Abstract
Studies of central nervous system myelination lack defined in vitro models which would effectively dissect molecular mechanisms of myelination that contain cells of the correct phenotype. Here we describe a co-culture of purified motoneurons and oligodendrocyte progenitor cells, isolated from rat embryonic spinal cord using a combination of immunopanning techniques. This model illustrates differentiation of oligodendrocyte progenitors into fully functional mature oligodendrocytes that myelinate axons. It also illustrates a contribution of axons to the rate of oligodendrocyte maturation and myelin gene expression. The defined conditions used allow molecular analysis of distinct stages of myelination and precise manipulation of inductive cues affecting axonal–oligodendrocyte interactions. This phenotypic in vitro myelination model can provide valuable insight into our understanding of demyelinating disorders, such as multiple sclerosis and traumatic diseases such as spinal cord injury where demyelination represents a contributing factor to the pathology of the disorder.
Keywords: Myelination, spinal cord, motoneurons, oligonucleotide, surface chemistry, oligodendrocyte precursor
Introduction
Mechanisms underlying oligodendrocyte maturation and the subsequent myelination of axons in the central nervous system (CNS) remain to be fully explored. During development of the spinal cord, oligodendocytes originate from the neuroepithelium through lineage restricted changes [1–3]. The oligodendrocyte progenitors (OPCs) are dividing, migratory cells with simple process bearing morphology, often bipolar. OPCs express gangliosides on their surface that are recognized by the monoclonal antibody A2B5 [2, 4–7] and, are initially localized in the ventral spinal cord at embryonic day 14 (E14) [2]. They then proliferate and migrate to become dispersed throughout the entire spinal cord. The maturation of OPCs is characterized by expression of the O4 surface sulfatide, which first appears at E16, and galactocerebroside (GC) immunoreactivity, which first appears at E18 [5]. Differentiated oligodendrocytes are postmitotic, extensively elaborated multipolar cells that express myelin basic protein (MBP) and upon maturation initiate the myelination of multiple axons. The outermost surface of myelin sheaths can be detected by expression of myelin oligodendrocyte glycoprotein (MOG) and is one sign of this functional maturation [8, 9].
It has been hypothesized that environmental cues, such as cell density along axons, axonal diameter and trophic support influence the maturation of oligodendrocyte precursor cells (OPCs) and production of the myelin sheath by mature oligodendrocytes [10–12]. Myelinated fibers are organized into domains, composed of the internode, the juxtaparanodal and paranodal regions, and the nodal membrane itself [13]. The generation of these domains and their accompanying enrichment in specific ion channels is essential for conduction of the saltatory action potential in CNS axons. In addition, a number of proteins show specific localization to these domains and play a vital role in their molecular organization. These include the contactin-associated protein (Caspr) which plays an essential role in tethering the paranodal loops to the axonal membrane, thereby segregating the paranodal and nodal domains [14].
In vitro model systems have proved invaluable in understanding molecular mechanisms that underlie myelination in the CNS. However, the complex multi-cellular nature of myelination has often rendered many of these models difficult to use or interpret. Such models include tissue slices, explants or aggregate cultures as well as co-cultures using purified cells [15–17]. Slice and aggregate cultures include various cell types and may be too complex to optically visualize and properly dissect cellular myelination mechanisms. Due to their ease of purification, researchers often utilize DRG neurons purified from the peripheral system, with OPCs prepared from the rat cortex [18–21] thereby limiting the applicability of this system to in vivo processes by not having the current cellular phenotypes. Furthermore, it has been suggested that the ability of oligodendrocytes to myelinate axons occurs only during a brief window early in their differentiation [20], such that only a small portion of the cells from the rat cortex fully differentiate into myelinating mature oligodendrocytes, further complicating the analysis of DRG/cortical OPC co-cultures. Finally, many of these myelinating co-culture systems require complex poorly defined substrates and serum thereby masking the effects of cell-substrate and various growth factors to OPC differentiation and myelination.
