Abstract
Beta-blockers are a class of drugs widely used to treat cardiac, respiratory and other ailments. They act by blocking beta-adrenergic receptor–mediated signalling. Studies in various cancers have shown that patients taking a beta-blocker have higher survival and lower recurrence and metastasis rates. This is supported by several preclinical and in vitro studies showing that adrenergic activation modulates apoptosis, promotes angiogenesis and other cancer hallmarks, and these effects can be abrogated by beta-blockers. These studies provide a rationale for the use of beta-blockers as adjuvants with cancer chemotherapy. However, all published studies so far are retrospective, and most do not take into account the specific beta-blocker used or address which is most likely to benefit cancer patients. The published epidemiological studies are correlative and have not examined the adrenergic receptor status of the tumours. Knowledge of the beta-adrenergic receptor status of tumour cells is essential in choosing the best beta-blocker for adjuvant therapy. A comprehensive, prospective study is necessary to prove definitively the utility of using beta-blockers with chemotherapy and to identify the specific betablocker most likely to benefit patients with cancer.
Keywords: Beta-blockers, beta-adrenergic receptor, catecholamines
1. Introduction
Chronic activation of the sympathetic nervous system is frequently associated with increased tumour burden and higher risk of recurrence; in fact, it has an impact on all the major hallmarks of cancer. Several retrospective epidemiological studies have shown that patients who are on a beta-blocker survive longer with lower rates of recurrence and metastasis in different cancers. Most studies show a survival benefit regardless of timing: that is, beta-blocker therapy may be beneficial before, during or after diagnosis and chemotherapy. A critical look at these studies will enable scientists and clinicians to evaluate the potential for use of beta-blockers as therapy for cancer.
Beta-adrenergic receptors are G-protein–coupled receptors that are ubiquitously expressed by normal cells. There are 3 sub-types of beta-adrenergic receptors, designated ADRB1, ADRB2 and ADRB3, which are encoded by genes located on chromosomes 10, 5 and 8, respectively. ADRBs are activated by catecholamines (such as noradrenaline and adrenaline) that are secreted largely in response to sympathetic nervous system stimulation, resulting in the “fight-or-flight” response. Although acute activation of adrenergic receptors has been shown to be beneficial to human health, prolonged activation is associated with decreased immune function, heart failure, arrhythmia and hypertension. Sustained activation can be a result of altered behavioural states such as those seen in depression, anxiety and chronic stress.
Beta-blockers primarily function by competitively blocking the activation of adrenergic receptors by noradrenaline and adrenaline. Beta-blockers are widely used to treat cardiac ailments such as hypertension and arrhythmia and respiratory disorders such as asthma. The U.S. Food and Drug Administration (FDA) and European Union’s European Medical Agency currently approve several betablockers. These differ in their beta-receptor specificity, their intrinsic sympathomimetic activity, their vasodilatory effects and their ability to cross the blood-brain barrier. Beta-blockers are divided into 3 categories: first-generation beta-blockers, such as propranolol, show equal antagonistic activity for both ADRB1 and ADRB2 receptors and are commonly referred as non-selective beta-blockers. The second generation of beta-blockers, such as atenolol, has higher affinity for the ADRB1 receptor and inhibits ADRB2 activity only at higher concentrations. The third generation of beta-blockers, such as nebivolol, differs from other generations of beta-blockers in their vasodilatory effects.
2. Adrenergic Signaling in Cancer
The deleterious effects of stress signalling have been supported by several clinical studies across a number of cancer types. Studies have shown that depression is rather common among patients with pancreatic, oropharyngeal, gynaecological, breast or lung cancer [1]. Moreover, diagnosis, treatment, surgery and recovery are all stressful situations for patients. In a study of 68 ovarian cancer patients, intratumoural noradrenaline level was significantly elevated in patients with low social support, whereas the plasma level of noradrenaline did not differ significantly from that of patients with a high level of social support [2]. Costanzo et al. showed that, in a cohort of 61 ovarian cancer patients, those with low social support had significantly higher levels of interleukin 6 (IL-6) in both peripheral blood and ascites than patients with high social support [3]. One of their interesting findings was that patients with a history of depression had higher levels of IL-6 in ascites. A similar study in breast cancer showed elevated IL-6 levels in patients with a history of major depression [4].
