Abstract
Background
The determination of rivaroxaban and apixaban from serum samples of patients may be beneficial in specific clinical situations when additional blood sampling for plasma and thus the determination of factor Xa activity is not feasible or results are not plausible.
Materials and methods
The primary aim of this study was to compare the concentrations of rivaroxaban and apixaban in serum with those measured in plasma. Secondary aims were the performance of three different chromogenic methods and concentrations in patients on treatment with rivaroxaban 10 mg od (n = 124) or 20 mg od (n = 94) or apixaban 5 mg bid (n = 52) measured at different time.
Results
Concentrations of rivaroxaban and apixaban in serum were about 20–25% higher compared with plasma samples with a high correlation (r = 0·79775–0·94662) using all assays (all P < 0·0001). The intraclass correlation coefficients were about 0·90 for rivaroxaban and 0·55 for apixaban. Mean rivaroxaban concentrations were higher at 2 and 3 h compared with 1 and 12 h after administration measured from plasma and serum samples (all P-values < 0·05) and were not different between 1 vs. 12 h (plasma and serum).
Conclusions
The results indicate that rivaroxaban and apixaban concentrations can be determined specifically from serum samples.
Keywords: Apixaban, chromogenic substrate methods, direct oral anticoagulants, plasma, rivaroxaban, serum
Introduction
The direct oral factor Xa inhibitors rivaroxaban and apixaban prevent venous thromboembolism following elective primary total hip and knee replacement surgery [1,2], recurrent venous thromboembolism in patients with acute venous thrombosis or pulmonary embolism [3,4] and cerebral and noncerebral embolism in patients with atrial fibrillation [5,6]. Rivaroxaban reduces recurrence of acute coronary syndromes in combination with acetylsalicylic acid and a thienopyridine [7]. These anticoagulants are given at fixed daily doses without need for laboratory-guided dose adjustments. If clinically indicated, current practice is to use factor Xa-specific chromogenic substrate assays to determine the drugs. For determination of rivaroxaban [8–13], and apixaban [14,15] activities, blood needs to be anticoagulated by sodium citrate during withdrawal to avoid clotting in vitro. However, determination of rivaroxaban or apixaban concentrations in blood may be required even if such pretest conditions are not met, for example, if serum is the only available test material. Therefore, the determination of drugs from serum may be desirable in specific clinical situations such as in renal failure, concomitant administration of inhibitors of P-glycoprotein-I [16–18], very elderly, bleeding or thrombotic complication while on anticoagulation, before operations, before initiation of thrombolytic therapy or for control of adherence to therapy [19–21], or if plasma activity determinations do not appear to be plausible.
Unlike plasma samples, in particular those containing citrate, serum samples are taken from all patients in acute and nonacute clinical situations. Upon adequate handling, clinical chemical analyses are performed on automated clinical chemistry analysers. Further to citrate addition, blood for the determination of coagulation parameters needs to be collected into plastic or siliconised glass tubes to inhibit mechanical coagulation activation in vitro. Potential pitfalls of blood drawing for coagulation parameters are incomplete filling of the tube resulting in wrong ratios of anticoagulant to blood leading to incorrect coagulation results and the activation of blood clotting during and after blood sampling due to incorrect handling. In addition, correct handling also includes centrifugation at given temperatures, analysis within a given time frame and specific procedures for freezing and thawing of samples [22].
The concentrations of drugs are predominantly determined in serum samples from patients using liquid chromatography techniques [14,23–25]. Drug levels or other clinical chemical parameters may be requested by clinicians after blood collection has been completed. Therefore, serum samples are often stored routinely for several days. This becomes important for patients with drug overdose or intoxication or for forensic purposes [26,27]. Rivaroxaban and apixaban bind directly to activated and nonactivated factor X, and they do not require cofactors. To determine the activities of both inhibitors in plasma – so far representing the only method of determination which is being accessible to clinical use – factor Xa is added in excess, and the residual factor Xa activity releases a chromophor in relation to the anticoagulant concentration. In a preliminary investigation, we reported on a photometric method to quantify the concentrations of new oral anticoagulants in serum samples of patients during treatment [28]. The aims of the study are the following: (i) a comparison of serum rivaroxaban and apixaban concentrations with those measured in plasma using several chromogenic methods, (ii) to compare the performance of three different chromogenic methods and (iii) to compare rivaroxaban and apixaban concentrations in patients measured at different time. Samples were obtained from patients on treatment with rivaroxaban 10 and 20 mg od and apixaban 5 mg bid.
Methods
Patients
Plasma and serum samples were obtained from patients before and after 4–6 days of treatment with rivaroxaban 10 mg od 12 h after intake of medication for prevention of thromboembolic complications after primary elective total knee (TKR) and hip replacement (THR) (n = 144). In a second group of patients (n = 74), plasma and serum samples were collected at 1, 2, 3 or 12 h after intake of rivaroxaban 20 mg od. They were treated to prevent systemic embolism in nonvalvular atrial fibrillation or recurrent events following venous thromboembolism. In a third group of patients with atrial fibrillation (n = 52) who received apixaban 5 mg bid, blood samples were collected at 2 h after administration. The studies were approved by the local ethics committee, and participants gave written informed consent prior to blood sampling.
