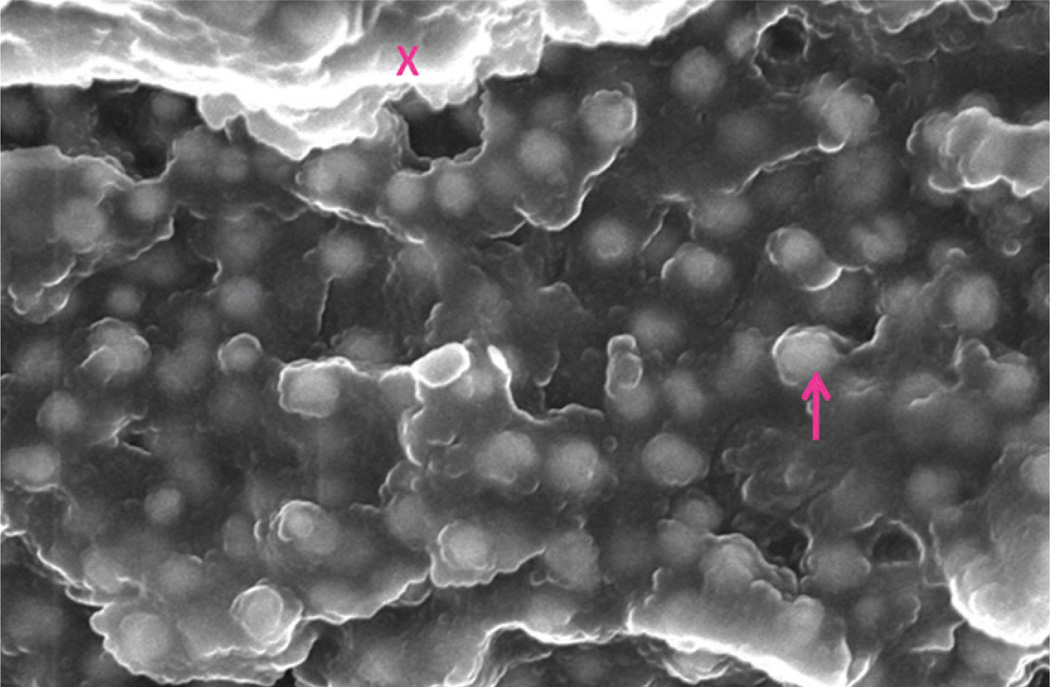
With respect to upgrading currently used inert IGRT biomaterials to smarter ones loaded with GNPs, a number of recent studies have demonstrated initial feasibility of achieving this where inert biomaterials were coated with biodegradable, biocompatible chitosan films containing nanoparticles loaded with a drug model for sustainable release as the polymer degrades [131]. A similar approach could be used to develop the smart RT biomaterial loaded with GNPs. Alternatively, GNPs can be incorporated in poly(d,l-lactide-co-glycolide) polymer millirods during the gel phase of production [132,133]. Figure 4 shows an electron microscopy image of such an RT biomaterial loaded with GNPs. In vivo studies using such biomaterials towards development of the RAID approach are currently ongoing.
Figure 4. Electron microscopy image (1 × 1 µM) of smart radiotherapy biomaterial loaded with stealth nanoparticles.
The arrow shows a gold nanoparticle embedded in the polymer matrix indicated by the ‘X’.
Customizable RT enhancement of wet age-related macular degeneration using GNPs
In a departure from customary investigations of GNPs as radiosensitizers for cancer treatment, Ngwa et al. recently demonstrated the feasibility of targeted RT with GNPs for treating wet age-related macular degeneration (AMD) [100]. AMD is the leading cause of blindness in developed countries for people over the age of 50 years [134,135], with an estimated annual financial burden of over US$343 billion [136]. The worldwide prevalence of the disease is expected to double in the next decade owing to population ageing. Photodynamic therapy and antiangiogenic pharmacotherapy currently represent the standard of care for most patients [134,137–141]. However, these therapies require repeated treatments or injections, putting the patient at risk for cataract formation, endophthalmitis, vitreous hemorrhage and retinal detachment, besides logistic difficulties and the patients’ discomfort [142,143]. Improving these standard treatments or developing other effective complementary treatment approaches would be of significant benefit to both patients and society.
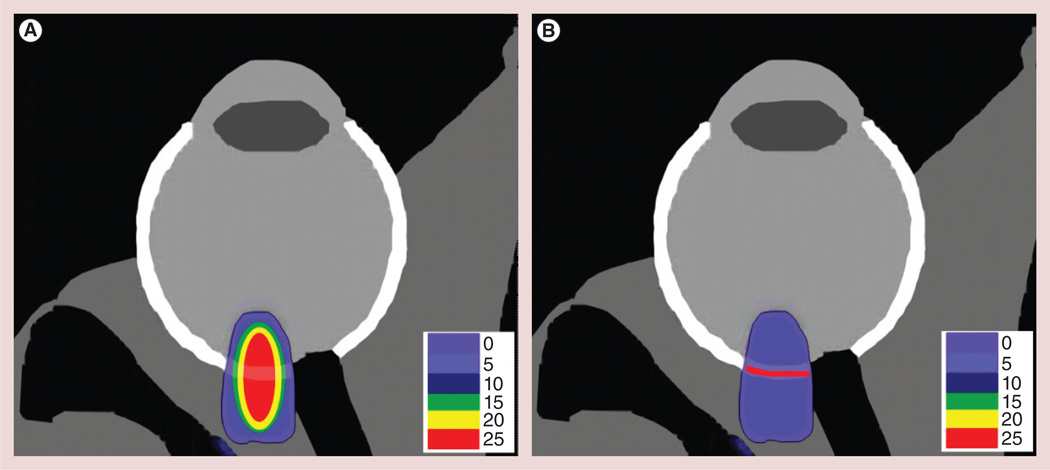
To this end, a noninvasive, kV stereotactic radiosurgery system has recently been developed and commercialized for the treatment of neovascular AMD (Oraya Therapy™; Oraya Therapeutics Inc., CA, USA) [144–146]. The rationale for such a RT approach is based on evidence that the rapidly proliferating neovascular endothelial cells that drive AMD development are relatively more radiosensitive than quiescent or less actively dividing cells [147,148]. The Oraya Therapy system can accurately deliver a radiation dose of up to 24 Gy to the radiosensitive choroidal neovasculature during a single treatment session, while maintaining dose limits to neighboring organs at risk [145,149]. A novel multimodality approach comprising Oraya Therapy of 16 or 24 Gy, and concomitant treatment with a VEGF inhibitor has also been developed [150] and implemented in clinical trials with no radiation-related adverse effects at 6 months [151–153].
