Abstract
A variety of strategies, have been applied to cancer treatment and the most recent one to become prominent is immunotherapy. This interest has been fostered by the demonstration that the immune system does recognize and often eliminate small tumors but tumors that become clinical problems block antitumor immune responses with immunosuppression orchestrated by the tumor cells. Methods to reverse this tumor-mediated immunosuppression will improve cancer immunotherapy outcomes. The immunostimulatory potential of nanoparticles (NPs), holds promise for cancer treatment. Phagocytes of various types are an important component of both immunosuppression and immunostimulation and phagocytes actively take up nanoparticles of various sorts, so NPs are a natural system to manipulate these key immune regulatory cells. NPs can be engineered with multiple useful therapeutic features, such as various payloads such as antigens and/or immunomodulatory agents including cytokines, ligands for immunostimulatory receptors or antagonists for immunosuppressive receptors. As more is learned about how tumors suppress antitumor immune responses the payload options expand further. Here we review multiple approaches to NP-based cancer therapies to modify the tumor microenvironment and stimulate innate and adaptive immune systems to obtain effective anti-tumor immune responses.
Introduction
This review discusses the confluence of two rapidly developing areas of cancer therapy, nanoparticles (NPs) and tumor immunology. The ability to produce NPs in the range of large proteins or protein complexes and combine multiple entities into these NPs has opened extensive new therapeutic possibilities for a variety of diseases, perhaps none more so than cancer. Currently, most clinically developed approaches depend on packaging clinically utilized chemotherapeutic agents in NPs and demonstrating improved efficacy in relation to toxicity 1–3. While these reformulations of existing drugs for improved delivery are the first NP cancer therapies to have an impact in the clinic, they will likely be followed in the coming years by much more complex and regulatable drug delivery systems.
The second area of cancer therapy that is rapidly progressing is immunotherapy, encompassing approaches to manipulate the patient’s immune system to attack the cancer. While this general approach is not new, the current sophisticated understanding of the immune system and the ability to assay immune changes in great detail has propelled this area into the forefront of current thinking about cancer therapy. Impressive clinical results on late stage patients that have failed prior therapies ensure the focus will remain on immunotherapy into the future. It is now clear that the immune system almost always can recognize and potentially attack tumors, despite their being so very similar to normal “self”, but in clinically identified cancer the tumor develops immunosuppressive systems that manipulate the immune system and protect it against anti-tumor immunity 4–6. The key to current immunotherapy strategies is modifying the tumor microenvironment such that the tumor-mediated immunosuppression is reduced, immune recognition of the tumor is supported and the immune system effectively attacks the tumor. There are many different immunotherapy strategies being developed and tested in preclinical and clinical models. It is quite likely that as the field matures the clinical approaches will combine multiple immunotherapy approaches, along with the current standard therapies of surgery, chemotherapy and radiation, in complex strategies to overcome a the complex challenges of cancer treatment. One ingredient in the mix of immunotherapies will be NPs-based approaches. This review is designed to provide the nonimmunologist with the basic concepts and strategies in NP-based immunotherapy and an understanding of the current status of this field and its future potential.
General Aspects of Nanoparticles
NPs are broadly defined as particles with a diameter of 10–200 nm and this scale entity has unique biological interaction potential. For scale appreciation, an immunoglobulin molecule is roughly 12 nm; the size of NPs ranges from individual proteins to large multiprotein complexes. The definition of NPs generally does not include individual proteins like immunoglobulins but rather focuses on artificially constructed multicomponent devices. Depending on a variety of NP and cell parameters, NPs can enter 7 and interact with cells in multiple ways 8. NP uptake by cells and NP-cell interactions are affected by parameters such as particle size and shape, surface charge, surface modification, and hydrophobicity/hydrophilicity 9–12. NPs can be engineered with a wide range of functional surface properties for utilization in a variety of biological tasks, including targeting immune cells to elicit innate and/or adaptive immune responses. This has led to the use of NPs in a variety of medical applications such as diagnostic devices, contrast agents, analytical tools, and drug delivery vehicles.
NPs utilized for therapeutic applications are composed of bioactive entities, such as small-molecule drugs, peptides, proteins, and nucleic acids, and the structural components (Fig. 1), such as lipids, polymers, metal, and carbon-based materials that serve as a variable platform. Together the “platform” and the “payload” formulate a nanocarrier delivery system. Additionally, surface-bound molecules can serve to help target the NPs to the desired cells (Fig. 1). NPs whose surfaces are not modified to prevent absorption of opsonins are generally ingested by phagocytic cells; thus, interaction with phagocytes is an inherent aspect of NP biology that must be considered for in vivo application and can be exploited in a variety of immunologically relevant ways. As noted, NPs are based on various inherently modifiable platforms and, therefore, can be tailored to manipulate specific biology, including immune biology. Immunostimulatory properties of NPs include stimulation of antigenicity, adjuvant activity, and inflammatory response.
Figure 1.
