Abstract
For many patients with neuropsychiatric illnesses, standard psychiatric treatments with mono or combination pharmacotherapy, psychotherapy, and transcranial magnetic stimulation are ineffective. For these patients with treatment resistant neuropsychiatric illnesses, a main therapeutic option is electroconvulsive therapy (ECT). Decades of research have found ECT to be highly effective; however, it can also result in adverse neurocognitive effects. Specifically, ECT results in disorientation after each session, anterograde amnesia for recently learned information, and retrograde amnesia for previously learned information. Unfortunately, the neurocognitive effects and underlying mechanisms of action of ECT remain poorly understood. The purpose of this paper is to synthesize the multiple moderating and mediating factors that are thought to underlie the neurocognitive effects of ECT into a coherent model. Such factors include demographic and neuropsychological characteristics, neuropsychiatric symptoms, ECT technical parameters, and ECT associated neurophysiological changes. Future research is warranted to evaluate and test this model, so that these findings may support the development of more refined clinical seizure therapy delivery approaches and efficacious cognitive remediation strategies to improve the utility of this important and widely used intervention tool for neuropsychiatric diseases.
Keywords: Electroconvulsive therapy, seizure therapy neuropsychology, memory, neural mechanism, electroencephalography, cognitive remediation
Introduction
For many patients with neuropsychiatric illnesses, standard psychiatric treatments with mono or combination pharmacotherapy, psychotherapy, transcranial magnetic stimulation (TMS) are ineffective. For example, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study found that antidepressant treatment with citalopram resulted in remission rates between 28–33% [1]. The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) found treatment with an antidepressant (paroxetine or bupropion) and a mood stabilizer (e.g., any US Food and Drug Administration approved antimanic agent) produced a 32% remission rate [2]. The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial studied second generation antipsychotic medications and found that a large majority (74%) of patients with schizophrenia discontinued those medications due to adverse side effects [3]. For these patients with major depressive disorder (MDD), bipolar disorder, or schizophrenia, whose diseases are classified as treatment resistant, a main therapeutic option is electroconvulsive therapy (ECT).
Decades of research have found that ECT is one of the most efficacious treatments for neuropsychiatric diseases, especially MDD [4]. The absolute number of patients who receive ECT is large, annually estimated at 1 million worldwide [5, 6]. Treatment with ECT produces rapid response and remission rates [7], and is safe for patients across the adult life span [8–10]. However, ECT also results in adverse neurocognitive effects [11–14]. Despite significant refinements in ECT practice, the adverse effects of ECT have remained and are a principal concern of both practitioners and patients. The ECT neurocognitive profile is primarily comprised of decreased orientation immediately after the ECT session, anterograde amnesia for recent information, and retrograde amnesia for long-term autobiographical and impersonal information [12]. Other neuropsychological domains that become inefficient or impaired include processing speed, attention, verbal fluency, and executive function (e.g., cognitive flexibility) [11]. Unlike the neurocognitive profile of Alzheimer’s disease that progressively worsens, this profile is transient in many cases [15]. Nonetheless, the adverse neurocognitive changes produced by ECT can persist for up to 6-months or longer and result in functional impairment, poor adherence, reduced clinical outcome, and increased relapse rates.
Unfortunately, as noted by the United States Food and Drug Administration (US FDA), underlying mechanisms of ECT neurocognitive effects remain poorly understood [5]. Indeed, Fraser et al. [16] in a systematic review of the past 20 years of ECT research reported that there exist no conceptual model that describes how ECT results in adverse neurocognitive effects. Though neuroimaging research [17] suggests that most cortical and subcortical regions are involved in ECT associated memory impairment, no conceptual model exists of how the cognitive effects of ECT develop in patients. Thus, the field is at the initial stage of model construction.
The purpose of this paper is to synthesize the multiple moderating and mediating factors that are thought to underlie the neurocognitive effects of ECT into a coherent model (see Figure 1). These factors include demographic and neuropsychological characteristics, neuropsychiatric symptoms, ECT technical parameters, and neurophysiological effects. Furthermore, future recommendations are provided to guide model testing and cognitive remediation strategy development. While ECT is beneficial for many neuropsychiatric diseases, the scope of this paper will be limited to major depressive disorder, bipolar disorder, and schizophrenia.
Figure 1. Conceptual Model of How Electroconvulsive Therapy Affects Neurocognitive Function.
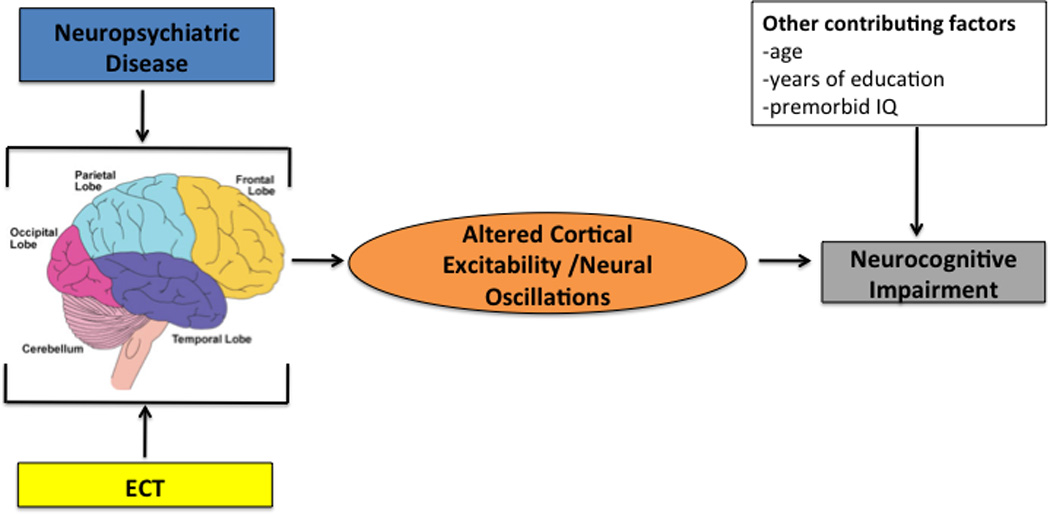
This figure shows the conceptual model of the relationship among neuropsychiatric disease, electroconvulsive therapy, altered cortical excitability/neural oscillations, demographic factors, and neurocognitive function.
ECT=Electroconvulsive Therapy
Demographic and Neuropsychological Factors
There has been limited investigation of the demographic and neuropsychological factors that may moderate the neurocognitive adverse effects of ECT. Such factors include age, education level, and premorbid intellectual ability, and cerebrovascular health, and are important to study based on their moderating role in normal aging and other neuropsychiatric and neurologic diseases including traumatic brain injury [18], Alzheimer’s disease [19], and Parkinson Disease [20]. Regarding ECT, early research found that age, but not depression severity or the number of ECT sessions, was significantly associated with change in memory performance [21]. Also, evidence has suggested that premorbid intellectual ability may affect neurocognitive outcome after ECT. One study [21] found that verbal intellectual ability was partially associated with change in memory performance after ECT. Another study [22] found that patients with major depressive disorder and psychotic features showed neurocognitive inefficiencies and impairment prior to treatment based on their premorbid intellectual estimated abilities. After the acute course, with the exception of phonemic fluency, most neurocognitive functions improved to be within normal limits. These findings highlight the need to interpret neurocognitive changes with respect to predicted estimates as based on premorbid intellectual ability. To our knowledge, there has been no evaluation of the relationship between years of education and neurocognitive changes after ECT. However, one study found that cognitive reserve moderated memory functions after ECT [23]. Specifically, that study found that after treatment with bilateral placement ECT, patients with high relative to those with low cognitive reserve (defined as a combination of years of education and occupational functioning level) showed better delayed recall and retention of learned information. Cerebrovascular health is an important determinant of neurocognitive function, that when poor can impair neurocognitive abilities [24, 25]. Indeed, cerebrovascular disease is associated with a wide spectrum of neurocognitive disorders including mild cognitive impairment, cortical dementias, and subcortical dementias, and is typically referred to as vascular cognitive impairment [26]. Also, cerebrovascular disease has been found to be a possible cause of depression in elderly adults [27–29]. While there is limited ECT research in patients with cerebrovascular disease, Brodaty et al. [30] suggested that underlying cerebrovascular disease may be a risk factor for cognitive adverse effects in elderly adults. However, recent evidence by Verwijk et al. [31] found ECT to be safe in elderly adults. They suggested that adverse neurocognitive effects that onset after ECT are associated with pre-existing neurocognitive impairment. Thus, when interpreting neurocognitive outcome related to ECT, demographic and neuropsychological characteristics, as well as cerebrovascular health, need to be included in the equation given their possible moderating effects.
Neuropsychiatric Disease Factors
Electroconvulsive therapy is effective for MDD, schizophrenia, and bipolar disorder. Each of these neuropsychiatric diseases can impede neurocognitive function before the initiation of treatment with ECT. As such, those impediments in neurocognitive function could moderate neurocognitive outcome after ECT.
Major Depressive Disorder
Converging evidence suggests that MDD is associated with inefficient, and at times impaired neurocognitive functions [32, 33]. Specifically, MDD has been found to result in poor processing speed, attention, learning and memory, and executive function. As MDD is a heterogeneous disorder comprised of a variety of depressive symptoms, the neurocognitive profile may vary from person to person, but it is commonly defined as a subcortical profile [34, 35]. This profile suggest that higher order cognitive functions such as executive abilities and memory become impaired due to inefficiencies in lower order cognitive function such as processing speed. For instance, Butters found in elderly adults with depression that decreased processing speed moderated impairments in language, memory, visuospatial, and executive functions [36].
