Abstract
Successful performance of a cognitive task depends upon both the quality of the sensory information and the processing resources available to perform that task. Thus, task performance can either be data-limited or process-limited (D. A. Norman and D. G. Bobrow, 1975). Using fMRI, we show that these conceptual distinctions are neurally dissociable: A parieto-frontal network involved in conscious perception is modulated by target interference manipulations that strain attentional processing, but not by equally difficult manipulations that limit the quality of target information. These results suggest that limitations imposed by processing capacity have distinct neural effects from those arising from the quality of sensory input, and provide empirical support for an influential neurobiological theory of consciousness (S. Dehaene, J.-P. Changeux, L. Naccache, J. Sackur, and C. Sergent, 2006).
Keywords: Process-limit, Data-limit, Attention, Consciousness, fMRI
Introduction
Any information processing system has limitations (Lloyd, 2000). In the context of human information processing, a distinction is made between two broad classes of limitations: those that result from the limited quality of information that is inputted into the system, i.e. data-based limits, and those that result from the limited processing capacities of that system, i.e. resource-based or process-based limits1 (Norman and Bobrow, 1975).
The distinction between process-based and data-based limitations has long maintained a foothold in the behavioral and computational literature (Garner, 1970; Lavie and DeFockert, 2003), and implicitly forms the essence of an influential theory on the neural basis of conscious perception (Dehaene et al., 2006). According to this theory, failures of conscious perception of sensory events may take place either because the quality of the sensory information is too impoverished to yield a supra-threshold percept in the brain, or because the central brain mechanisms that support attentional processing of the sensory event are too overloaded to operate on that event, even if its sensory quality is above threshold for conscious perception. Consistent with a role for attentional processes in limiting awareness, activity of the parieto-frontal attention network generally co-varies with conscious perception (Beck et al., 2001; Dehaene et al., 2006; Marois et al., 2004a; Rees et al., 2002). However, it is still unknown whether this parieto-frontal activation is specific to conditions that are limited by attentional processing capacities, or whether it generalizes to conditions where data is limited as well. Indeed, there is as of yet no clear neurobiological evidence to support a dissociation between process-based and data-based limitations of human cognition because these two conditions have never been directly contrasted within a single experiment.
The process-based versus data-based limitation account predicts that only under conditions in which attentional processing is strained, i.e. when its computational load is increased, will the parieto-frontal network be modulated. That is, when processing load of a given task increases, a larger amount of processing resource should be allocated or a processing device should be deployed for a longer period of time in order to meet the task demands, thereby increasing activation of brain regions implicated in capacity-limited processes. By contrast, manipulations limiting the quality of sensory input to the system would have a minimal impact on the network's processing capacity. The impoverishment of input data to be handled by the system does not impose any additional processing demand on the system. Hence, data-based limits should not modulate activation in the parieto-frontal network.
Here we tested this prediction using a variant of the attentional blink (AB) paradigm, which reveals a profound deficit in the conscious perception of targets embedded in a rapid serial visual presentation (RSVP) stream of distractor items when these targets are presented within a few hundred milliseconds of each other (Chun and Potter, 1995; Raymond et al., 1992). The AB is well suited to investigate the neural dissociation of process-based and data-based limitations because this paradigm allows these two limitations to be independently manipulated. Growing evidence shows that experimental factors taxing attentional processes interact with the AB (Dux et al., 2008, 2009), whereas degrading the quality of sensory input does not (Jannati et al., 2012; McLaughlin et al., 2001). Even though the exact cause of the AB is still under debate (for a review, see Dux and Marois, 2009), it is well-established that the AB reveals behavioral deficits primarily produced by increased processing load.
Processing can be strained by increasing distractor interference, as such a manipulation disrupts the attentional deployment for target selection (Chun and Potter, 1995; Di Lollo et al., 2005; Serences et al., 2005; Simons, 2000). The effects of limiting data input, in contrast, can be revealed by manipulating the duration of target presentation (Garner, 1970; Lavie and DeFockert, 2003; Norman and Bobrow, 1975). We hypothesize that the parieto-frontal network will be modulated by the distractor interference manipulation, but not by the stimulus duration manipulation.
Materials and methods
Experiment 1
Participants
Fifteen adults (aged 20–35; 5 males) participated in exchange for monetary compensation. The Vanderbilt Institutional Review Board approved the experimental protocol and written informed consent was obtained from each participant. One participant's data was excluded from the analysis due to failure to follow the task instructions and another because of excessive head motion (>10 mm).
Behavioral paradigm
The targets were randomly chosen among eight letters (B, N, Z, T, F, H, K, or L) in each trial. The distractors consisted of digits, excluding 0 and 1. All characters (white courier font on a black background) subtended 0.6°×1° of visual angle. Each trial's RSVP stream contained 11 frames (three target letters and eight digit distractors), with the first target presented anywhere between the third and the sixth frame. Similar to Kawahara et al.'s (2006) experimental design, three targets were presented in each trial of all conditions to reveal the transient effect of distractor presentation on attentional target selection (see Results) and to equate the number of targets to detect and respond to across conditions.
Three conditions (Continuous, Discontinuous, and Continuous-hard) were presented in each fMRI run (Fig. 1). In the Continuous condition, the three target letters were presented successively. This condition served to establish a reference pattern of behavioral performance and brain activation to which the two other conditions could be compared to assess their effectiveness in impairing performance and in modulating brain activity. In the Discontinuous condition, one of the eight digit distractors was inserted between the first and second targets. The stimulus presentation rate was constant across the Continuous and Discontinuous conditions for each individual participant, but it was adjusted (80–130 ms stimulus duration, 0 ms ISI) between participants, based on task performance in pilot testing, to yield about 80% correct performance. Finally, in a third condition (Continuous-hard), visual stimulation was identical to that of the Continuous condition, except that the duration of each target frame (90–160 ms for T1, 50–90 ms for T2, and 70–130 ms for T3) was adjusted independently after each fMRI run to match performance with the Discontinuous condition. Based upon behavioral pilot testing, in the first run, T1 duration for the Continuous-hard condition was lengthened by 16.7 ms (monitor refresh rate) and T2 duration was shortened by 33.3 ms, compared to the other conditions.