Conversely, several studies show survival benefits when catecholamine signalling is abrogated by a betablocker. In a study of 3561 patients with high-risk prostate cancer, of whom 1115 patients had used a beta-blocker before and after diagnosis, use of a beta-blocker was significantly associated with a delayed onset of disease, reduced risk of mortality from prostate cancer, a lower Gleason score and a lower rate of distant metastasis [5]. Independent survival benefits were also seen in patients who were prescribed an ADRB1-specific blocker although most pre-clinical studies show ADRB2 is the primary receptor for adrenergic signalling in cancer cells. The authors also showed that there was an additive survival benefit when a beta-blocker was combined with aspirin. In a study of patients with oral squamous carcinoma, expression of ADRB2 was associated with greater tumour size, higher rate of lymph node metastasis and advanced clinical stage [6]. A retrospective study done on patients with epithelial ovarian cancer showed that use of a beta-blocker increased both progression-free and overall survival when compared to patients who had never used a beta-blocker (56 months for users and 48 months for non-users for PFS and 57 months for users and 51 months for non-users for OS) [7].
Several clinical studies support the beneficial role of beta-blockers in breast cancer. In a cohort of 466 breast cancer patients, the 43 patients who were on a non-specific or ADRB1-targeted beta-blocker for hypertension had significantly prolonged survival and lower rates of metastasis and recurrence [8]. In another study, Barron et al. grouped breast cancer patients into those not on a beta-blocker, those on atenolol (beta-1 selective) and those on propranolol (non-selective beta blocker). When patients were matched by age, significant survival benefit was seen only among patients who were on propranolol. Past therapy with propranolol was also associated with lower tumour grade and lower incidence of metastasis than in patients not on a beta-blocker [9]. Use of a beta-blocker was associated with reduced risk of death in patients with malignant melanoma [10]. In a recent retrospective study in non–small cell lung cancer, use of a beta-blocker (ADRB1 specific or non-selective) during radiation therapy was associated with significantly better rates of overall survival, distant metastasis-free survival and progression-free survival [11]. In infantile haemangioma, propranolol is one of the most promising options for therapy, as it decreases expression of VEGF, which is vital in progression of this disease [12]. Propranolol is not approved by FDA for paediatric cancers, but it has been used to treat cardiac ailments in children and is documented to be well tolerated, with side effects that are seldom life threatening. There are currently clinical trials underway to assess propranolol, nadolol, timolol and other betablockers for treatment of haemangioma.
Although the clinical and preclinical data are compelling to support adjuvant use of beta-blockers in cancer chemotherapy, the findings have not been entirely concordant. Some studies have not shown a significant benefit of beta-blockers to cancer patients. In a retrospective study of a cohort of 1413 patients with triple-negative breast cancer, of whom 102 were on a beta-blocker, significant benefits of beta-blockers were seen in progression-free survival but not overall survival when adjusted for age, race, tumour stage and other clinical factors [13]. In a retrospective study using a cohort of 381 patients, with platinum-sensitive recurrent ovarian cancer from AGO phase 1 and phase 3 trials, a history of ADRB1 selective beta-blocker therapy (n=38) conferred no survival or response benefit [14].
3. Expert Opinion
Currently, beta-blockers are primarily prescribed to patients for cardiac indications. Nearly all the studies reviewed here show survival benefits for cancer patients who are taking one of these medications for another independent ailment (Table 1). All of these studies are retrospective and thus take into account only the historical record of beta-blocker usage by the patient. Most of the clinical studies have not specifically evaluated the type of beta-blocker associated with greatest survival benefit to patients. Prospective assessment of beta-blockers in cancer is needed to a) identify the patients who would benefit most from using a beta-blocker and b) identify the best beta-blocker for a specific tumour type based on ADRB expression. Use of a beta-blocker as single-agent therapy may not be sufficient, as aggressive tumours are driven by genetic instability, gene mutations and amplification of oncogenes that are likely to affect progression. The ideal use of a beta-blocker would be in combination with a chemotherapeutic drug, or anti-inflammatory medications (e.g., aspirin) or radiation, creating a synergism of effects to minimize tumour progression and recurrence. Moreover, a beta-blocker would be good adjuvant therapy for recovery after surgery, as adrenergic activation has been shown in preclinical studies to delay wound healing. Pre-clinical studies have also shown ADRBs are present on important immune cells such as macrophages; and suppression of macrophage activation by beta-blockers has shown to decrease metastasis in murine models.
