Abstract
Pancreatic polypeptide (PP) is a major agonist for neuropeptide Y4 receptors (NPY4R). While NPY4R has been identified in various tissues, the cells on which it is expressed and its function in those cells has not been clearly delineated. Here we report that NPY4R is present in all somatostatin-containing cells of tissues that we tested, including pancreatic islets, duodenum, hippocampus, and hypothalamus. Its agonism by PP decreases somatostatin secretion from human islets. Mouse embryonic hippocampal (mHippo E18) cells expressed NPY4Rs and their activation by PP consistently decreased somatostatin secretion. Furthermore, central injection of PP in mice induced c-Fos immunoreactivity in somatostatin-containing cells in the hippocampus compared with PBS-injected mice. In sum, our results identify PP as a pivotal modulator of somatostatin secretion.
Keywords: Pancreatic polypeptide, NPY4 receptor, Somatostatin secretion
1. Introduction
Pancreatic polypeptide (PP), gut derived peptide YY (PYY) and the neuronal derived peptide neuropeptide Y (NPY) belong to a family of structurally related peptides which have functions in both neural and endocrine signaling [1,2]. Relatively little is known about the functional significance of PP, a 36–amino acid peptide secreted by PP (or F) cells of the islets of Langerhans in the pancreas and released into the circulation. PP is also expressed in endocrine cells of both small and large intestine [3,4]. It shares considerable homology with the peptide sequences of PYY and NPY, and is reported to have effects on several gastrointestinal functions such as gastric motility, gallbladder contraction, and pancreatic exocrine secretion [5]. Previous studies have shown that PP appears to be involved in regulating food intake and energy balance [6]. Transgenic mice overexpressing PP gained less weight because of decreased food intake and this was accompanied by decreased fat mass [7]. Peripheral administration of PP to genetically obese ob/ob mice induced a state of negative energy balance because of decreased food intake and increased energy expenditure [8]. In humans, intravenous infusions of PP reduced food intake [9], and low circulating levels of PP have been observed in obese people [10-12]. PP secretion was thought to be primarily under vagal control [13], although other factors have also been shown to alter circulating PP concentrations [14]. Increasing plasma concentrations are seen after the ingestion of food and remain elevated for up to 6 hours [1,2,15]. In type 2 diabetic subjects, PP cells secrete excess PP and plasma PP levels are significantly elevated in the postprandial state, compared to non-diabetic subjects [16].
The biological effects of the NPY family peptides are exerted through the NPY receptor family, which consists of at least five distinct members (Y1, Y2, Y4, Y5, and Y6) [17]. As PP has the highest affinity for the NPY4 receptor (NPY4R), it is thought to be the major endogenous ligand for this receptor [18]. PP dose-dependently reduced food intake in fed and fasted mice, and this effect was completely abolished in NPY4R-null mice, indicating that the effect is entirely mediated by NPY4Rs [19]. NPY4R is a G protein-coupled receptor and its activation by PP inhibits forskolin-stimulated cAMP synthesis in several NPY4R-containing cell lines [18,20,21]. NPY4Rs are present in the peripheral organs, including the gastrointestinal tract, liver, pancreas, and heart [18,20,22-24]. Additionally, significant amounts of NPY4R mRNA and specific binding sites have been found in key areas of the brain including hypothalamus and hippocampus [20,23,24], underscoring the importance of the receptor to food intake. Because circulating PP levels are highest after eating, we investigated if PP, besides modulating food intake, is involved in locally regulating secretion from any of the islet cell types. We found that NPY4Rs are expressed in somatostatin-containing delta (δ) cells in islets of Langerhans, but not the other islet cell types. We then investigated if somatostatin-expressing cells in other organs, such as in duodenum and brain, also express NPY4R.
2. Materials and Methods
2.1. Materials and reagents
mHippo E18 cells were from CELLutions Biosystems Inc. The somatostatin EIA kit was from Phoenix Pharmaceuticals, human insulin ELISA from Mercodia, and pancreatic polypeptide (PP) was from BACHEM. Human islets were provided by the NIDDK-funded Integrated Islet Distribution Program (IIDP) at City of Hope. Frozen brain sections (40 μm) were from a 10 year old male rhesus macaque. Dr. Frederic B. Askin from the Department of Pathology at The Johns Hopkins University School of Medicine provided anonymous human tissues.
2.2. Cell culture and somatostatin and insulin secretion
mHippo E18 cells and insulin-secreting MIN6 cells were cultured in DMEM (Invitrogen) with 10% fetal bovine serum (FBS), 25 mM glucose, and 1% penicillin/streptomycin and maintained at 37°C with 5% CO2. Cells were seeded into 35 mm dishes, grown to confluency, washed 1× with PBS, and treated with 0.1 μM of PP or PBS as vehicle in triplicate for 48 hours. Media was collected and somatostatin levels were measured using a somatostatin EIA kit. For human islet experiments, we picked 100 size-matched islets per tube in Krebs buffer followed by 30 min incubation at 37°C. We pelleted the islets and treated with Krebs buffer containing 1 μM of PP or PBS. After 20 min incubation at 37°C, we collected the supernatant and somatostatin levels were measured using a somatostatin EIA kit and insulin was measured by ELISA according to the manufacturers’ instructions. Total protein concentration was measured by BSA assay (Pierce) for normalization.