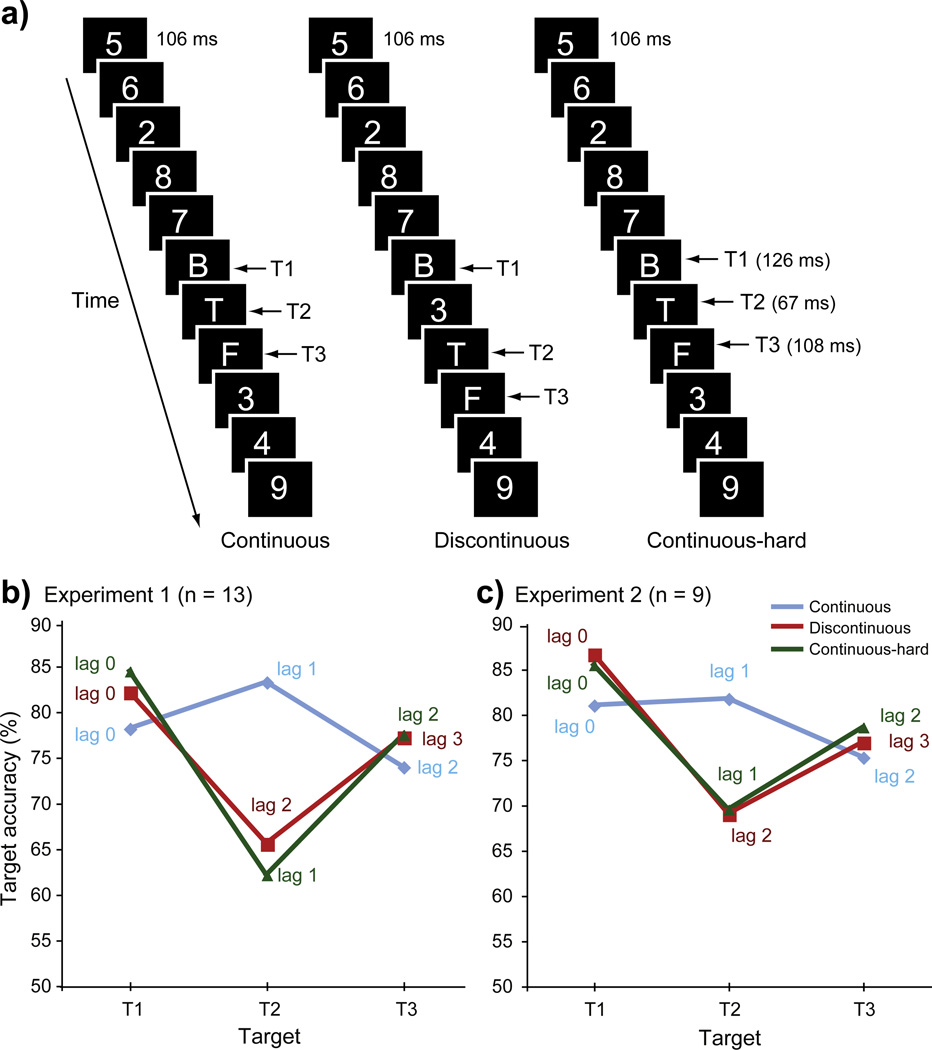
Fig. 1.
a) Experiment 1 trial design. In the Discontinuous condition, a digit distractor was inserted between T1 and T2. The Continuous-hard condition was identical to the Continuous condition except that the durations of T1, T2, and T3 were manipulated independently to match performance with the Discontinuous condition. b) Behavioral results in Experiment 1. c) Behavioral results in Experiment 2.
The RSVP was followed by the visual presentation of target response panels. The response panel for T1 appeared 2 s after the onset of the RSVP and listed the eight potential target letters in a row at fixation, with the letters colored red over a black background. Above the letter row the sentence ‘First Target?’ was also presented in red. Each letter was assigned to a distinct manual button press, mapped in a spatially congruent manner onto all the fingers except the thumbs. The T1 response panel remained visible for 2 s, followed by a 1-sec presentation for each of the T2 (green colored letters) and T3 (blue colored letters) response panels. The T1 panel was presented for a longer time because pilot testing showed that T1 RTs (mean= 1077 ms) were longer than T2 (283 ms) and T3 RTs (394 ms).This is probably because participants retrieved all three targets at the onset of the T1 response panel. Upon the onsets of the T2 and T3 response panels, the participants would then just have to select and execute the appropriate responses based upon already retrieved target identities.
Each trial lasted a total of 6 s, with trials separated by a blank interval of variable duration that followed an exponential distribution (23 trials×4 s, 10 trials×6 s, 4 trials×8 s, 2 trials×10 s, and 1 trial×12 s) to facilitate deconvolution analysis of the BOLD response. Each participant completed six such fMRI runs, each consisting of 20 Discontinuous trials, 10 Continuous trials, and 10 Continuous-hard trials. Thus, the number of trials in which a digit distractor followed T1 (Discontinuous) was the same as the number of trials in which another target followed T1 (Continuous and Continuous-hard). The presentation order of these three trial types was randomly intermixed, thereby ensuring that any activation differences between conditions were not due to preparatory or expectation effects.
fMRI methods
Anatomical 2D and 3D high-resolution T1-weighted images were acquired with conventional parameters on a 3T Philips scanner at the Van-derbilt University Institute of Imaging Sciences. Thirty-three 3.5 mm axial slices (0.5 mm skip; 3.75×3.75 mm in-plane) were taken parallel to the AC–PC line (TR, 2000 ms; TE, 35 ms; FA, 79°; FOV, 240 mm). The functional scan included 238 brain volumes. Imaging data were analyzed using Brain Voyager QX 1.10. Data preprocessing included 3D motion correction, slice scan time correction, linear trend removal, and spatial smoothing with an 8-mm (FWHM) Gaussian kernel. All functional data of each participant were aligned to the first functional run, and co-registered to that individual's anatomical T1-weighted image. Functional and anatomical data were transformed into standardized Talairach space.