Given that the Oraya Therapy system delivers kV energy x-rays and specifically targets the choroidal neovasculature, Ngwa et al. proposed and demonstrated in a theoretical study that GNPs would significantly enhance kV stereotactic radiosurgery for neovascular AMD [100], representing a departure from previous GNRT work, mainly focused on potential cancer therapy applications [42,101,154–156]. Justification for pursuing such a targeted RT with GNPs for wet AMD also comes from studies showing that nanoparticles can be specifically targeted to the AMD choroidal neovasculature [143,157,158]. Kim et al. have shown that passively targeted 50-nm GNPs administered intravenously could be safely distributed in different retinal layers depending on their size, while 100-nm GNPs would not penetrate the retinal–blood barrier [159]. The size of the GNPs could thus be customized to enable trapping of the GNPs in the fenestrated wet AMD neovasculature. However, active targeting methods such as those discussed in the section entitled ‘Design considerations of GNPs for targeted RT with GNPs’ above would be more appropriate. This was demonstrated in recent mouse studies where systemically administered functionalized nanoparticles could specifically target the neovascular AMD endothelial cells [134,143,157]. In ddition, a preclinical study investigating the use of GNPs for the photothermal treatment of choroidal neovascularization in AMD has been reported [160]. In that study, gold nanorods conjugated with RGD peptides were administered intravenously in a mouse model and shown to be localized in retinal endothelial cells. Further development of such choroidal neovascularization- targeting approaches for optimal application in humans would be valuable in potential development of this novel customizable RT enhancement with GNP modality. The customizable RT enhancement with GNP approach, under development by Ngwa et al. at Harvard Medical School (MA, USA), for enhanced treatment of AMD is illustrated in Figure 5.
Figure 5. Gold nanoparticle-aided Oraya Therapy™ (Oraya Therapeutics Inc., CA, USA) dose distribution.
(A) Spatial map of Oraya Therapy™ (Oraya Therapeutics Inc., CA, USA) dose distribution (in Gy) within the eye of a patient with an anterior–posterior diameter of the eye of approximately 24 mm. (B) Idealistic spatial map of Oraya Therapy dose distribution illustrating a possible treatment scenario when using gold nanoparticles. In this scenario, the directly administered Oraya Therapy dose is reduced by a factor of greater than 3 (corresponding to dose enhancement factor level), while the dose to the choroidal neovasculature is maintained due to dose enhancement by targeted gold nanoparticles. Such a scenario would lead to significantly reduced dose to neighboring healthy tissue. Reproduced with permission from [101] © IOP Publishing.
Future perspective
While the above emerging GNRT modalities show promise at this stage, a significant body of work remains to be performed before the discussed modalities can be possibly translated into the clinic. This includes more in vivo studies not only for kV energies, but also for MV-based EBRT. Synchronous development of advanced imaging modalities with GNPs will also be important, taking advantage of the fact that GNPs can serve as in vivo imaging contrast agents [41,161,162], allowing for useful delineation of the tumor vasculature and tumor volumes [162,163].
Following successful demonstration of enhanced therapeutic efficacy in vivo, further research and development on imaging would also greatly avail treatment planning towards potential clinical translation. It is expected that such imaging of GNPs could complement other imaging approaches currently being developed using MRI, ultrasound and so on [164]. Recently published work highlights the development of a new GNP imaging method that works by detecting the L-edge x-ray fluorescence emitted when gold is irradiated with kV energy x-rays. The technique is said to achieve increased detection sensitivity at greater depths than current optical modalities [165]. More of such developments would be a very useful advance for developing GNRT.
Going forward, there is also a need for more Monte Carlo simulations that can facilitate the development of treatment planning software tools. Such studies could also better elucidate mechanisms behind GNP-enhanced tumor cell killing and help reconcile the disparities between theory or calculations and experimental measurements. The development of treatment planning software tools for GNRT modalities would allow for correspondence between imaged GNP distribution and planned dose enhancement.
Further work is also necessary in understanding the behavior of GNPs in the tumor microenvironment taking into account physiological barriers in the tumor microenvironment. Such work will have to take into account the highly heterogeneous and continuously evolving nature of the tumor microenvironment, with expressed differences from one tumor to the next, from primary tumor to its metastasis, from 1 day to the next in the same tumor and the changes induced by treatment. Jain and Stylianopoulos have proposed some basic guidelines for the construction of nanotherapeutics based on approaches that reduce this heterogeneity, whose guidelines could benefit the development of GNRT [88]. One suggestion is that the size, shape and surface chemistry of a nanoparticle needs to be optimized for each tumor. In addition, the authors indicate that nanoparticles could be developed that respond to properties of the tumor microenvironment (e.g., low pH and partial oxygen pressure) or to external forces such as electric pulses, magnetic field, ultrasound, heat and light. Further more, the addition of targeting ligands should be carefully considered, as the addition increases the size and biological reactivity of the particles, which could limit penetration of the nanoparticles.
Although this review has been focused on GNRT with photons, some scientists are beginning to consider the use of other types of RT including electron beam therapy [104,166,167] and proton therapy [168]. In one study using 6 MeV electron beams, Chang et al. demonstrated that electron beam therapy with GNPs significantly retarded tumor growth and prolonged survival compared with the radiation alone controls (p < 0.05) [166]. The authors concluded that the results demonstrated the clinical potential of GNPs in improving the outcome of melanoma RT. Meanwhile, in one proton therapy study, prostate tumor cell killing was reportedly increased by approximately 15–20% for those cells containing internalized GNPs [169]. A proton beam was utilized to irradiate nanoparticles with a single Bragg peak set to occur inside a tumor volume (fully absorbed) or to occur after the beam had traversed the entire body. The dose-dependent increase in complete tumor regression was 37–62% in the fully-absorbed irradiation group or 50–100% in the traversing irradiation group, respectively, compared with the proton-alone control mice. The 1-year survival was 58–100% in the fully-absorbed irradiation group and the traversing irradiation group versus 11–13% in the proton-alone group. The dose-dependent increase of intracellular ROS level was 12–36% at 10 Gy compared with the proton-alone control cell. Therefore, the future of GNRT may incorporate RT modalities different from those employing photons. Other GNP-assisted mechanisms, such as hyperthermia and chemosensitization, may also be developed in synergy with GNRT [46,170].
In addition, while GNPs are the focus of this study, recent studies have investigated the use of other high-Z nanoparticles to enhance RT. Part of the motivation for considering other high-Z nanoparticles is the concern that if successfully translated, the additional costs from use of GNPs for GNRT would be significant. A further concern is the poor clearance of largesize GNPs when administered intravenously, due to potential long-term retention in the liver, spleen and other tissues [171]. For studies considering other high-Z nanoparticles, calculations in one study showed higher dose enhancement by bismuth nanoparticles relative to GNPs [172], which is expected given the higher Z-value for bismuth. Significant but lower dose enhancement was also predicted for platinum-based nanoparticles and gadolinium nanoparticles [173]. It is expected that such nanoparticles would be further investigated given their potential advantages in certain situations, for example, gadolinium nanoparticles when additional contrast for MRI may be useful.