In this study we have established a simple phenotypic myelinating co-culture system that overcomes many of the problems raised above. We have utilized motoneurons (EMNs) and OPCs purified from the same embryonic rat spinal cords (E15) to reflect the highest degree of in vivo relevance and compatibility. OPCs were selectively purified for a promyelinating phenotype by immunopanning with antibodies for the early OPC marker A2B5. OPCs and EMNs were co-cultured in a defined serum free medium, containing a minimum combination of growth factors required for neuronal growth [22]. This medium had also previously been shown to support Schwann cell survival, proliferation and myelination of motoneuron axons with concomitant formation of Nodes of Ranvier [23]. Co-cultures were plated and maintained on a non-degradable synthetic substrate N-1[3 (trimethoxysilyl) propyl] diethylenetriamine (DETA), which has previously been shown to promote the long term survival of motoneurons [22, 24] and their myelination by Schwann cells [23]. DETA forms a self-assembled monolayer on any hydroxalated surface and can be utilized in photolithographic patterning [25–27].
Material and Methods
DETA surface modification and characterization
Glass coverslips (VWR 48366067, 22×22 mm2, No. 1) were cleaned using 1:1 HCl–methanol and then soaked in concentrated H2SO4 for 2 h. The DETA (United Chemical Technologies Inc. T2910-KG) film was formed by the reaction of the cleaned surfaces with a 0.1% (v/v) mixture of the organosilane in freshly distilled toluene (VWR BDH1151). Coverslips were then boiled in deionized water and rinsed with acetone. The cleaned surfaces were heated to about 100°C in the organosilane mixture, rinsed with toluene, reheated to about 100°C in toluene, and then dried in the oven overnight (100°C).
Contact Angle Measurements and X-Ray Photoelectron Spectroscopy
Water contact angle measurements were measured with a Ramé-hart goniometer (Mountain Lakes, NJ). The contact angle of a static sessile drop (5 μl) of water was measured three times and averaged. The XPS characterization of a DETA surface was performed utilizing a Thermo ESCALAB 220i-XL X-Ray photoelectron spectrometer equipped with an aluminum anode and a quartz monochromator. The surface charge compensation was achieved by using a low-energy electron flood gun. Survey scans were recorded in order to determine the relevant elements (pass energy of 50 eV, step size= 1 eV). High resolution spectra were recorded for Si 2p, C 1s, N 1s, and O 1s (pass energy of 20 eV, step size= 0.1 eV). The spectrometer was calibrated against the reference binding energies of clean Cu, Ag and Au samples. In addition, the calibration of the binding energy (BE) scale was made by setting the C 1s BE of carbon in a hydrocarbon environment at 285 eV. N 1s and Si 2p peak deconvolution was performed with Avantage version 3.25 software, provided by Thermo Electron Corporation. DETA coverage was determined by monitoring the N 1s peaks intensity compared to the Si 2p signal. The values are reported as the mean ± SEM. Stable XPS readings and contact angles through the study indicated uniformity and reproducibility of the self-assembly of the DETA monolayer.
Animals
Dated pregnant Sprague–Dawley rats were housed in an animal facility at the University of Central Florida. All research was approved by the Institutional Animal Care and Use Committee at the University of Central Florida and conformed to NIH guidelines. Pregnant rats were anesthetized and sacrificed by inhalation of excess CO2 at embryonic day 15, embryos were removed by caesarean section and fetuses dissected under a stereomicroscope (Carl Zeiss, Stemi, 2000).