To create statistical parametric maps (SPMs) of BOLD activation, regressors were defined for each trial type and convolved with a double gamma function (SPM2, http://www.fil.ion.ucl.ac.uk/spm). Two group random-effects contrast analyses were performed to isolate brain regions preferentially activated by the Discontinuous condition. The first used a balanced contrast, with regression coefficients of 2 for the Discontinuous condition and − 1 for each of the Continuous and the Continuous-hard conditions. A voxel-wise statistical threshold of p<.005 was corrected for multiple comparisons using a cluster filter of five contiguous voxels (as determined via simulation using the Brain Voyager cluster threshold plug-in), yielding a map-wise error rate of p<0.05 (Forman et al., 1995). The second SPM was constructed using a conjunction of two contrasts (Nichols et al., 2005), (Discontinuous-Continuous) and (Discontinuous-Continuous-hard), using a voxel-wise statistical threshold of p<.05 with a cluster threshold of 11 contiguous voxels. Given that this conjunction contrast analysis is fairly conservative (Friston et al., 2005), we adopted a slightly more lenient threshold for the SPM to decrease the probability of Type II errors. For both SPMs, regions of interest (ROI) were defined as all contiguous supra-threshold voxels of distinct activation foci.
For ROI analysis, event-related timecourses of the BOLD signal for each participant and condition were estimated using a deconvolution analysis (using the eight volumes immediately following trial onsets). The Beta estimates for each volume were converted to % signal change relative to the mean Beta value of their run. The normalized Beta estimates were then averaged across participants, yielding group-averaged timecourses.
To statistically compare BOLD responses across conditions, BOLD amplitudes at the peak volume were contrasted using paired t-tests. The peak volume was derived by collapsing the timecourses of all the conditions and participants and determining the time point of greatest signal amplitude in the averaged response for each ROI (Todd and Marois, 2004). ROIs with bilateral foci in SPMs were first tested (using t tests) for hemispheric differences in activation. If none were found, the data were collapsed across hemispheres (Marois et al., 2004a).
Experiment 2
Nine participants were scanned (aged 19–28,4 males) in this experiment. All the behavioral and imaging protocols were identical to those of Experiment 1 except that the intervening distractor between T1 and T2 in the Discontinuous condition was selected from a different character category (keyboard symbols; @,#,%,&,<, >, +, =; see Di Lollo et al., 2005). Due to their relatively infrequent presentations, the keyboard symbols should act as ‘oddballs’ and therefore transiently capture attention, necessitating redeployment of attention towards the main task. Thus, this experimental manipulation, just as in the first experiment, should impose heavy demands on the neural mechanisms for attentional selection and transiently impair target detection performance. Trials of the two other conditions (Continuous and Continuous-hard) also included a keyboard symbol to equate for the presence of an oddball distractor across all conditions but, critically, this distractor was presented two to four frames after T3. Because the keyboard symbol in the Continuous and Continuous-hard conditions is shown after the attentional window for target selection, it should capture less attention and should affect target performance less than the intervening distractor in the Discontinuous condition.
The ROIs defined in Experiment 1 were probed in Experiment 2. Given that Experiment 2 aimed to replicate Experiment 1's directional effect of greater activity in the Discontinuous than in the other two conditions, one-tailed t-tests were used to test for statistical differences across conditions.
In addition to ROIs defined in Experiment 1, we probed regions previously implicated in the AB, using all the data from Experiments 1 and 2. Specifically, the coordinates of the center of mass of regions engaged in the AB – bilateral lateral prefrontal cortex and intra-parietal sulcus – were selected from a previous study (Marois et al., 2000), and ROIs were created by encompassing the center voxel and surrounding area up to 1 cm3. Then, a conjunction contrast was run on the mask created using those four ROIs, and the resulting SPM was corrected for multiple comparisons using a cluster filter of six contiguous voxel (as determined via simulation using the Brain Voyager cluster threshold plug-in). We also extracted timecourses from those ROIs and the peak amplitudes were compared across conditions.
Results
Experiment 1
Behavioral results
The experimental design, which was based on the three-target variant of the AB paradigm (Kawahara et al., 2006), consisted of three trial types: Continuous, Discontinuous, and Continuous-hard conditions. The Continuous trials included three consecutively presented target letters embedded in an RSVP stream of digit distractors. Under such condition, all three targets can easily be selected (Di Lollo et al., 2005). In contrast, the Discontinuous trials included an intervening distractor between targets that severely hampered the target selection process (Fig. 1). Such distractor interference is very transient, as only detection of the target that immediately follows the distractor is impaired; performance recovers if the following stimulus is another target (Kawahara et al., 2006). Both the initial disruption of the attentional settings for target selection as well as their eventual recovery should lead to a greater engagement of the brain regions supporting attentional selection in Discontinuous trials compared to the condition in which these attentional settings are unperturbed (Continuous trials).
In order to determine how the behavioral dissociation between process-based and data-based limitations is neurally instantiated, we also included the Continuous-hard condition, which is identical to the Continuous condition except that the durations of target presentations are reduced to match behavioral performance with the Discontinuous condition. While the interference manipulation of the Discontinuous condition should strain the brain's processing resources, the Continuous-hard condition should only affect the amount of data inputted into the system. As a result, activation differences between the Discontinuous and Continuous-hard conditions should reveal brain regions associated with process-based limitations, despite the two conditions being matched for accuracy (see Chee and Tan, 2010, for a similar logic of controlling for task performance across conditions in the interpretation of activation in the parieto-frontal attention network). By contrast, any brain regions that track task difficulty should show no activation differences between the Discontinuous and Continuous-hard conditions, but those two conditions should differ from the Continuous condition.
To analyze behavioral data acquired from the scanning session, target accuracy was entered into a 3×3 repeated-measures analysis of variance (ANOVA) with condition (Discontinuous, Continuous, and Continuous-hard) and target (T1, T2, and T3) as within-subject factors. The main effect of condition was significant, F(2, 24)=8.59, p<.01 (Fig. 1); target accuracy was generally worse for the Discontinuous and Continuous-hard conditions than for the Continuous condition. More specifically, the presence of a distractor following T1 in the Discontinuous condition disrupted attentional processing of T2 compared to T1, t(12)=4.83, p<.01. Such target disruption effects were very transient, as T3 performance was far superior to T2, t(12)=2.18, p<.001. A similar pattern was observed for the Continuous-hard condition; T2 accuracy was significantly lower than T1, t(12)=7.54, p<.01, and T3 accuracy, t(12)=8.15, p<.01. Importantly, performances between the Discontinuous and the Continuous-hard conditions were successfully matched: A 2×3 ANOVA with condition (Discontinuous, Continuous-hard) and target (T1, T2, and T3) as factors revealed no main effect of condition, F<1, though there was a marginal interaction, F(2, 24)=3.24, p>.057. This interaction was driven by the slightly worse performance in the Continuous-hard than in the Discontinuous condition, t(12)=2.23, p<.05, which indicates that, if anything, the Continuous-hard condition was slightly more difficult than the Discontinuous condition. Although the responses were unspeeded and offline, reaction times (RTs) were also analyzed in the same way as accuracy. There was no RT main effect of condition, p>.11. Although the mean RT for T1 was significantly longer than for T2 and T3 in all conditions (all ps<.01), this effect can be accounted for by the fact that T1 was the first response initiated on each trial (see Methods). The interaction between target and condition was not significant, p>.10, suggesting that the same pattern of RT result was observed across conditions.
