Abstract
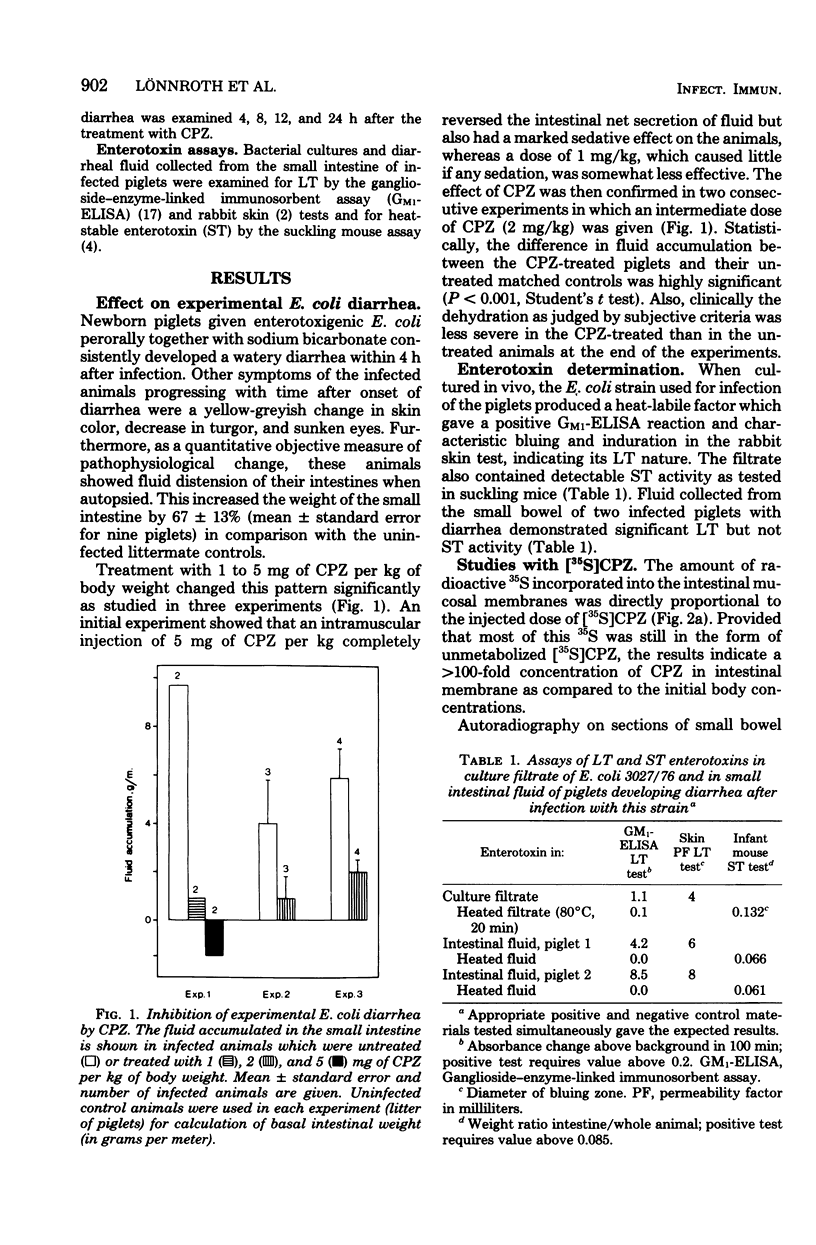
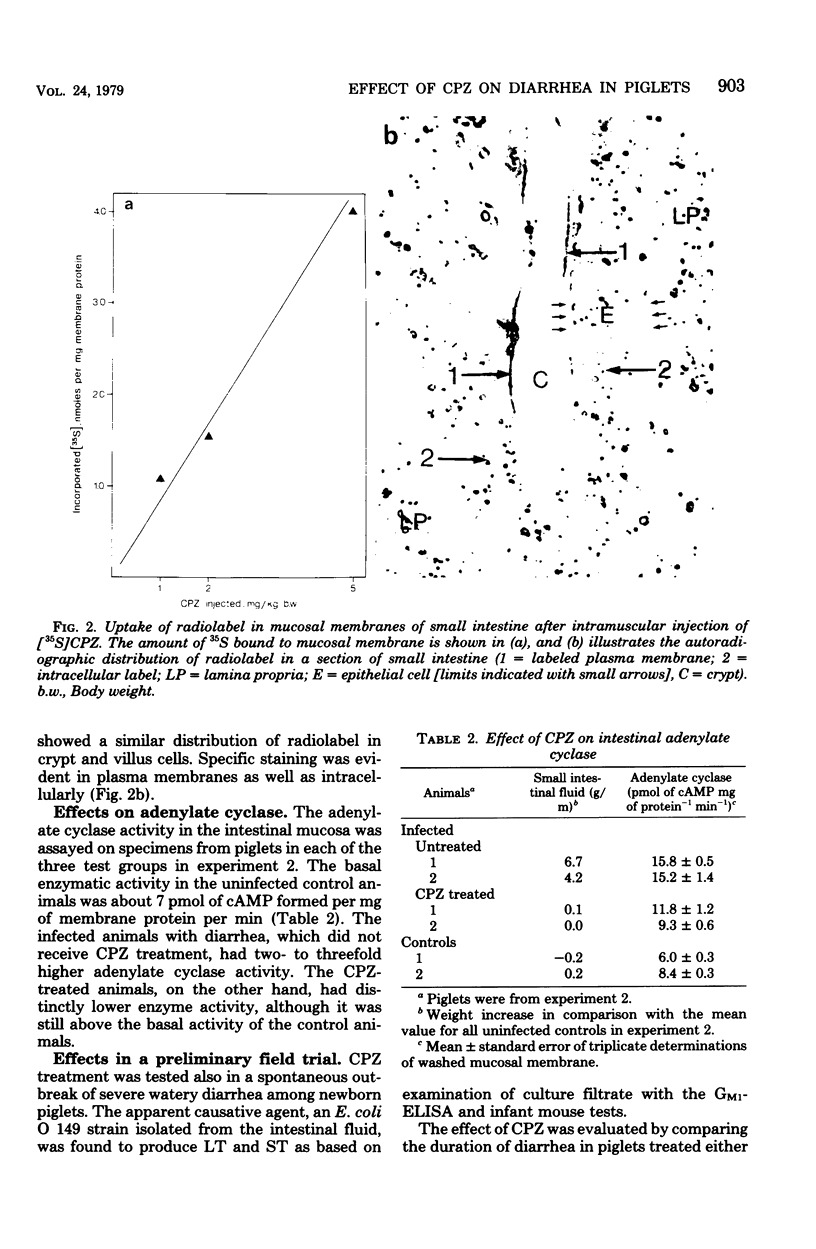
The effect of chlorpromazine (CPZ) on diarrhea caused by enterotoxigenic Escherichia coli was tested in piglets since CPZ has been shown to be a potent antagonist to enterotoxins in vitro in a cell system and in vivo in a mouse model. Experimental diarrhea was induced in three litters of newborn piglets which were infected by mouth with 2 × 109E. coli bacteria, which produce heat-labile (LT) and heat-stable (ST) enterotoxins. Treatment with CPZ given intramuscularly 1 h after the onset of diarrhea reversed fluid secretion in small intestine as well as dehydration, as judged by clinical criteria. A dose of 5 mg of CPZ per kg of body weight completely normalized the intestinal-fluid content measured 4 h after diarrhea developed, whereas 1 to 2 mg of CPZ per kg of body weight was somewhat less effective but still caused significant reduction of fluid (P < 0.001). Studies with radioactive [35S]CPZ showed preferential and dose-dependent uptake of 35S in the intestinal mucosa, the radioactivity being evenly distributed in the membranes of both crypt and villus cells. The enzyme adenylate cyclase, which probably mediates the cellular effects of LT, was shown to have two- to threefold higher activity in the infected than in the uninfected animals. This activation was reduced about 50% by the CPZ treatment (2 mg/kg of body weight). In a preliminary field trial the effect of CPZ was tested in a spontaneous outbreak of diarrhea in piglets due to enterotoxinogenic E. coli. The animals were treated either with oral electrolyte solution and standard antimicrobial agents only (controls) or with 1 mg of CPZ per kg of body weight intramuscularly in addition to this treatment. The mean duration of diarrhea in CPZ-treated animals was significantly shorter, 4.1 h (n = 23), than that in controls, 7.2 h (P < 0.05).