Purified embryonic motoneuron culture
Rat spinal cord motoneurons were purified from the ventral horn cords from embryonic day 15 (E15) embryos as described previously [28, 29]. Spinal cords were removed from the E15 pups and the ventral horn tissue was dissected out and digested in 0.05% trypsin–EDTA for 15 min in a 37°C water bath (Gibco 25300-120). Following incubation, the trypsin–EDTA was aspirated and the cells suspended in dissection media, containing 10% FBS, and the tissue gently triturated. The dissociated cell suspension was then centrifuged at 500g for 10 min at 4°C to pellet the cells. Next, the tissue was layered on a density gradient of Opti-prep (Sigma D1556) solution and centrifuged at 500g for 15 min at 4°C. After centrifugation, the resulting top two bands were collected in a 15 ml tube. The rest of the cells were pelleted and used for OPC isolation (Figure 1). The ventral horn cells were then applied to an immunopanning dish coated with goat affinity purified antibody to rat IgG and the low affinity nerve growth factor receptor p75 (Chemicon MAB365) in the dissection medium for 45 min. This positive selection process provides attachment for the motoneurons while the other cells remain in suspension. After immunopanning the non-adherent cells were aspirated and the adherent motoneurons were removed from the dish in dissection medium to a 15 ml tube. Lastly, the neurons were pelleted by centrifugation at 500g for 5 min and then resuspended in defined culture medium and plated at a cell density of 300 cells/mm2.
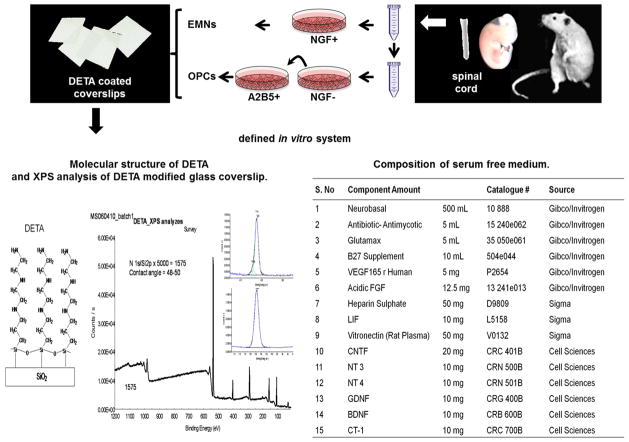
Figure 1. Schematic representation of co-cultures of OPCs with EMNs in defined in vitro system.
Glass coverslips were cleaned and prepared as outlined in the materials and methods section. A monolayer of DETA was formed on the coverslip and the degree of DETA coverage assessed using XPS measurements that compared the N 1s peak to the Si 2p signal. Spinal cords were dissected from E15 rats and enzymatically dissociated. Embryonic motoneurons (EMNs) were selected by immunopanning with antibodies to the low affinity NGF receptor P75. Non-adherent cells were further immunopanned against antibodies to A2B5 to isolate OPCs. OPCs and EMNs were co-cultured in a serum free-media whose components are listed.
Purification of embryonic spinal cord OPCs and co-culture with EMNs in serum-free medium
The dissociated spinal cord cell suspension was layered on a density gradient of Opti-prep (Sigma D1556) solution and centrifuged at 500g for 15 min at 4°C. After removal of the top two bands containing motoneurons as described above, the remaining cells were pelleted and used for OPC isolation. First, cells were applied to an immunopanning dish coated with goat affinity purified antibody to rat IgG and the low affinity nerve growth factor receptor p75 (Chemicon MAB365) in dissection medium for 45 min. This time, the negative selection process provided attachment for the neurons while the glia remained in suspension. After immunopanning the non-adherent cells were collected from the dish and transferred onto another immunopannning dish coated with A2B5 antibody to allow the positive selection of A2B5 expressing OPCs. After a 45 min incubation, the non-adherent cells were aspirated and the adherent OPCs were removed from the dish into dissection medium and centrifuged at 500g for 5 min. OPCs were then resuspended in culture medium and co-cultured with the EMNs isolated in the previous step at a cell density of 300 cells/mm2 or cultured alone at the same density in serum free culture medium. The composition of the medium is as described in Figure 1. Cultures were maintained with 1/2 volume fresh medium changed every 3 days. Cellular morphology was monitored using, phase-contrast images taken with a commercial Nikon Coolpix 990 camera and a Zeiss Axiovert S100 microscope. Pictures were analyzed using Scion Image Software (Scion Corp., Frederick, MD).