The mean duration of each stimulus frame was 106 ms in the Continuous and Discontinuous conditions. In the Continuous-hard condition, the stimulus duration was the same except for the three targets whose mean durations were 126 ms, 66 ms, and 108 ms for T1, T2, and T3, respectively. T1 duration in the Continuous-hard was longer than that of the other two conditions, t(12)=3.49, p<.01, while T2 duration was shorter, t(12)=6.61, p<.01 (T3 duration was similar across all conditions, p>.67). Importantly, the mean total duration of the three target presentations, and hence of the entire RSVP stream, was only 18 ms shorter in the Continuous-hard than in the Discontinuous condition (p=.06). It is therefore unlikely that any activation differences between these two conditions reflect differences in overall stimulus duration. This 18-ms difference was also present between the Continuous and Continuous-hard conditions, and that difference was sufficient to produce lower overall target accuracy in the latter condition (74.7% vs 78.5% in the Continuous condition; F(1, 12)=20.30, p<.001). The lower performance in the Continuous-hard condition that resulted from such a small overall decrease in target durations is due to the non-linear effects of presentation duration on target accuracy, as target detection performance precipitously declines in RSVPs with stimulus duration below 100 ms (Fisher, 1984; Todd et al., 2011).
fMRI results
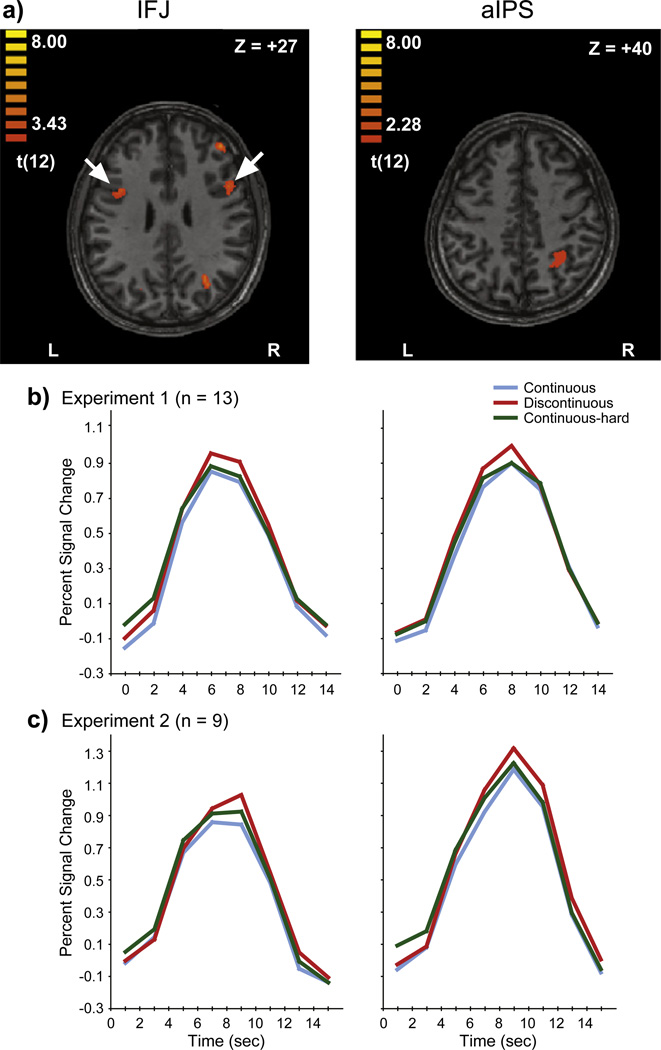
If the distinction between process-based and data-based limitations is reflected in brain activity, regions involved in process-based performance limitations would be expected to be more engaged by the Discontinuous condition than by either of the Continuous or Continuous-hard conditions. We searched for such regions in two SPM analyses (see Materials and methods). The first, a balanced contrast SPM analysis, revealed activations in several cortical regions (Table 1). Even though the balanced contrast is useful to identify brain activity more sensitive to one specific condition than to others (Marois et al., 2004b), it does not reveal whether these foci show an activation profile that corresponds to the expected pattern, namely greater activation in the Discontinuous than in the other two conditions. This is because this analysis is based on the comparative strength of regression coefficients across conditions, and does not indicate whether the timecourse of activity conforms to the predicted hemodynamic response profile. For example, an SPM analysis can reveal a statistically significant focus simply because that region is less deactivated in one condition than another. To examine this issue, we extracted and analyzed timecourses from the balanced contrast ROIs. Peak amplitude analysis of the timecourses extracted from these activation foci revealed that only the bilateral inferior frontal junction (IFJ) showed greater activity in the Discontinuous than both of the other conditions (Fig. 2) (paired t-test, Discontinuous–Continuous, t(12)=3.12, p<.01, Discontinuous– Continuous-hard, t(12)=3.03 p<.05, two-tailed), and no differences between the Continuous and Continuous-hard conditions (p=.54). The second SPM analysis, a conjunction SPM, also revealed activation in the same IFJ area identified by the balanced-contrast SPM, along with activation in an additional brain region, the right anterior intra-parietal sulcus (aIPS) (Table 1). Just like for the IFJ, the aIPS showed greater activity in the Discontinuous than in the other two conditions (Discontinuous– Continuous, t(12)=3.34, p<.01, Discontinuous–Continuous-hard, t(12)=2.53, p<.05), with no differences between Continuous and Continuous-hard conditions (p=.98). No other ROIs from either of the two SPM analyses showed this pattern of activation. We also searched for regions that showed greater activity for either of the Continuous or Continuous-hard conditions than for the Discontinuous condition, but no such regions were found. A direct contrast between the Continuous and Continuous-hard conditions did not reveal any significant activation foci, either.
Table 1.