Over the 20th century, following the findings by Wilhelm Röntgen and Marie Curie that spawned RT, the discipline developed from an experimental application of ionizing radiation to a highly sophisticated treatment modality for cancer. Whereas the emphasis in the past and ongoing research has been on refining techniques to ensure the accurate delivery of radiation, it has been indicated [174] that the future of RT may lie in working more with biologists to exploit the genetics and structure or the tumor microenvironment for better designed or effective personalized treatment. This review highlights the need for working with nanotechnologists as well. A PubMed search of the keywords ‘gold nanoparticles’and ‘radiotherapy’ reveals that over 42% of publications in this interdisciplinary area were published in the last 2 years, with the first listed work by Hainfeld et al. in 2004 [35]. The accelerating pace of work in this area in collaboration with nanotechnologists clearly signals that nanotechnology with GNPs will be at the cutting edge of research in the next years to further enhance therapeutic efficacy with RT. With this pace and continuous cross-disciplinary collaboration, it may be possible within the next decade to advance the emerging GNRT modalities highlighted in this review (and Figure 6) to clinical trials, and towards their establishment as more effective RT treatment options for patients with cancer and other diseases.
Figure 6. Three of the emerging approaches of gold nanoparticle-aided radiotherapy.
(A) Customizable radiotherapy enhancement with GNPs, (B) GNPs as vascular-distrupting agents and (C) radiotherapy application with in situ dose painting with GNPs.
AMD: Age-related macular degeneration; GNP: Gold nanoparticle.
Executive summary.
Design considerations of gold nanoparticles for targetedradiotherapy
Nanomedicine holds advantage over conventional medicine because nanoparticles can be designed to enable the preferential delivery of drugs or radiosensitizers to tumors owing to the enhanced permeability and retention effect, and the fact that nanoparticles can be programmed to sustainably release therapeutic agents including in situations that could benefit combination therapy. Gold nanoparticle (GNPs) can serve as both radiosensitizers and drug-delivery agents.
GNPs as radiosensitizers
There is now significant evidence of radiosensitization with GNPs, although the exact mechanisms of radiosensitization are not completely clear. There is need for more in vivo studies at clinically applicable energies, appropriate for the emerging GNP-aided radiotherapy (GNRT) modalities.
Emerging GNRT modalities
Promising emerging GNRT modalities include: brachytherapy or superficial radiotherapy, GNPs as tumor vascular-disrupting agents, radiotherapy application with in situ dose-painting using GNPs and customizable radiotherapy enhancement of wet age-related macular degeneration using GNPs.
Future perspective
It should be possible within the next decade to advance the emerging GNRT modalities highlighted in this review to clinical trials, and towards their establishment as more effective radiotherapy treatment options for patients with cancer and other diseases.
Acknowledgments
The authors acknowledge funding support from the Kayes Technology Research Awards; Mazonne Foundation; Joint Center for Radiation Therapy Foundation; DOD CDMRP W81XWH- 12-1-0064; and NIH/NCI R03 CA164645.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Detappe A, Tsiamas P, Ngwa W, Zygmanski P, Makrigiorgos M, Berbeco R. The effect of flattening filter free delivery on endothelial dose enhancement with gold nanoparticles. Med. Phys. 2013;40(3):031706. doi: 10.1118/1.4791671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsiamas P, Liu B, Cifter F, et al. Impact of beam quality on megavoltage radiotherapy treatment techniques utilizing gold nanoparticles for dose enhancement. Phys. Med. Biol. 2013;58(3):451–464. doi: 10.1088/0031-9155/58/3/451. [DOI] [PubMed] [Google Scholar]
- 3. Berbeco RI, Korideck H, Ngwa W, et al. DNA damage enhancement from gold nanoparticles for clinical MV photon beams. Radiat. Res. 2012;178(6):604–608. doi: 10.1667/RR3001.1. •• Provides experimental evidence of gold nanoparticle enhancement for different clinical MV photon beams. It is also the first experimental evidence of radiosensitization by gold nanoparticles during continuous low-dose-rate I125 brachytherapy.
- 4.Ngwa W, Korideck H, Kassis AI, et al. In vitro radiosensitization by gold nanoparticles during continuous low-dose-rate gamma irradiation with I-125 brachytherapy seeds. Nanomedicine. 2013;9(1):25–27. doi: 10.1016/j.nano.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006;65(4):1087–1096. doi: 10.1016/j.ijrobp.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 6.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer. 2011;11(4):239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 7.Barnett GC, West CM, Dunning AM, et al. Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat. Rev. Cancer. 2009;9(2):134–142. doi: 10.1038/nrc2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen PL, D’Amico AV, Lee AK, Suh WW. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: a systematic review of the literature. Cancer. 2007;110(7):1417–1428. doi: 10.1002/cncr.22941. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer. 2008;112(2):307–314. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 10.Westphalen AC, Coakley FV, Roach M, 3rd, McCulloch CE, Kurhanewicz J. Locally recurrent prostate cancer after external beam radiation therapy: diagnostic performance of 1.5-T endorectal MR imaging and MR spectroscopic imaging for detection. Radiology. 2010;256(2):485–492. doi: 10.1148/radiol.10092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephenson AJ, Eastham JA. Role of salvage radical prostatectomy for recurrent prostate cancer after radiation therapy. J. Clin. Oncol. 2005;23(32):8198–8203. doi: 10.1200/JCO.2005.03.1468. [DOI] [PubMed] [Google Scholar]
- 12.Zelefsky MJ, Yamada Y, Fuks Z, et al. Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2008;71(4):1028–1033. doi: 10.1016/j.ijrobp.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Uzzo RG, Hanks GE. Prostate cancer radiotherapy dose response: an update of the fox chase experience. J. Urol. 2004;171(3):1132–1136. doi: 10.1097/01.ju.0000111844.95024.74. [DOI] [PubMed] [Google Scholar]
- 14.Al-Mamgani A, Van Putten WL, Heemsbergen WD, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008;72(4):980–988. doi: 10.1016/j.ijrobp.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 15.Al-Mamgani A, Van Putten WL, Van Der Wielen GJ, Levendag PC, Incrocci L. Dose escalation and quality of life in patients with localized prostate cancer treated with radiotherapy: long-term results of the Dutch randomized dose-escalation trial (CKTO 96–10 trial) Int. J. Radiat. Oncol. Biol. Phys. 2010;79(4):1004–1012. doi: 10.1016/j.ijrobp.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Bachand F, Martin AG, Beaulieu L, Harel F, Vigneault E. An eight-year experience of HDR brachytherapy boost for localized prostate cancer: biopsy and PSA outcome. Int. J. Radiat. Oncol. Biol. Phys. 2009;73(3):679–684. doi: 10.1016/j.ijrobp.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Nutting CM, Corbishley CM, Sanchez-Nieto B, Cosgrove VP, Webb S, Dearnaley DP. Potential improvements in the therapeutic ratio of prostate cancer irradiation: dose escalation of pathologically identified tumour nodules using intensity modulated radiotherapy. Br. J. Radiol. 2002;75(890):151–161. doi: 10.1259/bjr.75.890.750151. [DOI] [PubMed] [Google Scholar]
- 18.Galvin JM, De Neve W. Intensity modulating and other radiation therapy devices for dose painting. J. Clin. Oncol. 2007;25(8):924–930. doi: 10.1200/JCO.2007.10.6716. [DOI] [PubMed] [Google Scholar]