Conflicting evidence exists regarding the different MDD characteristics that may underlie the associate changes in neurocognitive function. These characteristics include depression severity, the number of depressive episodes, and select depressive symptoms. Depression severity has been inconsistently associated with changes in neurocognitive function. A study in a cohort of patients with high depression severity referred for ECT found no association between depression severity as rated on the 24-item Hamilton Rating Scale and performance on measures of global cognitive function, visuospatial memory, and verbal learning and memory [37]. An earlier study found similar results in a cohort of patients with low depression severity as measured via self-report on the Beck Depression Inventory [38]. Though a meta-analysis suggested that high depression severity was related to poor processing speed, episodic memory, and executive abilities [39]. While it is suggested that recurrent is more clinically severe than single episode depression [40], studies have reported mixed findings with effects on neuropsychological function. For example, recurrent depression has been associated with decreased global cognitive function [41], attention and inhibition [42], and problem solving ability [43]. Conversely, other research has found no association between the number of depressive episodes and attention, memory, and executive functions [44].
Depressive symptom clusters (e.g., melancholic, atypical) and specific depressive symptoms too have been associated with inefficiencies in neurocognitive performance [45]. For example, patients with melancholic subtype MDD compared to those without melancholic features have been found to have worse set shifting abilities [46]. Likewise, patients with depression and psychotic features relative to those without psychotic features show poorer performance on measures of verbal fluency, immediate and delayed recall of both verbal and visual information, cognitive flexibility, and psychomotor speed [47]. Regarding specific depressive symptoms, McGirr et al. [48] showed that patients with high depression severity and high lethal suicidal ideation relative to those with low lethal suicidal ideation had impaired conceptual reasoning and problem solving ability. Similarly, the presence of insomnia has been linked to greater impaired neuropsychological abilities including psychomotor speed, learning and memory, semantic fluency, and complex problem solving and concept formation in elderly adults with depression [49, 50]. Thus, patients with MDD may have pre-existing neurocognitive inefficiencies or impairments that could moderate neuropsychological associated effects of ECT. Of clinical importance, patients with depression melancholic [51] or psychotic features [52] have been found to show high clinical response and remission with ECT.
Schizophrenia and Bipolar Depression
Compromised neurocognitive function is a central feature of schizophrenia that is already apparent at the time of the first psychotic episode [53, 54] and even earlier in the prodromal phases [55]. Profound deficits, as indexed by performance scores more than 2 standard deviations below accepted norms, in the areas of sustained visual attention, verbal memory, and executive abilities can be found in nearly 80% of the population, including those whose psychotic symptoms have remitted [56, 57]. Emerging evidence also points to similar neurocognitive dysfunction not being simply an artifact of depressive symptoms in bipolar depression [58]. Although during the euthymic phase there is a rebound of attention, verbal memory and executive functions reliant on speed, performance on neuropsychological tests may still be well below expected normative levels [59, 60]. These pervasive neurocognitive deficits in both schizophrenia and bipolar depression can further be seen in non-affected first-degree relatives who evidence mild deficits in these same domains [59]. As one might expect, these neurocognitive deficits are significantly related to poorer occupational and educational functioning, as patients with schizophrenia or bipolar depression struggle with navigating vocational or educational responsibilities [61]. Taken together, the consensus suggests there are significant trait-like core neurocognitive deficits in schizophrenia and bipolar depression that are not merely impacted by psychosis or mood states. Nevertheless, despite low cognitive reserve, when studying their response to ECT there is no convincing evidence at this time to suggest that symptom severity, baseline neurocognitive performance, or neuroleptic type and dosage contribute to the cognitive side effect profile in ECT for patients with schizophrenia or bipolar depression.
When examining the potential neurocognitive side effect of ECT in refractory schizophrenia, to date there are three main findings. The first is that ECT is generally deemed safe and efficacious. Results from recent studies suggest that the combination of bilateral ECT and antipsychotics is a useful and safe strategy for the treatment of refractory schizophrenia that leads to improvement of psychosis, quality of life, and social functioning [62–64]. Responders to ECT tended to be younger, with a shorter duration of illness, and lower baseline negative symptoms [65, 66]. However, these characteristics were not related to cognitive side effects. In a thorough review of 42 articles, Braga and Petrides [67] concluded that although no definitive conclusion about the combination of antipsychotics and ECT could be reached, the existing literature indicated that the combination was effective and the type and dosage of antipsychotics did not moderate any cognitive side effects from ECT. The second is that assessments of cognitive side effect profiles used in most of these studies have not been comprehensive. Many of these studies were retrospective (pulled data from existing medical records), and hence were limited to general and relatively insensitive measures such as the Mini Mental State Examination (MMSE), which is particularly problematic as it does not adequately measure the most common cognitive side effect of ECT (e.g., retrograde memory). The third is that in the few small studies that used more sensitive memory tests, the memory decline seemed to be transient. In the most comprehensive review to date, a 2005 Cochrane meta-analysis included 50 reports drawn from 26 separate trials [68]. This analysis indicated that there was very limited data to indicate that visual or verbal memory might decline after ECT in schizophrenia regardless of how antipsychotics were combined with ECT or the severity of psychosis. These findings were observed in both unilateral and bilateral ECT, and there was no indication that more treatments (i.e., 12 vs. 2) further impacted memory function.
There is evidence to suggest that maintenance electroconvulsive therapy (M-ECT) also has few cognitive sequelae in schizophrenia [69–71]. Rami et al. [72] conducted a small but well-controlled study examining the cognitive profile of schizophrenia patients undergoing M-ECT. Ten schizophrenia patients treated with M-ECT were matched for diagnosis, sex, and age with ten schizophrenia patients who had never been treated with ECT. Patients were treated with the standard bitemporal electrode placement. There was no significant difference on any cognitive measure between M-ECT patients and the control group, and no significant correlation was found between number of previous ECT treatments and any cognitive measure. In essence, patients with schizophrenia undergoing M-ECT could not be distinguished from matched patients on any of the cognitive measures. All in all, evidence for ECT in schizophrenia suggests the addition of ECT for patients who show limited response to antipsychotic medication may be considered an option with relatively few cognitive sequelae, although more controlled trials using comprehensive memory tests are necessary.
When examining the potential neurocognitive side effects of ECT in refractory bipolar depression, conclusions are more tenuous, mainly because there are so few studies that look exclusively at bipolar depression. Studies tend to include MDD and retrospectively separate out unipolar and bipolar depression. Also, we see the same limitation as in studies in schizophrenia--many studies are retrospective and use neither formal cognitive testing nor a specific measure of retrograde amnesia, relying instead on the MMSE [71, 73].
Culling together all the studies and reviews of ECT for bipolar depression, two main tentative conclusions emerge. One, it appears that patients with bipolar depression respond as well to ECT as patients with unipolar depression, with no difference in mental status change on the MMSE from either unilateral or bilateral ECT [74]. In cases where there were memory impairments following ECT such as the ability to recall names or retrieve recently acquired concepts, similarly as was found in schizophrenia, the decline was transient and disappeared within 6 months of ECT discontinuation regardless of antidepressant or depression severity. Two, stimulus dose relative to seizure threshold (SDRST) may be the best moderator of cognitive impairments associated with ECT in bipolar depression. In one of the most well-designed trials that examined the efficacy and side effects of ECT in unipolar and bipolar depression and fixed high dose versus titrated dose right unilateral (RUL) ECT, McCall et al. [75] found that SDRST explained the variance in ECT-related cognitive disturbance even after accounting for age, sex, and absolute stimulus intensity. Change on the MMSE was much more significant in the fixed dose group relative to the titrated dose group, while the fixed high dose group recalled a smaller percentage of autobiographical memories after ECT. In summary, although there have been few clinical investigations that conducted a formal review or meta-analysis of ECT exclusively in bipolar depression, the current perspective seems to be that ECT dose parameters may moderate memory more so than clinical features related to bipolar versus unipolar depression.
Electroconvulsive Therapy Treatment Technique and Stimulation Parameters
The most commonly studied ECT treatment technique and stimulation parameters with regard to neurocognitive outcome have been electrode placement, stimulus waveform, and stimulus train duration and frequency.
The first electrode placement used for stimulus delivery was bitemporal, followed by right unilateral and then bifrontal placement. Clinical research has consistently found that unilateral relative to bitemporal electrode placement has less neurocognitive adverse effects [76, 77]. Prior investigations have found that right unilateral relative to bilateral results in quicker time to reorientation [78], and less anterograde and retrograde amnesia. Also, elderly patients treated with right unilateral compared to those treated with bilateral placement ECT showed improved immediate recall of verbal information and better preservation of autobiographical information [79]. A recent meta analysis found significant advantages for unilateral relative to bilateral electrode configuration in the neurocognitive domains of long-term verbal and visual recall memory, and verbal paired associate memory [11]. There has been limited investigation with conflicting results of the neurocognitive effects of bifrontal placement. One study found that bifrontal placement had greater neurocognitive safety advantages relative to both bitemporal and right unilateral placement, but a recent a study [80] found no significant neurocognitive differences among the electrode placements. Sienaert et al. [81] also found equivalent neurocognitive effects between right unilateral and bifrontal electrode configuration when both were administered with ultrabrief pulse width. A recent systematic review and meta-analysis suggested that bifrontal placement may have less effects on memory functions relative to bitemporal or right unilateral, but that further research was warranted to characterize the full range of clinical, neurocognitive and functional outcomes [82]. A relatively new electrode configuration, focally electrical administered seizure therapy (FEAST), which combines asymmetric electrodes with a unidirectional stimulus train, may have beneficial implications for both clinical efficacy and neurocognitive outcome [83]. Findings in a pilot clinical study [84] suggested that FEAST resulted in quick reorientation after each treatment, and preserved global cognitive functioning and recollection of autobiographical information. Another experimental electrode placement, frontomedial, is even more effective in focusing the induced electric field in anterior frontal regions and sparing the hippocampus from electric field exposure [85]. Frontomedial ECT is in the early stags of clinical testing [86].