Brain regions isolated with the Balanced and Conjunction contrasts of Experiment 1. Except for the bilateral inferior frontal junction (IFJ) and right anterior intra-parietal sulcus (aIPS), none of the ROIs exhibited greater peak response in the Discontinuous than in both the Continuous and Continuous-hard conditions. However, the bilateral IPS, right precuneus, right middle temporal gyrus (MTG), and right inferior frontal gyrus (IFG) showed greater activation for the Discontinuous than for the Continuous condition (all ps<.05, two-tailed), but not than for the Continuous-hard condition (all ps>.1).
| ROI name | Mean t value |
Mean Talairach coordinates |
Volume (mm3) |
||
|---|---|---|---|---|---|
| X | Y | Z | |||
|
ROIs from the Balanced contrast of 2 (Discontinuous)–1 (Continuous)–1 (Continuous-hard) | |||||
| Left inferior frontal junction (IFJ) | 3.96 | −39 | 2 | 31 | 874 |
| Right inferior frontal junction (IFJ) | 3.86 | 46 | 8 | 27 | 1087 |
| Right inferior frontal gyrus-insula | 3.84 | 38 | 10 | 11 | 294 |
| Left cingulate | 3.60 | −4 | 13 | 44 | 139 |
| Left superior middle frontal gyrus | 3.70 | −22 | 10 | 50 | 233 |
| Right superior middle frontal gyrus | 3.84 | 35 | 12 | 49 | 359 |
| Right inferior middle frontal gyrus | 4.29 | 40 | 38 | 24 | 515 |
| Right postcentral gyrus | 3.79 | 40 | −39 | 54 | 595 |
| Left intraparietal sulcus | 4.06 | −24 | −70 | 34 | 397 |
| Right intraparietal sulcus | 3.95 | 28 | −63 | 31 | 511 |
| Right precuneus | 5.10 | 25 | −74 | 43 | 165 |
| Right middle temporal gyrus | 4.04 | 53 | −55 | 5 | 1080 |
| Left middle occipital gyrus | 3.78 | −54 | −58 | −6 | 137 |
| Right middle occipital gyrus | 3.75 | 47 | −64 | −2 | 166 |
|
ROIs from the Conjunction contrast (Discontinuous–Continuous and Discontinuous–Continuous-hard) | |||||
| Left inferior frontal junction (IFJ) | 2.53 | −34 | 4 | 40 | 307 |
| Right inferior frontal junction (IFJ) | 2.41 | 46 | 8 | 23 | 352 |
| Right anterior intraparietal sulcus (aIPS) | 3.15 | 28 | −49 | 40 | 347 |
Fig. 2.
a) SPM showing inferior frontal junction (IFJ) and anterior intra-parietal sulcus (aIPS) activation for the balanced contrast (left) and conjunction (right) analyses of Experiment 1. Arrows show IFJ. b,c) Activation timecourses in IFJ (left) and aIPS (right) ROIs in Experiment 1 (b) and Experiment 2 (c).
The results of Experiment 1 suggest that a network of prefrontal and parietal areas is particularly sensitive to the processing load of an attention-demanding task; activity in these brain regions increased with increased processing load. By contrast, modulations in data input did not have the same effect: while the parieto-frontal network was activated by the Continuous-hard condition, this activation was not different from that observed in the Continuous condition.
Experiment 2
A limitation of Experiment 1 is that the results of the timecourse analyses, which helped identify the ROIs that showed genuinely greater activation in the Discontinuous condition, were based on the same datasets used by the SPM analyses to isolate the ROIs. To address this issue of non-independence of statistical analyses, we carried out a second experiment aimed at replicating the results of Experiment 1 in a statistically independent dataset. The design of this new experiment, performed in a separate group of nine subjects, was as in the first experiment save for the intervening distractor identity, which now originated from a different category than the other RSVP distractors (see Methods). Based upon the results of Experiment 1 that only a subset of the frontal and parietal regions (IFJ and aIPS) was preferentially activated for the process-limiting (Discontinuous) condition, these regions served as functionally-defined, ‘a priori’ ROIs in Experiment 2. Thus, timecourses were extracted from a dataset (Expt 2) that was statistically independent from the dataset (Expt 1) used to define ROIs.
Behavioral results
As expected, target detection performance in Experiment 2 was similar to the pattern observed in the first experiment (Fig. 1). Specifically, there were no accuracy differences between all pairs of target order (all ps>.27) in the Continuous condition. By contrast, in the Discontinuous condition, T1 accuracy was higher than T2 accuracy, t(8)=8.88, p<.01, and T3 accuracy, t(8)=2.92, p<.05, and T3 accuracy was higher than T2, t(8)=2.45, p<.05. Thus, a significant deficit in mostly T2 performance was replicated in the Discontinuous condition of Experiment 2. Finally, there was no accuracy difference between the Discontinuous and Continuous-hard conditions (ps>.38).
fMRI results
Based upon the results of Experiment 1 indicating that only the bilateral IFJ and right aIPS were preferentially activated for the Discontinuous condition, we conducted two paired t-tests (Discontinuous greater than Continuous, Discontinuous greater than Continuous-hard) restricted to those regions. Given that these tests on a few a priori ROIs were planned comparisons designed into the experiment, corrections for multiple comparisons were not performed in order to preserve our sensitivity to detect differences across conditions (Rothman, 1990; Saville, 1990). Probing of the IFJ ROI (defined in Experiment 1) revealed greater activity for the Discontinuous condition than for the two other conditions (Discontinuous-Continuous, t(8)=2.00, p<.04, Discontinuous-Continuous-hard, t(8)=3.06, p<.01, one-tailed; Fig. 2), replicating Experiment 1’s results. Also just as in Experiment 1, the right aIPS showed greater activity for the Discontinuous than for the other conditions (Discontinuous-Continuous, t(8)=7.01, p<.01, Discontinuous-Continuous-hard. t(8)=1.98, p<.04, one-tailed).
Having replicated the Experiment 1 finding that the IFJ and aIPS were preferentially activated in the Discontinuous condition, we next extracted timecourses from the remaining Experiment 1 ROIs. In this exploratory analysis, which requires corrections for multiple comparisons, no region was more activated for the Discontinuous condition than for the other two conditions. This was so even if the statistical threshold was not corrected for multiple comparisons, which strengthens the notion that the IFJ and IPS are key brain regions involved in process-based limitations.