- 19.Kuban DA, Levy LB, Cheung MR, et al. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int. J. Radiat. Oncol. Biol. Phys. 2011;79(5):1310–1317. doi: 10.1016/j.ijrobp.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Heier JS, Boyer DS, Ciulla TA, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: year 1 results of the FOCUS study. Arch. Ophthalmol. 2006;124(11):1532–1542. doi: 10.1001/archopht.124.11.1532. [DOI] [PubMed] [Google Scholar]
- 21.Peeters ST, Heemsbergen WD, Van Putten WL, et al. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int. J. Radiat. Oncol. Biol. Phys. 2005;61(4):1019–1034. doi: 10.1016/j.ijrobp.2004.07.715. [DOI] [PubMed] [Google Scholar]
- 22.Litzenberg DW, Balter JM, Hadley SW, et al. Influence of intrafraction motion on margins for prostate radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006;65(2):548–553. doi: 10.1016/j.ijrobp.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 23.Li HS, Chetty IJ, Enke CA, et al. Dosimetric consequences of intrafraction prostate motion. Int. J. Radiat. Oncol. Biol. Phys. 2008;71(3):801–812. doi: 10.1016/j.ijrobp.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 24.Sovik A, Malinen E, Olsen DR. Strategies for biologic image-guided dose escalation: a review. Int. J. Radiat. Oncol. Biol. Phys. 2009;73(3):650–658. doi: 10.1016/j.ijrobp.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Burri RJ, Stone NN, Unger P, Stock RG. Long-term outcome and toxicity of salvage brachytherapy for local failure after initial radiotherapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010;77(5):1338–1344. doi: 10.1016/j.ijrobp.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 26.Allen GW, Howard AR, Jarrard DF, Ritter MA. Management of prostate cancer recurrences after radiation therapy-brachytherapy as a salvage option. Cancer. 2007;110(7):1405–1416. doi: 10.1002/cncr.22940. [DOI] [PubMed] [Google Scholar]
- 27.Ng CK, Touma NJ, Chalasani V, Moussa M, Downey DB, Chin JL. The pattern of prostate cancer local recurrence after radiation and salvage cryoablation. Can. Urol. Assoc. J. 2011;5(6):E125–E128. doi: 10.5489/cuaj.09116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen PL, Chen RC, Clark JA, et al. Patient-reported quality of life after salvage brachytherapy for radio-recurrent prostate cancer: a prospective Phase II study. Brachytherapy. 2009;8(4):345–352. doi: 10.1016/j.brachy.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beyer DC. Salvage brachytherapy after external-beam irradiation for prostate cancer. Oncology. 2004;18(2):151–158. [PubMed] [Google Scholar]
- 30.Beyer DC. Brachytherapy for recurrent prostate cancer after radiation therapy. Semin. Radiat. Oncol. 2003;13(2):158–165. doi: 10.1053/srao.2003.50015. [DOI] [PubMed] [Google Scholar]
- 31.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299(23):2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelefsky MJ, Fuks Z, Wolfe T, et al. Locally advanced prostatic cancer: long-term toxicity outcome after three-dimensional conformal radiation therapy – a dose-escalation study. Radiology. 1998;209(1):169–174. doi: 10.1148/radiology.209.1.9769828. [DOI] [PubMed] [Google Scholar]
- 33.Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med. Phys. 2006;33(10):3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 34.Herold DM, Das IJ, Stobbe CC, Iyer RV, Chapman JD. Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int. J. Radiat. Biol. 2000;76(10):1357–1364. doi: 10.1080/09553000050151637. [DOI] [PubMed] [Google Scholar]
- 35. Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004;49(18):N309–N315. doi: 10.1088/0031-9155/49/18/n03. • Highlights the pioneering in vivo evidence of gold nanoparticle-aided radiotherapy.
- 36.Cheung JY, Tang FH. The calculation of dose enhancement close to platinum implants for skull radiography. Health Phys. 2007;93(4):267–272. doi: 10.1097/01.HP.0000264450.81683.51. [DOI] [PubMed] [Google Scholar]
- 37.Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem. Biophys. Res. Commun. 2010;393(4):649–655. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee P, Bhattacharya R, Wang P, et al. Antiangiogenic properties of gold nanoparticles. Clin. Cancer Res. 2005;11(9):3530–3534. doi: 10.1158/1078-0432.CCR-04-2482. [DOI] [PubMed] [Google Scholar]
- 39.Shukla R, Bansal V, Chaudhary M, Basu A, Bhonde RR, Sastry M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir. 2005;21(23):10644–10654. doi: 10.1021/la0513712. [DOI] [PubMed] [Google Scholar]
- 40.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1(3):325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 41.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: a new x-ray contrast agent. Br. J. Radiol. 2006;79(939):248–253. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 42.Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. The Br. J. Radiol. 2012;85(1010):101–113. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hainfeld JF, Smilowitz HM, O’Connor MJ, Dilmanian FA, Slatkin DN. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine (Lond) 2013;8(10):1601–1609. doi: 10.2217/nnm.12.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar R, Korideck H, Ngwa W, Berbeco RI, Makrigiorgos GM, Sridhar S. Third generation gold nanoplatform optimized for radiation therapy. Transl. Cancer Res. 2013;2(4) doi: 10.3978/j.issn.2218-676X.2013.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hainfeld JF, Dilmanian FA, Slatkin DN, Smilowitz HM. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008;60(8):977–985. doi: 10.1211/jpp.60.8.0005. [DOI] [PubMed] [Google Scholar]
- 46.Hainfeld JF, Dilmanian FA, Zhong Z, Slatkin DN, Kalef-Ezra JA, Smilowitz HM. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol. 2010;55(11):3045–3059. doi: 10.1088/0031-9155/55/11/004. [DOI] [PubMed] [Google Scholar]
- 47.Cho SH, Jones BL, Krishnan S. The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/x-ray sources. Phys. Med. Biol. 2009;54(16):4889–4905. doi: 10.1088/0031-9155/54/16/004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roeske JC, Nunez L, Hoggarth M, Labay E, Weichselbaum RR. Characterization of the theorectical radiation dose enhancement from nanoparticles. Technol. Cancer Res. Treat. 2007;6(5):395–401. doi: 10.1177/153303460700600504. [DOI] [PubMed] [Google Scholar]
- 49.Zhang SX, Gao J, Buchholz TA, et al. Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: a Monte Carlo simulation study. Biomed. Microdevice. 2009;11:925–933. doi: 10.1007/s10544-009-9309-5. [DOI] [PubMed] [Google Scholar]
- 50.Jones BL, Krishnan S, Cho SH. Estimation of microscopic dose enhancement factor around gold nanoparticles by Monte Carlo Calculations. Med. Phys. 2010;37(7):3809–3816. doi: 10.1118/1.3455703. [DOI] [PubMed] [Google Scholar]
- 51.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 52.Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009;9(5):1909–1915. doi: 10.1021/nl900031y. [DOI] [PubMed] [Google Scholar]
- 53.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm. Res. 2009;26(1):235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 54.Decuzzi P, Ferrari M. The adhesive strength of non-spherical particles mediated by specific interactions. Biomaterials. 2006;27(30):5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 55.Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat. Res. 2010;173(6):719–728. doi: 10.1667/RR1984.1. [DOI] [PubMed] [Google Scholar]
- 56.Zhang XD, Wu D, Shen X, et al. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials. 2012;33(27):6408–6419. doi: 10.1016/j.biomaterials.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 57.Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc. Natl Acad. Sci. USA. 2007;104(29):11901–11904. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geng Y, Dalhaimer P, Cai S, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007;2(4):249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishiyama N. Nanomedicine: nanocarriers shape up for long life. Nat. Nanotechnol. 2007;2(4):203–204. doi: 10.1038/nnano.2007.88. [DOI] [PubMed] [Google Scholar]