2.3. Western blotting and densitometry
Cells and homogenized mouse liver were lysed in RIPA buffer (25 mM HEPES, 134 mM NaCl, 1% NP-40, 0.1% SDS, 1 mM sodium orthovanadate, 0.5% sodium deoxycholate, 100 mM NaF) supplemented with protease inhibitor cocktail set I and phosphatase inhibitor cocktail set II (Calbiochem) for 20 min ice with occasional vortexing, and then pelleted in a microcentrifuge at maximum speed for 20 min to remove insoluble material. Following a BCA protein assay, 50 μg of lysate was resolved on 4-12% Tris-glycine gels (Invitrogen) and blotted onto polyvinylidene difluoride (PVDF) membranes using the iBlot (Invitrogen). The blots were blocked in 5% milk/TBS-T for 1 h at room temperature and probed with anti-NPY4R (1:1000, Santa Cruz) or anti-GAPDH (1:500, Santa Cruz) antibodies in blocking buffer overnight at 4°C. Membranes were washed with TBS-T and the appropriate secondary antibody (1:5000, GE Heathcare) was added in 5% milk/TBS-T for 1 h at room temperature. Membranes were washed again with TBS-T and developed using ECL Plus (GE Heathcare). Western blot images were scanned, saved as Tiff files, inverted, and integrated density was analyzed using ImageJ software (National Institutes of Health). Values were normalized to GAPDH.
2.4. Transcranial stereotaxic injection of PP to the hippocampus
Two-month-old male B6C3F1/J mice were initially anesthetized with 4% isoflurane and then maintained on 1.5% isoflurane anesthesia for the duration of the surgery. During stereotaxic surgeries, mice were maintained on a heating pad to ensure constant body temperature. Mouse heads were secured in a stereotaxic apparatus and a longitudinal midsagittal cut was made with a sterile scalpel to expose the skull. Injections were performed through a small hole in the skull drilled along the anterior-posterior and mediolateral coordinates using a 1.0 mm drill head. Injections to the dorsal hippocampus were performed at the following coordinates, representing distances in mm from skull bregma: anterior-posterior −2.1, mediolateral +/− 1.35 and dorsoventral −2.1. PP (1 μM) was injected in a constant flow of 0.2 μl/min followed by 3 min to allow the fluid to be absorbed by the tissue. PP was stereotaxically injected into right side of the hippocampus. An equal volume of PBS was injected into the left hippocampus to serve as a control. Injections were performed using a CMA-400 automatic pump pushing two Hamilton syringes simultaneously. The syringes were connected via PE10 tubing to a 27 gauge needles. Following the injections, the skin was sutured and mice were placed in a separate cage. Exactly at 15, 30 and 60 min after PP injection, mice were perfused with 4% paraformaldehyde and the brains were rapidly dissected.
2.5. Immunostaining
Paraffin-embedded human islets and tissues were prepared as previously described (25). For frozen sections, tissues were rapidly dissected, fixed in 4% paraformaldehyde, immersed in 30% sucrose, embedded in O.C.T compound (Tissue-Tek) before freezing, and then sectioned at a thickness of 7 μm. After antigen unmasking (BioGenex), the tissues were blocked with 5% BSA/PBS for 1 h at room temperature and incubated overnight at 4°C with anti-insulin (1:500; Millipore), anti-glucagon (1:1000; Sigma), anti-PP (1:1000; Millipore), anti-NPY4R (1:100; Santa Cruz), anti-somatostatin (1:200; Santa Cruz) and anti-c-Fos (1:100; Santa Cruz) antibodies. After washing, tissues were incubated with the appropriate secondary antibodies (1:500, Jackson ImmunoResearch) along with TO-PRO-3 (1:5000, Molecular Probes), in some cases, for nuclear staining. Slides were viewed using a LSM-710 confocal microscope (Carl Zeiss MicroImaging).
2.6. Statistical analysis
Quantitative data are presented as the mean ± s.e.m. Differences between mean values were compared statistically by Student’s t-test. Comparisons were performed by using Graphpad Prism (GraphPad Software). A p value of < 0.05 was considered statistically significant.