Immunocytochemistry
Cells were washed with Hanks’ balanced salts solution and fixed in 4% paraformaldehyde for 18 min. Fixed cells were rinsed 3x with PBS, permeabilized with 0.5% Triton 100x in PBS for 7 min, and then blocked with 5% donkey serum for 1 hr. Incubation with the primary antibody took place overnight at 4°C. Primary antibodies used were mouse monoclonal A2B5 (MAB312, 1:250), O4 (MAB345, 1:100), MBP (1:25), MOG (1:100) and anti-neurofilament heavy chain (1:1000) (AB5539), all from Chemicon (Temecula, CA). The anti-CASPR antibody (1:500) (sc-14340) was obtained from Santa Cruz Biotechnology. After overnight incubation with primary antibodies, cells were rinsed 3x with PBS and incubated with an Alexa Fluor 488-conjugated anti-mouse IgG or Alexa Fluor 594 -conjugated anti-chicken IgG, diluted in PBS, for 2 hours at room temperature. Then, the secondary antibody solution was removed and the cells were rinsed 3x using PBS. Coverslips were mounted on glass slides under Vectashield mounting medium (H1000, Vector Laboratories, Burlingame, CA). For general visualization of cellular nuclei, the specimens were counterstained with DAPI. Immunoreactivity was observed and analyzed using an Ultra VIEW™LCI confocal imaging system (Perkin Elmer). Controls for aberrant primary antibody cross-reactivity consisted of cultures incubated with secondary antibody only.
Data analysis
Quantification was performed on cells at each stage of differentiation. At various time points after plating, 1 coverslip was randomly selected, and at least 10 random non-overlapping images were selected from areas covering over 40% of the surface area of the coverslip. All cells were counted and the percentage that also expressed particular differentiation markers (A2B5, O4 etc.) were calculated. At least three coverslips in each group were quantified and data were expressed as an average ± standard deviation (SD).
Results
Establishment of a Defined in vitro Myelination Model
Figure 1 outlines the stages involved in the development of the CNS myelinating co-culture model derived from the rat spinal cord. Spinal cord derived OPCs and EMNs were co-cultured on glass coverslips modified with the non-degradable substrate DETA. The structure of DETA is shown in Figure 1. This aminosilane contains multiple amines in its terminal group and forms a covalently bound, self-assembled monolayer on the surface of the glass coverslip. DETA’s structure is analogous to spermidine, a known growth factor [30, 31]. We have shown that DETA will support neuronal growth in a variety of conditions and for different cell types [23, 24, 27, 32]. Characterization of the DETA monolayer was carried out by determining the nitrogen/silicon content of the coverslip surface using XPS (Figure 1). Hydrophilicity of the coverslip surface was determined using a static contact angle measurement (Figure 1).
EMNs and OPCs were isolated from the spinal cords of embryonic day 15 rat embryos and EMNs separated by density gradient centrifugation. Bands containing EMNs were incubated on NGFR coated tissue culture dishes to isolate NGFR bound purified EMNs. The remaining cell suspension was centrifuged and used to isolate OPCs. Residual neurons were removed from the cell preparation by incubation on NGFR coated cell culture dishes and then unbound cells were transferred onto dishes coated with A2B5 monoclonal antibody for the selective adherence and purification of A2B5+ OPCs. OPCs bound to A2B5 were isolated and co-cultured with EMNs, in the serum free medium described in Figure 1, that has previously been shown to support the growth and maintenance of both EMNs [22, 24] and Schwann cells [23] in co-culture.
To determine the efficacy of the immunopanning technique for the isolation of A2B5+ immature OPCs, co-cultures prepared with A2B5 immunopanning were compared with those in which this process was omitted, and immunostained for A2B5+ cells after 5 days of co-culture (Figure 2). Results indicated that when the A2B5 immunopanning step was omitted, co-cultures contained 32.4 (+/−0.98) % of A2B5 expressing cells compared with 54.3 (+/−4.63) % of A2B5 positive cells (Figure 2B) in immunopanned co-cultures. Hence, selective immunopanning increased the number of A2B5+ cells present using the co-culture technique by approximately 1.5–2 fold, the presence of which is considered to enable a greater probability to proceed to myelination [20].
