Abstract
The prevalence of dental caries (tooth decay) among preschool children is increasing, driven partially by an earlier age of onset of carious lesions. The American Academy of Pediatrics recommends application of 5% sodium fluoride varnish at intervals increasing with caries risk status, as soon as teeth are present. However, the varnishes are marketed for treatment of tooth sensitivity and are regulated as medical devices rather than approved by the US Food and Drug Administration for prevention of dental caries (tooth decay). The objective of this research is to examine the safety of use in toddlers by characterizing the absorption and distribution profile of a currently marketed fluoride varnish. We measured urinary fluoride for 5 hours after application of fluoride varnish to teeth in 6 toddlers aged 12 to 15 months. Baseline levels were measured on a separate day. The urine was extracted from disposable diapers, measured by rapid diffusion, and extrapolated to plasma levels. The mean estimated plasma fluoride concentration was 13 μg/L (SD, 9 μg/L) during the baseline visit and 21 μg/L (SD, 8 μg/L) during the 5 hours after treatment. Mean estimated peak plasma fluoride after treatment was 57 μg/L (SD, 22 μg/L), and 20 μg/kg (SD, 4 μg/L) was retained on average. Retained fluoride was 253 times lower than the acute toxic dose of 5 mg/kg. Mean plasma fluoride after placement of varnish was within an SD of control levels. Occasional application of fluoride varnish following American Academy of Pediatrics guidance is safe for toddlers.
Keywords: fluorides, fluoride varnishes, pharmacokinetics, drug safety, toddler
Fluoride varnish (5% NaF) is effective in preventing dental caries (tooth decay)1 and is the standard of care. The American Dental Association,2 American Academy of Pediatric Dentistry,3 and American Academy of Pediatrics4 recommend application of varnish at intervals increasing with caries risk. Application is recommended every 6 months for children under 6 years of age who are at moderate risk and as often as every 3 months for children at high risk.5
Although fluoride varnish for preventing dental caries is becoming increasingly common, its use is “off label” and is not approved by the US Food and Drug Administration. These products were approved as medical devices “intended to coat a prepared cavity of a tooth before insertion of restorative materials” (CFR 872.3260), with approved indications of use including “treatment of hypersensitive teeth,” as “a cavity liner,” and on “sensitive root surfaces.” Fluoride varnish has not undergone the evaluation normally required for drugs. Despite other forms of topical fluoride having been shown to pose a toxicity risk,6 no systemic absorption or excretion data are available to evaluate safety in toddlers.
Varnishes are applied topically and left in place. The benefits are both topical and systemic.7 In studies of fluoride varnish in older children and adults, the teeth are isolated and dried to increase adherence. Fluoride from the varnishes is swallowed, absorbed from the gut, and deposited in the skeleton and teeth, with excess eliminated in the urine and stool. In toddlers it is not possible to completely isolate the teeth and more varnish is likely swallowed immediately after placement.
The major risks with ingesting fluorides are renal toxicity and fluorosis. Serum levels often accumulate to >50 μM (0.95 mg/L) fluoride during prolonged general anesthesia from fluorinated inhalation anesthetics.8,9 With prolonged serum >50 μM, the earliest adverse effects of systemic fluoride toxicity can be seen in less than half of studied patients, manifested as temporary decreased renal concentrating function.8–10 However, none of the patients in any of these studies developed clinical signs of nephrotoxicity.11 Generally, evidence suggests that fluorosis is the result of peak fluoride in the plasma rather than the quantity absorbed.12 Acute doses can result in sufficient fluoride being mobilized from the bone adjacent to the developing teeth to affect enamel development.12
Thus, the use of fluoride varnishes is clinically appropriate.13,14 Other forms of fluoride, especially those used chronically, are swallowed and may increase risk for adverse effects.15 Nevertheless, fluoride varnishes are incompletely effective in preventing dental caries and better agents are needed.16
The objective of this research is to use urinary levels of fluoride to characterize the absorption and distribution profile in toddlers for a currently marketed fluoride varnish. From these data we estimated the peak plasma fluoride concentration to allow for an estimate of the margin of safety.