- 60.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J. Control. release. 2007;121(1–2):3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan J, Shah S, Thomas A, Ou-Yang HD, Liu Y. The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluid. Nanofluidics. 2013;14(1–2):77–87. doi: 10.1007/s10404-012-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wong C, Stylianopoulos T, Cui J, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl Acad. Sci. USA. 2011;108(6):2426–2431. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang K, Ma H, Liu J, et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano. 2012;6(5):4483–4493. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 65.Chithrani DB. Intracellular uptake, transport, and processing of gold nanostructures. Mol. Membr. Biol. 2010;27(7):299–311. doi: 10.3109/09687688.2010.507787. [DOI] [PubMed] [Google Scholar]
- 66.Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7(6):1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 67.Caldorera-Moore M, Guimard N, Shi L, Roy K. Designer nanoparticles: incorporating size, shape and triggered release into nanoscale drug carriers. Expert Opin. Drug Deliv. 2010;7(4):479–495. doi: 10.1517/17425240903579971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chithrani DB. Nanoparticles for improved therapeutics and imaging in cancer therapy. Recent Pat. Nanotechnol. 2010;4(3):171–180. doi: 10.2174/187221010792483726. [DOI] [PubMed] [Google Scholar]
- 69.Hauck TS, Ghazani AA, Chan WC. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small. 2008;4(1):153–159. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 70.Zhang G, Yang Z, Lu W, et al. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30(10):1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl Acad. Sci. USA. 2010;107(3):1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yao L, Daniels J, Moshnikova A, et al. pHLIP peptide targets nanogold particles to tumors. Proc. Natl Acad. Sci. USA. 2013;110(2):465–470. doi: 10.1073/pnas.1219665110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol. 2005;23(11):1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 74.Kang B, Mackey MA, El-Sayed MA. Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J. Am. Chem. Soc. 2010;132(5):1517–1519. doi: 10.1021/ja9102698. [DOI] [PubMed] [Google Scholar]
- 75.Sokolov K, Follen M, Aaron J, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63(9):1999–2004. [PubMed] [Google Scholar]
- 76.Brown SD, Nativo P, Smith J, et al. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010;132(13):4678. doi: 10.1021/ja908117a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dabbas S, Kaushik RR, Dandamudi S, Kuesters M, Campbell RB. Importance of the lipoosomal cationic lipid content and type in tumor vascular targeting: physicochemical characterization and in vitro studies using human promary and transformed endothelial cells. Endothelium. 2008;15:189–201. doi: 10.1080/10623320802228583. [DOI] [PubMed] [Google Scholar]
- 78.Cai W, Shin DW, Chen K, et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006;6(4):669–676. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 79.Diagaradjane P, Orenstein-Cardona JM, Colon-Casasnovas NE, et al. Imaging epidermal growth factor receptor expression in vivo: pharmacokinetic and biodistribution characterization of a bioconjugated quantum dot nanoprobe. Clin. Cancer Res. 2008;14(3):731–741. doi: 10.1158/1078-0432.CCR-07-1958. [DOI] [PubMed] [Google Scholar]
- 80.McCarthy JR, Weissleder R. Multifunctional magnetic nanoparticles for targeted imaging and therapy. Adv. Drug Deliv. Rev. 2008;60(11):1241–1251. doi: 10.1016/j.addr.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qian X, Peng XH, Ansari DO, et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008;26(1):83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 82.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338(6109):903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grieneisen ML, Zhang M. Nanoscience and nanotechnology: evolving definitions and growing footprint on the scientific landscape. Small. 2011;7(20):2836–2839. doi: 10.1002/smll.201100387. [DOI] [PubMed] [Google Scholar]
- 84.Tripathi RM, Shrivastav A, Shrivastav BR. Biogenic gold nanoparticles: as a potential candidate for brain tumor directed drug delivery. Artif. Cells Nanomed. Biotechnol. 2014 doi: 10.3109/21691401.2014.885445. [DOI] [PubMed] [Google Scholar]
- 85.Pandey S, Mewada A, Thakur M, Shah R, Oza G, Sharon M. Biogenic gold nanoparticles as fotillas to fire berberine hydrochloride using folic acid as molecular road map. Mater. Sci. Eng. C Mater. Biol. Appl. 2013;33(7):3716–3722. doi: 10.1016/j.msec.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Lee SM, Kim HJ, Kim SY, et al. Drug-loaded gold plasmonic nanoparticles for treatment of multidrug resistance in cancer. Biomaterials. 2014;35(7):2272–2282. doi: 10.1016/j.biomaterials.2013.11.068. [DOI] [PubMed] [Google Scholar]
- 87.Cheng Y, Doane TL, Chuang CH, Ziady A, Burda C. Near infrared light-triggered drug generation and release from gold nanoparticle carriers for photodynamic therapy. Small. 2014;10(9):1799–1804. doi: 10.1002/smll.201303329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 90.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 91.Magzoub M, Jin S, Verkman AS. Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J. 2008;22(1):276–284. doi: 10.1096/fj.07-9150com. [DOI] [PubMed] [Google Scholar]
- 92.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60(9):2497–2503. [PubMed] [Google Scholar]
- 93.Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Begum M, Abbulu K, Sudhakar M. Flurbiprofen-loaded stealth liposomes: studies on the development, characterization, pharmacokinetics, and biodistribution. J. Young Pharm. 2012;4(4):209–219. doi: 10.4103/0975-1483.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harrington KJ, Mohammadtaghi S, Uster PS, et al. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin. Cancer Res. 2001;7(2):243–254. [PubMed] [Google Scholar]
- 96.Zhang SX, Gao J, Buchholz TA, et al. Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: a Monte Carlo simulation study. Biomed. Microdevice. 2009;11:925–933. doi: 10.1007/s10544-009-9309-5. [DOI] [PubMed] [Google Scholar]
- 97.Ngwa W, Makrigiorgos GM, Berbeco RI. Applying gold nanoparticles as tumor-vascular disrupting agents during brachytherapy: estimation of endothelial dose enhancement. Phys. Med. Biol. 2010;55(21):6533–6548. doi: 10.1088/0031-9155/55/21/013. [DOI] [PubMed] [Google Scholar]
- 98.Ngwa W, Makrigiorgos GM, Berbeco RI. Gold nanoparticle-aided brachytherapy with vascular dose painting: estimation of dose enhancement to the tumor endothelial cell nucleus. Med. Phys. 2012;39(1):392–398. doi: 10.1118/1.3671905. [DOI] [PubMed] [Google Scholar]
- 99.Berbeco RI, Ngwa W, Makrigiorgos GM. Localized dose enhancement to tumor blood vessel endothelial cells via megavoltage x-rays and targeted gold nanoparticles: new potential for external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(1):270–276. doi: 10.1016/j.ijrobp.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 100. Ngwa W, Makrigiorgos GM, Berbeco RI. Gold nanoparticle enhancement of stereotactic radiosurgery for neovascular age-related macular degeneration. Phys. Med. Biol. 2012;57(20):6371–6380. doi: 10.1088/0031-9155/57/20/6371. •• Highlights a potential new approach for treatment of retinal diseases using gold nanoparticles and the Oraya Therapy™ system (Oraya Therapeutics Inc., CA, USA).