Preparation of samples
Blood was collected into plastic tubes containing 3·8% sodium citrate (1/9, v/v, citrate/blood) to obtain platelet-poor plasma and into plastic tubes containing kaolin to obtain serum during the same venipuncture. Latter samples remained for 30 min at room temperature to allow serum formation in kaolin-containing tubes. They were centrifuged for 30 min at 1800 g and 4 °C, and several aliquots of the supernatant were [29] frozen with liquid nitrogen and kept at −72 °C until analysed.
Origin and quality of anticoagulants
Apixaban was kindly supplied by Bristol-Meyers-Squibb (Plainsboro,NJ, USA). Rivaroxaban was purified from commercially available Xarelto® tablets, and its purity was characterised by analytical methods as described [30].
Analysis of serum and plasma samples
Three chromogenic substrate assays were used for the determination of drug concentrations from serum [31] and plasma samples. The chromogenic assay in serum was performed as described for plasma. Following the addition of the chromogenic substrate, factor Xa was incubated in excess with the serum sample for 2 min (Coamatic and HemosIL assays) or 10 min (S2222 chromogenic substrate assay). The S2222 chromogenic substrate assay was performed as described using human factor Xa [71 nkat/mL, both reagents from Haemochrom, Essen, Germany, lower limit of detection (LOD) for plasma and serum about 25 ng/mL] [32]. Coamatic (Haemochrom, Essen, Germany, LOD for plasma and serum about 9 ng/mL) and HemosIL (Instrumentation Laboratory GmbH, Kirchheim, Germany, LOD for plasma and serum about 27 ng/mL, limit of quantification about three times higher for all methods in plasma and serum) chromogenic substrate assays were performed as described by the manufacturers. All assays were run on the microtiter plate system Multiskan FC connected to the software program SkanIt 3.1 (Thermo Fisher Scientific, Langenselbold, Germany). Standard curves were computed using serial concentrations of rivaroxaban and apixaban ranging from 0 to 500 ng/mL added to normal human plasma or serum pooled from 20 volunteers.
Statistical analysis
All statistical calculations have been performed with SAS software, release 9.3 (SAS Institute Inc., Cary, NC, USA). Quantitative variables are given as mean and standard deviation (SD). Furthermore, Pearson's correlation coefficient has been assessed to estimate the strength of correlation.
To compare mean values and error variances of plasma and serum concentrations, the t-test for two paired samples and the test of Maloney and Rastogi have been performed, respectively. The latter test indicates whether serum and plasma measurements differ regarding their precision. It is based on the correlation between the sum and the difference of the two measurements and thus is equivalent to Bland–Altman analysis which is based on the correlation between measurements' mean and difference. Finally, the intraclass correlation coefficient was used to determine the strength of agreement between two measurement methods.
Mean values of two independent groups (e.g. control vs. treatment) have been compared using the two-sample t-test.
To evaluate changes over time, anova for repeated measurements has been performed using the SAS procedure PROC MIXED. In the case of a statistically significant test result, Scheffe tests have been performed for pairwise comparisons. Statistical significance was assumed for test results with P < 0·05.
Results
Rivaroxaban and apixaban standard curves in plasma and serum
The serial dilutions of rivaroxaban and apixaban added to serum and plasma of six volunteers gave correlation coefficients [optical density (405 nm) vs. concentration] for rivaroxaban of r = 0·993 with the S2222 assay, r = 0·998 for the Coamatic assay and r = 0·9925 for the HemosIL assay and for apixaban of r = 0·9993 for the S2222 assay, r = 0·9980 for the Coamatic assay and r = 0·9961 for the HemosIL assay (all P < 0·0001).
Control values of patients not on treatment with an anticoagulant
The mean concentrations of rivaroxaban (n = 137, Table 1) in serum samples of controls were higher compared with those in plasma samples. The S2222 assay showed higher values compared with the other assays, which themselves were not different from each other (Table 1). The correlation of plasma and serum values was rather low (r = 0·437) but statistically significant with the HemosIL assay, and values did not correlate for the other assays. The precision was higher for the Coamatic assay compared with the other assays using plasma and serum samples (Maloney-Rastogi test, Table 2). For apixaban, the mean, SD and variance values of plasma and serum samples of controls did not differ (Table 4), and values of plasma and serum samples did not correlate (Table 4).
Table 1.