There have been three primary waveforms used in clinical ECT practice including sine wave, brief pulse (bidirectional, rectangular pulse width between 0.5–2 ms), and ultrabrief pulse (bidirectional, rectangular pulse width less than 0.5 ms). Sine wave was the first stimulus wave form used in ECT [87]. Though a preclinical study in mice found equivalent effects on neurocognitive function between sine wave and brief pulse width [88], clinical research has suggested that sine wave produces worse neurocognitive outcome. For instance, early work by Weiner et al. [89] found that patients treated with sine wave ECT versus brief pulse ECT showed significantly poorer performance on neurocognitive measures of verbal learning and memory, complex visuospatial recall, impersonal memory for famous events, and autobiographical memory recall. More recently, in a community based study, Sackeim et al. [14] found that patients treated with sine wave relative to those treated with brief or ultrabrief pulse width ECT showed significantly decreased global cognitive function, processing speed, sustained attention, and recall of autobiographical memory. Those two studies provide conclusive evidence that sine wave results in deleterious neurocognitive effects (note that ECT devices are no longer made with sine wave capability). For current clinical ECT practice, clinicians use both brief and ultrabrief pulse waveform. Clinical evidence has suggested that ultrabrief relative to brief pulse waveform may have neurocognitive advantages. For example, patients treated with ultrabrief wave form have been found to show faster reorientation time [90] and less anterograde amnesia for verbally learned information and retrograde amnesia for autobiographical information [91]. Specific to autobiographical memory, six months after completion of acute course ECT, patients treated with ultrabrief relative to those treated with brief pulse, showed better preservation of autobiographical information [14]. Further, as measured on the Kopelman Autobiographical Memory Interview, patients treated with ultrabrief pulse ECT showed improved recall of early childhood semantic memories, whereas patients treated with brief pulse ECT showed decreased recall of autobiographical information [92]. While most studies support the neurocognitive advantages of ultrabrief relative to brief pulse ECT, a recent study [93] suggested that they may be equivalent in their effects on neurocognitive outcome.
In addition to electrode placement and stimulus shape and pulse width, other technical factors that contributes to neurocognitive outcome are the stimulus train parameters (e.g., train duration and frequency). The commonly used seizure threshold titration procedure for determining ECT dosage adjusts the train duration and/or frequency, while holding fixed the pulse width and pulse amplitude. Treatment dose is then determined by multiples of the initiation titrated seizure threshold. Squire et al. [94] noted that the amount of charge used to generate the seizure may be related to the adverse neurocognitive effects. Holding the pulse width and amplitude constant, McCall et al. [75] and Sackeim et al. [78] found that patients treated with higher energy doses relative to those with lower doses showed longer times to reorientation. A meta analytic study found a significant negative association between electrical dose and learning and delayed recall of verbal information [95]. Andrade and Bolwig postulated that higher ECT associated dosage may result in a hypertensive surge that breaches the blood brain barrier, which then leads to adverse neurocognitive effects. Indeed, electrical dose, as determined by titration in the stimulus train duration/frequency domain, is an important consideration in the provision of ECT. Computational modeling has suggested that dose is dependent on a combination of multiple factors including unique ECT stimulus parameters (e.g., pulse shape, pulse amplitude, pulse width, train frequency and duration) and electrode configuration [96–99].
The major driver of focality of stimulation is the amplitude of the stimulus pulse (current, measured in milliamperes). Traditionally, pulse current amplitdute is kept at 0.8 or 0.9 Amps for all patients. Recent work suggests that seizures can be induced with much lower current amplitudes, and that this simple manipulation may be a powerful means of increasing the cognitive safety of seizure therapy. This concept is well illustrated in the case of magnetic seizure therapy (MST) which induces seizures with much lower induced current levels than ECT[100], and which has been demonstrated to induce markedly lower cognitive side effects than even ultrabrief pulse RUL ECT [101, 102]. Besides using magnetic induction, the current amplitude of ECT can be lowered. Commercial devices allow the current to be lowered to 500 mA, and preliminary studies show that seizures can be induced at that level. Pre-clinical and computational modeling work suggests that going even lower, and individualizing current amplitude for each subject may be an effective way of sparing brain regions important for cognition from unnecessary field exposure. [85, 100, 103, 104]. In addition, current amplitude adjustment could be a means of compensation for interindividual anatomical variation, thus reducing variability in clinical outcome [97].
In clinical practice, ECT parameters are used together to generate a therapeutic seizure, thus combinations of select parameters may generate differential neurocognitive effects. Collectively, clinical evidence at this time has suggested that the combination of sine wave form and bitemporal electrode configuration may result in the worse neurocognitive outcome, and a combination of ultra brief pulse wave form and right unilateral or more focal electrode configuration may be relatively more cognitively benign. Further research is warranted to guide evidence-based practice in the section of optimal ECT parameter combinations.
Underlying Neurophysiological Factors
While ECT results in neurocognitive impairment, the mechanisms of action remain unknown [12]. To date, no clinical investigation has demonstrated a direct link between underlying neurophysiological changes related to ECT and observed neurocognitive outcome. Most research has focused on the effects of ECT on cortical structural, functional, and neural dynamic changes [105]. As ECT has been most associated with impaired memory functions, most studies have centered on ECT associated structural changes to the hippocampus. In brief, preclinical ECT models have been found to be safe due to the absence of histological lesions in cortical and hippocampal regions [106]. Ende et al. [107] found no changes in the hippocampus secondary to ECT, and particularly noted an absence of atrophy or cell death. Likewise, Scalia et al. [108] reported on the neuropathological examination of a 92 year old woman following a total of 91 ECT treatments lifetime and found no evidence of pathology attributable to ECT. On the other hand, preclinical research found that ECT produced neurogenesis in the hippocampus, particularly in the dentate gyrus [109]. Also, clinical research found increased hippocampal volume secondary to ECT [110] that returns to baseline within 6-months, but that change was unassociated with both clinical and neurocognitive outcome [111]. Dukart et al. [112] found significant increased gray matter volume in the right hemisphere hippocampal complex and subgenual cortex after right unilateral ECT. Also, they found significant decreased gray matter volume in the prefrontal cortex. Those specific changes in gray matter volume were associated with decreased depression severity.
Regarding function and connectivity effects, Perrin et al. [113] found that ECT decreased regulation of connectivity in key neural circuits including medial cortex structures (i.e., anterior cingulate), dorsolateral prefrontal cortex, supermarginal gyrus, angular gyrus, and the somatosensory association cortex. The decreased neural connectivity occurred in conjunction with decreased depression severity. However, the study reported no formal statistical association between change in neural connectivity and clinical outcome, nor was there any report of change in neurocognitive function. Bealle et al. [114] found normalization of GABA levels and significantly decreased blood oxygenation level dependent contrast in the orbitofrontal cortex after ECT, which may have been associated with changes in both depression severity and working memory. However, there was no neurocognitive information collected before and after the ECT course, which limited the analysis of neurocognitive moderating factors. For further information, see a recent systematic review by Abbott et al. [115] on ECT effects on cortical structures.
Regarding neural dynamics, clinical research found an association between changes in neural oscillation and antidepressant outcome with ECT. Specifically, midictal amplitude and postictal suppression on electroencephalography (EEG) were associated with a greater therapeutic response to ECT [116]. However, research to find a link between changes in neural oscillations and neurocognitive outcome associated with ECT has been inconclusive [117], with the exception of one study [118]. That study evaluated changes in resting-state background EEG during the ECT course and found that increased delta and theta power in the anterior frontal temporal region were associated with disorientation, and that the ratio of delta and theta power was associated with decreased global cognitive function. Those findings have yet to be replicated; however, they are consistent with work indicating that increased delta and theta EEG activity in the resting-state background EEG serve as biomarkers that distinguish elderly adults with normal neurocognitive function from those with mild cognitive impairment or Alzheimer’s disease [119, 120]. Those prior ECT studies may have been limited by the available EEG technology, which included a limited number of recording channels (e.g., 19) that resulted in poor spatial resolution [121] and insufficient analytic methods [122].
Preclinical rodent models found that ECT disrupted long term potentiation (LTP), a mechanism for learning and memory, through its increase in cortical excitability [123–125]. For example, Hesse and Teyler [124] showed that low frequency electroconvulsive shock stimuli temporarily disrupted in part or in whole LTP in the CA1 and CA3 hippocampal regions. Importantly, they also showed that LTP was re-established with additional low frequency electroconvulsive shock stimuli. In rodent hippocampal slices, Moore et al. [126] found an inverse relationship between electrical stimulation time and magnitude of LTP in the CA1 and CA3 regions, but that neuroplasticity returned to baseline 1 hour after cessation of the electrical stimulation. Taking that study one step further, Barr et al. [125] studied different electrical stimulation frequencies and their effects on LTP in rodent hippocampal slices. They found that high frequency theta burst stimulation did not induce, but rather inhibited durability of LTP. These findings may provide a mechanistic link as to how ECT results in transient disruption of neurocognitive functions. Indeed, at 0.8–0.9 Amps, the train of pulses given during ECT represents a tetanic stimulus of nearly the entire brain, based on our modeling work [127], which would be expected to saturate LTP in hippocampus and other brain regions globally.