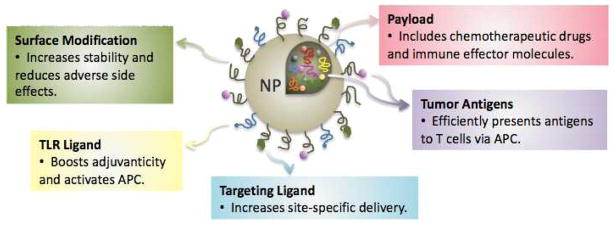
Schematic diagram of structure of nanoparticle platforms for therapeutic applications including surface modification, TLR ligands, targeting ligands, tumor antigen load, and payload composed of therapeutic entities. NP, nanoparticle; TLR, toll-like receptor
The development of a variety of NPs over the last decade has led to developmental research in cancer therapeutics. Multiple nanotechnology approaches to treat cancer by awakening and strengthening the immune system are being intensively researched in preclinical models and clinical trials, and a limited number are already FDA-approved for some cancers. Applications of nanomaterials for immune-mediated cancer therapy are still generally preclinical, but the progress is substantial and serves as the basis for this review.
Nanoparticles loaded with tumor antigens can augment tumor-specific immune response
The immune responses elicited by tumor antigens are often weak, both because the tumor antigens are in effect modified self antigens, and because the tumors mediate local immunosuppression. Tumors that become a clinical issue have invariably developed systems to suppress anti-tumor responses of the immune system, for example, by recruitment of immunosuppressive leukocytes. The “payload” of NPs can include tumor antigens and, since NPs are efficiently taken up by phagocytes that can present antigen to T cells, this can be an effective delivery system for tumor antigens in anti-tumor vaccines. If done properly, the NP can both deliver the tumor antigen and stimulate phagocytic cells to become activated and switch from an immunosuppressive cell, with poor antigen presentation, to an immunostimulatory antigen-presenting cell (APC). Support for this idea come from reports that NPs can improve antigenicity/immunogenicity of conjugated weak antigens to augment immune responses toward such antigens 13. Importantly NPs can stimulate responses by T cells, the central cell type in many anti-tumor immune responses (Fig. 2). For example, human mature dendritic cells (DCs) loaded with poly DL-lactide-co-glycolide NPs (PLGA-NPs) encapsulating “melanoma antigen recognized by T cells 1” (MART-1) generated greater cytototoxic activity of the tumor antigen-specific cytotoxic T lymphocytes (CTLs) than that induced by human DCs pulsed with free MART-1 peptide 14. Also, liposomes loaded with the melanoma antigens gp100 and TRP-2 were taken up by dendritic cells in B16 melanoma-challenged mice and resulted in increased immunostimulatory cytokines, IFN-γ and TNF-α in the tumor microenvironment, as well as increased CTL activity against B16 tumors 15. This approach has been used in breast cancer as well, in which uptake of Her-2/neu-decorated liposomes by APCs protected mice from challenge with Her-2/neu positive tumors 16.
Figure 2.
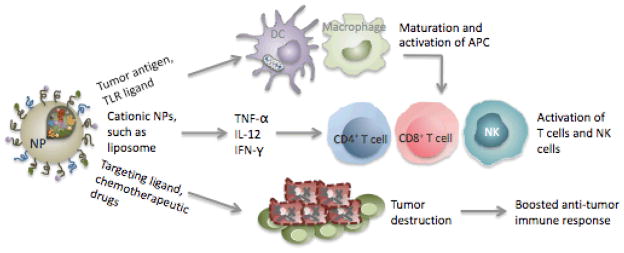
Advantages of nanoparticles used as therapeutic regimens for cancer therapy. Use of NPs with potent adjuvant activity or TLR ligand-encapsulated NPs as a platform to efficiently deliver tumor antigen to APCs can generate optimal delivery of tumor antigen and full activation of APCs that result in an effective adaptive immune response. The immunostimulatory activity of NPs is capable of inducing secretion of inflammatory cytokines, such as TNF-α, IL-12, and IFN-γ, which are crucial for stimulation of T cells and recruitment of NK cells. Inclusion of targeting ligands on the surface of NPs can cause selective destruction of tumor cells. NP-mediated delivery of chemotherapeutic drugs can offer effective drug delivery and controlled release, leading to enhanced safety and improved chemotherapy efficacy for tumor treatment. NP, nanoparticle; DC, dendritic cell; APC, antigen-presenting cell; NK, natural killer.