Concentration of rivaroxaban (ng/mL) in controls and patients on treatment with 10 and 20 mg od 12 h after medication, and P-values for comparisons between plasma and serum. Assays and doses and correlations between plasma vs. serum
| Plasma | Serum | Plasma | Serum | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | Serum | Plasma vs. serum | Control vs. treatment | 10 vs. 20 mg | Plasma vs. serum Pearson correlation and intraclass correlation (ICC) | Plasma vs. serum Maloney Rastogi | |||||||
| n | Mean [ng/mL] | SD [ng/mL] | Mean [ng/mL] | SD [ng/mL] | P-value t-test | P-value two-sample t-test | P-value two-sample t-test | P-value two-sample t-test | P-value two-sample t-test | r | ICC | P-value | |
| Control | |||||||||||||
| Coamatic | 137 | 9·8 | 8·1 | 4·5 | 9·6 | < 0·0001 | 0·1465 | 0·0941 | |||||
| HemosIL | 137 | 10·8 | 11·3 | 4·9 | 11·5 | < 0·0001 | 0·4370 | 0·2346 | |||||
| S2222 | 137 | 14·3 | 11·5 | 17·2 | 16·7 | 0·0271 | 0·2504 | < 0·0001 | |||||
| 10 mg od | |||||||||||||
| Coamatic | 120 | 52·9 | 38·8 | 63·6 | 49·5 | < 0·0001 | < 0·0001 | < 0·0001 | 0·9434 | 0·8887 | < 0·0001 | ||
| HemosIL | 120 | 56 | 40·2 | 64·9 | 54·9 | 0·0003 | < 0·0001 | < 0·0001 | 0·8796 | 0·8560 | 0·0005 | ||
| S2222 | 121 | 67·8 | 41 | 82·6 | 40·4 | < 0·0001 | < 0·0001 | < 0·0001 | 0·8074 | 0·7584 | 0·4930 | ||
| 20 mg od | |||||||||||||
| Coamatic | 99 | 182·7 | 119·2 | 226·7 | 156·1 | < 0·0001 | < 0·0001 | < 0·0001 | < 0·0001 | < 0·0001 | 0·9466 | 0·8549 | < 0·0001 |
| HemosIL | 99 | 185·6 | 119·2 | 197·5 | 145·2 | 0·0109 | < 0·0001 | < 0·0001 | < 0·0001 | < 0·0001 | 0·9113 | 0·8922 | 0·0004 |
| S2222 | 99 | 233·8 | 113·1 | 270·1 | 138·3 | < 0·0001 | < 0·0001 | < 0·0001 | < 0·0001 | < 0·0001 | 0·9129 | 0·8596 | < 0·0001 |
Table 2.
Inter-assay comparisons for patients on treatment with rivaroxaban 10 and 20 mg od
| Plasma | Serum | |||||||
|---|---|---|---|---|---|---|---|---|
| t-test for paired samples | Maloney Rastogi | Pearson correlation (r) Intraclass correlation (ICC) | t-test for paired samples | Maloney Rastogi | Pearson correlation (r) Intraclass correlation (ICC) | |||
| P-value | P-value | r | ICC | P-value | P-value | r | ICC | |
| Control | ||||||||
| Coamatic-HemosIL | 0·3431 | < 0·0001 | 0·2913 | 0·7291 | 0·0706 | 0·8632 | ||
| Coamatic-S2222 | 0·0002 | < 0·0001 | 0·0626 | < 0·0001 | < 0·0001 | 0·0574 | ||
| HemosIL-S2222 | 0·0043 | 0·8369 | 0·2496 | < 0·0001 | 0·0013 | 0·0687 | ||
| 10 mg od | ||||||||
| Coamatic-HemosIL | 0·0043 | 0·1801 | 0·9573 | 0·9582 | 0·3270 | 0·0096 | 0·9492 | 0·9470 |
| Coamatic-S2222 | < 0·0001 | 0·1764 | 0·8932 | 0·8385 | < 0·0001 | < 0·0001 | 0·8749 | 0·8337 |
| HemosIL-S2222 | < 0·0001 | 0·6136 | 0·8786 | 0·8466 | < 0·0001 | 0·0085 | 0·8377 | 0·8203 |
| 20 mg od | ||||||||
| Coamatic-HemosIL | 0·1215 | 0·5290 | 0·9482 | 0·9466 | < 0·0001 | 0·0773 | 0·9263 | 0·9073 |
| Coamatic-S2222 | < 0·0001 | 0·3334 | 0·9111 | 0·7874 | < 0·0001 | 0·0003 | 0·9653 | 0·9200 |
| HemosIL-S2222 | < 0·0001 | 0·2876 | 0·8721 | 0·7672 | < 0·0001 | 0·3363 | 0·9532 | 0·8379 |
Table 4.
Concentration of apixaban (ng/mL) in controls and patients on treatment, and P-values for comparisons between plasma and serum. Assays and correlations between plasma vs. serum
| Plasma vs. serum | Plasma | Serum | Plasma vs. serum | Plasma vs. serum | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plasma | Serum | Control vs. treatment | Malony Rastogi | Pearson correlation and ICC | |||||||
| n | Mean ng/mL | SD | Mean ng/mL | SD | P-value t-test for paired samples | P value two–sample t-test | P-value | r | ICC | ||
| Control | |||||||||||
| Coamatic | 72 | 16·7 | 17·7 | 15·3 | 15·1 | 0·6146 | 0·1893 | 0·0346 | |||
| HemosIL | 72 | 24·9 | 19·7 | 19·9 | 19·3 | 0·1358 | 0·8754 | −0·0412 | |||
| S2222 | 48 | 16·8 | 23·2 | 24·4 | 18·1 | 0·0808 | 0·0957 | −0·0107 | |||
| Apixaban | |||||||||||
| Coamatic | 43 | 199·1 | 66 | 282·1 | 144·5 | < 0·0001 | < 0·0001 | < 0·0001 | < 0·0001 | 0·9399 | 0·5347 |
| HemosIL | 43 | 208·1 | 77 | 314·3 | 148·4 | < 0·0001 | < 0·0001 | < 0·0001 | < 0·0001 | 0·9301 | 0·5151 |
| S2222 | 52 | 211·4 | 68·4 | 284·7 | 130·4 | < 0·0001 | < 0·0001 | < 0·0001 | < 0·0001 | 0·7977 | 0·5147 |
Patients on treatment with rivaroxaban 10 mg od
Concentrations of rivaroxaban in plasma and serum were significantly higher during treatment (n = 121) compared with controls using all chromogenic assay (Table 1). Mean and SD values are shown in Table 1 and as Boxplots in Fig. 1 for plasma and serum samples for all assays. Despite the higher mean and variance values for serum samples, there was a strong correlation between plasma and serum values (Table 1). All chromogenic assays measured 15–20% higher concentrations in serum samples compared with plasma samples (P-values Table 1). S2222 assay determined about 20% higher values in serum and plasma compared with the other two chromogenic assays (P-values Table 2). The precision of the assays did not differ in plasma samples and was lower for the Coamatic assay in serum samples compared with HemosIL and S2222 assays and was lower for the HemosIL compared with the S2222 assay (Maloney-Rastogi test, P-values Table 2). The correlation of the concentrations of rivaroxaban in plasma and serum samples between assays ranged from r = 0·8377–0·9482 (Table 2). The ICC supported the results of the other statistical examinations.