Those preclinical findings are concordant with clinical research by Squire et al. [128] who found that the neurocognitive process of consolidation was immediately disrupted after the ECT induced seizure. This disruption in consolidation may follow a similar time course as the disruption and normalization of LTP. More recent clinical research [129] showed that ECT also disrupted the neurocognitive process of reconsolidation. Specifically, after a single ECT session, patients were unable to recall emotional valenced stories that had been reactivated. A recent study by Casarotto et al. [130] may provide useful information that connects ECT to altered neural dynamics that underlie changes in cognitive function. In that study, the authors found that depressed humans treated with ECT showed increased cortical excitability in the fronto-temporal cortices, which is indicative of synaptic potentiation. As found in ECT preclinical models, such cortical excitability may produce adverse neurocognitive effects because it blocks hippocampal and neocortical long-term potentiation (LTP) through saturation [123, 124, 131]. Unfortunately, Casarotto et al. did not conduct neurocognitive assessments and thus was unable to show a direct link between changes in both neurocognitive function and neurophysiology. Thus, further research is warranted to provide a direct link between ECT associated changes in neural dynamics and neurocognitive outcome.
Synthesizing the Multiple Factors into a Coherent Model and Future Directions
Though neuroimaging research [17] suggests that most cortical and subcortical regions are involved in ECT associated neurocognitive impairment, no conceptual model exists of how the cognitive effects of ECT develop in patients with MDD. Adding further complication, standard clinical ECT practice does not incorporate neurocognitive measurements to assess adverse neurocognitive effects, which means that ECT clinical decisions are made without such critical information [132, 133]. Thus, the field is at the initial stage of model construction. We have synthesized available information from preclinical and clinical investigations to form a coherent model (see Figure 1) that provides a link between ECT associated changes in neural mechanisms and neurocognitive outcome. This model takes into account multiple factors (see Table 1) including demographic and neuropsychological characteristics, neuropsychiatric symptoms, ECT technical parameters, and ECT associated neurophysiological changes. In this model, moderating factors include the demographic, neuropsychological, and neuropsychiatric variables. The mediating factors include the ECT parameters, associated neurophysiological changes, and associated changes in clinical symptoms.
Table 1.
Factors that May Affect the Neurocognitive Effects of Electroconvulsive Therapy
| Factor | Variable |
|---|---|
| Demographic and Neuropsychological Factors |
|
| Neuropsychiatric Factors |
|
| Electroconvulsive Therapy Treatment Technique and Stimulation Parameters1 |
|
| Neurophysiological Factors |
|
see Peterchev et al. [96] for a comprehensive review of electroconvulsive therapy stimulus parameters.
This model can be used to guide the necessary variables (e.g., cohort composition, neurocognitive outcome variables) to address specific hypotheses and to help identify which variable constructs act as moderators, mediators, and in rare cases, both. As such, both clinicians and researchers can begin to implement measurement-based care by documenting the neuropsychiatric symptomatology and neurocognitive functional status both at baseline and after completion of ECT. The neuropsychiatric symptoms can be documented with semi-structured diagnostic interviews and both observer-rated and self-rated neuropsychiatric symptom severity inventories. Regarding neurocognitive function and outcome, these can be characterized with a variety of neurocognitive tools. For instance, Porter et al. [132] recommended a neurocognitive battery comprised of measures of global cognitive function, verbal learning and memory, autobiographical memory recall, psychomotor processing speed, and orientation should be administer before and after the acute ECT course. Given the moderating effects of premorbid intellectual function [22], such a measure should also be included in the neurocognitive battery. Also, as neuropsychiatric diseases could negatively impact cognitive processing speed and attention, two neurocognitive functions that underscore higher-order cognitive processes, those too should be measured. Recently, Martin et al. [134] found that a very brief battery of cognitive measures was feasible to administer after each ECT session that provided useful information regarding immediate change in neurocognitive function.
This novel model establishes a framework to conduct future investigations to directly test hypotheses about the association between ECT and changes in neurocognitive function, with a direct examination of both moderating and mediating factors. For instance, an important question that can be addressed with the model and the collection of the above information is the complex relationship between the rapid change in clinical outcome (e.g., response, remission) associated with ECT and neurocognitive status. For instance, patients with neuropsychiatric disease often present with inefficient, or at times, impaired neurocognitive abilities. Thus, treatment of the disease to remission could result in normalization of those neurocognitive functions. However, this is not always the case. In elderly adults, neurocognitive impairment may result in the depressive syndrome, and certain cognitive domains including memory and executive function may remain impaired, despite the absence of depressive symptoms [135]. Both Manning et al. [136] and Morimoto et al. [137] found that elderly adults with executive dysfunction were slower to benefit from antidepressant medication. These findings suggest that neurocognitive deficits may moderate the clinical outcome. A recent meta-analysis substantiated a link between executive dysfunction and depression, but was unable to specify causality [138].
Across the adult life span, there appears to be a disconnect between change in clinical outcome and neurocognitive status associate with a spectrum of antidepressant therapies. Greer et al. [139] found that after treatment with duloxetine, patients with depression showed improved performance on measures of cognitive processing speed, affective decision making, and both verbal and visual memory, which was independent of clinical outcome. A recent meta-analysis further affirmed that antidepressant pharmacotherapy may have specific cognitive enhancing properties that are independent of clinical effects [140]. Antidepressant treatment with deep brain stimulation (DBS) applied to the subcallosal cingulate, change in clinical outcome in patients with unipolar or bipolar depression was unrelated to stability or change in cognitive outcome [141]. Prior research with ECT too has suggested that change in clinical outcome is unrelated to change in neurocognitive status [22, 142, 143]. A permutation of ECT antidepressant strategy that has received limited research is its combination with psychotropic medication. Sackeim et al. [144] found differential clinical and neurocognitive effects depending on the antidepressant medication that was combined with RUL ECT. For instance, the combination of ECT with nortriptyline resulted in better efficacy and less neurocognitive adverse effects, but combination with venlafaxine resulted in slightly reduced clinical efficacy and a greater degree of cognitive adverse effects. As there is inconsistent information within varying combinations of ECT practice, continued examination of the relationship between clinical efficacy and neurocognitive function after ECT is warranted and can be tested with the proposed model.
The model is also useful to test direct hypotheses of causality. One such causal explanation (see Figure 2) of how ECT results in neurocognitive adverse effects is that it temporarily disrupts LTP [124]. That disruption in LTP then leads to decreased learning and recall of information during that specific time period in which LTP was disrupted. The model provides the framework to test the novel hypothesis that ECT may increase cortical excitability in distinct cortical regions that leads to disrupted LTP, which underscores the transient impairment in learning and memory. To maximize internal validity, the neuropsychiatric illness would be MDD, and the ECT treatment parameters (e.g., RUL, ultra-brief pulse, dose titrated) would be fixed. The clinical variables would be documented with depression symptom severity scales and diagnostic instruments, and the change in memory function would be objectively rated on standardized neurocognitive metrics. The neurophysiological changes would be assessed with EEG, to document neural activity before, during, and after the seizure. Integrated EEG and TMS, as demonstrated by Casarotto et al. [130], could be used to examine ECT associated alterations in cortical excitability and LTP.
Figure 2. Proposed Causal Model of How Electroconvulsive Therapy Affects Memory Function.
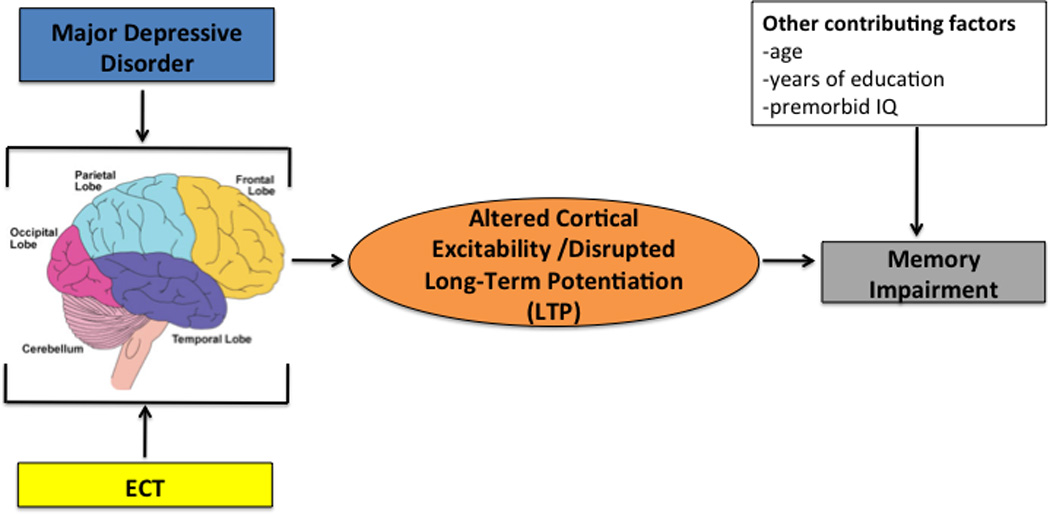
This figure shows a causal model of how electroconvulsive therapy negatively impacts memory function in patients with major depressive disorder through its intermediate alterations in cortical excitability and disruption of long-term potentiation (LTP).
ECT=Electroconvulsive Therapy
Through addressing this important question of how ECT results in transient disruption of neurocognitive functions, we can then devise preventive, as well as cognitive remediation (CR) strategies. Given the current limited understanding of underlying neural mechanisms at this time, it is prudent to begin development of CR strategies. Cognitive remediation is a programmatic, evidence-based behavioral treatment that was initially developed in the early 1960s to treat cognitive impairments associated with acquired brain injury [145]. Since then, CR has been widely studied and applied in various neurologic and neuropsychiatric disorders including stroke [146], dementia [147], and schizophrenia [148–150]. Also, there is emerging literature on the efficacy of CR in ameliorating memory deficits associated with seizure disorder [151]. Although ECT-related memory impairments are not identical to the neuropsychological sequelae of seizure disorder, overall, the neurocognitive pattern of ECT-related memory impairments can be fairly consistent with memory loss associated with temporal lobe epilepsy (TLE), with possible disruption of storage and/or retrieval processes dependent on the hippocampus, parahippocampal gyrus, and related diencephalic structures [152, 153]. Of relevance is that ECT and TLE patients show preserved priming, skill acquisition, and other types of procedural memory abilities. The disturbance is one of memory consolidation and/or retrieval, with strong evidence that retrograde amnestic effects of ECT may have frontal lobe involvement [154, 155].
