Abstract
Background and Objectives
In cocaine vaccine studies, only a minority of subjects made strong antibody responses. To investigate this issue, IgG and IgM antibody responses to cocaine and to cholera toxin B (CTB—the carrier protein used to enhance immune responses to cocaine) were measured in sera from the 55 actively vaccinated subjects in a Phase IIb randomized double-blind placebo-controlled trial (TA-CD 109).
Methods
Isotype specific ELISAs were used to measure IgG and IgM anti-cocaine and anti-CTB antibody in serial samples collected prior to and at intervals after immunization. We assessed IgG anti-cocaine responses of patients with pre-vaccination IgM anti-cocaine antibodies. Competitive inhibition ELISA was used to evaluate antibody specificity.
Results and Conclusions
Before immunization, 36/55 subjects had detectable IgM antibodies to cocaine, and 9 had IgM levels above the 95% confidence limit of 11 µg/ml. These nine had significantly reduced peak IgG anti-cocaine responses at 16 weeks, and all were below the concentration (40 µg/ml) considered necessary to discourage recreational cocaine use. The IgG anti-CTB responses of these same subjects were also reduced.
Scientific Significance
Subjects who develop an IgM antibody response to cocaine in the course of repeated recreational exposure to this drug are significantly less likely to produce high levels of IgG antibodies from the cocaine conjugate vaccine. The failure may be due to recreational cocaine exposure induction of a type 2 T-cell independent immune response. Such individuals will require improved vaccines and are poor candidates for the currently available vaccine.
INTRODUCTION
Vaccines are being developed to immunize individuals addicted to various substances with the expectation that when and if sufficiently high levels of circulating antibody to these drugs are attained, the targeted substance will be retained in the blood, slowing brain uptake. In effect we postulate that high levels of circulating IgG antibody will significantly alter the pharmacological activity of the targeted substance, thereby inhibiting the reinforcing response from limited drug exposure. Consequently addicted subjects may stop using the psychoactive drugs. As reported by Martell et al.,1 a conjugate vaccine made by linking the cocaine hapten norcocaine to cholera toxin B (CTB) can elicit high level antibody responses, sufficient to cause responding individuals to voluntarily reduce their use of cocaine. However, we found that only 21 of the 55 subjects immunized with this cocaine CTB vaccine made 40 µg/ml or more IgG anti-cocaine antibody. Since at least 40 µg/ml anti-cocaine antibody with an average affinity of 100 nM or less is required to significantly inhibit uptake of cocaine by the brain,2 it was clear that this vaccine was likely to be effective in only a subset of the cocaine users enrolled in this study. Indeed the frequency of cocaine free urine samples was significantly increased in those who had 40 µg/ml or more anti-cocaine antibody.1 Further study of the sera collected in this research found that some of the enrolled subjects already had IgM anti-cocaine antibodies, even before they were immunized. The current investigations were designed to evaluate the impact of preexisting immunity to cocaine on the response to this vaccine; IgM or IgG antibody to cocaine in pre-immunization serum was taken as evidence that the research subject had previously made an immune response to cocaine, presumably as a consequence of self-administration of this drug in such a way that adducts to native proteins were formed. We postulated that the conjugate vaccine would boost the preexisting immunity with the result that subjects with IgM anti-cocaine antibodies prior to vaccination would develop very high levels of antibody following immunization.