3. Results
3.1. NPY4R is expressed in somatostatin-containing cells
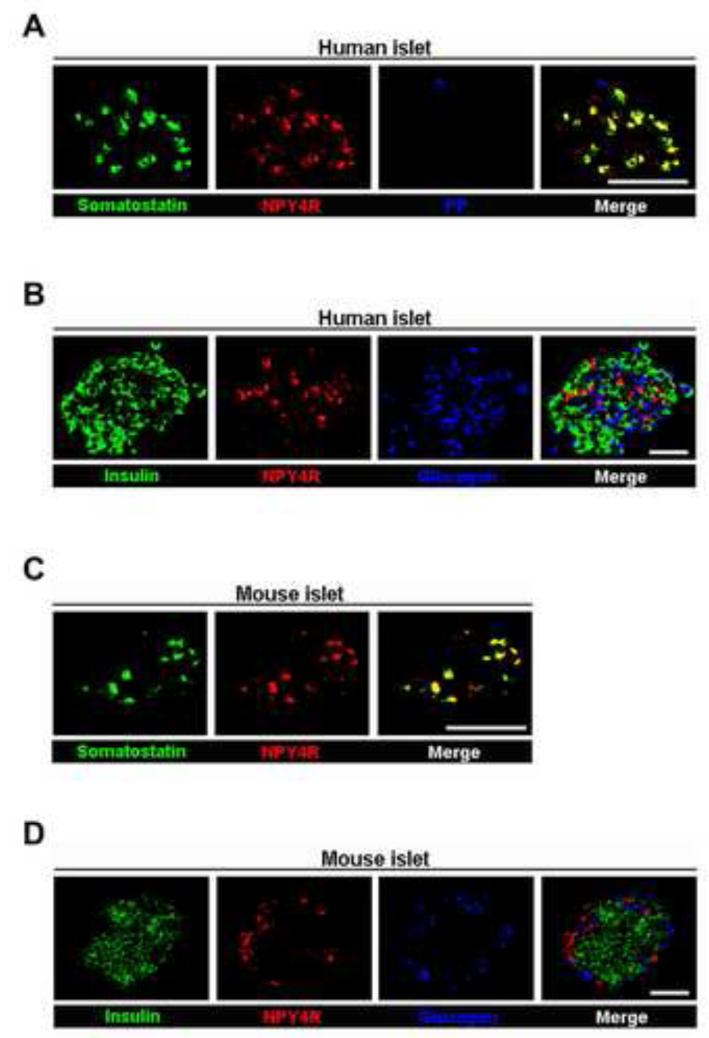
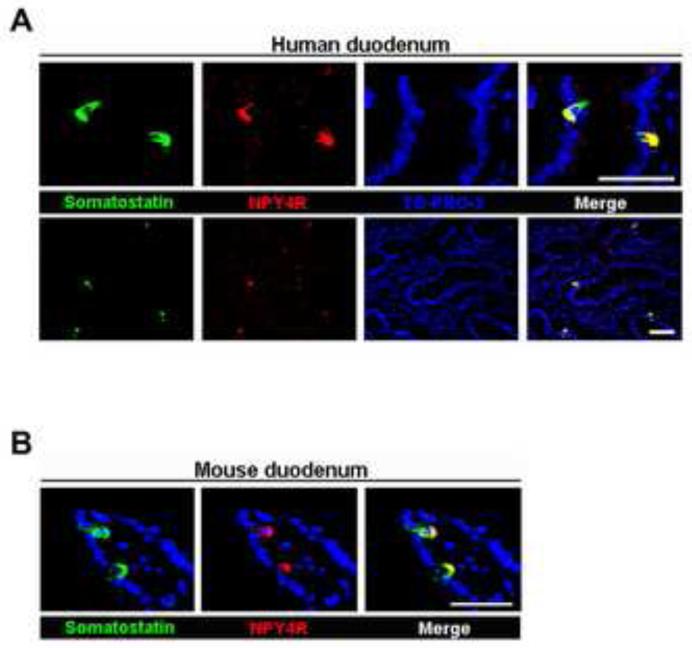
Somatostatin-containing cells are dispersed throughout the gut, pancreas, and brain [26-28]. We first looked for evidence of the presence of NPY4Rs in both human and mouse islets. By immunostaining, NPY4Rs were colocalized with somatostatin-containing cells (Fig. 1A), but were absent from PP (F), insulin-containing (β), and glucagon-containing (α) cells in human islets (Fig. 1A and B). Consistent with this result, NPY4Rs were only present in somatostatin-containing cells in mouse islets (Fig. 1C and D). We also confirmed their presence in the somatostatin-containing cells in both human and mouse duodena by immunostaining (Fig. 2A and B).
Fig. 1.
The colocalization of NPY4R with somatostatin-containing cells in pancreatic islets. (A) Immunofluorescent images showing that NPY4R (red) is colocalized with somatostatin-containing cells (green), but not PP cells (blue), in human islets. (B) Immunofluorescent images showing that NPY4R (red) is not colocalized with insulin (green) or glucagon (blue) in human islets. (C) Immunofluorescent images showing that NPY4R (red) is colocalized with somatostatin-containing cells (green) in mouse islets. (D) Immunofluorescent images showing that NPY4R (red) is not colocalized with insulin (green) or glucagon (blue) in mouse islets. Scale bar = 50 μm.
Fig. 2.