Nanoparticles with adjuvant activity enhance antigen presentation
Effective anti-tumor immune response requires the activation of adaptive immunity and, in most vaccines, this is done by addition of an adjuvant. APCs, such as dendritic cells (DCs), are required for the activation of adaptive immune responses by presenting antigens to T cells. However, unless given a clear “danger” signal to mature and activate the APCs, tumor antigen presentation by APCs is ineffective and tumor-resident APCs mediate immunosuppression rather than immunostimulation 17. A productive strategy is using NPs to modulate immunosuppressive APCs into immunostimulatory phenotypes in the tumor microenvironment by including adjuvants in the payload. Such an approach has the advantage that phagocytic APCs tend to ingest NPs preferentially as compared to other cells 18. APCs express a broad variety of receptors that recognize pathogen-associated constituents, such as toll-like receptors (TLRs), and activation of such receptors are crucial for activating APCs and stimulating an effective adaptive immune response 19. Signaling through TLRs activates APCs into cells that more efficiently process and present the antigens they carry and better stimulate antigen specific T cells, by expression of cytokines and costimulatory molecules (Fig. 3). Substances that activate TLRs or other “danger” signal receptors are used in vaccines as adjuvants to stimulate an innate immune response through which a more effective adaptive immune response is generated.
Figure 3.
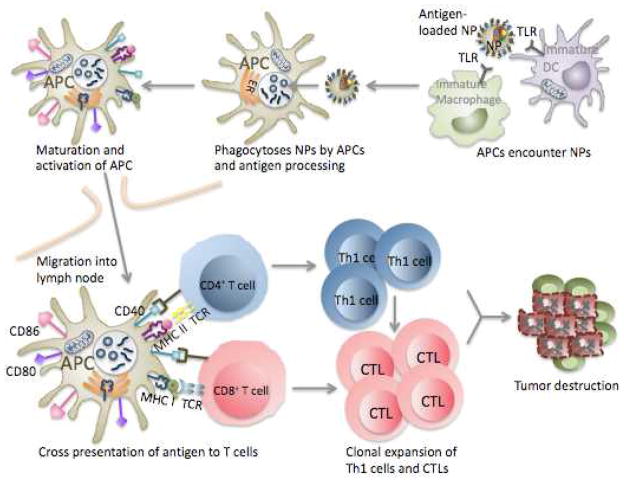
Schematic overview of the immune response elicited by a therapeutic nanoparticle. NP uptake by immature APCs after encounter with NPs or TLR ligand-conjugated NPs entrapped with tumor antigens induces efficient delivery of antigen to APCs. Maturation and upregulation of co- stimulatory molecules CD80, CD86, and CD40 following antigen uptake and processing within APCs promotes their migration to lymph nodes where they can activate T cells. Cross presentation of tumor antigens through MHC class I and class II on APCs to CD8+ or CD4+ T cells, respectively, stimulates T cells. CD8+ T cells undergo proliferation and differentiate into CTLs whereas CD4+ T cells differentiate into T-helper 1 (Th1) cells that can enhance anti-tumor CTL immune response at the tumor site. CTLs can cause tumor destruction by direct lysis of tumor cells. NP, nanoparticle; APC, antigen-presenting cell; TLR, toll-like receptor; DC, dendritic cell; ER, endoplasmic reticulum; MHC, major histocompatibility complex; TCR, T cell receptor; CTL, cytotoxic T lymphocyte; Th1, T-helper 1.
Some lipids used to generate NPs are directly immunostimulatory. Polyethylenimine (PEI) is a positively charged polymer that has been widely used to form liposome nanoparticles, and recent reports indicate that this cationic polymer has significant anti-tumor immune activity triggered by adjuvant activity due to activation of TLRs 13,20,21. PEI is one of a family of organic polycations used as nucleic acid complexing/condensation reagents in vitro and in vivo, most of which have not been analyzed for TLR interaction. PEI by itself triggered robust TLR5 activation and encapsulation of immunostimulatory siRNA further stimulated APCs within the tumor microenvironment 21. Administration of siRNA-PEI NPs to deliver siRNA targeting the immunosuppressive cell surface receptor PD-L1 induced knockdown of PD-L1 and changed the immunosuppressive phenotype of human and mouse ovarian cancer-associated DCs to an immunostimulatory phenotype that resulted in enhanced antigen presentation and increased numbers of tumor-reactive CD8+ T cells in the tumor microenvironment, as well as improved survival in mouse models 21. PEI-based NPs encapsulating antisense RNA against miR-155 effectively modified immunosuppressive cancer-associated DCs into immunostimulatory cells via reduced miR-155 and TLR5 activation, thus boosting anti-tumor immune responses 22. PEI-complexed delivery of siRNA against essential oncogenes pleiotrophin (PTN) and HER-2/neu resulted in significant reduction of tumor growth in glioblastoma xenografts 23 and breast cancer models 24. PEI is not the only NP generating polymer that stimulates TLRs. Use of polymethyl vinyl ether-co-maleic anhydride (PVMA)-coated NPs as an adjuvant also has been demonstrated to activate DCs through TLR2/4 stimulation 25.
NP containing agonists that stimulate TLRs are effective in functional maturation of DCs and their ability to prime T cells 19,26 Inclusion of TLR ligands on the surface of NPs gives pathogen-like properties to these particles that enable recognition by conventional pathogen-specific routes 27. Such recognition stimulates pro-inflammatory signaling pathways 28–30, which boosts response to antigens. TLR agonist-loaded NPs containing tumor antigens, consequently can induce stronger antigen-specific immune responses than similar amount of soluble antigens with or without adjuvants. For example, DCs exposed to PLGA-NPs containing antigens and TLR4 or TLR9 ligands induced greater immune response in comparison to DCs that were treated with soluble antigen alone or antigen and TLR ligands 31.