METHODS
Subjects
Cocaine and opiate addicted subjects in a double blind, placebo controlled cocaine vaccination trial (identification below) were treated with methadone for their opiate addiction, as well as counseling for their substance abuse. Urine samples were collected three times per week for cocaine use monitoring, and sera were collected for antibody testing at biweekly to monthly intervals throughout the study. The sera studied here were from the 55 research subjects actively immunized with succinylnorcocaine covalently linked to CTB (SNC-rCTB) and adsorbed onto the adjuvant, aluminum hydroxide; the other subjects in this study were given placebo vaccinations and their sera were not studied here. Sera were stored at −80°C, and analyzed by ELISA for IgG and IgM anti-cocaine antibodies. The results of the IgG responses of subjects enrolled in this 24-week Phase IIb randomized double-blind placebo-controlled trial (Trial Registration: Protocol ID: NIDA-15477-1; Clinical trials.gov ID: NCT00142857; Short Title: TA-CD Vaccine for Cocaine Dependence; http://clinicaltrials.gov/ct/show/NCT00142857?order=2) were reported previously.1
Enzyme-Linked Immunosorbent Assay
To measure specific anti-cocaine antibody, ELISA plates (Immulon 2HB, Daigger, Vernon Hills, IL) were coated overnight in carbonate buffer (.05 M, pH 9.6) using succinylnorcocaine conjugates prepared with a heterologous carrier protein [bovine serum albumin (BSA)]. Background antibody binding to the carrier alone (which was very low in most samples) was subtracted from every sample to ensure that the results reflect antibodies specific for the hapten, cocaine. A standard curve of bound human IgG or IgM was included in each ELISA plate in order to calculate the quantity of specific antibody. Samples were added to plates in twofold serial dilutions starting at 1:1,000 in PBS–tween (.1%) and incubated for 2 hours. After washing with PBS–tween, goat anti-human IgG or IgM conjugated to horseradish peroxidase (MP Biomedicals, Irvine, CA) was then incubated in the plates for 30 minutes. The plates were again washed and then substrate (tetramethylbenzidine, Sigma, St. Louis, MO) was incubated in the plates for 45 minutes. Reactions were stopped with 1 M HCl; the optical density (OD) was measured on a microplate reader (TELAC, Research Triangle Park, NC). Background for the ELISA with buffers only was ~.1 OD in all assays. OD readings in the linear range of the standard curve were used to calculate the concentration of antibody from appropriate dilutions. If a serum sample did not have readings within the standard curve, samples were reassayed at a higher dilution. The specificity of the IgG ELISA was validated using the humanized monoclonal antibody to cocaine, 2E2,3 a gift of Dr. Andrew Norman, University of Cincinnati College of Medicine. The same strategy was used to create and analyze ELISAs to measure IgG and IgM specific antibodies to CTB, except that GM1-ganglioside (Sigma) was first bound to the wells to enable capture of CTB as the target antigen for antibody binding.4
Competitive Inhibition Assay
We tested pooled serum at dilutions that fell well within the linear range of the ELISA to measure the inhibition of antibody binding when sera were pre-incubated for 30 minutes with a range of concentrations of cocaine or control drugs including methamphetamine, benzoylecgonine, or bupivacaine. Inhibition was evaluated by comparing the OD produced in the ELISA when drugs were added to the ELISA to the OD produced when the same dilution was tested in wells with added PBS. The 50% inhibitory concentration (CI50) is that concentration that reduced the resulting OD to half the baseline level.
Data Analysis
Correlation analyses used Spearman ranks and Pearson approaches, and other statistical tests, including comparisons of means by Student’s t-test or non-parametric analyses were performed using Statistica 6.
RESULTS
Pre-immunization IgM Anti-cocaine Antibodies
Pre-immunization serum samples were screened for IgM and IgG antibodies capable of recognizing cocaine conjugated to BSA. To measure cocaine specific antibodies, these same sera were also tested for reactivity to unconjugated BSA; the quantity of antibody to BSA was subtracted from that measured using the cocaine BSA conjugate to give the amount of cocaine specific antibody. Only 1 of the 55 immunized subjects had IgG anti-cocaine antibodies in pre-immunization sera as reported previously,1 but 36 had detectable IgM anti-cocaine antibodies. The mean ±95% confidence limits for IgM anti-cocaine antibody in their pre-immunization sera was 4.66 ± 6.42 µg/ml. Nine of 55 subjects had IgM anti-cocaine antibodies that were above the 95% confidence limit and none of these developed IgG anti-cocaine levels of 40 or more µg/ml (Fig. 1). Thus, despite our initial expectation that preexisting immune recognition of cocaine in the form of IgM anti-cocaine antibodies might augment the vaccine response, we found that having these IgM anti-cocaine antibodies prior to immunization is associated with a significantly lower IgG antibody response to the vaccine, as shown by the regression line in Fig. 1 and supported by both Pearson and Spearman correlations (p < .001).