- 101.Lechtman E, Chattopadhyay N, Cai Z, Mashouf S, Reilly R, Pignol JP. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys. Med. Biol. 2011;56(15):4631–4647. doi: 10.1088/0031-9155/56/15/001. [DOI] [PubMed] [Google Scholar]
- 102.Leung MK, Chow JC, Chithrani BD, Lee MJ, Oms B, Jaffray DA. Irradiation of gold nanoparticles by x-rays: Monte Carlo simulation of dose enhancements and the spatial properties of the secondary electrons production. Med. Phys. 2011;38(2):624–631. doi: 10.1118/1.3539623. [DOI] [PubMed] [Google Scholar]
- 103.Zygmanski P, Liu B, Tsiamas P, et al. Dependence of Monte Carlo microdosimetric computations on the simulation geometry of gold nanoparticles. Phys. Med. Biol. 2013;58(22):7961–7977. doi: 10.1088/0031-9155/58/22/7961. [DOI] [PubMed] [Google Scholar]
- 104.Rahman WN, Bishara N, Ackerly T, et al. Enhancement of radiation effects by gold nanoparticles for superficial radiation therapy. Nanomedicine. 2009;5(2):136–142. doi: 10.1016/j.nano.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 105.Kong T, Zeng J, Wang X, et al. Enhancement of radiation cytotoxicity in breast-cancer cells by localized attachment of gold nanoparticles. Small. 2008;4(9):1537–1543. doi: 10.1002/smll.200700794. [DOI] [PubMed] [Google Scholar]
- 106.Jain S, Coulter JA, Hounsell AR, et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int. J. Radiat. Oncol. Biol. Phys. 2011;79(2):531–539. doi: 10.1016/j.ijrobp.2010.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu CJ, Wang CH, Chen ST, et al. Enhancement of cell radiation sensitivity by pegylated gold nanoparticles. Phys. Med. Biol. 2010;55(4):931–945. doi: 10.1088/0031-9155/55/4/002. [DOI] [PubMed] [Google Scholar]
- 108.Roa W, Zhang X, Guo L, et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology. 2009;20(37):375101. doi: 10.1088/0957-4484/20/37/375101. [DOI] [PubMed] [Google Scholar]
- 109.Butterworth KT, McMahon SJ, Taggart LE, Prise MK. Radiosensitization by gold nanoparticles: effective at megavoltage energies and potential role of oxidative stress. Transl. Cancer Res. 2013;2(4):269–279. [Google Scholar]
- 110.Butterworth KT, Coulter JA, Jain S, et al. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: potential application for cancer therapy. Nanotechnology. 2010;21(29):295101. doi: 10.1088/0957-4484/21/29/295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Misawa M, Takahashi J. Generation of reactive oxygen species induced by gold nanoparticles under x-ray and UV Irradiations. Nanomedicine. 2011;7(5):604–614. doi: 10.1016/j.nano.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 112.Pan Y, Leifert A, Ruau D, et al. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2009;5(18):2067–2076. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- 113.Chompoosor A, Saha K, Ghosh PS, et al. The role of surface functionality on acute cytotoxicity, ROS generation and DNA damage by cationic gold nanoparticles. Small. 2010;6(20):2246–2249. doi: 10.1002/smll.201000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Al Zaki A, Joh D, Cheng Z. Gold-loaded polymeric micelles for computed tomography-guided radiation therapy treatment and radiosensitization. ACS Nano. 2014;8(1):104–112. doi: 10.1021/nn405701q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bobyk L, Edouard M, Deman P, et al. Photoactivation of gold nanoparticles for glioma treatment. Nanomedicine. 2013;9(7):1089–1097. doi: 10.1016/j.nano.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 116.Joh DY, Sun L, Stangl M, et al. Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS One. 2013;8(4):e62425. doi: 10.1371/journal.pone.0062425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brun E, Sanche L, Sicard-Roselli C. Parameters governing gold nanoparticle x-ray radiosensitization of DNA in solution. Colloids Surf. B Biointerfaces. 2009;72(1):128–134. doi: 10.1016/j.colsurfb.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 118.Ngwa W, Berbeco RI, Makrigiorgos GM. Gold nanoparticle-aided brachytherapy with vascular dose painting: Estimation of dose enhancement to the tumor endothelial cell nucleus. Med. Phys. 2012;39(1):392–398. doi: 10.1118/1.3671905. [DOI] [PubMed] [Google Scholar]
- 119.Makrigiorgos GM, Adelstein SJ, Kassis AI. Cellular radiation dosimetry and its implications for estimation of radiation risks. Illustrative results with technetium 99m-labeled microspheres and macroaggregates. JAMA. 1990;264(5):592–595. [PubMed] [Google Scholar]
- 120.Makrigiorgos GM, Ito S, Baranowska-Kortylewicz J, et al. Inhomogeneous deposition of radiopharmaceuticals at the cellular level: experimental evidence and dosimetric implications. J. Nucl. Med. 1990;31(8):1358–1363. [PubMed] [Google Scholar]
- 121.Denekamp J. Endothelial cell proliferation as a novel approach to targeting tumour therapy. Br. J. Cancer. 1982;45(1):136–139. doi: 10.1038/bjc.1982.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Denekamp J. Vascular endothelium as the vulnerable element in tumours. Acta Radiol. Oncol. 1984;23(4):217–225. doi: 10.3109/02841868409136015. [DOI] [PubMed] [Google Scholar]
- 123.Gridelli C, Rossi A, Maione P, et al. Vascular disrupting agents: a novel mechanism of action in the battle against non-small cell lung cancer. Oncologist. 2009;14(6):612–620. doi: 10.1634/theoncologist.2008-0287. [DOI] [PubMed] [Google Scholar]
- 124.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 125.Boerman OC, Sharkey RM, Blumenthal RD, Aninipot RL, Goldenberg DM. The presence of a concomitant bulky tumor can decrease the uptake and therapeutic efficacy of radiolabeled antibodies in small tumors. Int. J. Cancer. 1992;51(3):470–475. doi: 10.1002/ijc.2910510322. [DOI] [PubMed] [Google Scholar]
- 126.Siemann DW, Shi W. Targeting the tumor blood vessel network to enhance the efficacy of radiation therapy. Semin. Radiat. Oncol. 2003;13(1):53–61. doi: 10.1053/srao.2003.50005. [DOI] [PubMed] [Google Scholar]