These findings indicate that NPs engineered to include adjuvant activity have considerable potential for cancer immunotherapy. The adjuvant activity of NPs is thought to extend beyond direct stimulation of TLR or other pathogen receptors and to have contributions from repetitive antigen display, like the surface of a pathogen, a “depot effect” that is created by controlled, slow release of the antigen from the NPs, and antigen stabilization in the in vivo environment provided by insoluble NPs 32–34. However, the contribution of these multiple mechanisms and potential of NP-mediated adjuvant activity in cancer immunotherapy are just being explored.
Virus-like nanoparticles as a cancer immunotherapy platform
Viruses are essentially natural nanoparticles since they are in the proper size range and have a repetitive multicomponent nature. Virus-like particles (VLPs), a type of biological NPs, are self-assembling particles composed of one or several viral structural proteins but are non-infectious by lacking the viral genome. There are VLP vaccines that have already been approved by the FDA to prevent infection by viruses that cause cancer, such as VLP vaccines against human papilloma virus (HPV) that causes human cervical cancer, and a VLP vaccine that protects against hepatitis B virus (HBV) infection, which can lead to liver cancer. There is growing interest in using VLPs in vaccine technology against infectious disease or tumors since the VLPs not only can carry a “payload” but appear to be recognizable by the immune system as a pathogen and can activate APCs 35. Like other NPs, VLPs also can be engineered to incorporate exogenous molecules, such as antigens, giving them potential value for immunotherapy, and as vaccines for a range of cancers and other diseases. For example, single dose vaccination with murine polyomavirus virus-like particles (MPyV-VLPs) containing VP2 and Her-2/neu stimulated anti-tumor immunity that inhibited the growth of a Her-2/neu-expressing tumor line in Balb/c mice 36. Immunization with a VLP delivering murine Trop2, a cell surface glycoprotein overexpressed in pancreatic cancer, increased activation and tumor infiltration of T cells and significantly reduced tumor growth in a mouse pancreatic cancer model 37. Taken together, these results support significant potential of VLPs for cancer immunotherapy, but as with many NP-mediated therapies, VLP technology for cancer treatment still needs to be validated and adopted clinically.
Nanoparticles may induce immunostimulatory cytokine production
Clearly, each NP used in therapy must be evaluated for safety and there are significant concerns about potential safety problems with NP usage in humans. One issue with NPs for clinical use is that some NPs can trigger immunostimulatory responses mediated by the production of inflammatory cytokines (Fig. 2). Secretion of inflammatory cytokines has been shown for a variety of nanomaterials themselves including gold colloids, dendrimers, polymers, and lipid NPs, 34,38–42. Nanoparticle size, surface charge and composition have been demonstrated to be important for immunostimulatory activity. For example, smaller NPs ranging from <40 to 50 nm have been shown to be very effective in stimulating both humoral immunity and MHC-I restricted CD8+ T cell immunity 38–40. Cationic liposomes have also been reported to better facilitate secretion of inflammatory cytokines such as TNF-α, IL-12, and IFN-γ, and increase the expression of CD80/CD86 activation markers on the surface of DCs than anionic or neutral NPs 9,43,44.
For most uses inflammation induction would be a negative side effect but that is not clear for tumor immunotherapy where it may be what is desired and has to be managed. Although inflammation induced by immunostimulatory NPs can cause tissue damage and other potential toxic effects, the immunostimulatory properties of NPs still support their potential application in cancer immunotherapy. For example, poly DL-lactic acid (PLA)-biodegradable NPs conjugated with anti-Neu/anti-CD40 antibodies mediated increased recognition of Neu-expressing tumor cells by APCs but not Neu− tumor cells 45. Intratumoral injection of PLA-NPs conjugated with the anti-neu/anti-CD40 in subcutaneous Her2/neu+ TUBO mammary tumor model also induced a strong pro-inflammatory response including significantly elevated levels of IL-6, IL-12, INF-γ, and TNF-α and rejected the primary tumor or significantly delayed tumor growth. Mice that rejected the primary tumors also had protection against tumor rechallenge 45. While immune-mediated toxicity is a potential side effect of any cancer immunotherapy, there is no reason to expect that such toxicity mediated by NP-based therapies will be worse or more difficult to control than similar challenges with other immunotherapies. Virtually every cancer therapy employed has to balance damage to tumors with damage to the normal tissues, so this is not a unique problem.