To further examine whether brain regions other than the IFJ and aIPS would be preferentially activated by the Discontinuous condition, we repeated the balanced and conjunction contrasts in the same manner as in Experiment 1, but we combined participants’ data from both experiments (n=22) to increase statistical power. The statistical thresholds for both of these SPMs were corrected for multiple comparisons, using a cluster filter of nine contiguous voxels (Forman et al., 1995). Besides the IFJ and aIPS foci, the balanced contrast SPM revealed activation sites in superior medial frontal/anterior cingulate cortex (Talairach coordinates: 0, 18, 43; Fig. 3) and the left hemisphere counterpart of the aIPS (−29, −45, 39). The conjunction contrast SPM revealed significant activation foci only in the left (−42, 1, 33) and right IFJ (47, 7, 24), and left (−29, −47, 38) and right aIPS (29, −50, 39), which were similar to those defined from Experiment 1 alone. Taken together, these findings provide further evidence that the IFJ and aIPS are involved in process-based limitations.
Fig. 3.
SPM showing superior medial frontal cortex for the balanced contrast of the Discontinuous condition versus the Continuous and Continuous-hard conditions, created by combining data of Experiment 1 and Experiment 2 (n=22).
An additional analysis determined whether the IFJ and aIPS regions were the only ones of those activated by the experimental task to show a differential activation pattern in the Discontinuous and Continuous-hard conditions. Specifically, an open contrast SPM was created by collapsing data from both experiments and assigning a regression coefficient of 1 to each condition, implicitly contrasting all epochs of task performance against the ITIs (baseline). This contrast, thresholded at p<.05, corrected using a cluster filter of five contiguous voxels, revealed activation foci in the bilateral IFJ and anterior IPS similar to those identified by the balanced and conjunction contrast SPMs from Experiment 1 participants alone (Table 2). The timecourse analysis of the IFJ and aIPS from this pooled dataset showed that activations in these ROIs were greater for the Discontinuous than for the other conditions (IFJ: Discontinuous-Continuous, t(21)=3.46, p=.0024, Discontinuous-Continuous-hard. t(21) = 2.93, p = .0080. aIPS: Discontinuous–Continuous, t(21)=6.12, p=.0007, Discontinuous–Continuous-hard. t(21)=2.83, p=.0010). In addition to the IFJ and aIPS, the posterior IPS, frontal eyefields (FEF), anterior cingulate (AC), and anterior insula (AI) were isolated by the open contrast, but the timecourse analyses revealed that none of these regions showed significantly greater activity in the Discontinuous condition than in the other conditions (ps>.17). Finally, when the conjunction contrast was restricted to regions masked by the open contrast, the resulting SPM (p<.05, corrected using a cluster filter of six contiguous voxels), showed significant activation foci only in the left (−44, 0, 35) and right IFJ (48, 10, 21), and left (−29, −47, 39) and right aIPS (32, −46, 39), consistent with the timecourse analyses and further attesting to the pivotal involvement of these two brain regions in process-based attention.
Table 2.
Brain regions isolated with the Open contrast. The bilateral IFJ and aIPS showed greater activation for the Discontinuous than for the Continuous and Continuous-hard conditions (all ps<.05, two-tailed).
| ROIs from the Open contrast (N=22) | |||||
|---|---|---|---|---|---|
| ROI name | Mean t value |
Mean Talairach Coordinates |
Volume (mm3) |
||
| X | Y | Z | |||
| Left inferior frontal junction (IFJ) | 11.77 | −47 | 4 | 23 | 1303 |
| Right inferior frontal junction (IFJ) | 6.14 | 45 | 4 | 26 | 1889 |
| Left anterior intraparietal sulcus (aIPS) | 9.67 | −30 | −45 | 42 | 1163 |
| Right anterior intraparietal sulcus (aIPS) | 7.54 | 27 | −45 | 42 | 1001 |
| Left posterior intraparietal sulcus (pIPS) | 11.04 | −24 | −59 | 42 | 1307 |
| Right posterior intraparietal sulcus (pIPS) | 9.53 | 23 | −57 | 45 | 1309 |
| Left frontal eyefield | 4.29 | −31 | −5 | 43 | 1625 |
| Right frontal eyefield | 3.79 | 28 | −6 | 47 | 1263 |
| Left anterior cingulate | 4.06 | −6 | 5 | 46 | 1292 |
| Right anterior cingulate | 3.95 | 5 | 9 | 43 | 1281 |
| Left anterior insula | 5.10 | −29 | 20 | 2 | 1317 |
| Right anterior insula | 4.04 | 31 | 15 | 2 | 1208 |
In a separate analysis, we examined whether the parieto-frontal areas (IFJ, ±47, 9, 32; IPS, ±29, −59, 50) previously implicated in the AB (Marois et al., 2000, 2004a) showed greater activity in the Discontinuous condition. To do so, an ROI mask that includes the lateral prefrontal and dorsal parietal regions engaged in these AB studies was first applied, and the conjunction contrast was run on this mask (see Methods). The resulting SPM (p<.05, corrected for multiple comparisons using a cluster filter of six contiguous voxels), revealed significant activational foci in the left (−49, 4, 32) and right lateral prefrontal (46, 7, 27), and right dorsal parietal regions (31, −58, 50). Timecourse analyses of these prefrontal and dorsal parietal regions revealed greater activity in the lateral prefrontal cortex for the Discontinuous condition (Discontinuous-Continuous, t(21)=2.49, p<.05, Discontinuous-Continuous-hard. t(21)=3.01, p<.01, two-tailed). The parietal region (IPS) also showed a similar pattern (Discontinuous-Continuous, t(21)=3.48, p<.01, Discontinuous-Continuous-hard. t(21)=2.05, p=.050, two-tailed). These findings suggest that the parieto-frontal attention network implicated in the AB is also sensitive to increased demands for attentional control (see also Marois et al., 2004a).
Discussion
The key finding of the present study is that bilateral IFJ and anterior IPS are preferentially engaged under process-limiting conditions compared to data-limiting conditions, even when these two conditions are equated for task performance. Although the superior medial frontal/anterior cingulate cortex also demonstrated a similar pattern of activation based on the data collapsed across experiments, this result must be validated in a statistically independent confirmatory experiment. While the activity of IFJ and aIPS was preferentially associated with process-based limitations, no region was found to be preferentially activated under data-based limitations. This single dissociation is predicted from the original framework of process-based versus data-based limits; only the former strains attentional processing while the latter, which is independent of processing resources, does not impose any additional processing demands on the system (Norman and Bobrow, 1975). Even though data-based limitations cannot be resolved by additional effort exerted by the attentional system following a brief stimulus presentation, we surmise that there might be a mechanism that attempts to compensate for the impoverishment of data to be processed under conditions in which subjects know ahead of the stimulus presentation that it is a Data-limited trial (e.g. by blocking trial types). Under such condition, increasing the level of arousal or top–down attention to prepare for tasks of greater difficulty might contribute to alleviating behavioral costs induced by data-based limitations.