The colocalization of NPY4R with somatostatin-containing cells in duodenum. Immunofluorescent images showing that NPY4R (red) colocalized with somatostatin (green) in human (A) and mouse (B) duodena. TO-PRO-3 (blue) was used to stain nuclei. Scale bar = 50 μm.
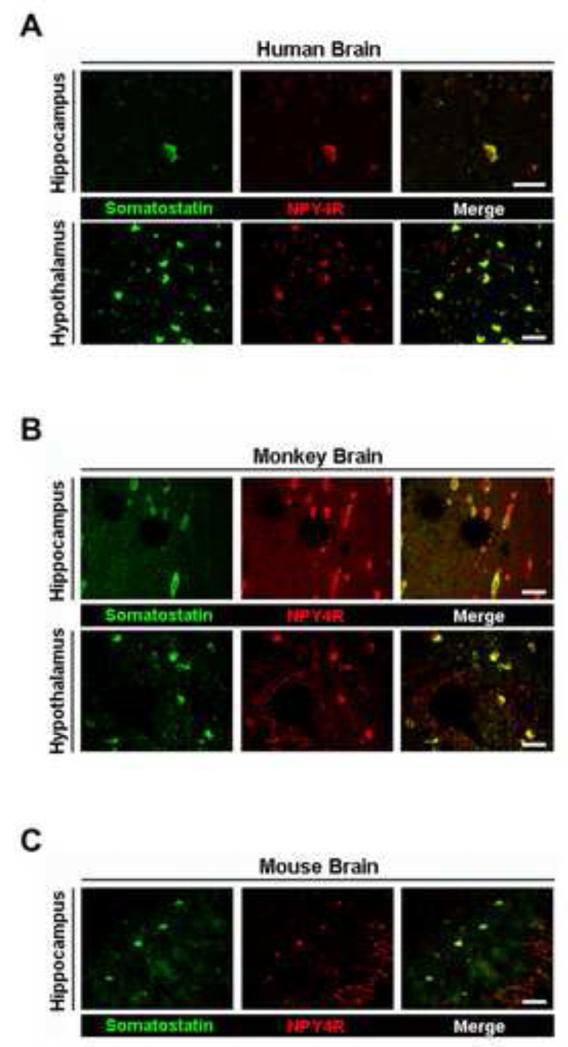
As somatostatin is also found in neural tissues including hypothalamus and hippocampus [29,30], we evaluated the presence of NPY4R in somatostatin-containing cells in brain. NPY4Rs were expressed in somatostatin-containing cells in the hippocampus and hypothalamus of both human and monkey brains (Fig. 3A and B) and in the hippocampus of mouse brain (Fig. 3C).
Fig. 3.
The colocalization of NPY4R with somatostatin-containing cells in brain. Immunofluorescent images showing that NPY4R (red) colocalized with somatostatin (green) in human (A) and monkey (B) hypothalamus and hippocampus and mouse (C) hippocampus. Scale bar = 50 μm.
3.2. PP inhibits somatostatin secretion
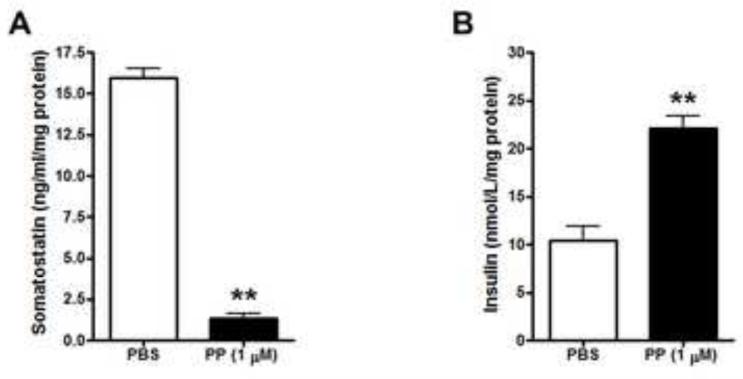
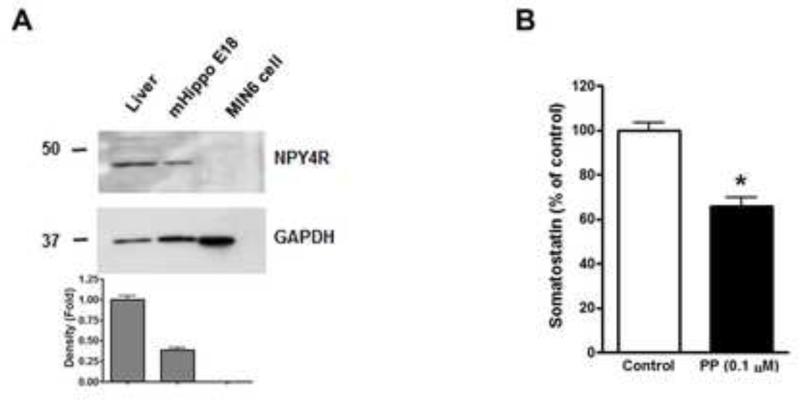
To investigate whether PP modulates somatostatin secretion, we treated human islets with 1 μM PP and measured somatostatin and insulin levels in the medium. PP treatment led to decreased somatostatin secretion from human islets (Fig. 4A). Conversely insulin secretion from human islets was significantly increased by PP (Fig. 4B), which is consistent with the fact that somatostatin inhibits insulin release from pancreatic islets [31,32]. To further confirm that PP modulates somatostatin secretion in other somatostatin-containing cells besides pancreatic δ cells, we used mouse embryonic hippocampal (mHippo E18) cells. Western blot analysis confirmed that NPY4Rs are expressed in mouse liver, as expected, and mHippo E18 cells, but lacking in MIN6 cells (Fig. 5A), as is true of β cells in situ (Fig. 1). Consistent with the results in isolated islets (Fig. 4A), somatostatin secretion from mHippo E18 cells was significantly decreased by 0.1 μM PP (Fig. 5B), confirming the inhibitory action of PP on somatostatin secretion in a receptor-dependent manner.