Nanoparticles can serve as a platform for delivering cytokines or other immune mediators
NPs can provide a useful and multifunctional platform for delivering many therapeutic molecules for cancer treatment (Fig. 2). NP-mediated delivery of immune effector molecules, such as cytokines can extend circulatory half-life of molecules which in turn enables the use of reduced concentrations with less toxicities. IL-2 is systemically administered for treating some tumors, but has considerable toxicity 46,47. Combination delivery of TGF-β inhibitor and IL-2 encapsulated in a liposome, either intratumorally or intravenously, increased activation of CD8+ T cells and natural killer (NK) cells and significantly delayed tumor growth without the adverse side effects observed generally in systemic IL-2 administration 46. Delivery of IL-2 by liposome nanocomplex significantly enhanced therapeutic effect of IL-2 in a variety of other tumor models, including liver cancer 48, melanoma 49, and lung cancer 50. In all cases, liposomal NP-mediated IL-2 delivery increased half-life of IL-2 in circulation and reduced systemic toxicity by using lower doses than that of free IL-2, and resulted in significant reduction in tumor growth 48–50. Liposomal IL-2 nanocomplexes also lead to increased infiltration of Gr-1+ inflammatory cells and increased influx of CD3+ T cells within the tumors 49,50.
Utilizing NPs as a vehicle for delivery of immunostimulatory cytokines has been demonstrated with other NPs and cytokines, as well. Irradiated B16 melanoma cells supplemented with liposomal IFN-γ induced protection against B16 challenge mediated by increased tumor-specific CTLs activity 51. There are clinical trials ongoing whereby patients with metastatic melanoma and colon adenocarcinoma are infused with recombinant human TNF-α bound to colloidal gold NPs. Clinical use of TNF-α-gold NPs provided greater TNF-α accumulation in patient tumors as assessed by biopsy 24 hours post-infusion and resulted in massive vascular leakage in tumors 52.
Nanoparticle-mediated hyperthermia activates the immune system
Another area of interest for immune-mediated anti-tumor nanotechnology is the use of NPs that are dormant by themselves but can be activated using external energy sources. Metallic particles, such as iron-, silver-, and gold-based NPs, can be excited by external energy sources, such as an alternating magnetic field, infrared light, or radiofrequency electromagnetic field, and can produce heat in vivo 53–55. Carbon nanotubes, which are non-metallic polymer-based NPs with good thermal properties can also generate heat following absorption of infrared radiation 56,57. Direct administration of heatable NPs into the tumors and/or application of external energy sources specifically in tumors can induce nanoparticle-mediated local hyperthermia.
Properly applied hyperthermia is immunogenic. Cancer cells experiencing stress from hyperthermia induce heat shock proteins (HSPs) as a part of the defense mechanisms. HSPs released after heat-induced necrosis are immunostimulatory 58. HSPs are known to activate APCs through TLR signaling pathways. For example, HSP70 bound to TLR2 and TLR4 on DCs resulted in increased production of inflammatory cytokines, such as TNF-α, IL-1β, and IL-12 59,60. Several reports demonstrated that HSPs induced cross-presentation of cancer antigen to prime cytotoxic CD8+ T cells and resulted in improved adaptive anti-tumor immune response 61–64. Heat and HSPs can also effectively improve the immunogenicity of cancer cells. For example, HSP70 stimulated by hyperthermia (43°C for 1 hour) increased the expression of MHC class I antigen on rat T-9 glioma cells resulting in enhanced tumor recognition by CTLs 65. Fever-range thermal stress (39.5°C for 6 hours) has also been demonstrated to increase tumor cell susceptibility to NK cells via increased expression of NK target molecule like MICA that is reported to have heat shock response elements in the promoter 66. These results suggest that developing novel cancer treatment based on NP-mediated hyperthermia in combination with immunotherapy has potential to enhance anti-tumor immunity.
Conclusion
The positive impact the immune system can have on treating cancer has not been fully developed and immunotherapy is not yet applied as a frontline strategy for treatment of most cancers (current exceptions are certain types of bladder cancer and kidney cancer). As doctors and scientists have learned about the role of the immune system in controlling cancer, immunotherapy has become an important part of cancer treatment research. NP-based cancer immunotherapy can contribute to cancer immunotherapy by providing useful features such as large payloads, accommodation of multiple targeting ligands, co-delivery of antigens and immunomodulatory agents, inherent adjuvant activity, preferential targeting of phagocytic immune cells, stability in vitro and in vivo, and sustained release of the therapeutic payload. Clinical results in cancer patients and reports in animal cancer models described in this review provide proof that NPs can have enhanced anti-cancer effects with reduced side effects owing to more specifically targeted localization in tumors via improved pharmacokinetics and pharmacodynamics and effective immunostimulation. However, current NP technologies require optimization before they may be utilized for broad use clinically. The success of NPs for cancer immunotherapy largely depends on the development of new and better targeting modalities to support preferential accumulation of NPs in tumors rather than other tissues.67. Further challenges involve the obvious need to combine multiple therapies in the clinic and the complexities of determining optimal combinatorial strategies. Yet the application of NPs to treat cancer is actively being investigated and is producing exciting results. The work is still in progress and so far the clinical impact of nanotechnology on cancer immunotherapy remains in the exploratory stage. While our understanding of NPs therapeutic effects on the immune system improves, and emerging research holds great promise for cancer treatment, further mechanistic investigations of NP-mediated immunostimulatory effects are required to develop its true therapeutic potential.