FIGURE 1.
Significant (r2 = .20, p = .0007, Pearson correlation) reciprocal relationship between the quantity of IgM anti-cocaine antibody in pre-immunization sera and IgG anti-cocaine antibodies attained by the same individuals 16 weeks after immunization.
To evaluate whether the antibodies detected by ELISA were specific for cocaine, we performed competitive inhibition studies on four sera containing high IgM anti-cocaine antibody levels. Figure 2 shows that binding of the IgM anti-cocaine antibodies in one of these sera could be inhibited by free cocaine in a dose dependent manner. The IC50, that is the concentration of free cocaine that inhibited antibody binding by 50%, was 4.2 mM. Binding of IgM antibodies to the norcocaine–BSA target could not be inhibited by methamphetamine. The average cocaine IC50 for the four sera with high IgM anti-cocaine concentrations we studied was 3.25 ± .91 mM.
FIGURE 2.
Competitive inhibition assay to test specificity of the IgM antibodies that react with cocaine. In the case shown in the figure the serum, diluted 1:2,000, was incubated for 1-hour with increasing concentrations of cocaine (diamonds) or methamphetamine (squares), and then assayed by ELISA for reactivity with the cocaine conjugate. The resulting optical densities (ODs) at various concentrations were divided by the maximum OD observed with no drug competitor. These results were expressed as the percent residual antibody binding plotted against the drug concentration.
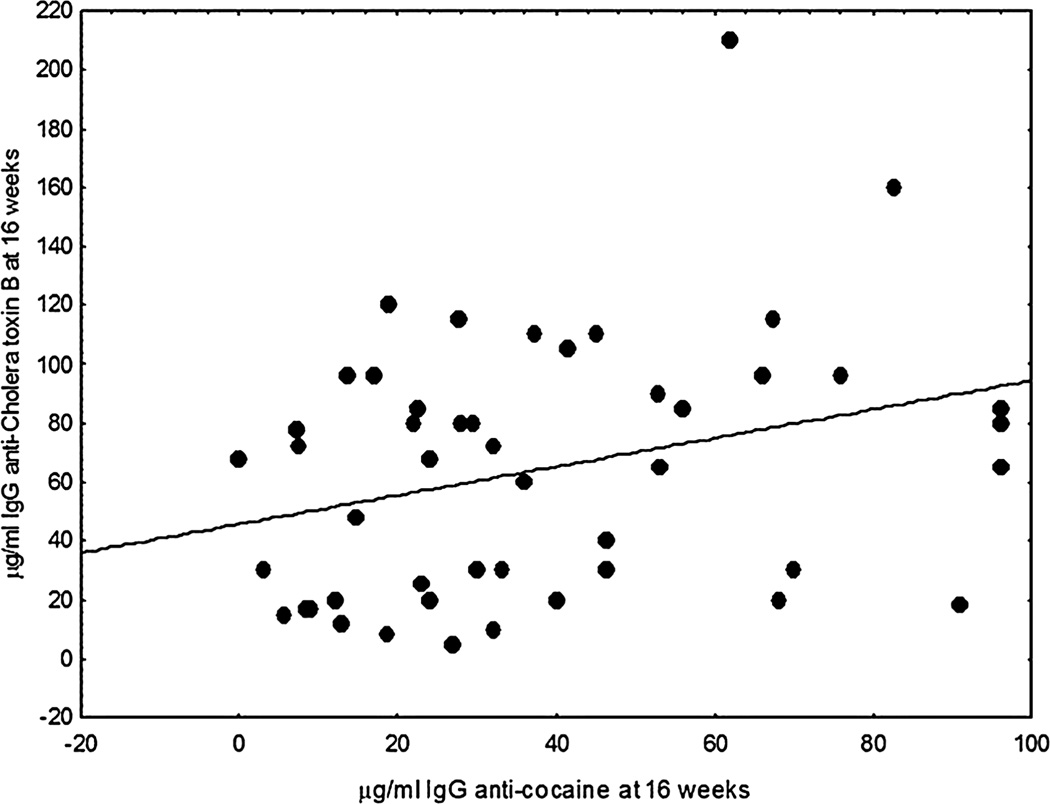
To evaluate whether measurement of pre-immunization IgM anti-cocaine antibodies provided a prognostic indicator for the response specifically to the cocaine moiety of the cocaine CTB vaccine or whether it was an indicator of the general response to immunization, we looked at the relationship between the IgM anti-cocaine antibodies in pre-immunization sera and the peak responses to the CTB component of the vaccine. The data in Fig. 3 shows that high levels of IgM anti-cocaine antibodies in pre-immunization sera were also significantly associated with a relatively lower response to the carrier protein, CTB, as supported by both Pearson and Spearman correlations (p < .01).