Fig. 4.
Effects of PP on somatostatin and insulin secretion in human islets. Levels of somatostatin (A) and insulin (B) secreted into the media from 100 size matched human islets incubated with 1 μM PP or PBS for 20 min were analyzed and normalized to total protein concentration. Data are shown as the mean ± s.e.m. **P < 0.01.
Fig. 5.
Effects of PP on somatostatin secretion in mouse embryonic hippocampal (mHippo E18) cells. (A) Western blot analysis of NPY4R expression in total lysates prepared from mouse liver, mHippo E18 cells, and MIN6 cells. Mouse liver and MIN6 cells were used as a positive and negative control, respectively, for NPY4R. Signals on Western blots were quantified by densitometry and shown in the bottom panel. (B) Somatostatin secretion from mHippo E18 cells incubated with 1 μM PP for 48 hours. Data are shown as the mean ± s.e.m. from three independent experiments. *P < 0.05.
3.3. PP activates somatostatin-containing cells in the hippocampus of mouse
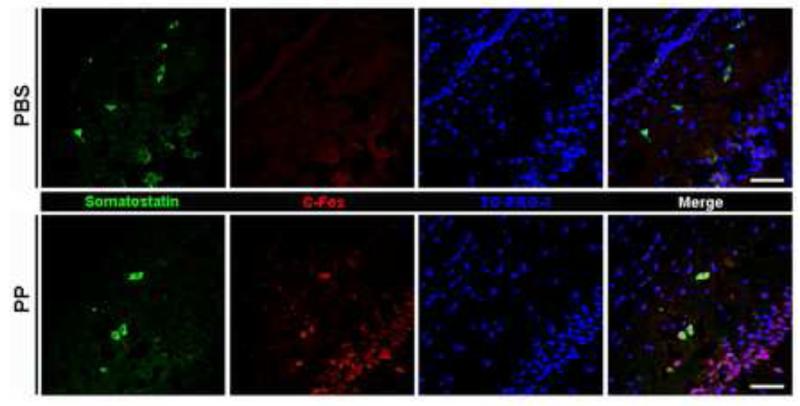
The evidence shown above is not a direct measure of neuronal activation and does not directly show functionally active NPY4Rs. To determine whether NPY4Rs are activated in somatostatin-containing cells in vivo, we investigated the expression of the neuronal activation marker c-Fos in somatostatin-containing cells of mouse brain as early as 30 minutes after PP injection into the hippocampus. No c-Fos immunoreactivity was seen in somatostatin-containing cells in PBS-injected controls, while after PP injection, c-Fos was easily visualized in those cells (Fig. 6).
Fig. 6.
c-Fos immunoreactivity in the hippocampus of PP-injected mice. Representative images showing somatostatin (green) and c-Fos (red) immunoreactivity in mouse hippocampus 30 minutes after injection with PBS (top panel) or PP (bottom panel). TO-PRO-3 (blue) was used to stain nuclei. Scale bar = 50 μm.
4. Discussion
Here we report the first evidence that NPY4R is present in mammalian somatostatin-containing cells, including δ cells in islets of Langerhans. While we do not have isolated islets from a NPY4R null mouse to provide direct evidence, it is likely that PP is activating NPY4R, leading to a decrease in somatostatin secretion. As somatostatin-containing δ cells are distributed widely throughout the nervous system, particularly hippocampus and hypothalamus, and in the gut [26-28,33], we tested those tissues for NPY4R expression and found that NPY4Rs are expressed in all somatostatin-containing cells that we tested. In pancreatic islets, NPY4Rs are expressed only in δ cells, and not in the other cell types (F, β, and α cells). Such degree of specificity led us to examine its functional significance to islet physiology.
PP treatment led to increased insulin secretion from isolated human islets, most likely because it decreased somatostatin secretion, a known inhibitor of insulin secretion [31,32]. We therefore present evidence for the functional significance of increased PP secretion within islets: it decreases somatostatin secretion that acts as a brake on insulin secretion. Elevated PP levels in type 2 diabetes [16] likely represent a compensatory response in an effort to restore normal blood glucose by decreasing somatostatin secretion within islets.