Table 1.
Selected clinical studies of beta-blocker effects in cancer patients
| Tumor site | Study name | Total patients (patients on beta-blockers) |
|---|---|---|
| Prostate | Grytli et al. (2013) | 3561 (1115) |
| Lung | Wang et al. (2013) | 722 (155) |
| Ovarian | Diaz et al. (2012) | 248 (23) |
| Breast | Melhem-Bertrandt et al. (2011) | 1413 (102) |
| Breast | Barron et al. (2011) | 5801 (propranolol:70, atenolol: 525) |
| Melanoma | Lemeshow et al. (2011) | 4179 (372) |
| Breast | Powe et al. (2010) | 466 (43) |
| Recurrent Ovarian Cancer | Heitz et al. (2013) | 381 (38) |
While using a non-selective beta-blocker such as propranolol might be a safe option for blocking adrenergic activation, having knowledge of the ADRB status of the tumour could help to tailor therapy to a specific beta-blocker to maximize benefit and decrease side effects. For example, while atenolol conferred survival benefit in pancreatic cancer, it failed to show a significant benefit in ovarian cancer. Furthermore, there is a subset of tumour types for which beta-blocker use could adversely affect outcome (figure 1). A patient is most likely to benefit from a beta-blocker as chemotherapy adjuvant if the tumour cells express one or more ADRB receptors. Hence, ADRB expression status could serve as a biomarker for selecting patients most likely to benefit from a specific beta-blocker. The FDA and European Union already document the complete biological effects and recommended safe doses of beta-blockers and hence translation to the clinic as adjuvant for chemotherapy could be readily achieved.
Figure 1.
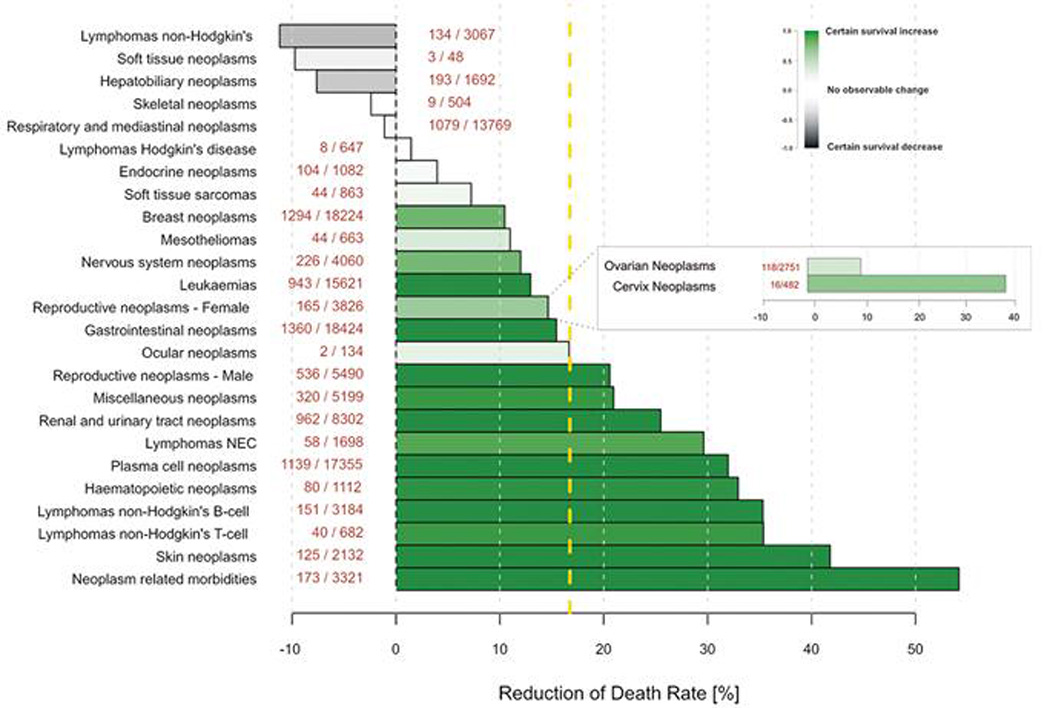
Impact of using beta-blockers on survival of patients across cancer types. Cancer-related mortality, estimated based on data from the FDA Adverse Event Reporting System. Bars indicate mortality rates for each cancer, bars to left indicate decreased survival on beta-blockers and bars to right show survival benefits on using beta-blocker (number of patients are included within parentheses). Mortality reduction over all cancer types is indicated by the dashed yellow line at 17% [15].