FIGURE 3.
IgM anti-cocaine antibodies in pre-immunization sera were reciprocally correlated (p = .0126, Spearman correlation) with the levels of IgG anti-CTB antibodies attained 16 weeks after immunization by these same individuals.
Despite the impaired CTB responses in people with IgM anti-cocaine antibodies in pre-immunization sera, comparisons of maximal IgG responses to cocaine and CTB suggest that the carrier protein generally performed effectively. As expected it stimulated IgG responses to both vaccine components, especially in subjects whose capacity to respond had not been impaired by a prior spontaneous IgM response to cocaine (Fig. 4), as supported by both Pearson and Spearman correlations (p < .05).
FIGURE 4.
IgG anti-cocaine and IgG anti-CTB antibody responses were significantly correlated (p = .0316, Spearman correlation).
DISCUSSION
Contrary to expectation, self-immunization with cocaine prior to participation in this study did not prime vaccine recipients to make a secondary response with high level production of IgG antibody following inoculation with the vaccine. Instead, the presence of IgM antibody appeared to be a marker for a poor IgG antibody response to the vaccine. The cocaine CTB vaccine stimulated sufficiently high concentrations of IgG anti-cocaine to diminish or stop cocaine intake among only 38% of vaccine recipients.1 Thirty-five percent of the vaccinees made <20 µg/ml antibody, a concentration well below that needed to interfere with the central nervous system effects of cocaine and therefore the incentive to use this drug.5 The data in the present report suggest possible reasons why a substantial fraction of habitual cocaine users might fail to respond or to respond suboptimally to the cocaine CTB vaccine: (1) repeated use of cocaine may stimulate a T-cell independent immune response to this drug. (2) Poor responses to both CTB and cocaine may reflect cocaine’s known global impairment of immune responsivity. (3) Contaminants in street drugs are commonly present, such as levamisole,6 which is an immunomodulator commonly associated in this context with the induction of pathogenic autoantibodies, for example, IgM that recognizes cardiolipin, as well as IgG antibodies against various nuclear antigens.7,8
There are several reasons to support the hypothesis that cocaine use may have elicited a T independent antigen response in those subjects having pre-immunization IgM in their sera. First, cocaine can acylate lysines of native human serum proteins in chronic users to create antigenic structures recognizable by immune cells.9 Such native protein adducts are poor immunogens in comparison to foreign carrier proteins. Secondly, T-cell independent antigens directly activate B cells in either of two ways. Type 1 T independent antigens, for example bacterial lipopolysaccharides or poly-inosinic:cytidilic acid (poly-I-C), directly activate B cells and stimulate them to divide because they interact with and activate both antigen receptors and toll-like receptors on or within B cells, generating stimuli that are sufficient to cause B cells to produce abundant quantities of antibody. In contrast, Type 2 T independent responses are most often stimulated by polysaccharides, that typically display repeating antigenic subunits that simultaneously engage many identical antigen receptors on the B-cell surface.10 Recent studies suggest that some Type 2 T independent antigens, especially those that stimulate B-1b cells,11,12 are protein based, like the antigen created in habitual cocaine users by cocaine acylation of lysine in self proteins.9
A signature feature of Type 2 T independent antigen stimulated immune responses is that a second or subsequent antigenic exposure fails to elicit a recall or “booster” response.10,13 Considering that no subjects in this study who had 11 µg/ml or more IgM anti-cocaine antibody prior to immunization made more than 20 µg/ml IgG antibody after repeated stimulation with cocaine CTB vaccine (in fact, 9/16 patients with these low levels of IgG antibody had anti-cocaine IgM > 11 µg/ml), we infer that recreational cocaine may have induced a Type 2 T independent antibody response in these subjects. In the research that demonstrated that methyl esters of cocaine may generate a complete antigen by acylating the amino group of lysines on albumin and α2 macroglobulin, Deng et al.9 reported that plasma from two of seven chronic cocaine users contained an immunoglobulin that reacted with cocaine. This is the mechanism we postulate may be responsible for causing cocaine to become a “self” antigen in the habitual cocaine users we studied.