Gut-islet interactions have been well studied because incretin hormones (GIP, GLP-1) released from scattered enteroendocrine cells regulate glucose-mediated insulin secretion. We now show the converse in an islet-gut interaction through PP regulation of somatostatin-containing enteroendocrine cells. Somatostatin inhibits release of a large number of gastrointestinal endocrine hormones (CCK, secretin, gastrin, GIP, and GLP-1) but levels of all of these actually increase in the circulation after eating [34-36]. Hence suppression of somatostatin postprandially in the gut is a vital necessity. So gut-islet interactions via hormones are bidirectional. Additionally, somatostatin is known to inhibit pancreatic exocrine secretion, hepatic bile secretion, and gall bladder emptying [34,37,38]. These three effects need to be disinhibited after eating; a role PP now seems to be responsible for via local inhibition of somatostatin secretion.
Uncovering that PP, actively secreted after eating, may directly activate somatostatin-containing neurons in the brain is very interesting. We used c-Fos as a marker for neuronal activation by PP of somatostatin-containing cells because it is rapidly increased by many physiologic and pharmacologic stimuli in the central nervous system. Although somatostatin-positive cells comprise a relatively small subpopulation of hippocampal interneurons, they are anatomically positioned to mediate a powerful inhibitory drive on granule and pyramidal cell activity [39]. Nearly all somatostatin-expressing neurons throughout the hippocampus colocalize with GAD, and the vulnerability of these cells is thought to contribute to memory impairment in a variety of conditions, including Alzheimer Disease [40] and animal models of age-related neurodegeneration [41]. Consistent with this proposal, recent evidence indicates that somatostatin protein expression is dramatically decreased in the hilus of the dentate gyrus in a rat model of cognitive aging, selectively among aged individuals that display deficits in spatial memory dependent on the hippocampus [42]. Our data suggests that somatostatin-containing cells are dynamically regulated in the hippocampus and are highly responsive to the metabolic state of the animals, such as the fed/fasted state, a factor not previously appreciated. That somatostatin-expressing neurons are uniquely activated by a specific islet hormone, PP, demonstrates a direct peripheral to central connectivity previously not appreciated in this population of cells. It would be of interest to investigate if pharmacological levels of PP might protect against loss of somatostatin-containing cells in animal models of age-related neurodegeneration.
In summary, PP secretion is increased locally within islets after eating. There, it suppresses somatostatin secretion and consequently, a brake on insulin secretion is lifted. This allows the full capability of β cells to respond to incretins and glucose and release insulin as appropriate to the fed state. At the same time, PP secretion into the circulation increases and it then inhibits somatostatin secretin in the gut, again locally disinhibiting all the inhibitory functions of somatostatin on hormone secretion and dynamic gut-pancreatic-bile functions. Most intriguingly, PP activates somatostatin-containing cells in the brain. The full significance of this awaits further investigation.
Highlights.
NPY4Rs were expressed on somatostatin-containing cells of tissues.
PP inhibited somatostatin secretion from human islets and cells.
Central injection of PP activated somatostatin-containing cells in the hippocampus.
Our results identify PP as a pivotal modulator of somatostatin secretion.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging (NIA)/NIH. W.K was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future (NRF-2012R1A1A1041352) and the Ministry of Education (No. 2009-0093826).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Schwartz TW. Pancreatic polypeptide: a hormone under vagal control. Gastroenterology. 1983;85(6):1411–1425. [PubMed] [Google Scholar]
- [2].Gehlert DR. Multiple receptors for the pancreatic polypeptide (PP-fold) family: physiological implications. Proc Soc Exp Biol Med. 1998;218(1):7–22. doi: 10.3181/00379727-218-44263. [DOI] [PubMed] [Google Scholar]