Figure 2. A2B5 expression at day 5 of co-culture.

The degree of OPC enrichment by immunopanning was assessed by immuostaining with antibodies to A2B5 in co-cultures with or without A2B5 immunopanning. Quantitation of these results revealed a purification of 1.5–2 fold for OPCs in the culture. Scale bar 100 um.
Examination of the differentiation rate of OPCs in co-culture
To evaluate differentiation of spinal cord OPCs in the co-culture system they were stained with antibodies to the lineage markers O4 and MBP. MBP is a major constituent of the myelin sheath and hence a marker for mature oligodendrocytes. Neuronal axons were identified by neuron specific neurofilament heavy chain antibody. To determine the contribution of neuronal interactions to the differentiation of OPCs, similar experiments were carried out at identical time-points and cell densities with A2B5 purified OPCs incubated in the absence of EMNs (Figure 3). Although cells were initially negative for both O4 and MBP markers, at 10 days of co-culture, newly derived oligodendrocytes exhibited elaborate processes (Figure 3A) and 53.4% (+/−4.16) of cells expressed O4 and 28.67% (+/−5.03) of cells stained positive for MBP (Figure 3B) in the presence of EMNs. In contrast, only 32.3% of OPCs cultured in the absence of EMNs exhibited O4 expression and no cells were observed to express MBP after 10 days in culture (Figures 3A and 3B). These results indicated that presence of EMNs axons promoted differentiation of OPCs and expression of myelin genes.
Figure 3. Expression of lineage specific markers at day 10 of culture.

To determine if EMN axons affected the rate of OPC development, OPCs were either cultured alone for 10 days in co-culture media or in the presence of EMNs in the same media. Cultures were stained with antibodies to O4 to detect early development stage oligodendrocytes or with MBP for mature oligodendrocytes. EMN axons were detected with antibodies to MBP. Quantitation of this staining revealed 53.4% (+/− 4.16%) of cells expressing O4 in co-cultures with a further 28.67% (+/− 5.03%) of cells expressing MBP. In contrast no MBP staining was observed in OPCs cultured alone and only 32.3% (+/−4.1%) of cells expressed the O4 marker. Scale bars = 20 μm.
Defined System Characterization
To further evaluate oligodendrocyte maturation and MN myelination in our co-culture system, cells were stained after 20 days for the presence of compact myelin segments using antibodies against MBP and the myelin oligodendrocyte glycoprotein (MOG), which is expressed on the oligodendrocyte cell surface and concentrates on the outermost surface of myelin sheaths. To identify nodes of Ranvier immunostaining was also done with the paranodal protein Caspr (Contactin-Associated Protein). Neuronal axons were detected by NFH staining. As a control, immunostained cultures were examined where OPCs were plated in the absence of EMNs axons (Figure 4). Results indicated that at day 20 in culture, when oligodendrocytes were cultured alone, 26.7% (+/− 2.52) cells expressed O4, and 38.7% (+/−3.51) of cells expressed MBP as mature oligodendrocytes. As expected, cultures were negative for myelin segments or Caspr expression. However, in OPCs-EMNs co-cultures at 20 days, 32.3% (+/−5.51) expressed O4, 23.16% (+/−0.85) of cells were positive for MBP and 22.8% (+/−1.06) of cells formed myelin segments, positive for MOG, the majority of which had CASPR positive staining at their ends as would be expected for developing paranodes (Figure 4).
Figure 4. Formation of Myelin segments after 20 days in culture.