Sidebar. Basic modes of nanoparticle mediated immune modulation.
Preferred uptake by phagocytic antigen presenting cells
Tumor antigen deliviery and depots
NP platforms with Inherent immunostimulatory adjuvant activity
Viral-like particles
Delivery of cytokines or chemokines
Targeted generation of local hyperthermia
Contributor Information
Mee Rie Sheen, Department of Microbiology and Immunology, Geisel School of Medicine at Dartmouth, Hanover, NH 03755.
Patrick H. Lizotte, Department of Microbiology and Immunology, Geisel School of Medicine at Dartmouth, Hanover, NH 03755
Seiko Toraya-Brown, Department of Microbiology and Immunology, Geisel School of Medicine at Dartmouth, Hanover, NH 03755.
Steven Fiering, Department of Microbiology and Immunology, Geisel School of Medicine at Dartmouth, Hanover, NH 03755. Department of Genetics, Geisel School of Medicine at Dartmouth, Hanover, NH 03755. Norris Cotton Cancer Center, Lebanon, NH 03756.
References
- 1.O’Brien S, Schiller G, Lister J, Damon L, Goldberg S, Aulitzky W, Ben-Yehuda D Stock W, Coutre S, Douer D, et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia. J Clin Oncol. 2013;31:676–683. doi: 10.1200/JCO.2012.46.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stathopoulos GP, Antoniou D, Dimitroulis J, Michalopoulou P, Bastas A, Marosis K, Stathopoulos J, Provata A, Yiamboudakis P, Veldekis D, et al. Liposomal cisplatin combined with paclitaxel versus cisplatin and paclitaxel in non-small-cell lung cancer: a randomized phase III multicenter trial. Ann Oncol. 2010;21:2227–2232. doi: 10.1093/annonc/mdq234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amadori D, Milandri C, Comella G, Saracchini S, Salvagni S, Barone C, Bordonaro R, Gebbia V, Barbato A, Serra P, et al. A phase I/II trial of non-pegylated liposomal doxorubicin, docetaxel and trastuzumab as first-line treatment in HER-2-positive locally advanced or metastatic breast cancer. Eur J Cancer. 2011;47:2091–2098. doi: 10.1016/j.ejca.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Ullrich E, Koch J, Cerwenka A, Steinle A. New prospects on the NKG2D/NKG2DL system for oncology. Oncoimmunology. 2013;2:e26097. doi: 10.4161/onci.26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 6.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Burgess R. Medical applications of nanoparticles and nanomaterials. Stud Health Technol Inform. 2009;149:257–283. [PubMed] [Google Scholar]
- 8.Mailander V, Landfester K. Interaction of nanoparticles with cells. Biomacromolecules. 2009;10:2379–2400. doi: 10.1021/bm900266r. [DOI] [PubMed] [Google Scholar]
- 9.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 10.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumari A, Yadav SK. Cellular interactions of therapeutically delivered nanoparticles. Expert Opin Drug Deliv. 2011;8:141–151. doi: 10.1517/17425247.2011.547934. [DOI] [PubMed] [Google Scholar]
- 13.Wegmann F, Gartlan KH, Harandi AM, Brinckmann SA, Coccia M, Hillson WR, Kok WL, Cole S, Ho LP, Lambe T. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat Biotechnol. 2012;30:883–888. doi: 10.1038/nbt.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma W, Chen M, Kaushal S, McElroy M, Zhang Y, Ozkan C, Bouvet M, Kruse C, Grotjahn D, Ichim T, Minev B. PLGA nanoparticle-mediated delivery of tumor antigenic peptides elicits effective immune responses. Int J Nanomedicine. 2012;7:1475–1487. doi: 10.2147/IJN.S29506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M. Suppression of melanoma growth and metastasis by DNA vaccination using an ultrasound-responsive and mannose-modified gene carrier. Mol Pharm. 2011;8:543–554. doi: 10.1021/mp100369n. [DOI] [PubMed] [Google Scholar]
- 16.Roth A, Rohrbach F, Weth R, Frisch B, Schuber F, Wels WS. Induction of effective and antigen-specific antitumour immunity by a liposomal ErbB2/HER2 peptide-based vaccination construct. Br J Cancer. 2005;92:1421–1429. doi: 10.1038/sj.bjc.6602526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghiringhelli F, Puig PE, Roux S, Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toraya-Brown S, Sheen MR, Baird JR, Barry S, Demidenko E, Turk MJ, Hoopes PJ, Conejo-Garcia JR, Fiering S. Phagocytes mediate targeting of iron oxide nanoparticles to tumors for cancer therapy. Integr Biol (Camb) 2012;5:159–171. doi: 10.1039/c2ib20180a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck B, Dorfel D, Lichtenegger FS, Geiger C, Lindner L, Merk M, Schendel DJ, Subklewe M. Effects of TLR agonists on maturation and function of 3-day dendritic cells from AML patients in complete remission. J Transl Med. 2011;9:151. doi: 10.1186/1479-5876-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Z, Yang Y, Jiang Y, Shao J, Sun X, Chen J, Dong L, Zhang J. Anti-tumor immune responses of tumor-associated macrophages via toll-like receptor 4 triggered by cationic polymers. Biomaterials. 2013;34:746–755. doi: 10.1016/j.biomaterials.2012.09.062. [DOI] [PubMed] [Google Scholar]