- [3].Cox HM. Neuropeptide Y receptors; antisecretory control of intestinal epithelial function. Auton Neurosci. 2007;133(1):76–85. doi: 10.1016/j.autneu.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [4].Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46(6):261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kojima S, Ueno N, Asakawa A, Sagiyama K, Naruo T, Mizuno S, Inui A. A role for pancreatic polypeptide in feeding and body weight regulation. Peptides. 2007;28(2):459–463. doi: 10.1016/j.peptides.2006.09.024. [DOI] [PubMed] [Google Scholar]
- [6].Inui A. Neuropeptide Y feeding receptors: are multiple subtypes involved? Trends Pharmacol Sci. 1999;20(2):43–46. doi: 10.1016/s0165-6147(99)01303-6. [DOI] [PubMed] [Google Scholar]
- [7].Ueno N, Inui A, Iwamoto M, Kaga T, Asakawa A, Okita M, Fujimiya M, Nakajima Y, Ohmoto Y, Ohnaka M, Nakaya Y, Miyazaki JI, Kasuga M. Decreased food intake and body weight in pancreatic polypeptide-overexpressing mice. Gastroenterology. 1999;117(6):1427–1432. doi: 10.1016/s0016-5085(99)70293-3. [DOI] [PubMed] [Google Scholar]
- [8].Asakawa A, Inui A, Yuzuriha H, Ueno N, Katsuura G, Fujimiya M, Fujino MA, Niijima A, Meguid MM, Kasuga M. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124(5):1325–1336. doi: 10.1016/s0016-5085(03)00216-6. [DOI] [PubMed] [Google Scholar]
- [9].Berntson GG, Zipf WB, O’Dorisio TM, Hoffman JA, Chance RE. Pancreatic polypeptide infusions reduce food intake in Prader-Willi syndrome. Peptides. 1993;14(3):497–503. doi: 10.1016/0196-9781(93)90138-7. [DOI] [PubMed] [Google Scholar]
- [10].Glaser B, Zoghlin G, Pienta K, Vinik AI. Pancreatic polypeptide response to secretin in obesity: effects of glucose intolerance. Horm Metab Res. 1988;20(5):288–292. doi: 10.1055/s-2007-1010817. [DOI] [PubMed] [Google Scholar]
- [11].Reinehr T, Enriori PJ, Harz K, Cowley MA, Roth CL. Pancreatic polypeptide in obese children before and after weight loss. Int J Obes (Lond) 2006;30(10):1476–1481. doi: 10.1038/sj.ijo.0803393. [DOI] [PubMed] [Google Scholar]
- [12].Jia BQ, Taylor IL. Failure of pancreatic polypeptide release in congenitally obese mice. Gastroenterology. 1984;87(2):338–343. [PubMed] [Google Scholar]
- [13].Taylor IL, Impicciatore M, Carter DC, Walsh JH. Effect of atropine and vagotomy on pancreatic polypeptide response to a meal in dogs. Am J Physiol. 1978;235(4):E443–E447. doi: 10.1152/ajpendo.1978.235.4.E443. [DOI] [PubMed] [Google Scholar]
- [14].Linnestad P, Schrumpf E. Pancreatic polypeptide release stimulated by food, secretin and cholecystokinin in chronic pancreatitis. Scand J Gastroenterol. 1983;18(3):385–389. doi: 10.3109/00365528309181611. [DOI] [PubMed] [Google Scholar]
- [15].Adrian TE, Bloom SR, Bryant MG, Polak JM, Heitz PH, Barnes AJ. Distribution and release of human pancreatic polypeptide. Gut. 1976;17(12):940–944. doi: 10.1136/gut.17.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Floyd JC, Jr, Fajans SS, Pek S, Chance RE. A newly recognized pancreatic polypeptide; plasma levels in health and disease. Recent Prog Horm Res. 1976;33:519–570. doi: 10.1016/b978-0-12-571133-3.50019-2. [DOI] [PubMed] [Google Scholar]
- [17].Blomqvist AG, Herzog H. Y-receptor subtypes--how many more? Trends Neurosci. 1997;20(7):294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- [18].Lundell I, Blomqvist AG, Berglund MM, Schober DA, Johnson D, Statnick MA, Gadski RA, Gehlert DR, Larhammar D. Cloning of a human receptor of the NPY receptor family with high affinity for pancreatic polypeptide and peptide YY. J Biol Chem. 1995;270(49):29123–29128. doi: 10.1074/jbc.270.49.29123. [DOI] [PubMed] [Google Scholar]
- [19].Balasubramaniam A, Mullins DE, Lin S, Zhai W, Tao Z, Dhawan VC, Guzzi M, Knittel JJ, Slack K, Herzog H, Parker EM. Neuropeptide Y (NPY) Y4 receptor selective agonists based on NPY(32-36): development of an anorectic Y4 receptor selective agonist with picomolar affinity. J Med Chem. 2006;49(8):2661–2665. doi: 10.1021/jm050907d. [DOI] [PubMed] [Google Scholar]
- [20].Bard JA, Walker MW, Branchek TA, Weinshank RL. Cloning and functional expression of a human Y4 subtype receptor for pancreatic polypeptide, neuropeptide Y, and peptide YY. J Biol Chem. 1995;270(45):26762–26765. doi: 10.1074/jbc.270.45.26762. [DOI] [PubMed] [Google Scholar]
- [21].Yan H, Yang J, Marasco J, Yamaguchi K, Brenner S, Collins F, Karbon W. Cloning and functional expression of cDNAs encoding human and rat pancreatic polypeptide receptors. Proc Natl Acad Sci U S A. 1996;93(10):4661–4665. doi: 10.1073/pnas.93.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Barrios VE, Sun J, Douglass J, Toombs CF. Evidence of a specific pancreatic polypeptide receptor in rat arterial smooth muscle. Peptides. 1999;20(9):1107–1113. doi: 10.1016/s0196-9781(99)00106-0. [DOI] [PubMed] [Google Scholar]