There are clinical trials currently underway that combine chemotherapy with beta-blockers to study effects on cardiac function, angiogenesis and targeting of stress modulators in ovarian, lung, and breast cancers. That beta-blockers are promising candidates for chemotherapy adjuvants is supported by several studies. However, additional prospective clinical studies with well-defined controls and adequate sample sizes are required. A patient’s likelihood of benefit from a beta-blocker needs to be assessed and the specific agent carefully selected for each patient depending on ADRB status and tumour type.
Outcome associated with beta-blockers.
Reduced risk of mortality, lower Gleason score, less distant metastases and delayed onset of disease; difference in overall survival was 39 months (p=0.001)
Prolonged distant metastasis-free survival (p<0.01), disease-free survival (p< 0.01) and overall survival (p=0.01)
Prolonged both progression-free and overall survival; difference in overall survival was 8 months (p=0.02)
Prolonged recurrence-free survival in patients with triple-negative breast tumour (p=0.027)
Significantly lower risk of breast cancer–specific mortality (propranolol)
Lower mortality rate
Reduced rates of metastasis development (p=0.026) and tumour recurrence (p=0.001); longer disease-free interval (p=0.01)
No benefits in both overall survival (p=0.15) and progression-free survival (p=0.65)
References
- 1.Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 2.Lutgendorf SK, DeGeest K, Dahmoush L, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2011;25(2):250–255. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costanzo ES, Lutgendorf SK, Sood AK, et al. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104(2):305–313. doi: 10.1002/cncr.21147. [DOI] [PubMed] [Google Scholar]
- 4.Soygur H, Palaoglu O, Akarsu ES, et al. Interleukin-6 levels and HPA axis activation in breast cancer patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1242–1247. doi: 10.1016/j.pnpbp.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Grytli HH, Fagerland MW, Fosså SD, et al. Association Between Use of β-Blockers and Prostate Cancer–Specific Survival: A Cohort Study of 3561 Prostate Cancer Patients with High-Risk or Metastatic Disease. European Urology. (0) doi: 10.1016/j.eururo.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Shang ZJ, Liu K, Liang de F. Expression of beta2-adrenergic receptor in oral squamous cell carcinoma. J Oral Pathol Med. 2009;38(4):371–376. doi: 10.1111/j.1600-0714.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 7.Diaz ES, Karlan BY, Li AJ. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol. 2012 doi: 10.1016/j.ygyno.2012.07.102. [DOI] [PubMed] [Google Scholar]
- 8.Powe DG, Voss MJ, Zanker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1(7):628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barron TI, Connolly RM, Sharp L, et al. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. 2011;29(19):2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 10.Lemeshow S, Sørensen HT, Phillips G, et al. β-Blockers and Survival among Danish Patients with Malignant Melanoma: A Population-Based Cohort Study. Cancer Epidemiology Biomarkers & Prevention. 2011;20(10):2273–2279. doi: 10.1158/1055-9965.EPI-11-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang HM, Liao ZX, Komaki R, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Annals of Oncology. 2013 doi: 10.1093/annonc/mds616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leaute-Labreze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 13.Melhem-Bertrandt A, Chavez-MacGregor M, Lei X, et al. Beta-Blocker Use Is Associated With Improved Relapse-Free Survival in Patients With Triple-Negative Breast Cancer. Journal of Clinical Oncology. 2011;29(19):2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heitz F, du Bois A, Harter P, et al. Impact of beta blocker medication in patients with platinum sensitive recurrent ovarian cancer-a combined analysis of 2 prospective multicenter trials by the AGO Study Group, NCIC-CTG and EORTC-GCG. Gynecol Oncol. 2013 doi: 10.1016/j.ygyno.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Armaiz-Pena GN, Allen JK, Cruz A, et al. Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013;4:1403. doi: 10.1038/ncomms2413. [DOI] [PMC free article] [PubMed] [Google Scholar]