Figure 1.
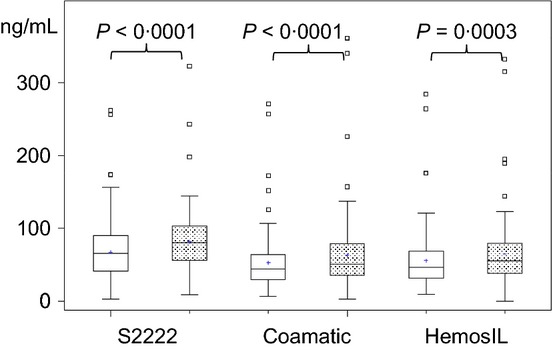
Boxplots of plasma (open boxes) and serum (stippled boxes) concentrations of rivaroxaban at 12 h after administration of 10 mg rivaroxaban od for 4–6 days in patients with elective hip or knee replacement surgery.
Patients on treatment with rivaroxaban 20 mg od
Plasma and serum values were strongly correlated in all assays (Table 1). Mean concentrations and variances of rivaroxaban in plasma samples were lower compared with serum samples using all chromogenic assays (Table 1). The interassay comparisons showed no differences between plasma values and variances determined by the Coamatic and HemosIL assays (Table 2). The other intercomparisons of plasma and serum concentrations showed differences, but variances were not different except a lower variance of results of serum concentrations for Coamatic compared with S2222 assay (Table 2). The correlation between assays was strong for plasma and serum samples ranging from 0·8721 to 0·9653 (Table 2).
The concentrations of rivaroxaban in plasma and serum were different across all time points with all assays (t-test, Table 3). Detailed analysis using Scheffe test showed that in plasma, the concentrations between the different time points were not different using the S2222 chromogenic substrate assay. In contrast, values in serum samples were higher at 2 and 3 h after intake compared with 1 and 12 h (Table 3). Using the HemosIL and Coamatic chromogenic assays, concentrations were higher at 2 and 3 h compared with 1 and 12 h in plasma as well as in serum samples (Table 3). The concentrations of rivaroxaban were higher in serum samples compared with plasma samples with all chromogenic assays (Table 3). Values in plasma and serum samples were higher using the S2222 compared with the results using the Coamatic and HemosIL chromogenic assays (Table 3, Fig. 2a–c).
Table 3.
Concentrations in plasma and serum samples at different time points in patients on treatment with rivaroxaban 20 mg od using three chromogenic substrate assays
 |
Figure 2.
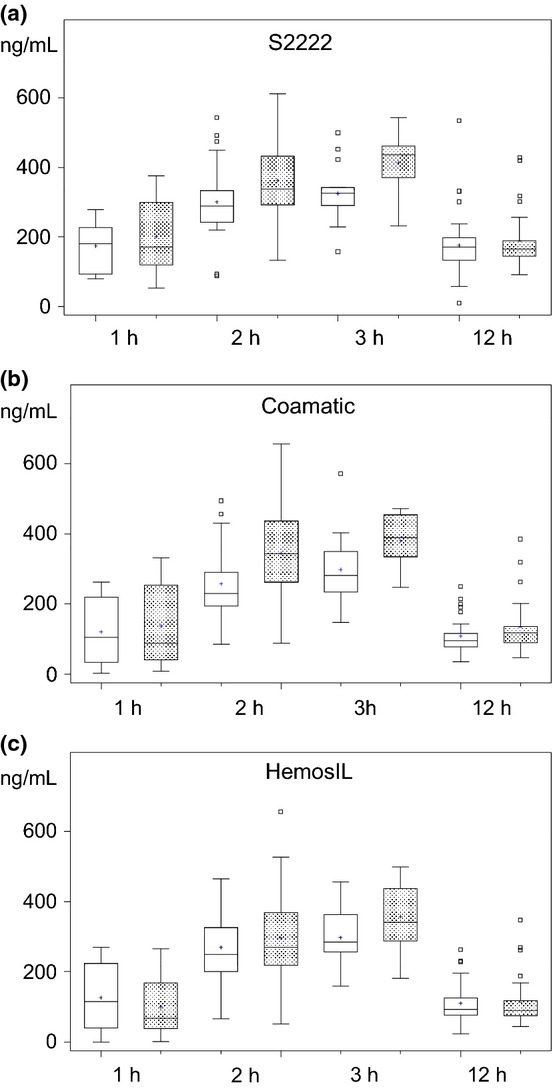
(a–c) Boxplots (S2222 chromogenic substrate method, (a) Coamatic chromogenic substrate assay, (b) and HemosIL chromogenic substrate assay, (c) of plasma (open boxes) and serum (stippled boxes) concentrations of rivaroxaban at 1, 2, 3 and 12 h following administration of 20 mg rivaroxaban od at steady state levels. Samples were taken from patients at different outpatient visits at indicated time intervals after intake of medication. P-values are given in Table 3.