Co-cultures of OPCs and EMNs were cultured for 20 days in co-culture media in parallel with OPCs alone, before staining with antibodies to O4 (not shown), MBP and NFH. To detect myelin segments cultures were also stained with antibodies to MOG and to the paranodal marker CASPR to assess the clustering of nodal components. Quantitation of these results revealed that 26.7% (+/−2.52%) of OPCs cultured alone expressed the O4 marker and 38.7% (+/− 3.51%) expressed MBP. As might be expected no evidence of myelin segment formation was visible. In contrast, cells in co-culture with EMNs at 20 days showed 32.3% (+/−5.51) of cells expressing O4 and 23.2% (+/−0.85%) of cells expressing MBP with 22.8% (+/−1.06) of cells forming myelin segments positive for MOG and the paranodal marker CASPR. Scale bars 20 μm.
Discussion
In this paper we describe a phenotypic in vitro model for studying spinal cord CNS myelination under defined conditions. Advantages of this system are: 1) Both the neuronal population (motoneurons) and OPC population are derived from the same source, namely the developing (E15) rat spinal cord, and can be harvested simultaneously and co-prepared by differential immunopanning. 2) The system uses a chemically defined serum-free medium and substrate (DETA) which allows for rigid control of experimental conditions and allows the effect of each growth factor or component of the media to be individually assessed for its role in myelination and exquisite control of the staging of the process, with particular regard to the proliferation and differentiation of OPCs. Using this system we have successfully demonstrated functional myelination in vitro, and shown that the presence of motoneuron axons promotes the differentiation of OPCs into mature MBP-expressing oligodendrocytes.
An important component of this in vitro system is the use of the artificial substrate DETA which forms a self-assembling monolayer on the coverslip the quality of which can be characterized using XPS to ensure reproducibility. DETA carries multiple amines in its terminal group, which means that at physiological pH DETA provides a hydrophilic surface that promotes cellular attachment. DETA also promotes long-term cell survival because it is non-digestible by matrix proteases secreted by cells [24, 33]. We have observed that DETA is a permissive substrate for a large number of cell types including neurons and glia and have already observed the myelination of sensory neurons [26] and motoneurons by Schwann cells on this substrate [23]. Hence, DETA lends itself to the long-term co-culture of the cell types required for efficient myelin formation.
Another advantage of this system is its physiological relevance, in that we are incubating spinal cord motoneurons with the same OPCs that these neurons would be exposed to in vivo. OPCs purified from the E15 rat spinal cord express the cell surface marker A2B5 but are negative for the more mature oligodendroglial marker O4. This provides an advantage over other models such as the use of DRG neurons or the use of cortical OPCs commonly isolated from day 0–2 neonatal rat pups. These cortical OPCs are in more advanced differentiation stage, already expressing O4 and do not demonstrate robust myelination of axons in vitro [1]. Watkins et al (2008) have shown that OPCs have a narrow window of competency for myelination characterized by expression of the A2B5 surface marker. By selectively immunopanning for A2B5 expression we have successfully isolated OPCs within this window as evidenced by their successful functional myelination of motoneuron axons. Another advantage of this model is that it avoids the need to form cellular aggregates or use organotypic methods, allowing for greater clarification in optical procedures. A number of factors, both soluble and membrane bound, have been reported to regulate the differentiation of OPCs [34]. Our results indicate that OPCs cultured alone will differentiate and express MBP. However, this process is stimulated when OPCs are grown in the presence of their corresponding neurons and axons. Whether this requires physical contact between the OPC and the axon or the release of soluble factors by the neuron remains to be established.
Conclusion
We anticipate that the use of this simple, but highly controlled CNS myelination system will allow further probing of the mechanisms involved in OPC differentiation and myelination and will offer insights into demyelinating diseases such as multiple sclerosis, especially for creating new phenotypic assays for drug screening. In addition to demyelinating diseases, the loss of myelin is implicated in traumatic diseases such as SCI and traumatic brain injury (TBI). The loss of oligodendrocytes and OPCs often occurs secondary to the major injury as a result of the toxic inflammatory environment. The loss of this cellular pool and the demyelination of surviving axons contributes to the pathology associated with SCI and TBI and limits the extent of any potential recovery. Hence, it is important to develop therapeutics that limit the extent of oligodendrocyte and OPC loss as a result of inflammation in response to brain injury as well. To this end, the culture system described in this manuscript could be utilized as a functional platform to develop and evaluate new therapeutics to improve functional recovery in SCI and other traumatic conditions where demyelination plays a pathological role.