Given the similarities between epilepsy and ECT-induced seizures, techniques and basic research theories on the neuropsychological rehabilitation of epilepsy may be useful to the remediation of memory deficits in ECT. The most pertinent factors associated with cognitive recovery in epilepsy (and presumably ECT) seem to be (a) intervening pre and post-ictally, with CR applied as proximal to the induced seizure episode as possible, (b) engaging patients to achieve adequate treatment dosage of cognitive training pre and post-ictally, (c) incorporating psycho-education to inform the patient about effects of depression and ECT on memory, and (d) providing precise memory training targeting retrieval and consolidation deficits [152, 155]. Although these guiding principles have been somewhat successful in reducing cognitive impairments associated with TLE, CR techniques for memory training in seizures have only recently been applied to help patients regain their memory after ECT. To date, there is only a single published CR study in ECT. Choi et al. [156] designed and piloted a novel memory training program specifically tailored to target the neurocognitive effects of ECT based on findings from basic and experimental paradigms for memory consolidation and retrieval in TLE. The training program was designed to specifically target anterograde and retrograde memory that may be compromised following ECT, and to help patients regain their general memory skills immediately following ECT. Compared to patients randomized to an active control (puzzles), those who received memory training had significantly greater recovery of retrograde and anterograde memory following RUL ECT.
While the above finding is certainly encouraging, it is the first such trial in the emergent field of behavioral interventions targeting cognitive adverse effects associated with ECT. Similar to the stage of empirical research in combined ECT and psychotherapy [157], additional large and well-designed trials of CR for ECT are needed to more definitively examine the efficacy of such interventions, along with their precise mechanisms of action. The ultimate goal of this line of research is to develop a safe and effective behavioral strategy to minimize the potential adverse memory side effects of ECT so that ECT may be a more easily tolerated treatment for patients who need this therapeutic option.
In summary, ECT is an invaluable and highly effective neurotherapeutic intervention, that also results in adverse neurocognitive effects. While those neurocognitive effects and underlying mechanisms of action of ECT remain poorly understood, we propose the synthesis of multiple moderating and mediating factors into a coherent, and testable model. Future research is warranted to evaluate, test, and revise this model as needed. The generated information could be used to guide both clinical ECT practice, inform the development of new seizure therapies to reduce cognitive risk, and develop cognitive remediation strategies to improve long-term outcomes.
Acknowledgments
This manuscript was supported in part by grants from the National Institutes of Health / National Institute of Mental Health (K23 MH087739 and K23 MH086755).
References
- 1.Rush AJ, et al. Bupropion-SR, Sertraline, or Venlafaxine-XR after Failure of SSRIs for Depression. New England Journal of Medicine. 2006;354(12):1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 2.Sachs GS, et al. Effectiveness of Adjunctive Antidepressant Treatment for Bipolar Depression. New England Journal of Medicine. 2007;356(17):1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JA, et al. Effectiveness of Antipsychotic Drugs in Patients with Chronic Schizophrenia. New England Journal of Medicine. 2005;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 4.Lisanby SH. Electroconvulsive Therapy for Depression. New England Journal of Medicine. 2007;357:1939–1945. doi: 10.1056/NEJMct075234. [DOI] [PubMed] [Google Scholar]
- 5.Weiner RD, et al. Electroconvulsive therapy device classification: Response to the FDA advisory panel hearing and recommendations. Journal of Clinical Psychiatry. 2013;74(1):38–42. doi: 10.4088/JCP.12cs08260. [DOI] [PubMed] [Google Scholar]
- 6.Leiknes KA, Schweder LJ, Hoie B. Contemporary use and practice of electroconvulsive therapy worldwide. Brain and Behavior. 2012;2(3):283–345. doi: 10.1002/brb3.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husain MM, et al. Speed of response and remission in major depressive disorder with acute Electroconvulsive therapy (ECT): A consortium for research in ECT (CORE) report. Journal of Clinical Psychiatry. 2004;65(4):485–491. doi: 10.4088/jcp.v65n0406. [DOI] [PubMed] [Google Scholar]
- 8.Oudega ML, et al. White matter hyperintensities and cognitive impairment during electroconvulsive therapy in severely depressed elderly patients. American Journal of Geriatric Psychiatry. 2014;22(2):157–166. doi: 10.1016/j.jagp.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Weiner RD, Prudic J. Electroconvulsive Therapy in the United States: How Often Is It Used? Biological Psychiatry. 2013;73(2):105–106. doi: 10.1016/j.biopsych.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Manly DT, Oakley SP, Bloch RM. Electroconvulsive therapy in old-old patients. American Journal of Geriatric Psychiatry. 2000;8(3):232–236. [PubMed] [Google Scholar]
- 11.Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: A systematic review and meta-analysis. Biological Psychiatry. 2010;68:568–577. doi: 10.1016/j.biopsych.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 12.McClintock S, Staub B, Husain M. The effects of electroconvulsive therapy on neurocognitive function in elderly adults. Annals of Long-Term Care: Clinical Care and Aging. 2011;19(3):32–38. [Google Scholar]
- 13.Squire LR, Slater PC, Chace PM. Retrograde amnesia: Temporal gradient in very long term memory following electroconvulsive therapy. Science. 1975;187(4171):77–79. doi: 10.1126/science.1109228. [DOI] [PubMed] [Google Scholar]
- 14.Sackeim HA, et al. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology. 2007;32(1):244–54. doi: 10.1038/sj.npp.1301180. [DOI] [PubMed] [Google Scholar]
- 15.Squire LR, Miller PL. Diminution of anterograde amnesia following electroconvulsive therapy. British Journal of Psychiatry. 1974;125:490–495. doi: 10.1192/bjp.125.5.490. [DOI] [PubMed] [Google Scholar]
- 16.Fraser LM, O'Carroll RE, Ebmeier KP. The effect of electroconvulsive therapy on autobiographical memory: A systematic review. Journal of ECT. 2008;24(1):10–17. doi: 10.1097/YCT.0b013e3181616c26. [DOI] [PubMed] [Google Scholar]
- 17.Nobler MS, Sackeim HA. Neurobiological correlates of the cognitive side effects of electroconvulsive therapy. Journal of ECT. 2008;24(1):40–45. doi: 10.1097/YCT.0b013e31815d6957. [DOI] [PubMed] [Google Scholar]
- 18.Millis SR, et al. Long-term neuropsychological outcome after traumatic brain injury. Journal of Head Trauma Rehabilitation. 2001;16(4):343–355. doi: 10.1097/00001199-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Stern Y, et al. Influence of education and occupation on the incidence of Alzheimer's Disease. JAMA. 1994;271(13):1004–1010. [PubMed] [Google Scholar]
- 20.Glatt SL, et al. Risk factors for dementia in Parksinon's Disease: Effect of education. Neuroepidemiology. 1996;15:20–25. doi: 10.1159/000109885. [DOI] [PubMed] [Google Scholar]
- 21.Squire LR, Chace PW. Memory functions six to nine months after electroconvulsive therapy. Archives of General Psychiatry. 1975;32(12):1557–1564. doi: 10.1001/archpsyc.1975.01760300095008. [DOI] [PubMed] [Google Scholar]
- 22.Bayless JD, et al. Pre- and post-electroconvulsive therapy multidomain cognitive assessment in psychotic depression: Relationship to premorbid abilities and symptom improvement. Journal of ECT. 2010;26(1):47–52. doi: 10.1097/YCT.0b013e3181ac8ec2. [DOI] [PubMed] [Google Scholar]
- 23.Legendre SA, et al. The influcence of cognitive reserve on memory following electroconvulsive therapy. Journal of Neuropsychiatry & Clinical Neurosciences. 2003;15:333–339. doi: 10.1176/jnp.15.3.333. [DOI] [PubMed] [Google Scholar]
- 24.Garrett KD, et al. The neuropsychological profile of vascular cognitive impairment—no dementia: comparisons to patients at risk for cerebrovascular disease and vascular dementia. Archives of Clinical Neuropsychology. 2004;19(6):745–757. doi: 10.1016/j.acn.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 25.DeCarli C, et al. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Archives of Neurology. 2001;58(4):643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 26.Roman GC, et al. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. Journal of the Neurological Sciences. 2004;226(1):2, 81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulos GS, et al. 'Vascular Depression' Hypothesis. Archives of General Psychiatry. 1997;54(10):915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin RC, O'Brien J. Vascular basis of late-onset depressive disorder. British Journal of Psychiatry. 2002;180:157–160. doi: 10.1192/bjp.180.2.157. [DOI] [PubMed] [Google Scholar]
- 29.Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18(9):963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brodaty H, et al. A prospective follow-up study of ECT outcome in older depressed patients. Journal of Affective Disorders. 2000;60(2):101–111. doi: 10.1016/s0165-0327(99)00169-x. [DOI] [PubMed] [Google Scholar]
- 31.Verwijk E, et al. Short- and long-term neurocognitive functioning after electroconvulsive therapy in depressed elderly: A prospective naturalistic study. International Psychogeriatrics. 2014;26(2):315–324. doi: 10.1017/S1041610213001932. [DOI] [PubMed] [Google Scholar]