T-cell independent antigens differ from conjugated T-cell dependent antigens created by binding a hapten to an immunogenic carrier protein. The conjugate vaccine molecule is taken up by B cells expressing surface immunoglobulin M proteins that recognize and bind the hapten component, for example, norcocaine, of the composite antigen. The carrier protein, in this case CTB, is processed and then presented on MHC class II molecules to stimulate cognate helper T cells to provide signals required to stimulate cocaine specific B lymphocytes to multiply and produce IgG antibodies. Even with these processes, high IgG antibody responses require additional costimulatory signals, usually provided by adjuvants, with alum being most commonly used for clinical vaccines.
In vitro and in vivo experimental studies in animals demonstrate that the antibodies initially induced by Type 2 T independent responses block activation of antigen reactive B cells following second and subsequent exposure to antigen.10,13–15 The mechanism(s) responsible for this inhibition remain unknown; however, antigen masking by the IgM antibodies produced in response to the initial stimulus and activation of inhibitory FcγIIB receptors on antigen presenting cells or B lymphocytes during or after the initial immune response have been ruled out as possible explanations.10,13 After initial exposure to the antigen and the development of a Type 2 T independent response, the inability to respond to subsequent antigenic stimulation cannot be overcome by simply modifying the antigen in such a way that it becomes T-cell dependent,15 at least with adjuvants like alum, because alum, which is the adjuvant usually used with human vaccines (and the adjuvant used with the TA-CD clinical trial), is thought to costimulate primarily through IL-1.16 Similarly, modifying the dose or timing of booster immunizations is unlikely to affect this immunoregulatory bias, although manipulating these variables can markedly affect the affinity and magnitude of the immune responses in primary immunizations.17–19 This raises the possibility that immunization with Type 2 T independent antigens engenders, among other responses, a stimulus that induces regulatory T cells. The inhibitory effect of regulatory T cells can potentially be overcome by using other adjuvants that strongly costimulate through other signaling molecules like the toll like receptors (TLR) and thereby should enhance the antibody response.20 Some of these adjuvants are now in clinical use (eg, monophosphoryl lipid A21 and MF5922), but are restricted to certain commercial vaccines. Nonetheless, many other new developments in the vaccine field23 may also provide sufficient positive stimuli to overcome the reduced responses in patients with preexisting IgM against cocaine. Alternative methods now include blocking inhibitory signaling, such as with monoclonal antibodies against CTLA4, which has been beneficial in prostate cancer, or ex vivo antigen loading and stimulation of dendritic cells, for example, which has allowed anticancer vaccines to overcome the immunosuppressive cells in certain cancers to achieve effective tumor killing.24
Another possible explanation for the failure of some cocaine users to respond adequately to the cocaine CTB vaccine is that chronic exposure to cocaine suppresses immune responses.25–29 The experimental evidence includes data showing that the function of monocyte-derived macrophages, which can act as antigen presenting cells, as well as responses of B cells and T lymphocytes, are depressed by cocaine.27 While none of these studies have specifically examined the effect of chronic cocaine use on induced antibody responses to antigen conjugates containing cocaine, as for example the cocaine CTB conjugate used in this study, one can infer from the effects of this drug on many elements of the immune response system that the antibody response to this antigen in cocaine users may be less than in subjects who have never used this drug recreationally. In future studies of cocaine conjugate vaccines, it will be of interest to investigate whether the immunosuppressive effects associated with chronic exposure to cocaine can be overcome by stimulating the host with better adjuvants and perhaps more effective carriers that aggressively activate the immune system to a greater extent than the alum used to stimulate responses to cocaine CTB.