The values in plasma and serum 12 h after administration of rivaroxaban 10 mg od were lower compared with 20 mg od using all chromogenic substrate assays (Table 1).
Patients on treatment with apixaban 5 mg bid
In controls, the mean values and variances of the concentrations of apixaban were not significantly different in plasma and serum samples using all assays (Table 4). Correlations were strong for plasma and serum values with all assays (Table 4).
During treatment with apixaban, plasma concentrations were higher compared with control values (Table 4). Values are given as Boxplots for plasma and serum concentration as determined with all chromogenic assays (Fig. 3). Correlations of plasma and serum concentrations were strong using all assays (Table 4). Concentrations and variances were lower in plasma compared with serum samples using all assays (Table 4). The ICC values were lower somewhat than the Pearson's correlation coefficients due to the higher mean concentrations of apixaban in serum compared with plasma.
Figure 3.
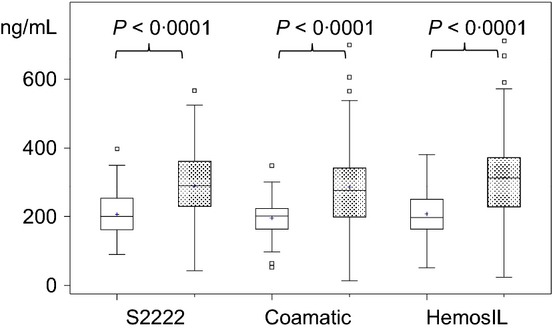
Boxplots of plasma (open boxes) and serum (stippled boxes) concentrations at 12 h following administration of 5 mg apixaban bid at months 3–6 in patients with atrial fibrillation or prevention of recurrent venous thromboembolism. Concentrations were measured using different chromogenic substrate assays.
The inter-assay comparisons of the mean values (t-test) and of the variances (Maloney-Rastogi test) of plasma and serum concentrations were rather strong using plasma and serum samples (Table 5). Mean values of the concentrations of apixaban were lower with the Coamatic assay for both plasma and serum samples compared with the other assays (Table 5). Precision was higher for Coamatic compared with the other assays using plasma samples. Using serum samples, however, S2222 assay was more precise than Coamatic or HemosIL assay (Table 5). The ICC confirmed the high interassay correlations for plasma and serum samples.
Table 5.
Inter-assay comparisons for patients on treatment with apixaban
| Plasma | Serum | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| t-test for paired samples | Maloney Rostogi | Pearson correlation and ICC | t-test for paired samples | Maloney Rostogi | Pearson correlation and ICC | ||||
| n | P-value | P-value | r | P-value | P-value | P-value | r | ||
| Control | |||||||||
| Coamatic-HemosIL | 72 | < 0·0001 | 0·2783 | 0·5860 | 0·0114 | 0·0087 | 0·6433 | ||
| Coamatic-S2222 | 48 | 0·9773 | 0·0623 | −0·0499 | 0·0002 | < 0·0001 | 0·0456 | ||
| HemosIL-S2222 | 48 | 0·0155 | 0·6602 | −0·1217 | 0·1427 | 0·1142 | −0·1609 | ||
| Apixaban | |||||||||
| Coamatic-HemosIL | 53 | 0·0012 | < 0·0001 | 0·9663 | 0·9377 | < 0·0001 | 0·3720 | 0·9632 | 0·9473 |
| Coamatic-S2222 | 53 | 0·0250 | 0·0005 | 0·8316 | 0·8167 | 0·6186 | 0·0046 | 0·9196 | 0·9032 |
| HemosIL-S2222 | 53 | 0·8477 | 0·0032 | 0·8353 | 0·8429 | 0·0045 | 0·0005 | 0·9257 | 0·8906 |
Discussion
The present data indicate that rivaroxaban and apixaban can be determined from serum samples of patients on treatment and that values correlate well with those determined from plasma samples. The concentrations following intake of 20 mg rivaroxaban, which were obtained from different patients at different days, showed that they were significantly higher at 2 and 3 h compared with 1 and 12 h after application in plasma as well as serum samples. Serum samples are preferred for determination of drugs because of the absence of many proteins which may interfere with the determination such as fibrinogen and other coagulation factors [26,27,33]. Oral direct factor Xa inhibitors require only factor Xa to act on an enzyme which is added in excess to serum samples in the present assay. An enzyme-specific substrate releases a chromophor quantitatively from the substrate in competition with the factor Xa inhibitor [34,35]. Therefore, the oral direct factor Xa inhibitors can be determined in serum samples, and the adoption of plasma samples is not prerequisite.
In real life studies, the concentration of rivaroxaban varied widely in small groups of patients using plasma samples and a chromogenic substrate assay [36] and coagulation based assays [37]. However, using high-performance liquid chromatography mass-spectrometry as detection method, concentrations were significantly different 2, 12 and 24 h after intake of 10 mg rivaroxaban od [38]. In our study after intake of 20 mg rivaroxaban od we also found significant different concentrations in plasma as well as in serum samples of treated patients between 1 vs. 2 or 3 and 1 or 2 vs. 12 hours using chromogenic assays. Despite some differences between assays in our study, all three chromogenic substrate tests confirm the feasibility to determine rivaroxaban also from serum samples following administration of 10 or 20 mg rivaroxaban od.