- 32.McClintock SM, et al. Association between depression severity and neurocognitive function in major depressive disorder: A review and synthesis. Neuropsychology. 2010;24(1):9–34. doi: 10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- 33.Papakostas GI. Cognitive symptoms in patients wtih major depressive disorder and their implications for clinical practice. Journal of Clinical Psychiatry. 2014;75(1):8–14. doi: 10.4088/JCP.13r08710. [DOI] [PubMed] [Google Scholar]
- 34.Massman PJ, et al. The subcortical dysfunction hypothesis of memory deficits in depression: Neuropsychological validation in a subgroup of patients. Journal of Clinical and Experimental Neuropsychology. 1992;14(5):687–706. doi: 10.1080/01688639208402856. [DOI] [PubMed] [Google Scholar]
- 35.Mesholam-Gately R, et al. Verbal learning and memory in older adults in minor and major depression. Archives of Clinical Neuropsychology. 2012;27:196–207. doi: 10.1093/arclin/acr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butters MA, Whyte E, Nebes RD. The nature and determinants of neuropsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 37.McClintock SM, et al. Evaluation of the effects of depression severity on global cognitive function and memory. CNS Spectrums. 2010;15(5):304–313. doi: 10.1017/s109285290002753x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trichard C, et al. Time course of prefrontal lobe dysfunction in severely depressed in-patients: A longitudinal neuropsychological study. Psychological Medicine. 1995;25:79–85. doi: 10.1017/s0033291700028105. [DOI] [PubMed] [Google Scholar]
- 39.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders. 2009;119(1):3, 1–8. doi: 10.1016/j.jad.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Paradis AD, et al. Major depression in the transition to adulthood: The impact of active and past depression in young adult functioning. The Journal of Nervous and Mental Disease. 2006;194:318–323. doi: 10.1097/01.nmd.0000217807.56978.5b. [DOI] [PubMed] [Google Scholar]
- 41.Kessing LV. Cognitive impairment in the euthymic phase of affective disorder. Psychological Medicine. 1998;28:1027–1038. doi: 10.1017/s0033291798006862. [DOI] [PubMed] [Google Scholar]
- 42.Stordal KI, et al. Impairment across executive functions in recurrent major depression. Nordic Journal of Psychiatry. 2004;58:41–47. doi: 10.1080/08039480310000789. [DOI] [PubMed] [Google Scholar]
- 43.Heaton RK, et al. Wisconsin Card Sorting Test Manual: Revised and Expanded. Lutz, FL: Psychological Assessment Resources, Inc.; 1993. [Google Scholar]
- 44.Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: Evidence of modest impairment. Biological Psychiatry. 2001;50:35–43. doi: 10.1016/s0006-3223(00)01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elliott R. The neuropsychological profile in unipolar depression. Trends in Cognitive Sciences. 1998;2:447–454. doi: 10.1016/s1364-6613(98)01235-2. [DOI] [PubMed] [Google Scholar]
- 46.Michopoulos I, et al. Neuropsychological and hypothalamic–pituitary-axis function in female patients with melancholic and non-melancholic depression. European archives of psychiatry and clinical neuroscience. 2008;258(4):217–225. doi: 10.1007/s00406-007-0781-8. [DOI] [PubMed] [Google Scholar]
- 47.Basso MR, Bornstein RA. Neuropsychological deficits in psychotic versus nonpsychotic unipolar depression. Neuropsychology. 1999;13(1):69–75. doi: 10.1037//0894-4105.13.1.69. [DOI] [PubMed] [Google Scholar]
- 48.McGirr A, et al. Deterministic learning and attempted suicide among older depressed individuals: Cognitive assessment using the Wisconsin Card Sorting Task. Journal of Psychiatric Research. 2012;46:226–232. doi: 10.1016/j.jpsychires.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naismith SL, et al. Sleep disturbance relates to neuropsychological functioning in late-life depression. Journal of Affective Disorders. 2011;132(1–2):139–145. doi: 10.1016/j.jad.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 50.Naismith SL, et al. Does sleep disturbance mediate neuropsychological functioning in older people with depression? Journal of Affective Disorders. 2009;116(1–2):139–143. doi: 10.1016/j.jad.2008.11.017. [DOI] [PubMed] [Google Scholar]
Supplemental References
- 51.Hickie I, et al. Prediction of ECT response: validation of a refined sign-based (CORE) system for defining melancholia. The British Journal of Psychiatry. 1996;169(1):68–74. doi: 10.1192/bjp.169.1.68. [DOI] [PubMed] [Google Scholar]
- 52.Petrides G, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17(4):244–53. doi: 10.1097/00124509-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Nuechterlein KH, et al. Individual placement and support for individuals with recent-onset schizophrenia: integrating supported education and supported employment. Psychiatric Rehabilitation Journal. 2008;31(4):340–349. doi: 10.2975/31.4.2008.340.349. [DOI] [PubMed] [Google Scholar]
- 54.Corcoran C, et al. Trajectory to a first episode of psychosis: a qualitative research study with families. Early Intervention in Psychiatry. 2007;1(4):308–315. doi: 10.1111/j.1751-7893.2007.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophrenia Research. 2005;74(1):15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Fiszdon JM, et al. Impact of intellectual status on response to cognitive task training in patients with schizophrenia. Schizophrenia Research. 2006;87(1):3, 261–269. doi: 10.1016/j.schres.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Bowie CR, Harvey PD. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatric Clinics of North America. 2005;28(3):613–633. doi: 10.1016/j.psc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Bowie CR, et al. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. American Journal of Psychiatry. 2010;167(9):1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harvey PD, et al. Cognition and disability in bipolar disorder: lessons from schizophrenia research. Bipolar disorders. 2010;12(4):364–375. doi: 10.1111/j.1399-5618.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 60.Rubinsztein J, et al. Cognitive impairment in remission in bipolar affective disorder. Psychological medicine. 2000;30(05):1025–1036. doi: 10.1017/s0033291799002664. [DOI] [PubMed] [Google Scholar]
- 61.Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia Research. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Chanpattana W, Kramer BA. Acute and maintenance ECT with flupenthixol in refractory schizophrenia: Sustained improvements in psychopathology, quality of life, and social outcomes. Schizophrenia Research. 2003;63(1):189–193. doi: 10.1016/s0920-9964(02)00330-4. [DOI] [PubMed] [Google Scholar]
- 63.Chanpattana W, Somchai-Chakabhand ML. Combined ECT and neuroleptic therapy in treatment-refractory schizophrenia: Prediction of outcome. Psychiatry Research. 2001;105(1):107–115. doi: 10.1016/s0165-1781(01)00321-3. [DOI] [PubMed] [Google Scholar]
- 64.Chanpattana W, et al. Continuation ECT in treatment-resistant schizophrenia: A controlled study. Journal of ECT. 1999;15(3):178–192. [PubMed] [Google Scholar]
- 65.Saperstein AM, Fiszdon JM, Bell MD. Intrinsic motivation as a predictor of work outcome after vocational rehabilitation in schizophrenia. The Journal of nervous and mental disease. 2011;199(9):672–677. doi: 10.1097/NMD.0b013e318229d0eb. [DOI] [PubMed] [Google Scholar]
- 66.Tremeau F, et al. Inpatients with schizophrenia report impaired situational motivation but intact global and social motivation. Psychiatry Research. 2013;210(1):43–49. doi: 10.1016/j.psychres.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 67.Braga RJ, Petrides G. The combined use of electroconvulsive therapy and antipsychotics in patients with schizophrenia. Journal of ECT. 2005;21(2):75–83. doi: 10.1097/01.yct.0000165500.60784.05. [DOI] [PubMed] [Google Scholar]
- 68.Tharyan P, Adams CE. Electroconvulsive therapy for schizophrenia. Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD000076.pub2. [DOI] [PubMed] [Google Scholar]
- 69.Choi J, Fiszdon JM, Medalia A. Expectancy-value theory in persistence of learning effects in schizophrenia: role of task value and perceived competency. Schizophrenia bulletin. 2010;36(5):957–965. doi: 10.1093/schbul/sbq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Logsdon RG, et al. Quality of life in Alzheimer's disease: Patient and caregiver reports. Journal of Mental Health and Aging. 1999;5(1):21–32. [Google Scholar]
- 71.Russell JC, et al. Long-term maintenance ECT: a retrospective review of efficacy and cognitive outcome. The journal of ECT. 2003;19(1):4–9. doi: 10.1097/00124509-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 72.Rami L, et al. Absence of additional cognitive impairment in schizophrenia patients during maintenance electroconvulsive therapy. Schizophrenia Bulletin. 2004;30(1):185–189. doi: 10.1093/oxfordjournals.schbul.a007062. [DOI] [PubMed] [Google Scholar]
- 73.Pagnin D, et al. Efficacy of ECT in depression: a meta-analytic review. The journal of ECT. 2004;20(1):13–20. doi: 10.1097/00124509-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 74.Grunhaus L, et al. Response to ECT in major depression: are there differences between unipolar and bipolar depression? Bipolar Disorders. 2002;4(s1):91–93. doi: 10.1034/j.1399-5618.4.s1.40.x. [DOI] [PubMed] [Google Scholar]
- 75.McCall WV, et al. Titrated Moderately Suprathreshold vs Fixed High-Dose Right Unilateral Electroconvulsive Therapy: Acute Antidepressant and Cognitive Effects. Archives of General Psychiatry. 2000;57(5):438–444. doi: 10.1001/archpsyc.57.5.438. [DOI] [PubMed] [Google Scholar]