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess M. N., Bywater R. J., Cowley C. M., Mullan N. A., Newsome P. M. Biological evaluation of a methanol-soluble, heat-stable Escherichia coli enterotoxin in infant mice, pigs, rabbits, and calves. Infect Immun. 1978 Aug;21(2):526–531. doi: 10.1128/iai.21.2.526-531.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Regulation of active ion transport in the small intestine. Ciba Found Symp. 1976;(42):109–127. doi: 10.1002/9780470720240.ch7. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Lange S., Lönnroth I. Reversal of cyclic AMP-mediated intestinal secretion in mice by chlorpromazine. Gastroenterology. 1978 Dec;75(6):1103–1108. [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Kimbert D. V. Cyclic nucleotides and their role in gastrointestinal secretion. Gastroenterology. 1974 Nov;67(5):1023–1064. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lönnroth I., Holmgren J., Lange S. Chlorpromazine inhibits cholera toxin-induced intestinal hypersecretion. Med Biol. 1977 Jun;55(3):126–129. [PubMed] [Google Scholar]
- Sack R. B. Human diarrheal disease caused by enterotoxigenic Escherichia coli. Annu Rev Microbiol. 1975;29:333–353. doi: 10.1146/annurev.mi.29.100175.002001. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Sharp G. W. Action of cholera toxin on fluid and electrolyte movement in the small intestine. Annu Rev Med. 1973;24:19–23. doi: 10.1146/annurev.me.24.020173.000315. [DOI] [PubMed] [Google Scholar]