- 127.Cooney MM, Van Heeckeren W, Bhakta S, Ortiz J, Remick SC. Drug insight: vascular disrupting agents and angiogenesis – novel approaches for drug delivery. Nat. Clin. Pract. Oncol. 2006;3(12):682–692. doi: 10.1038/ncponc0663. [DOI] [PubMed] [Google Scholar]
- 128.Skliarenko JV, Lunt SJ, Gordon ML, Vitkin A, Milosevic M, Hill RP. Effects of the vascular disrupting agent ZD6126 on interstitial fluid pressure and cell survival in tumors. Cancer Res. 2006;66(4):2074–2080. doi: 10.1158/0008-5472.CAN-05-2046. [DOI] [PubMed] [Google Scholar]
- 129.Van Heeckeren WJ, Bhakta S, Ortiz J. Promise of new vascular-disrupting agents balanced with cardiac toxicity: is it time for oncologists to get to know their cardiologists. J. Clin. Oncol. 2006;24(10):1485–1488. doi: 10.1200/JCO.2005.04.8801. [DOI] [PubMed] [Google Scholar]
- 130.Cormack RA, Sridhar S, Suh WW, D’Amico AV, Makrigiorgos GM. Biological in situ dose painting for image-guided radiation therapy using drug-loaded implantable devices. Int. J. Radiat. Oncol. Biol. Phys. 2010;76(2):615–623. doi: 10.1016/j.ijrobp.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 131.Nagesha DK, Tada DB, Stambaugh CK, et al. Radiosensitizer-eluting nanocoatings on gold fiducials for biological in-situ image-guided radio therapy (BIS-IGRT) Phys. Med. Biol. 2010;55(20):6039–6052. doi: 10.1088/0031-9155/55/20/001. [DOI] [PubMed] [Google Scholar]
- 132.Dong Y, Chin SF, Blanco E, et al. Intratumoral delivery of beta-lapachone via polymer implants for prostate cancer therapy. Clin. Cancer Res. 2009;15(1):131–139. doi: 10.1158/1078-0432.CCR-08-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qian F, Szymanski A, Gao J. Fabrication and characterization of controlled release poly(d,l-lactide-co-glycolide) millirods. J. Biomed. Mater. Res. 2001;55(4):512–522. doi: 10.1002/1097-4636(20010615)55:4<512::aid-jbm1044>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 134.Takeda A, Baffi JZ, Kleinman ME, et al. CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature. 2009;460(7252):225–230. doi: 10.1038/nature08151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull. World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 136.The Global Economic Cost of Visual Impairment. London, UK: AMD Alliance International; 2010. [Google Scholar]
- 137.Axer-Siegel R, Ehrlich R, Yassur Y, et al. Photodynamic therapy for age-related macular degeneration in a clinical setting: visual results and angiographic patterns. Am. J. Ophthalmol. 2004;137(2):258–264. doi: 10.1016/j.ajo.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 138.Yip PP, Woo CF, Tang HH, Ho CK. Triple therapy for neovascular age-related macular degeneration using single-session photodynamic therapy combined with intravitreal bevacizumab and triamcinolone. Br. J. Ophthalmol. 2009;93(6):754–758. doi: 10.1136/bjo.2008.150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 140.Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can. J. Ophthalmol. 2005;40(3):352–368. doi: 10.1016/S0008-4182(05)80078-X. [DOI] [PubMed] [Google Scholar]
- 141.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65. e55. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 142.Bressler NM. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology. 2009;116(10 Suppl.):S15–S23. doi: 10.1016/j.ophtha.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 143.Salehi-Had H, Roh MI, Giani A, et al. Utilizing targeted gene therapy with nanoparticles binding alpha v beta 3 for imaging and treating choroidal neovascularization. PLoS One. 2011;6(4):e18864. doi: 10.1371/journal.pone.0018864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Petrarca R, Jackson TL. Radiation therapy for neovascular age-related macular degeneration. Clin. Ophthalmol. 2011;5:57–63. doi: 10.2147/OPTH.S16444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hanlon J, Lee C, Chell E, et al. Kilovoltage stereotactic radiosurgery for age-related macular degeneration: assessment of optic nerve dose and patient effective dose. Med. Phys. 2009;36(8):3671–3681. doi: 10.1118/1.3168554. [DOI] [PubMed] [Google Scholar]
- 146.Moshfeghi DM, Kaiser PK, Gertner M. Stereotactic low-voltage x-ray irradiation for age-related macular degeneration. Br. J. Ophthalmol. 2011;95(2):185–188. doi: 10.1136/bjo.2009.163907. [DOI] [PubMed] [Google Scholar]
- 147.Fine SL, Maguire MG. It is not time to abandon radiotherapy for neovascular age-related macular degeneration. Arch. Ophthalmol. 2001;119(2):275–276. [PubMed] [Google Scholar]
- 148.Bonnaud S, Niaudet C, Pottier G, et al. Sphingosine-1-phosphate protects proliferating endothelial cells from ceramide-induced apoptosis but not from DNA damage-induced mitotic death. Cancer Res. 2007;67(4):1803–1811. doi: 10.1158/0008-5472.CAN-06-2802. [DOI] [PubMed] [Google Scholar]
- 149.Hanlon J, Firpo M, Chell E, Moshfeghi DM, Bolch WE. Stereotactic radiosurgery for AMD: a Monte Carlo-based assessment of patient-specific tissue doses. Invest. Ophthalmol. Vis. Sci. 2011;52(5):2334–2342. doi: 10.1167/iovs.10-6421. [DOI] [PubMed] [Google Scholar]
- 150.Taddei PJ, Chell E, Hansen S, Gertner M, Newhauser WD. Assessment of targeting accuracy of a low-energy stereotactic radiosurgery treatment for age-related macular degeneration. Phys. Med. Biol. 2010;55(23):7037–7054. doi: 10.1088/0031-9155/55/23/S06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Canton VM, Quiroz-Mercado H, Velez-Montoya R, et al. 16-Gy low-voltage x-ray irradiation with ranibizumab therapy for AMD: 6-month safety and functional outcomes. Ophthalmic Surg. Lasers Imaging. 2011;42(6):468–473. doi: 10.3928/15428877-20110804-01. [DOI] [PubMed] [Google Scholar]