IFJ and IPS activity has previously been observed in several attentional manipulations (Asplund et al., 2010; Brass et al., 2005; Corbetta and Shulman, 2002), but none had directly tested whether these areas are modulated solely or primarily by processing demands rather than by difficulty. While other brain imaging studies have found parieto-frontal cortex activation with different manipulations of task difficulty (Chee and Tan, 2010; Heekeren et al., 2004; Marois et al., 2000, 2004b), these difficulty manipulations did not categorically dissociate between process-based and data-based limitations because they either did not involve both types of limitations, failed to equate for task difficulty and time-on-task, or they involved degrading target detection performance using procedures that can affect both the quality of the target data and its processing (e.g. noise addition, lateral or spatial masking). By contrast, the present manipulation of data limitation was categorically distinct from the manipulation of process limitation, yet it generated similar performance costs to the process manipulation despite only minute changes in overall stimulus duration.
The minimal difference of stimulus presentation durations across conditions likely explains why no significant brain activation differences were observed between the Continuous-hard and Continuous conditions. While it is possible, if not likely, that such activation differences would appear if the stimulus duration and task performance differences were increased between these two conditions, our results clearly show that when task performance is matched between the Continuous-hard (data-based limit) and Discontinuous (process-based limit) conditions, only the latter manipulation modulates the parieto-frontal network compared to the Continuous condition. As such, our findings provide support for a dissociation between process-based and data-based limitations in the human brain, as predicted from classic behavioral work (Jannati et al., 2012; McLaughlin et al., 2001; Norman and Bobrow, 1975).
The current results also fit well with recent evidence that a parietofrontal network composed of the IFJ and IPS is a key neural substrate for attentional control (see Asplund et al., 2010). These regions, especially the IFJ, have been implicated in working memory encoding (Todd et al., 2011), response selection (Dux et al., 2006, 2009; Sigman and Dehaene, 2008), and in their shared limitations (Marti et al., 2012; Tombu et al., 2011). The engagement of the IFJ in these capacity-limited processes might reflect its general involvement in attentional allocation to meet task demands.
The present study also has important implications for models of attentional limits to conscious perception. First, the results inform accounts of the attentional blink (AB). The fact that brain regions previously implicated in attention control (Asplund et al., 2010; Corbetta and Shulman, 2002; Kastner et al., 2007) were modulated by process manipulations in the present experiments lends credence to the hypothesis that the AB ultimately reveals limitations in the control of attention (Di Lollo et al., 2005; Dux and Marois, 2009; Nieuwenstein et al., 2009; Olivers et al., 2007). These attentional control accounts of the AB have argued against the hypothesis that the AB results from depletion of attentional resources for the consolidation of visual items into working memory (Chun and Potter, 1995; Ouimet and Jolicoeur, 2007). This is because even when working memory encoding was heavily taxed by presenting multiple targets successively, as in our Continuous condition, these studies have observed no AB (Di Lollo et al., 2005; Kawahara et al., 2006; Nieuwenstein and Potter, 2006). By contrast, a severe AB was observed when a distractor, or even a blank interval, was inserted between targets. These latter manipulations are thought to tax the control mechanisms for switching attention from one target to another (Di Lollo et al., 2005; Kawahara et al., 2006; Nieuwenstein et al., 2009). Although our current results are highly consistent with loss of attentional control models of the AB, they are not inconsistent with working memory encoding models either (Chun and Potter, 1995; Ouimet and Jolicoeur, 2007), as a similar IFJ region has been directly implicated in working memory encoding (Todd et al., 2011). These seemingly contradictory findings can be reconciled by positing that WM encoding is an attention-demanding process, and that manipulations that increase WM encoding load will correspondingly increase the attentional, and therefore neural, demands for encoding of task-relevant items (Dux and Marois, 2009; see also Martens and Wyble, 2010).
Finally, the results are also highly consistent with Dehaene et al.’s (2006) proposition that the parieto-frontal attention network may only limit awareness under conditions where this network's capacity is overloaded by task processing demands, as they demonstrate that robust modulation of the parieto-frontal attention network is observed only when processing resources are strained. Furthermore, our results situate that model in a broader cognitive framework in which information processing – including information processing for conscious perception – can either be data-limited or process-limited (Norman and Bobrow, 1975).
Acknowledgments
This work was supported by an NIMH grant R01 MH70776 to R.M. and a P30-EY008126 grant to the VVRC.
Footnotes
The term ‘resources’ is used to denote flexible, energetic forms of commodities necessary for processing (Kok, 1997). However, in the distinction between resource-limit and data-limit, resource-limit may not only imply limitations originating from finite amount of flexible resource, but also limits from scarcity or temporary unavailability of processing devices. Hence, we favor the term ‘process-based limit’ in this article as it clearly conveys that what is ultimately limited is the system’s ability to process information, in contrast to limitations in the amount or quality of data to be processed (data-based limit).
The authors have no conflict of interest.
Contributor Information
Suk Won Han, Email: suk.w.han@vanderbilt.edu.
René Marois, Email: rene.marois@vanderbilt.edu.