Acknowledgments
This work was supported by the National Institute for Health grant numbers R01EB009429 and R01NS050452.
Footnotes
The authors confirm that no competing financial interests exist and there has been no financial support for this research that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis H, Gonzales M, Bhargava N, Stancescu M, Hickman JJ, Lambert S. Rat cortical oligodendrocyte-embryonic motoneuron co-culture: An in vitro axon-oligodendrocyte interaction model. J Biomater Tissue Eng. 2012;2:206–214. doi: 10.1166/jbt.2012.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall A, Giese NA, Richardson WD. Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGF alpha-receptors. Development. 1996;122:4085–4094. doi: 10.1242/dev.122.12.4085. [DOI] [PubMed] [Google Scholar]
- 3.Richardson WD, Pringle NP, Yu WP, Hall AC. Origins of spinal cord oligodendrocytes: possible developmental and evolutionary relationships with motor neurons. Dev Neurosci. 1997;19:58–68. doi: 10.1159/000111186. [DOI] [PubMed] [Google Scholar]
- 4.Eisenbarth GS, Walsh FS, Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979;76:4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fok-Seang J, Miller RH. Distribution and differentiation of A2B5+ glial precursors in the developing rat spinal cord. J Neurosci Res. 1994;37:219–235. doi: 10.1002/jnr.490370208. [DOI] [PubMed] [Google Scholar]
- 6.Ono K, Bansal R, Payne J, Rutishauser U, Miller RH. Early development and dispersal of oligodendrocyte precursors in the embryonic chick spinal cord. Development. 1995;121:1743–1754. doi: 10.1242/dev.121.6.1743. [DOI] [PubMed] [Google Scholar]
- 7.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 8.Breithaupt C, Schubart A, Zander H, Skerra A, Huber R, Linington C, et al. Structural insights into the antigenicity of myelin oligodendrocyte glycoprotein. Proc Natl Acad Sci U S A. 2003;100:9446–9451. doi: 10.1073/pnas.1133443100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solly SK, Thomas JL, Monge M, Demerens C, Lubetzki C, Gardinier MV, et al. Myelin/oligodendrocyte glycoprotein (MOG) expression is associated with myelin deposition. Glia. 1996;18:39–48. doi: 10.1002/(SICI)1098-1136(199609)18:1<39::AID-GLIA4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Bambakidis NC, Miller RH. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J. 2004;4:16–26. doi: 10.1016/j.spinee.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Leach MK, Redmond SA, Chong SY, Mellon SH, Tuck SJ, et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat Methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, et al. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolswijk G, Balesar R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 2003;126:1638–1649. doi: 10.1093/brain/awg151. [DOI] [PubMed] [Google Scholar]
- 15.Lubetzki C, Demerens C, Anglade P, Villarroya H, Frankfurter A, Lee VM, et al. Even in culture, oligodendrocytes myelinate solely axons. Proc Natl Acad Sci U S A. 1993;90:6820–6824. doi: 10.1073/pnas.90.14.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Notterpek LM, Bullock PN, Malek-Hedayat S, Fisher R, Rome LH. Myelination in cerebellar slice cultures: development of a system amenable to biochemical analysis. J Neurosci Res. 1993;36:621–634. doi: 10.1002/jnr.490360603. [DOI] [PubMed] [Google Scholar]
- 17.Thomson CE, Hunter AM, Griffiths IR, Edgar JM, McCulloch MC. Murine spinal cord explants: a model for evaluating axonal growth and myelination in vitro. J Neurosci Res. 2006;84:1703–1715. doi: 10.1002/jnr.21084. [DOI] [PubMed] [Google Scholar]