- 76.Sackeim HA, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. New England Journal of Medicine. 1993;328:839–846. doi: 10.1056/NEJM199303253281204. [DOI] [PubMed] [Google Scholar]
- 77.Squire LR, Slater PC. Bilateral and unilateral ECT: Effects on verbal and nonverbal memory. American Journal of Psychiatry. 1978;135(11):1316–1320. doi: 10.1176/ajp.135.11.1316. [DOI] [PubMed] [Google Scholar]
- 78.Sackeim HA, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425–34. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- 79.O'Connor DW, et al. Cognition in elderly patients receiving unilateral and bilateral electroconvulsive therapy: A prospective, naturalistic comparison. Journal of Affective Disorders. 2010;124:235–240. doi: 10.1016/j.jad.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 80.Kellner CH, et al. Bifrontal, bitemporal, and right unilateral electrode placement in ECT: randomized trial. British Journal of Psychiatry. 2010;196:226–234. doi: 10.1192/bjp.bp.109.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sienaert P, et al. Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: cognitive side-effects. Journal of Affective Disorders. 2010;122(1–2):60–67. doi: 10.1016/j.jad.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 82.Dunne RA, McLoughlin DM. Systematic review and meta-analysis of bifrontal electroconvulsive therapy versus bilateral and unilateral electroconvulsive therapy in depression. World Journal of Biological Psychiatry. 2012;13(4):248–258. doi: 10.3109/15622975.2011.615863. [DOI] [PubMed] [Google Scholar]
- 83.Spellman T, Peterchev AV, Lisanby SH. Focal electrically administered seizure therapy: a novel form of ECT illustrates the roles of current directionality, polarity, and electrode configuration in seizure induction. Neuropsychopharmacology. 2009;34(8):2002–2010. doi: 10.1038/npp.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nahas Z, et al. A feasibility study of a new method for electrically producing seizures in man: Focal electrically administered seizure therapy [FEAST] Brain Stimulation. 2013;6(3):403–408. doi: 10.1016/j.brs.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Lee WH, et al. Elecrtric field characteristics of electroconvulsive therapy with individualized current amplitude: A preclinical study. Conference Proceedings-IEEE Engineering in Medicine and Biology Society. 2013;2013:2082–3085. doi: 10.1109/EMBC.2013.6610192. [DOI] [PubMed] [Google Scholar]
- 86.Rosa MA, et al. Fronto-medial electrode placement with low current amplitude: A case report. Journal of ECT. 2012;28:146. [Google Scholar]
- 87.Weiner RD. The first ECT devices. Convulsive Therapy. 1988;4(1):50–61. [PubMed] [Google Scholar]
- 88.Spanis CW, Squire LR. Memory and convulsive stimulation: Effects of stimulus waveform. American Journal of Psychiatry. 1981;138(9):1177–1181. doi: 10.1176/ajp.138.9.1177. [DOI] [PubMed] [Google Scholar]
- 89.Weiner RD, et al. Effects of stimulus parameters on cognitive side effects. Annals of the New York Academy of Sciences. 1986;462:315–325. doi: 10.1111/j.1749-6632.1986.tb51266.x. [DOI] [PubMed] [Google Scholar]
- 90.Sackeim HA, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimulation. 2008;1(2):71–83. doi: 10.1016/j.brs.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loo CK, et al. A comparison of RUL ultrabrief pulse (0.3ms) ECT and standard RUL ECT. The International Journal of Neuropsychopharmacology. 2008;11(7):883–890. doi: 10.1017/S1461145708009292. [DOI] [PubMed] [Google Scholar]
- 92.Mayur P, Byth K, Harris A. Autobiographical and subjective memory with right unilateral high-dose 0.3-millisecond ultrabrief-pulse and 1-millisecond brief-pulse electroconvulsive therapy. Journal of ECT. 2013;29(4):277–282. doi: 10.1097/YCT.0b013e3182941baf. [DOI] [PubMed] [Google Scholar]
- 93.Spaans H-P, et al. Efficacy and cognitive side effects after brief pulse and ultrabrief pulse right unilateral electroconvulsive therapy for major depression: A randomized, double-blind, controlled study. Journal of Clinical Psychiatry. 2013;74:e1029–e1036. doi: 10.4088/JCP.13m08538. [DOI] [PubMed] [Google Scholar]
- 94.Squire LR. Memory functions as affected by electroconvulsive therapy. Annals New York Academy of Sciences. 1986;462:307–314. doi: 10.1111/j.1749-6632.1986.tb51265.x. [DOI] [PubMed] [Google Scholar]
- 95.Semkovska M, et al. Unilateral brief-pulse electroconvulsive therapy and cognition: Effects of electrode placement, stimulus dosage and time. Journal of Psychiatric Research. 2011;45(6):770–780. doi: 10.1016/j.jpsychires.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 96.Peterchev AV, et al. Electroconvulsive Therapy Stimulus Parameters: Rethinking Dosage. Journal of ECT. 2010;26(3):159–174. doi: 10.1097/YCT.0b013e3181e48165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Deng ZD, Lisanby SH, Peterchev AV. Controlling stimulation strength and focality in electroconvulsive therapy via current amplitude and electrode size and spacing: Comparison with magnetic seizure therapy. Journal of ECT. 2013;29(4):325–335. doi: 10.1097/YCT.10.1097/YCT.0b013e3182a4b4a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Quante A, et al. Effects of 3 different stimulus intensities of ultrabrief stimuli in right unilateral electroconvulsive therapy in major depression: A randomized, double-blind pilot study. Journal of Psychiatric Research. 2011;45(2):174–178. doi: 10.1016/j.jpsychires.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 99.Lisanby SH, et al. Magnetic seizure therapy of major depression. Archives of General Psychiatry. 2001;58(3):303–305. doi: 10.1001/archpsyc.58.3.303. [DOI] [PubMed] [Google Scholar]
- 100.Deng ZD, Lisanby SH, Peterchev AV. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: A finite element study. Journal of Neural Engineering. 2011;8(1):016007. doi: 10.1088/1741-2560/8/1/016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lisanby SH, et al. Safety and Feasibility of Magnetic Seizure Therapy (MST) in Major Depression: Randomized Within-Subject Comparison with Electroconvulsive Therapy. Neuropsychopharmacology. 2003;28(10):1852–1865. doi: 10.1038/sj.npp.1300229. [DOI] [PubMed] [Google Scholar]
- 102.Kirov G, et al. Quick recovery of orientation after magnetic seizure therapy for major depressive disorder. The British Journal of Psychiatry. 2008;193(2):152–155. doi: 10.1192/bjp.bp.107.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rosa MA, et al. Seizure induction with low-amplitude-current (0.5 A) electroconvulsive therapy. Journal of ECT. 2011;27(4):341–342. doi: 10.1097/YCT.0b013e31822149db. [DOI] [PubMed] [Google Scholar]
- 104.Won Hee L, et al. Stimulation strength and focality of electroconvulsive therapy with individualized current amplitude: A preclinical study. in Engineering in Medicine and Biology Society (EMBC), 2012 Annual International Conference of the IEEE; 2012. [DOI] [PubMed] [Google Scholar]
- 105.Devanand D, et al. Does ECT alter brain structure? American Journal of Psychiatry. 1994;151(7):957–970. doi: 10.1176/ajp.151.7.957. [DOI] [PubMed] [Google Scholar]
- 106.Dwork AJ, et al. Absence of Histological Lesions in Primate Models of ECT and Magnetic Seizure Therapy. American Journal of Psychiatry. 2004;161(3):576–578. doi: 10.1176/appi.ajp.161.3.576. [DOI] [PubMed] [Google Scholar]
- 107.Ende G, et al. The hippocampus in patients treated with electroconvulsive therapy: A proton magnetic resonance spectroscopic imaging study. Archives of General Psychiatry. 2000;57(10):937–943. doi: 10.1001/archpsyc.57.10.937. [DOI] [PubMed] [Google Scholar]
- 108.Scalia J, et al. Neuropathologic examination after 91 ECT treatments in a 92-year-old woman with late-onset depression. Journal of ECT. 2007;23(2):96–98. doi: 10.1097/YCT.0b013e31804bb99d. [DOI] [PubMed] [Google Scholar]
- 109.Perera TD, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. Journal of Neuroscience. 2007;27(18):4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nordanskog P, et al. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: A volumetric magnetic resonance imaging study. Journal of ECT. 2010;26(1):62–67. doi: 10.1097/YCT.0b013e3181a95da8. [DOI] [PubMed] [Google Scholar]
- 111.Nordanskog P, et al. Hippocampal volume in relation to clinical and cognitive outcome after electroconvulsive therapy in depression. Acta Psychiatrica Scandinavica. doi: 10.1111/acps.12150. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dukart J, et al. Elecroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proceedings of the National Academy of Sciences. 2014;111(3):1156–1161. doi: 10.1073/pnas.1321399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Perrin JS, et al. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proceedings of the National Academy of Sciences. 2012;109(14):5464–5468. doi: 10.1073/pnas.1117206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beall EB, et al. Effects of electroconvulsive therapy on brain functional activation and connectivity in depression. Journal of ECT. 2012;28(4):234–241. doi: 10.1097/YCT.0b013e31825ebcc7. [DOI] [PubMed] [Google Scholar]
- 115.Abbott CC, et al. A Review of Longitudinal Electroconvulsive Therapy: Neuroimaging Investigations. Journal of Geriatric Psychiatry and Neurology. 2014;27(1):33–46. doi: 10.1177/0891988713516542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krystal AD, et al. Changes in seizure threshold over the course of electroconvulsive therapy affect therapeutic response and are detected by ictal EEG ratings. Journal of Neuropsychiatry & Clinical Neurosciences. 1998;10:178–186. doi: 10.1176/jnp.10.2.178. [DOI] [PubMed] [Google Scholar]