The effect of apixaban spiked at increasing concentrations in pooled citrated normal human platelet-poor plasma substantially prolonged coagulation times of clotting assays and varied between methods used [39–41]. The variability of the results of chromogenic methods was reduced using methods adapted to a specific coagulation platform based on data of an international collaborative study [30]. Pharmacokinetic studies in healthy volunteers resulted in peak levels at steady state conditions using high-performance liquid chromatography from serum samples [40,42] as we found in our patients using chromogenic substrate assays. Our data from the chromogenic substrate assay correlated well between serum and plasma samples. However, plasma values were significantly lower than serum values with all assays. The values of the interassay and intraclass correlation coefficients were high with rivaroxaban for the three chromogenic assays support the validity of this determination from serum samples despite minor systematic differences between the three tests. These values were lower for the comparison of apixaban in plasma and serum samples using all chromogenic assays.
The values for rivaroxaban and apixaban determined in serum samples were about 20–25% higher compared with plasma samples.
The concentrations of rivaroxaban or apixaban may require determination in specific clinical situation such as deterioration of renal function, in the elderly or oldest population, before surgery or in acute clinical situations, during recurrent events or during bleeding complications or to check adherence to therapy [19,20]. Such determinations may be ordered also with some time delay when plasma samples are not at all or not any more available (too long storage period). Serum samples may be an alternative to measure rivaroxaban and apixaban in such situations.
Limitations of the present investigations are that the determination of rivaroxaban and apixaban in serum samples remains to be validated in clinical studies. However, even the validation of the plasma assays for both anticoagulants is still missing in relation to the incidences of thrombotic or bleeding events. A strength of this study is that the determination of rivaroxaban and apixaban in serum samples involves commercially available methods which can be adapted to current coagulation platforms following the description for plasma samples.
In conclusion, the determination of rivaroxaban and apixaban from serum samples of patients offers an additional method to determine their concentrations in specific clinical situations. They can be performed with all chromogenic assays, but some differences may exist between them indicating requirement of standardization of every chromogenic assay. An adaptation of the chromogenic assays to current coagulation platforms seems feasible and may even improve the results, but yet remains to be tested.
Acknowledgments
The research was supported by a grant of the Dietmar Hopp foundation. For technical assistance, we would like to thank Mrs. Christina Giese, Antje Hagedorn and Inge Träger.
Conflicts of interest
JH: recipient of the grant from the Dietmar Hopp foundation. The other authors do not have to declare conflicts of interest for this manuscript.
Address
Department of Clinical Pharmacology, Medical Faculty Mannheim, Ruprecht-Karls University Heidelberg, Maybachstr. 14, 68169 Mannheim, Germany (J. Harenberg, S. Krämer, S. Du, S. Zolfaghari, M. Wehling); Department of Orthopedic and Trauma Surgery, Medical Faculty Mannheim, Ruprecht-Karls University Heidelberg, Theodor Kutzer Ufer 1-3, 68167 Mannheim, Germany (A. Schulze); Inorganic Chemistry Institute, Ruprecht-Karls-University Heidelberg, Im Neuenheimeer Feld, 69116 Heidelberg, Germany (R. Krämer); Department of Biometry and Statistics, Medical Faculty Mannheim, Ruprecht-Karls University Heidelberg, Ludolf Krehl Strasse 4, 68167 Mannheim, Germany (C. Weiss); University of Birmingham Centre for Cardiovascular Sciences City Hospital Birmingham, B187QH, UK (G. Y. H. Lip).
References
- 1.Adam SS, McDuffie JR, Lachiewicz PF, Ortel TL, Williams JW. Comparative effectiveness of new oral anticoagulants and standard thromboprophylaxis in patients having total hip or knee replacement: a systematic review. Ann Intern Med. 2013;159:275–84. doi: 10.7326/0003-4819-159-4-201308200-00008. [DOI] [PubMed] [Google Scholar]
- 2.Castellucci LA, Cameron C, Le Gal G, Rodger MA, Coyle D, Wells PS, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta-analysis. BMJ. 2013;347:f5133. doi: 10.1136/bmj.f5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox BD, Kahn SR, Langleben D, Eisenberg MJ, Shimony A. Efficacy and safety of novel oral anticoagulants for treatment of acute venous thromboembolism: direct and adjusted indirect meta-analysis of randomised controlled trials. BMJ. 2012;345:e7498. doi: 10.1136/bmj.e7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prins MH, Lensing AW, Bauersachs R, van Bellen B, Bounameaux H, Brighton TA, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11:21. doi: 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kansal AR, Sharma M, Bradley-Kennedy C, Clemens A, Monz BU, Peng S, et al. Dabigatran versus rivaroxaban for the prevention of stroke and systemic embolism in atrial fibrillation in Canada. Comparative efficacy and cost-effectiveness. Thromb Haemost. 2012;108:672–82. doi: 10.1160/TH12-06-0388. [DOI] [PubMed] [Google Scholar]