- [23].Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11(4):1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- [24].Larsen PJ, Kristensen P. Central Y4 receptor distribution. Radioactive ribonucleotide probe in situ hybridization with in vitro receptor autoradiography. Methods Mol Biol. 2000;153:185–198. doi: 10.1385/1-59259-042-X:185. [DOI] [PubMed] [Google Scholar]
- [25].Fiori JL, Shin YK, Kim W, Krzysik-Walker SM, González-Mariscal I, Carlson OD, Sanghvi M, Moaddel R, Farhang K, Gadkaree SK, Doyle ME, Pearson KJ, Mattison JA, de Cabo R, Egan JM. Resveratrol prevents β-cell dedifferentiation in nonhuman primates given a high-fat/high-sugar diet. Diabetes. 2013;62(10):3500–3513. doi: 10.2337/db13-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Benali N, Ferjoux G, Puente E, Buscail L, Susini C. Somatostatin receptors. Digestion. 2000;62(Suppl 1):27–32. doi: 10.1159/000051852. [DOI] [PubMed] [Google Scholar]
- [27].Møller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim Biophys Acta. 2003;1616(1):1–84. doi: 10.1016/s0005-2736(03)00235-9. [DOI] [PubMed] [Google Scholar]
- [28].Pintér E, Helyes Z, Szolcsányi J. Inhibitory effect of somatostatin on inflammation and nociception. Pharmacol Ther. 2006;112(2):440–456. doi: 10.1016/j.pharmthera.2006.04.010. [DOI] [PubMed] [Google Scholar]
- [29].Krulich L, Dhariwal AP, McCann SM. Stimulatory and inhibitory effects of purified hypothalamic extracts on growth hormone release from rat pituitary in vitro. Endocrinology. 1968;83(4):783–790. doi: 10.1210/endo-83-4-783. [DOI] [PubMed] [Google Scholar]
- [30].Saito T, Iwata N, Tsubuki S, Takaki Y, Takano J, Huang SM, Suemoto T, Higuchi M, Saido TC. Somatostatin regulates brain amyloid beta peptide Abeta42 through modulation of proteolytic degradation. Nat Med. 2005;11(4):434–439. doi: 10.1038/nm1206. [DOI] [PubMed] [Google Scholar]
- [31].D’Alessio DA, Sieber C, Beglinger C, Ensinck JW. A physiologic role for somatostatin 28 as a regulator of insulin secretion. J Clin Invest. 1989;84(3):857–862. doi: 10.1172/JCI114246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mandarino L, Stenner D, Blanchard W, Nissen S, Gerich J, Ling N, Brazeau P, Bohlen P, Esch F, Guillemin R. Selective effects of somatostatin-14, -25 and -28 on in vitro insulin and glucagon secretion. Nature. 1981;291(5810):76–77. doi: 10.1038/291076a0. [DOI] [PubMed] [Google Scholar]
- [33].Bates CM, Kegg H, Grady S. Expression of somatostatin in the adult and developing mouse kidney. Kidney Int. 2004;66(5):1785–1793. doi: 10.1111/j.1523-1755.2004.00953.x. [DOI] [PubMed] [Google Scholar]
- [34].Harris AG. Somatostatin and somatostatin analogues: pharmacokinetics and pharmacodynamic effects. Gut. 1994;35(3 Suppl):S1–S4. doi: 10.1136/gut.35.3_suppl.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60(4):470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moss CE, Marsh WJ, Parker HE, Ogunnowo-Bada E, Riches CH, Habib AM, Evans ML, Gribble FM, Reimann F. Somatostatin receptor 5 and cannabinoid receptor 1 activation inhibit secretion of glucose-dependent insulinotropic polypeptide from intestinal K cells in rodents. Diabetologia. 2012;55(11):3094–3103. doi: 10.1007/s00125-012-2663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van Liessum PA, Hopman WP, Pieters GF, Jansen JB, Smals AG, Rosenbusch G, Kloppenborg PW, Lamers CB. Postprandial gallbladder motility during long term treatment with the long-acting somatostatin analog SMS 201-995 in acromegaly. J Clin Endocrinol Metab. 1989;69(3):557–562. doi: 10.1210/jcem-69-3-557. [DOI] [PubMed] [Google Scholar]
- [38].Magnusson I, Einarsson K, Angelin B, Nyberg B, Bergström K, Thulin L. Effects of somatostatin on hepatic bile formation. Gastroenterology. 1989;96(1):206–212. doi: 10.1016/0016-5085(89)90782-8. [DOI] [PubMed] [Google Scholar]
- [39].Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6(4):347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [40].Kumar U. Expression of somatostatin receptor subtypes (SSTR1-5) in Alzheimer’s disease brain: an immunohistochemical analysis. Neuroscience. 2005;134(2):525–538. doi: 10.1016/j.neuroscience.2005.04.001. [DOI] [PubMed] [Google Scholar]
- [41].Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30(41):13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Spiegel AM, Koh MT, Vogt NM, Rapp PR, Gallagher M. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol. 2013;521(15):3508–3523. doi: 10.1002/cne.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]