- 21.Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009;119:2231–2244. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, Anadon-Arnillas J, Harwood NM, Korc M, Fiering SN, et al. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72:1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grzelinski M, Urban-Klein B, Martens T, Lamszus K, Bakowsky U, Höbel S, Czubayko F, Aigner A. RNA interference-mediated gene silencing of pleiotrophin through polyethylenimine-complexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum Gene Ther. 2006;17:751–766. doi: 10.1089/hum.2006.17.751. [DOI] [PubMed] [Google Scholar]
- 24.Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–466. doi: 10.1038/sj.gt.3302425. [DOI] [PubMed] [Google Scholar]
- 25.Camacho AI, Da Costa Martins R, Tamayo I, de Souza J, Lasarte JJ, Mansilla C, Esparza I, Irache JM, Gamazo C. Poly(methyl vinyl ether-co-maleic anhydride) nanoparticles as innate immune system activators. Vaccine. 2011;29:7130–7135. doi: 10.1016/j.vaccine.2011.05.072. [DOI] [PubMed] [Google Scholar]
- 26.Bal SM, Slutter B, Verheul R, Bouwstra JA, Jiskoot W. Adjuvanted, antigen loaded N-trimethyl chitosan nanoparticles for nasal and intradermal vaccination: adjuvant- and site-dependent immunogenicity in mice. Eur J Pharm Sci. 2012;45:475–481. doi: 10.1016/j.ejps.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta. 2002;1589:1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen DN, Mahon KP, Chikh G, Kim P, Chung H, Vicari AP, Love KT, Goldberg M, Chen S, Krieg AM, et al. Lipid-derived nanoparticles for immunostimulatory RNA adjuvant delivery. Proc Natl Acad Sci U S A. 2012;109:E797–803. doi: 10.1073/pnas.1121423109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamayo I, Irache JM, Mansilla C, Ochoa-Reparaz J, Lasarte JJ, Gamazo C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin Vaccine Immunol. 2010;17:1356–1362. doi: 10.1128/CVI.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castillo PM, Herrera JL, Fernandez-Montesinos R, Caro C, Zaderenko AP, Mejías JA, Pozo D. Tiopronin monolayer-protected silver nanoparticles modulate IL-6 secretion mediated by Toll-like receptor ligands. Nanomedicine (Lond) 2008;3:627–635. doi: 10.2217/17435889.3.5.627. [DOI] [PubMed] [Google Scholar]
- 31.Chithrani BD, Chan WC. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007;7:1542–1550. doi: 10.1021/nl070363y. [DOI] [PubMed] [Google Scholar]
- 32.O’Hagan DT, De Gregorio E. The path to a successful vaccine adjuvant--‘the long and winding road’. Drug Discov Today. 2009;14:541–551. doi: 10.1016/j.drudis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 34.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology. 2010;151:458–465. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crisci E, Barcena J, Montoya M. Virus-like particles: the new frontier of vaccines for animal viral infections. Vet Immunol Immunopathol. 2012;148:211–225. doi: 10.1016/j.vetimm.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tegerstedt K, Franzen AV, Andreasson K, Joneberg J, Heidari S, Ramqvist T, Dalianis T. Murine polyomavirus virus-like particles (VLPs) as vectors for gene and immune therapy and vaccines against viral infections and cancer. Anticancer Res. 2005;25:2601–2608. [PubMed] [Google Scholar]
- 37.Cubas R, Zhang S, Li M, Chen C, Yao Q. Chimeric Trop2 virus-like particles: a potential immunotherapeutic approach against pancreatic cancer. J Immunother. 2011;34:251–263. doi: 10.1097/CJI.0b013e318209ee72. [DOI] [PubMed] [Google Scholar]
- 38.Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, McKenzie IF, Plebanski M. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173:3148–3154. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 39.Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm. 2007;4:73–84. doi: 10.1021/mp060096p. [DOI] [PubMed] [Google Scholar]
- 40.Scholer N, Hahn H, Muller RH, Liesenfeld O. Effect of lipid matrix and size of solid lipid nanoparticles (SLN) on the viability and cytokine production of macrophages. Int J Pharm. 2002;231:167–176. doi: 10.1016/s0378-5173(01)00882-1. [DOI] [PubMed] [Google Scholar]
- 41.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 42.Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, Gabrielsson S. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett. 2006;6:1682–1686. doi: 10.1021/nl060860z. [DOI] [PubMed] [Google Scholar]