References
- Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral pre-frontal cortex in goal-directed and stimulus-driven attention. Nat. Neurosci. 2010;13:507–513. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DM, Rees G, Frith CD, Lavie N. Neural correlates of change detection and change blindness. Nat. Neurosci. 2001;4:645–650. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn. Sci. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Tan JC. Lapsing when sleep deprived: neural activation characteristics of resistant and vulnerable individuals. Neuroimage. 2010;51:835–843. doi: 10.1016/j.neuroimage.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. J. Exp. Psychol. Hum. Percept. Perform. 1995;21:109–127. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux J-P, Naccache L, Sackur J, Sergent C. Conscious, precon-scious, and subliminal processing:a testable taxonomy. Trends Cogn. Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Kawahara J-I, Ghorashi SMS, Enns JT. The attentional blink: resource depletion or temporary loss of control. Psychol. Res. 2005;69:191–200. doi: 10.1007/s00426-004-0173-x. [DOI] [PubMed] [Google Scholar]
- Dux PE, Marois R. The attentional blink: a review of data and theory. Atten. Percept. Psychophys. 2009;71:1683–1700. doi: 10.3758/APP.71.8.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Ivanoff J, Asplund CL, Marois R. Isolation of a central bottleneck of information processing with time-resolved fMRI. Neuron. 2006;52:1109–1120. doi: 10.1016/j.neuron.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Asplund CL, Marois R. An attentional blink for sequentially presented targets: evidence in favor of resource depletion accounts. Psychon. Bull. Rev. 2008;15:809–813. doi: 10.3758/pbr.15.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, Asplund CL, Marois R. Both exogenous and endogenous target salience manipulations support resource depletion accounts of the attentional blink: a reply to Olivers, Spalek, Kawahara & Di Lollo. Psychon. Bull. Rev. 2009;16:219–224. doi: 10.3758/PBR.16.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dux PE, et al. Training improves multitasking performance by increasing the speed of information processing in human prefrontal cortex. Neuron. 2009;63:127–138. doi: 10.1016/j.neuron.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DL. Central capacity limits in consistent mapping, visual search tasks: four channels or more. Cogn. Psychol. 1984;16:449–484. doi: 10.1016/0010-0285(84)90017-3. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Garner WR. The stimulus in information processing. Am. Psychol. 1970;25:350–358. [Google Scholar]
- Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431:859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- Jannati A, Spalek TM, Lagroix HE, Di Lollo V. The attentional blink is not affected by backward masking of T2, T2-mask SOA, or level of T2 impoverishment. J. Exp. Psychol. Hum. Percept. Perform. 2012;38:161–168. doi: 10.1037/a0025985. [DOI] [PubMed] [Google Scholar]
- Kastner S, DeSimone K, Konen CS, Szczepanski SM, Weiner KS, Schneider KA. Topographic maps in human frontal cortex revealed inmemory-guided saccade and spatial working-memory tasks. J. Neurophysiol. 2007;97:3494–3507. doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- Kawahara J-I, Kumada T, Di Lollo V. The attentional blink is governed by a temporary loss of control. Psychon. Bull. Rev. 2006;13:886–890. doi: 10.3758/bf03194014. [DOI] [PubMed] [Google Scholar]
- Kok A. Event-related-potential (ERP) reflections of mental resources: a review and synthesis. Biol. Psychol. 1997;45:19–56. doi: 10.1016/s0301-0511(96)05221-0. [DOI] [PubMed] [Google Scholar]
- Lavie N, DeFockert JW. Contrasting effects of sensory limits and capacity limits in visual selective attention. Percept. Psychophys. 2003;65:202–212. doi: 10.3758/bf03194795. [DOI] [PubMed] [Google Scholar]
- Lloyd S. Ultimate physical limits to computation. Nature. 2000;406:1047–1054. doi: 10.1038/35023282. [DOI] [PubMed] [Google Scholar]
- Marois R, Chun MM, Gore JC. Neural correlates of the attentional blink. Neuron. 2000;28:299–308. doi: 10.1016/s0896-6273(00)00104-5. [DOI] [PubMed] [Google Scholar]
- Marois R, Chun MM, Gore JC. A common parieto-frontal network is recruited under both low visibility and high perceptual interference conditions. J. Neurophysiol. 2004a;92:2985–2992. doi: 10.1152/jn.01061.2003. [DOI] [PubMed] [Google Scholar]
- Marois R, Yi D-J, Chun MM. The neural fate of consciously perceived and missed events in the attentional blink. Neuron. 2004b;41:465–472. doi: 10.1016/s0896-6273(04)00012-1. [DOI] [PubMed] [Google Scholar]
- Martens S, Wyble B. The attentional blink: past, present, and future of a blind spot in perceptual awareness. Neurosci. Biobehav. Rev. 2010;34:947–957. doi: 10.1016/j.neubiorev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti S, Sigman M, Dehaene S. A shared cortical bottleneck underlying attentional blink and psychological refractory period. Neuroimage. 2012;59:2883–2898. doi: 10.1016/j.neuroimage.2011.09.063. [DOI] [PubMed] [Google Scholar]
- McLaughlin EN, Shore DI, Klein RM. The attentional blink is immune to masking-induced data limits. Q. J. Exp. Psychol. 2001;54:169–196. doi: 10.1080/02724980042000075. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J-B. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Potter MC. Temporal limits of selection and memory encoding: a comparison of whole versus partial report in rapid serial visual presentation. Psychol. Sci. 2006;17:471–475. doi: 10.1111/j.1467-9280.2006.01730.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenstein MR, Potter MC, Theeuwes J. Unmasking the attentional blink. J. Exp. Psychol. Hum. Percept. Perform. 2009;35:159–169. doi: 10.1037/0096-1523.35.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman DA, Bobrow DG. On data-limited and resource-limited processes. Cogn. Psychol. 1975;4:44–64. [Google Scholar]
- Olivers CNL, Van Der Stigchel S, Hulleman J. Spreading the sparing: against a limited-capacity account of the attentional blink. Psychol. Res. 2007;71:126–139. doi: 10.1007/s00426-005-0029-z. [DOI] [PubMed] [Google Scholar]
- Ouimet C, Jolicoeur P. Beyond task 1 difficulty: the duration of T1 encoding modulates the attentional blink. Vis. Cogn. 2007;15:290–304. [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink. J. Exp. Psychol. Hum. Percept. Perform. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat. Rev. Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Saville DJ. Multiple comparison procedures: the practical solution. Am. Stat. 1990;44:174–180. [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychol. Sci. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Sigman M, Dehaene S. Brain mechanisms of serial and parallel processing during dual-task performance. J. Neurosci. 2008;28:7585–7598. doi: 10.1523/JNEUROSCI.0948-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ. Attentional capture and inattentional blindness. Trends Cogn. Sci. 2000;4:147–155. doi: 10.1016/s1364-6613(00)01455-8. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Han SW, Harrison S, Marois R. The neural correlates of visual working memory consolidation: a time-resolved fMRI study. Neuropsychologia. 2011;49:1527–1536. doi: 10.1016/j.neuropsychologia.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombu MN, Asplund CL, Dux PE, Godwin D, Martin JW, Marois R. A Unified attentional bottleneck in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13426–13431. doi: 10.1073/pnas.1103583108. [DOI] [PMC free article] [PubMed] [Google Scholar]