- 152.Moshfeghi AA, Canton VM, Quiroz-Mercado H, et al. 16-Gy low-voltage x-ray irradiation followed by as-needed ranibizumab therapy for AMD: 6-month outcomes of a “radiation-first” strategy. Ophthalmic Surg. Lasers Imaging. 2011;42(6):460–467. doi: 10.3928/15428877-20110804-03. [DOI] [PubMed] [Google Scholar]
- 153.Canton VM, Quiroz-Mercado H, Velez-Montoya R, et al. 24-Gy low-voltage x-ray irradiation with ranibizumab therapy for neovascular AMD: 6-month safety and functional outcomes. Ophthalmic Surg. Lasers Imaging. 2012;43(1):20–24. doi: 10.3928/15428877-20111129-01. [DOI] [PubMed] [Google Scholar]
- 154.Gokeri G, Kocar C, Tombakoglu M. Monte Carlo simulation of microbeam radiation therapy with an interlaced irradiation geometry and an Au contrast agent in a realistic head phantom. Phys. Med. Biol. 2010;55(24):7469–7487. doi: 10.1088/0031-9155/55/24/006. [DOI] [PubMed] [Google Scholar]
- 155.Van Den Heuvel F, Locquet JP, Nuyts S. Beam energy considerations for gold nano-particle enhanced radiation treatment. Phys. Med. Biol. 2010;55(16):4509–4520. doi: 10.1088/0031-9155/55/16/S06. [DOI] [PubMed] [Google Scholar]
- 156.Garnica-Garza HM. Contrast-enhanced radiotherapy: feasibility and characteristics of the physical absorbed dose distribution for deep-seated tumors. Phys. Med. Biol. 2009;54(18):5411–5425. doi: 10.1088/0031-9155/54/18/004. [DOI] [PubMed] [Google Scholar]
- 157.Singh SR, Grossniklaus HE, Kang SJ, Edelhauser HF, Ambati BK, Kompella UB. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16(5):645–659. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Iezzi R, Guru BR, Glybina IV, Mishra MK, Kennedy A, Kannan RM. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials. 2012;33(3):979–988. doi: 10.1016/j.biomaterials.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 159.Kim JH, Kim KW, Kim MH, Yu YS. Intravenously administered gold nanoparticles pass through the blood–retinal barrier depending on the particle size, and induce no retinal toxicity. Nanotechnology. 2009;20(50):505101. doi: 10.1088/0957-4484/20/50/505101. [DOI] [PubMed] [Google Scholar]
- 160.Farjo KM, Ma JX. The potential of nanomedicine therapies to treat neovascular disease in the retina. J. Angiogenes Res. 2010;2:21. doi: 10.1186/2040-2384-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Korideck H, Ngwa W, Makrigiorgos GM, Berbeco RI. The Quantification of gold nanoparticles as contrast agents for small animal volumetric studies. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(2 Suppl.):S887–S888. doi: 10.1016/j.ijrobp.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 162.Reuveni T, Motiei M, Romman Z, Popovtzer A, Popovtzer R. Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study. Int. J. Nanomedicine. 2011;6:2859–2864. doi: 10.2147/IJN.S25446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Popovtzer R, Agrawal A, Kotov NA, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8(12):4593–4596. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mason RP, Zhao D, Liu L, Trawick ML, Pinney KG. A perspective on vascular disrupting agents that interact with tubulin: preclinical tumor imaging and biological assessment. Integr Biol (Camb) 2011;3(4):375–387. doi: 10.1039/c0ib00135j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ricketts K, Guazzoni C, Castoldi A, Gibson AP, Royle GJ. An x-ray fluorescence imaging system for gold nanoparticle detection. Phys. Med. Biol. 2013;58(21):7841–7855. doi: 10.1088/0031-9155/58/21/7841. [DOI] [PubMed] [Google Scholar]
- 166.Chang MY, Shiau AL, Chen YH, Chang CJ, Chen HH, Wu CL. Increased apoptotic potential and dose-enhancing effect of gold nanoparticles in combination with single-dose clinical electron beams on tumor-bearing mice. Cancer Sci. 2008;99(7):1479–1484. doi: 10.1111/j.1349-7006.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zheng Y, Hunting DJ, Ayotte P, Sanche L. Radiosensitization of DNA by gold nanoparticles irradiated with high-energy electrons. Radiat. Res. 2008;169(1):19–27. doi: 10.1667/RR1080.1. [DOI] [PubMed] [Google Scholar]
- 168.Polf JC, Bronk LF, Driessen WH, Arap W, Pasqualini R, Gillin M. Enhanced relative biological effectiveness of proton radiotherapy in tumor cells with internalized gold nanoparticles. Applied Phys. Lett. 2011;98(19):193702. doi: 10.1063/1.3589914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim JK, Seo SJ, Kim HT, et al. Enhanced proton treatment in mouse tumors through proton irradiated. Phys. Med. Biol. 2012;57(24):8309–8323. doi: 10.1088/0031-9155/57/24/8309. [DOI] [PubMed] [Google Scholar]
- 170.Hebert EM, Debouttiere PJ, Lepage M, Sanche L, Hunting DJ. Preferential tumour accumulation of gold nanoparticles, visualised by magnetic resonance imaging: radiosensitisation studies in vivo and in vitro. Int. J. Radiat. Biol. 2010;86(8):692–700. doi: 10.3109/09553001003746067. [DOI] [PubMed] [Google Scholar]
- 171.Hainfeld JF, O’Connor MJ, Lin P, Qian L, Slatkin DN, Smilowitz HM. Infrared-transparent gold nanoparticles converted by tumors to infrared absorbers cure tumors in mice by photothermal therapy. PLoS One. 2014;9(2):e88414. doi: 10.1371/journal.pone.0088414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hossain M, Su M. Nanoparticle location and material dependent dose enhancement in x-ray radiation therapy. J. Phys. Chem. 2012;116(43):23047–23052. doi: 10.1021/jp306543q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bahreyni Toossi MT, Ghorbani M, Mehrpouyan M, Akbari F, Sobhkhiz Sabet L, Soleimani Meigooni A. A Monte Carlo study on tissue dose enhancement in brachytherapy: a comparison between gadolinium and gold nanoparticles. Australas. Phys. Eng. Sci. Med. 2012;35(2):177–185. doi: 10.1007/s13246-012-0143-3. [DOI] [PubMed] [Google Scholar]
- 174.Bernier J, Hall EJ, Giaccia A. Radiation oncology: a century of achievements. Nat. Rev. Cancer. 2004;4(9):737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]