- 6.Harenberg J, Marx S, Wehling M. Head-to-head or indirect comparisons of the novel oral anticoagulants in atrial fibrillation: what's next? Thromb Haemost. 2012;108:407–9. doi: 10.1160/TH12-07-0463. [DOI] [PubMed] [Google Scholar]
- 7.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 8.Harenberg J, Marx S, Weiss C, Kraemer R, Samama M, Schulman S. Report of the Subcommittee of Control of Anticoagulation on the determination of the anticoagulant effects of rivaroxaban. J Thromb Haemost. 2012;10:1433–6. doi: 10.1111/j.1538-7836.2012.04784.x. [DOI] [PubMed] [Google Scholar]
- 9.Samama MM, Amiral J, Guinet C, Perzborn E, Depasse F. An optimised, rapid chromogenic assay, specific for measuring direct factor Xa inhibitors (rivaroxaban) in plasma. Thromb Haemost. 2010;104:1078–9. doi: 10.1160/TH10-03-0204. [DOI] [PubMed] [Google Scholar]
- 10.Samama MM, Contant G, Spiro TE, Perzborn E, Guinet C, Gourmelin Y, et al. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost. 2012;107:379–87. doi: 10.1160/TH11-06-0391. [DOI] [PubMed] [Google Scholar]
- 11.Asmis LM, Alberio L, Angelillo-Scherrer A, Korte W, Mendez A, Reber G, et al. Rivaroxaban: quantification by anti-FXa assay and influence on coagulation tests A study in 9 Swiss laboratories. Thromb Res. 2012;129:492–8. doi: 10.1016/j.thromres.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Nagler M, Bachmann LM, Alberio L, Angelillo-Scherrer A, Asmis LM, Korte W, et al. Variability between laboratories performing coagulation tests with identical platforms: a nationwide evaluation study. Thromb J. 2013;11:6. doi: 10.1186/1477-9560-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baglin T. The role of the laboratory in treatment with new oral anticoagulants. J Thromb Haemost. 2013;11(Suppl 1):122–8. doi: 10.1111/jth.12227. [DOI] [PubMed] [Google Scholar]
- 14.He K, Luettgen JM, Zhang D, He B, Grace JE, Jr, Xin B, et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor. Eur J Drug Metab Pharmacokinet. 2011;36:129–39. doi: 10.1007/s13318-011-0037-x. [DOI] [PubMed] [Google Scholar]
- 15.Becker RC, Yang H, Barrett Y, Mohan P, Wang J, Wallentin L, et al. Chromogenic laboratory assays to measure the factor Xa-inhibiting properties of apixaban–an oral, direct and selective factor Xa inhibitor. J Thromb Thrombolysis. 2011;32:183–7. doi: 10.1007/s11239-011-0591-8. [DOI] [PubMed] [Google Scholar]
- 16.Hartter S, Sennewald R, Nehmiz G, Reilly P. Oral bioavailability of dabigatran etexilate (Pradaxa((R))) after co-medication with verapamil in healthy subjects. Br J Clin Pharmacol. 2013;75:1053–62. doi: 10.1111/j.1365-2125.2012.04453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76:455–66. doi: 10.1111/bcp.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang D, He K, Herbst JJ, Kolb J, Shou W, Wang L, et al. Characterization of efflux transporters involved in distribution and disposition of apixaban. Drug Metab Dispos. 2013;41:827–35. doi: 10.1124/dmd.112.050260. [DOI] [PubMed] [Google Scholar]
- 19.Harenberg J, Marx S, Erdle S, Kramer R. Determination of the anticoagulant effects of new oral anticoagulants: an unmet need. Expert Rev Hematol. 2012;5:107–13. doi: 10.1586/ehm.11.79. [DOI] [PubMed] [Google Scholar]
- 20.Samama MM, Amiral J, Guinet C, Le Flem L, Seghatchian J. Monitoring plasma levels of factor Xa inhibitors: how, why and when? Expert Rev Hematol. 2013;6:155–64. doi: 10.1586/ehm.13.11. [DOI] [PubMed] [Google Scholar]
- 21.Tripodi A. The laboratory and the direct oral anticoagulants. Blood. 2013;121:4032–5. doi: 10.1182/blood-2012-12-453076. [DOI] [PubMed] [Google Scholar]
- 22.Lippi G, Salvagno GL, Montagnana M, Lima-Oliveira G, Guidi GC, Favaloro EJ. Quality standards for sample collection in coagulation testing. Semin Thromb Hemost. 2012;38:565–75. doi: 10.1055/s-0032-1315961. [DOI] [PubMed] [Google Scholar]
- 23.Juenke JM, Brown PI, Urry FM, Johnson-Davis KL, McMillin GA. Simultaneous UPLC-MS/MS assay for the detection of the traditional antipsychotics haloperidol, fluphenazine, perphenazine, and thiothixene in serum and plasma. Clin Chim Acta. 2013;423:32–4. doi: 10.1016/j.cca.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Hu ZY, Parker RB, Herring VL, Laizure SC. Conventional liquid chromatography/triple quadrupole mass spectrometry based metabolite identification and semi-quantitative estimation approach in the investigation of in vitro dabigatran etexilate metabolism. Anal Bioanal Chem. 2013;405:1695–704. doi: 10.1007/s00216-012-6576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohde G. Determination of rivaroxaban–a novel, oral, direct Factor Xa inhibitor–in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872:43–50. doi: 10.1016/j.jchromb.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Wu AH, McKay C, Broussard LA, Hoffman RS, Kwong TC, Moyer TP, et al. National academy of clinical biochemistry laboratory medicine practice guidelines: recommendations for the use of laboratory tests to support poisoned patients who present to the emergency department. Clin Chem. 2003;49:357–79. doi: 10.1373/49.3.357. [DOI] [PubMed] [Google Scholar]