- 117.Perera TD, et al. Seizure Expression During Electroconvulsive Therapy: Relationships with Clinical Outcome and Cognitive Side Effects. Neuropsychopharmacology. 2004;29(4):813–825. doi: 10.1038/sj.npp.1300377. [DOI] [PubMed] [Google Scholar]
- 118.Sackeim HA, et al. Electrophysiological correlates of the adverse cognitive effects of electronconvulsive therapy. Journal of ECT. 2000;16(2):110–120. doi: 10.1097/00124509-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 119.Prichep LS, et al. Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiology of Aging. 1994;15(1):85–90. doi: 10.1016/0197-4580(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 120.Prichep LS, et al. Prediction of longitudinal cognitive decline in normal elderly with subjective complaints using electrophysiological imaging. Neurobiology of Aging. 2006;27(3):471–481. doi: 10.1016/j.neurobiolaging.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 121.Luber B, et al. Quantitative EEG during seizures induced by electroconvulsive therapy: Relations to treatment modality and clinical features. II. Topographic analyses. Journal of ECT. 2000;16(3):229–243. doi: 10.1097/00124509-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 122.Nobler MS, et al. Quantitative EEG during seizures Induced by electroconvulsive therapy: Relations to treatment modality and clinical features. I. Global analyses. Journal of ECT. 2000;16(3):211–228. doi: 10.1097/00124509-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 123.Teyler TJ, Discenna P. Long-term potentiation as a candidate mnemonic device. Brain Research Reviews. 1984;7(1):15–28. doi: 10.1016/0165-0173(84)90027-4. [DOI] [PubMed] [Google Scholar]
- 124.Hesse GW, Teyler TJ. Reversible loss of hippocampal long term potentiation following electroconvulsive seizures. Nature. 1976;264:562–564. doi: 10.1038/264562a0. [DOI] [PubMed] [Google Scholar]
- 125.Barr DS, et al. Induction and reversal of long-term potentiation by low- and high-intensity theta pattern stimulation. Journal of Neuroscience. 1995;15(7):5402–5410. doi: 10.1523/JNEUROSCI.15-07-05402.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moore SD, Barr DS, Wilson WA. Seizure-like activity disrupts LTP in vitro. Neuroscience Letters. 1993;163:117–119. doi: 10.1016/0304-3940(93)90243-e. [DOI] [PubMed] [Google Scholar]
- 127.Lee WH, et al. Regional electric field induced by electroconvulsive therapy in a realistic finite element head model: Influence of white matter anisotropic conductivity. Neuroimage. 2012;59(3):2110–2123. doi: 10.1016/j.neuroimage.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Squire LR, Cohen NJ, Zouzounis JA. Preserved memory in retrograde amnesia: Sparing of a recently acquired skill. Neuropsychologia. 1984;22(2):145–152. doi: 10.1016/0028-3932(84)90057-5. [DOI] [PubMed] [Google Scholar]
- 129.Kroes MC, et al. An electroconvulsive therapy procedure impairs reconsolidation of episodic memories in humans. Nature Neuroscience. 2014;17(2):204–206. doi: 10.1038/nn.3609. [DOI] [PubMed] [Google Scholar]
- 130.Casarotto S, et al. Assessing the effects of electroconvulsive therapy on cortical excitability by means of transcranial magnetic stimulation and electroencephalography. Brain Topography. 2013;26:326–337. doi: 10.1007/s10548-012-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Trepel C, Racine RJ. Long-term potentiation in the neocortex of the adult, freely moving rat. Cerebral Cortex. 1998;8:719–729. doi: 10.1093/cercor/8.8.719. [DOI] [PubMed] [Google Scholar]
- 132.Porter RJ, Douglas K, Knight RG. Monitoring of cognitive effects during a course of electroconvulsive therapy: Recommendations for clinical practice. Journal of ECT. 2008;24(1):25–34. doi: 10.1097/YCT.0b013e31815d9627. [DOI] [PubMed] [Google Scholar]
- 133.Bennett DM, et al. Usefulness of treatment reports for electroconvulsive therapy. Journal of ECT. 2013;29(3):210–213. doi: 10.1097/YCT.0b013e3182887baa. [DOI] [PubMed] [Google Scholar]
- 134.Martin DM, et al. A new early cognitive screening measure to detect cognitive side-effects of electroconvulsive therapy? Journal of Psychiatric Research. 2013;47:1967–1974. doi: 10.1016/j.jpsychires.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 135.Butters MA, et al. Changes in cognitive functioning following treatment of late-life depression. American Journal of Psychiatry. 2000;157(12):1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 136.Manning KJ, et al. Executive Functioning Complaints and Escitalopram Treatment Response in Late-Life Depression. The American Journal of Geriatric Psychiatry. doi: 10.1016/j.jagp.2013.11.005. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morimoto SS, et al. Executie function and short-term remission of geriatric depression: The role of semantic strategy. American Journal of Geriatric Psychiatry. 2011;19(2):115–122. doi: 10.1097/JGP.0b013e3181e751c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Snyder HR. Major depressive disorder is associated iwth broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin. 2013;139(1):81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Greer TL, et al. Does duloxetine improve cognitive function independently of its antidepressant effect in patients with major depressive disorder and subjective reports of cognitive dysfunction. Depression Research and Treatment. doi: 10.1155/2014/627863. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Keefe RSE, et al. Cognitive effects of pharmacotherapy for major depressive disorder: A systematic review. Journal of Clinical Psychiatry. doi: 10.4088/JCP.13r08609. In Press. [DOI] [PubMed] [Google Scholar]
- 141.Moreines JL, et al. Neurocognitive function before and after subcallosal cingulate deep brain stimulation in patients with treatment-resistant depression. Depression and Anxiety. doi: 10.1002/da.22263. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Squire LR, Slater PC, Miller PL. Retrograde amnesia and bilateral electroconvulsive therapy: Long-term follow-up. Archives of General Psychiatry. 1981;38(1):89–95. doi: 10.1001/archpsyc.1981.01780260091010. [DOI] [PubMed] [Google Scholar]
- 143.Smith GE, et al. A randomized controlled trial comparing the memory effects of continuation electroconvulsive therapy versus continuation pharmacotherapy: results from the Consortium for Research in ECT (CORE) study. J Clin Psychiatry. 2010;71(2):185–93. doi: 10.4088/JCP.08m04797gre. [DOI] [PubMed] [Google Scholar]
- 144.Sackeim HA, et al. Effect of Concomitant Pharmacotherapy on Electroconvulsive Therapy Outcomes: Short-term Efficacy and Adverse Effects. Archives of General Psychiatry. 2009;66(7):729–737. doi: 10.1001/archgenpsychiatry.2009.75. [DOI] [PubMed] [Google Scholar]
- 145.Cicerone KD, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Archives of physical medicine and rehabilitation. 2005;86(8):1681–1692. doi: 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 146.Lincoln N, Majid M, Weyman N. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database Syst Rev. 2000;4 doi: 10.1002/14651858.CD002842. [DOI] [PubMed] [Google Scholar]
- 147.Choi J, Twamley EW. Cognitive Rehabilitation Therapies for Alzheimer’s Disease: A Review of Methods to Improve Treatment Engagement and Self-Efficacy. Neuropsychology review. 2013:1–15. doi: 10.1007/s11065-013-9227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McGurk SR, et al. A meta-analysis of cognitive remediation in schizophrenia. American Journal of Psychiatry. 2007;164(12):1791–802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Twamley EW, et al. Development and pilot testing of a novel compensatory cognitive training intervention for people with psychosis. American Journal of Psychiatric Rehabilitation. 2008;11(2):144–163. doi: 10.1080/15487760801963678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wykes T, et al. Effects on the brain of a psychological treatment: cognitive remediation therapy functional magnetic resonance imaging in schizophrenia. The British Journal of Psychiatry. 2002;181(2):144–152. doi: 10.1017/s0007125000161872. [DOI] [PubMed] [Google Scholar]
- 151.Engelberts N, et al. The Effectiveness of Cognitive Rehabilitation for Attention Deficits in Focal Seizures: A Randomized Controlled Study. Epilepsia. 2002;43(6):587–595. doi: 10.1046/j.1528-1157.2002.29401.x. [DOI] [PubMed] [Google Scholar]
- 152.Ponds RW, Hendriks M. Cognitive rehabilitation of memory problems in patients with epilepsy. Seizure. 2006;15(4):267–273. doi: 10.1016/j.seizure.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 153.Bayley PJ, Hopkins RO, Squire LR. The Fate of Old Memories after Medial Temporal Lobe Damage. J. Neurosci. 2006;26(51):13311–13317. doi: 10.1523/JNEUROSCI.4262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shulman MB, Barr W. Treatment of memory disorders in epilepsy. Epilepsy & Behavior. 2002;3(5) Supplement 1:30–34. doi: 10.1016/s1525-5050(02)00509-7. [DOI] [PubMed] [Google Scholar]
- 155.Mangaoang MA, Lucey JV. Cognitive rehabilitation: assessment and treatment of persistent memory impairments following ECT. Adv Psychiatr Treat. 2007;13(2):90–100. [Google Scholar]
- 156.Choi J, et al. A conceptual introduction to cognitive remediation for memory deficits associated with right unilateral electroconvulsive therapy. Journal of ECT. 2011;27(4):286–291. doi: 10.1097/YCT.0b013e31821d3ab3. [DOI] [PubMed] [Google Scholar]
- 157.McClintock SM, et al. A systematic review of the combined use of electroconvulsive therapy and psychotherapy for depression. Journal of ECT. 2011;27(3):236–243. doi: 10.1097/YCT.0b013e3181faaeca. [DOI] [PMC free article] [PubMed] [Google Scholar]