Acknowledgments
This paper reports novel studies of sera collected during a clinical trial supported by the National Institute on Drug Abuse (NIDA), grants 1 R01 DA15477, K05-DA 0454 (Dr. Kosten), and P50-DA12762 and the Veterans Affairs Mental Illness Research, Education, and Clinical Center (New England MIRECC). Additional studies of these sera were supported by the Department of Veterans Affairs and NIDA grants DA023898, and DA030338 (Dr. Orson).
We thank Bangyi Mao for excellent technical assistance.
Footnotes
Declaration of Interest
Celtic Pharmaceuticals supplied the cocaine-CTB vaccine and gave Dr. Kosten travel fees for consultative services. No contractual publication constraint applies to this research. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- 1.Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence: A randomized double-blind placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orson FM, Kinsey BM, Singh RAK, et al. Substance abuse vaccines. Ann N Y Acad Sci. 2008;1141:257–269. doi: 10.1196/annals.1441.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paula S, Tabet MR, Farr CD, et al. Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J Med Chem. 2004;47:133–142. doi: 10.1021/jm030351z. [DOI] [PubMed] [Google Scholar]
- 4.Jani D, Meena LS, Rizwan-ul-Haq QM, et al. Expression of cholera toxin B subunit in transgenic tomato plants. Transgenic Res. 2002;11:447–454. doi: 10.1023/a:1020336332392. [DOI] [PubMed] [Google Scholar]
- 5.Orson FM, Kinsey BM, Singh RAK, et al. The future of vaccines in the management of addictive disorders. Curr Psychiatry Rep. 2007;9:381–387. doi: 10.1007/s11920-007-0049-z. [DOI] [PubMed] [Google Scholar]
- 6.Larocque A, Hoffman RS. Levamisole in cocaine: Unexpected news from an old acquaintance. Clin Toxicol (Phila) 2004;50:231–241. doi: 10.3109/15563650.2012.665455. [DOI] [PubMed] [Google Scholar]
- 7.Graf J, Lynch K, Yeh CL, et al. Purpura, cutaneous necrosis, and antineutrophil cytoplasmic antibodies associated with levamisole-adulterated cocaine. Arthritis Rheum. 2011;63:3998–4001. doi: 10.1002/art.30590. [DOI] [PubMed] [Google Scholar]
- 8.Chan AL, Louie S, Leslie KO, et al. Cutting edge issues in Goodpasture’s disease. Clin Rev Allergy Immunol. 2011;41:151–162. doi: 10.1007/s12016-010-8222-2. [DOI] [PubMed] [Google Scholar]
- 9.Deng SX, Bharat N, Fischman MC, et al. Covalent modification of proteins by cocaine. Proc Natl Acad Sci USA. 2002;99:3412–3416. doi: 10.1073/pnas.042700599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203:305–310. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alugupalli KR. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr Top Microbiol Immunol. 2008;319:105–130. doi: 10.1007/978-3-540-73900-5_5. [DOI] [PubMed] [Google Scholar]
- 12.Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol. 2011;187:5183–5195. doi: 10.4049/jimmunol.1101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodeur PH, Wortis HH. Regulation of thymus-independent responses: Unresponsiveness to a second challenge of TNP-Ficoll is mediated by hapten-specific antibodies. J Immunol. 1980;125:1499–1505. [PubMed] [Google Scholar]
- 14.Hosokawa T. Studies on B-cell memory. II. T-cell independent antigen can induce B-cell memory. Immunology. 1979;38:291–299. [PMC free article] [PubMed] [Google Scholar]