- 43.Tan Y, Li S, Pitt BR, Huang L. The inhibitory role of CpG immunostimulatory motifs in cationic lipid vector-mediated transgene expression in vivo. Hum Gene Ther. 1999;10:2153–2161. doi: 10.1089/10430349950017149. [DOI] [PubMed] [Google Scholar]
- 44.Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112:13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 45.Dominguez AL, Lustgarten J. Targeting the tumor microenvironment with anti-neu/anti-CD40 conjugated nanoparticles for the induction of antitumor immune responses. Vaccine. 2010;28:1383–1390. doi: 10.1016/j.vaccine.2009.10.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, et al. Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11:895–905. doi: 10.1038/nmat3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siegel JP, Puri RK. Interleukin-2 toxicity. J Clin Oncol. 1991;9:694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- 48.Kanaoka E, Takahashi K, Yoshikawa T, Jizomoto H, Nishihara Y, Uchida N, Maekawa R, Hirano K. A significant enhancement of therapeutic effect against hepatic metastases of M5076 in mice by a liposomal interleukin-2 (mixture) J Control Release. 2002;82:183–187. doi: 10.1016/s0168-3659(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 49.Neville ME, Robb RJ, Popescu MC. In situ vaccination against a non-immunogenic tumour using intratumoural injections of liposomal interleukin 2. Cytokine. 2001;16:239–250. doi: 10.1006/cyto.2001.0963. [DOI] [PubMed] [Google Scholar]
- 50.Skubitz KM, Anderson PM. Inhalational interleukin-2 liposomes for pulmonary metastases: a phase I clinical trial. Anticancer Drugs. 2000;11:555–563. doi: 10.1097/00001813-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 51.van Slooten ML, Storm G, Zoephel A, Küpcü Z, Boerman O, Crommelin DJ, Wagner E, Kircheis R. Liposomes containing interferon-gamma as adjuvant in tumor cell vaccines. Pharm Res. 2000;17:42–48. doi: 10.1023/a:1007514424253. [DOI] [PubMed] [Google Scholar]
- 52.Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Jr, Gannon WE, Walker M, Seidel GD, Yuldasheva N, Tamarkin L. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16:6139–6149. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: an in vivo study. Jpn J Cancer Res. 1998;89:463–469. doi: 10.1111/j.1349-7006.1998.tb00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu KW, Huang CC, Hwu JR, Su WC, Shieh DB, Yeh CS. A new photothermal therapeutic agent: core-free nanostructured Au x Ag1-x dendrites. Chemistry. 2008;14:2956–2964. doi: 10.1002/chem.200800114. [DOI] [PubMed] [Google Scholar]
- 55.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torti SV, Byrne F, Whelan O, Levi N, Ucer B, Schmid M, Torti FM, Akman S, Liu J, Ajayan PM, et al. Thermal ablation therapeutics based on CN(x) multi-walled nanotubes. Int J Nanomedicine. 2007;2:707–714. [PMC free article] [PubMed] [Google Scholar]
- 57.Gannon CJ, Cherukuri P, Yakobson BI, Cognet L, Kanzius JS, Kittrell C, Weisman RB, Pasquali M, Schmidt HK, Smalley RE, Curley SA. Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field. Cancer. 2007;110:2654–2665. doi: 10.1002/cncr.23155. [DOI] [PubMed] [Google Scholar]
- 58.Todryk SM, Melcher AA, Dalgleish AG, Vile RG. Heat shock proteins refine the danger theory. Immunology. 2000;99:334–337. doi: 10.1046/j.1365-2567.2000.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 60.Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- 61.Suzue K, Zhou X, Eisen HN, Young RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci U S A. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–1408. [PubMed] [Google Scholar]
- 63.Moroi Y, Mayhew M, Trcka J, Hoe MH, Takechi Y, Hartl FU, Rothman JE, Houghton AN. Induction of cellular immunity by immunization with novel hybrid peptides complexed to heat shock protein 70. Proc Natl Acad Sci U S A. 2000;97:3485–3490. doi: 10.1073/pnas.070550797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noessner E, Gastpar R, Milani V, Brandl A, Hutzler PJ, Kuppner MC, Roos M, Kremmer E, Asea A, Calderwood SK, Issels RD. Tumor-derived heat shock protein 70 peptide complexes are cross-presented by human dendritic cells. J Immunol. 2002;169:5424–5432. doi: 10.4049/jimmunol.169.10.5424. [DOI] [PubMed] [Google Scholar]
- 65.Ito A, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Augmentation of MHC class I antigen presentation via heat shock protein expression by hyperthermia. Cancer Immunol Immunother. 2001;50:515–522. doi: 10.1007/s00262-001-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol. 2007;82:1322–1331. doi: 10.1189/jlb.1106699. [DOI] [PubMed] [Google Scholar]
- 67.Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release. 2001;74:47–61. doi: 10.1016/s0168-3659(01)00309-1. [DOI] [PubMed] [Google Scholar]