- 18.Callizot N, Combes M, Steinschneider R, Poindron P. A new long term in vitro model of myelination. Exp Cell Res. 2011;317:2374–2383. doi: 10.1016/j.yexcr.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Gingras M, Le Beaulieu M-M, Gagnon V, Durham HD, Berthod F. In vitro study of axonal migration and myelination of motor neurons in a three-dimensional tissue-engineered model. Glia. 2008;56:354–364. doi: 10.1002/glia.20617. [DOI] [PubMed] [Google Scholar]
- 20.Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60:555–569. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liazoghli D, Roth AD, Thostrup P, Colman DR. Substrate micropatterning as a new in vitro cell culture system to study myelination. ACS Chem Neurosci. 2012;3:90–95. doi: 10.1021/cn2000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das M, Bhargava N, Gregory C, Riedel L, Molnar P, Hickman JJ. Adult rat spinal cord culture on an organosilane surface in a novel serum-free medium. In Vitro Cell Dev Biol Anim. 2005;41:343–348. doi: 10.1007/s11626-005-0006-2. [DOI] [PubMed] [Google Scholar]
- 23.Rumsey JW, Das M, Stancescu M, Bott M, Fernandez-Valle C, Hickman JJ. Node of Ranvier formation on motoneurons in vitro. Biomaterials. 2009;30:3567–3572. doi: 10.1016/j.biomaterials.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das M, Molnar P, Devaraj H, Poeta M, Hickman J. Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnol Prog. 2003;19:1756–1761. doi: 10.1021/bp034076l. [DOI] [PubMed] [Google Scholar]
- 25.Ravenscroft MS, Bateman KE, Shaffer KM, Schessler HM, Jung DR, Schneider TW, et al. Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane- modified surfaces. J Am Chem Soc. 1998;120:12169–12177. [Google Scholar]
- 26.Rumsey JW, McAleer C, Das M, Bhalkikar A, Wilson K, Stancescu M, et al. Myelination and node of Ranvier formation on sensory neurons in a defined in vitro system. In Vitro Cell Dev Biol - Anim. 2013;49:608–618. doi: 10.1007/s11626-013-9647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaffner AE, Barker JL, Stenger DA, Hickman JJ. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. J Neurosci Methods. 1995;62:111–119. doi: 10.1016/0165-0270(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 28.Camu W, Henderson CE. Purification of embryonic rat motoneurons by panning on a monoclonal-antibody to the low-affinity ngf receptor. J Neurosci Methods. 1992;44:59–70. doi: 10.1016/0165-0270(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 29.Camu W, Henderson CE. Rapid purification of embryonic rat motoneurons: an in vitro model for studying MND/ALS pathogenesis. J Neurol Sci. 1994;124 (suppl):73–74. doi: 10.1016/0022-510x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 30.Eisenberg T, Knauer H, Schauer A, Büttner S, Ruckenstuhl C, Carmona-Gutierrez D, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 31.Kaeberein M. Spermidine surprise for a long life. Nat Cell Biol. 2009;11:1277–1278. doi: 10.1038/ncb1109-1277. [DOI] [PubMed] [Google Scholar]
- 32.Stenger DA, Pike CJ, Hickman JJ, Cotman CW. Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Res. 1993;630:136–147. doi: 10.1016/0006-8993(93)90651-3. [DOI] [PubMed] [Google Scholar]
- 33.Das M, Molnar P, Gregory C, Riedel L, Hickman JJ. Long-term culture of embyonic rat cardiomyocytes on an organosilane surface in a serum free medium. Biomaterials. 2004;25:5643–5647. doi: 10.1016/j.biomaterials.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Kotter MR, Stadelmann C, Hartung HP. Enhancing remyelination in disease--can we wrap it up? Brain. 2011;134:1882–1900. doi: 10.1093/brain/awr014. [DOI] [PubMed] [Google Scholar]