- 15.Lindroth K, Mastache EF, Roos I, et al. Understanding thymus-independent antigen-induced reduction of thymus-dependent immune responses. Immunology. 2004;112:413–419. doi: 10.1111/j.1365-2567.2004.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelka K, Latz E. Getting closer to the dirty little secret. Immunity. 2011;34:455–458. doi: 10.1016/j.immuni.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisen HN, Siskind GW. Variations in affinities of antibodies during the immune response. Biochemistry. 1964;3:996–1008. doi: 10.1021/bi00895a027. [DOI] [PubMed] [Google Scholar]
- 18.Goidl EA, Paul WE, Siskind GW, et al. The effect of antigen dose and time after immunization on the amount and affinity of anti-hapten antibody. J Immunol. 1968;100:371–375. [PubMed] [Google Scholar]
- 19.Paul WE, Thorbecke GJ, Siskind GW, et al. The effect of dose in tolerance induction on the subsequent response to a cross-reactive antigen. Immunology. 1969;17:85–92. [PMC free article] [PubMed] [Google Scholar]
- 20.Mosca F, Tritto E, Muzzi A, et al. Molecular, cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA. 2008;105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandepapeliere P, Horsmans Y, Moris P, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008;26:1375–1386. doi: 10.1016/j.vaccine.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 22.Baudner BC, Ronconi V, Casini D, et al. MF59 emulsion is an effective delivery system for a synthetic TLR4 agonist (E6020) Pharm Res. 2009;26:1477–1485. doi: 10.1007/s11095-009-9859-5. [DOI] [PubMed] [Google Scholar]
- 23.Wilson-Welder JH, Torres MP, Kipper MJ, et al. Vaccine adjuvants: Current challenges and future approaches. J Pharm Sci. 2009;98:1278–1316. doi: 10.1002/jps.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boikos SA, Antonarakis ES. Immunotherapy for prostate cancer enters its golden age. Clin Med Insights Oncol. 2012;6:263–273. doi: 10.4137/CMO.S7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhillon NK, Williams R, Peng F, et al. Cocaine-mediated enhancement of virus replication in macrophages: Implications for human immunodeficiency virus-associated dementia. J Neurovirol. 2007;13:483–495. doi: 10.1080/13550280701528684. [DOI] [PubMed] [Google Scholar]
- 26.Friedman H, Pross S, Klein TW. Addictive drugs and their relationship with infectious diseases. FEMS Immunol Med Microbiol. 2006;47:330–342. doi: 10.1111/j.1574-695X.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 27.Irwin MR, Olmos L, Wang M, et al. Cocaine dependence and acute cocaine induce decreases of monocyte proinflammatory cytokine expression across the diurnal period: Autonomic mechanisms. J Pharmacol Exp Ther. 2007;320:507–515. doi: 10.1124/jpet.106.112797. [DOI] [PubMed] [Google Scholar]
- 28.Pellegrino TC, Dunn KL, Bayer BM. Mechanisms of cocaine-induced decreases in immune cell function. Int Immunopharmacol. 2001;1:665–675. doi: 10.1016/s1567-5769(00)00051-5. [DOI] [PubMed] [Google Scholar]
- 29.XuW, Flick T, Mitchel J, et al. Cocaine effects on immunocompetent cells: An observation of in vitro cocaine exposure. Int J Immunopharmacol. 1999;21:463–472. doi: 10.1016/s0192-0561(99)00023-5. [DOI] [PubMed] [Google Scholar]