- 27.Bush DM. The U.S. Mandatory Guidelines for Federal Workplace Drug Testing Programs: current status and future considerations. Forensic Sci Int. 2008;174:111–9. doi: 10.1016/j.forsciint.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Harenberg J, Kraemer S, Du S, Giese C, Schulze A, Kraemer R, et al. Determination of direct oral anticoagulants from human serum samples. Semin Thromb Hemost. 2014;40:129–34. doi: 10.1055/s-0033-1363462. [DOI] [PubMed] [Google Scholar]
- 29.Harenberg J, Marx S, Dahl OE, Marder VJ, Schulze A, Wehling M, et al. Interpretation of endpoints in a network meta-analysis of new oral anticoagulants following total hip or total knee replacement surgery. Thromb Haemost. 2012;108:903–12. doi: 10.1160/TH12-07-0482. [DOI] [PubMed] [Google Scholar]
- 30.Harenberg J, Marx S, Weiss C, Kramer R, Samama M, Schulman S. Report of the Subcommittee of Control of Anticoagulation on the determination of the anticoagulant effects of rivaroxaban. J Thromb Haemost. 2012;10:1433–6. doi: 10.1111/j.1538-7836.2012.04784.x. [DOI] [PubMed] [Google Scholar]
- 31.Harenberg J, Kraemer R. 2011. Direct factor Xa inhibitors, International Patent Application PCT/EP2011/005586, 2011.
- 32.Harenberg J, Kramer R, Giese C, Marx S, Weiss C, Wehling M. Determination of rivaroxaban by different factor Xa specific chromogenic substrate assays: reduction of interassay variability. J Thromb Thrombolysis. 2011;32:267–71. doi: 10.1007/s11239-011-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu ZY, Laizure SC, Meibohm B, Herring VL, Parker RB. Simple and sensitive assay for quantification of oseltamivir and its active metabolite oseltamivir carboxylate in human plasma using high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry: improved applicability to pharmacokinetic study. J Pharm Biomed Anal. 2013;72:245–50. doi: 10.1016/j.jpba.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aurell L, Friberger P, Karlsson G, Claeson G. A new sensitive and highly specific chromogenic peptide substrate for factor Xa. Thromb Res. 1977;11:595–609. doi: 10.1016/0049-3848(77)90018-4. [DOI] [PubMed] [Google Scholar]
- 35.Harenberg J, Krämer R, Giese C, Marx S, Weiss C, Wehling M. Determination of rivaroxaban by different factor Xa specific chromogenic substrate assays: reduction of interassay variability. J Thromb Thrombolysis. 2011;32:267–71. doi: 10.1007/s11239-011-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samama MM, Guinet C, Le Flem L, Ninin E, Debue JM. Measurement of dabigatran and rivaroxaban in primary prevention of venous thromboembolism in 106 patients, who have undergone major orthopedic surgery: an observational study. J Thromb Thrombolysis. 2013;35:140–6. doi: 10.1007/s11239-012-0803-x. [DOI] [PubMed] [Google Scholar]
- 37.Mani H, Hesse C, Stratmann G, Lindhoff-Last E. Rivaroxaban differentially influences ex vivo global coagulation assays based on the administration time. Thromb Haemost. 2011;106:156–64. doi: 10.1160/TH10-10-0667. [DOI] [PubMed] [Google Scholar]
- 38.Mueck W, Eriksson BI, Bauer KA, Borris L, Dahl OE, Fisher WD, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban - an oral, direct factor Xa inhibitor - in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008;47:203–16. doi: 10.2165/00003088-200847030-00006. [DOI] [PubMed] [Google Scholar]
- 39.Samama MM, Martinoli JL, LeFlem L, Guinet C, Plu-Bureau G, Depasse F, et al. Assessment of laboratory assays to measure rivaroxaban–an oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103:815–25. doi: 10.1160/TH09-03-0176. [DOI] [PubMed] [Google Scholar]
- 40.Frost C, Nepal S, Wang J, Schuster A, Byon W, Boyd RA, et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol. 2013;76:776–86. doi: 10.1111/bcp.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douxfils J, Dogne JM, Mullier F, Chatelain B, Ronquist-Nii Y, Malmstrom RE, et al. Comparison of calibrated dilute thrombin time and aPTT tests with LC-MS/MS for the therapeutic monitoring of patients treated with dabigatran etexilate. Thromb Haemost. 2013;110:543–9. doi: 10.1160/TH13-03-0202. [DOI] [PubMed] [Google Scholar]
- 42.Harenberg J, Du S, Weiss C, Krämer R, Hoppensteadt D, Walenga J. working party: methods to determine apixaban of the Subcommittee on Control of Anticoagulation of the International Society of Thrombosis and Haemostasis. Report of the Subcommittee on Control of Anticoagulation on the determination of the anticoagulant effects of apixaban: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:801–4. doi: 10.1111/jth.12547. [DOI] [PubMed] [Google Scholar]
- 43.Upreti VV, Wang J, Barrett YC, Byon W, Boyd RA, Pursley J, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76:908–16. doi: 10.1111/bcp.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]