Patients and Methods
Six healthy toddlers between 12 and 15 months of age who had at least 2 teeth were recruited from the University of Washington Center for Pediatric Dentistry. Children who had an allergy or recent stomatitis were excluded. The University of Washington Institutional Review Board approved the study and informed consent was obtained.
Varnish
The varnish studied contains 5% NaF, 48% w/v rosin, 26.23% C2H6O, 10.0% CaSO40.2(H2O), 5.8% Na2HPO4, 2.0% glycerin, plus beeswax and high intensity sweetener (Enamel Pro, Premier Dental Products, Plymouth Meeting, PA). Analyses confirmed the fluoride concentration (4.89%; SD, 0.17%).
Procedure
Participants had 2 visits. The day before, parents were given fluoride-free water to make up formula and juice. They were instructed to refrain from brushing the child's teeth with fluoridated toothpaste. Visits were scheduled before the child’s usual feeding time.
At the treatment visit, the teeth were dried and varnish applied. The amount of the fluoride applied was the difference between the calculated amount put on the brush from the weight and concentration minus the amount measured in the brush after application.
A modification of the “gauze/cotton ball method” was used to collect the urine in which an absorbent pad (Kotex Lightdays Liners, Kimberly Clark, Neenah, WI) was substituted for cotton wool.17 Unexposed pads contained inconsequential amounts of fluoride. In pretests, 92% to 97% of the fluoride from urine-soaked pads was recovered.
On arrival the child was weighed and parents were instructed to commence feeding. After the first urination, varnish was applied and urine collection began. A similar procedure was followed for the control collection. Diapers were checked every 10 minutes. When a diaper was wet it was taken off, replaced, and the time noted. A few samples were noted to be damp at the next 10- or 20-minute interval, with significantly more volume. These diapers were pooled in analyses. Urine volume was determined by the difference in weight of the diaper and pad before and after use.
Fluoride was determined by the diffusion and detection method.18,19 After a minimal amount of urine was taken for creatinine determination, each pad was divided in half and reweighed. One half was assessed for fluoride first, allowing for sorted measuring of second halves.20 Analyses were performed in duplicate and concentrations averaged. Standard measurements of creatinine were carried out for participants D, E, and F. Retained fluoride was estimated by subtracting the additional fluoride measured at the treatment visit above that at the control visit from the applied fluoride.
Extrapolation to Plasma
Extrapolation was used because measurement of plasma fluoride would not be permitted under current ethical standards. Mean plasma fluoride concentration was estimated by the total recovered urine fluoride divided by time between diapers, weight of the participant, and the expected renal clearance of fluoride.21,22 This formula is a rearrangement of the calculation for plasma clearance. Expected renal clearance was taken as the average net plasma fluoride clearance measured during the 5 hours after feeding of a 0.25-mg fluoride supplement to 17 children 3 to 13 months of age (1.09 mL kg−1 min−1).21 Estimates of the peak plasma levels used the same participant urinary data as above, multiplied by the ratio of peak plasma fluoride levels to urinary excretion (2.6 × 103 kg*min/L).21 To assess the relationship of fluoride excretion to time, the time lapsed with each diaper for participant F was noted and adjusted by measured creatinine per average for the second through the last diaper of that day.
Results
Six participants, 3 female, 12 to 15 months were studied. Mean weight and age were 9.8 kg and 14 months, respectively. Mean varnish applied was 10.7 mg (0.22 mg fluoride). Saliva contamination obfuscated measurement for the first participant. The Environmental Protection Agency Oral Reference Dose no observable adverse effect level for daily fluoride exposure (60 μg/kg)23 was not exceeded for any child. Mean dose of applied fluoride was 23 μg/kg body weight, with non-urinated fluoride retention of 20 μg/kg after 5 hours.
Excretion
Recovered fluoride averaged 73 μg fluoride on the treatment day and 43 μg fluoride on the control day. More fluoride was retrieved during the control day than the treatment day for participant E (Table 1). Excessively high urine creatinine was recovered in the first diaper from participant F, indicating 95 minutes of urinary holding before varnish application. Urine fluoride for this time-point was corrected (Supplemental Table 1) by removing the amount of fluoride that would have built up during this amount of time on the control day. The collection times shown reflect the last diaper wetted before the end of the study period, on the treatment day. One child remained for an additional 140 minutes as the child was not urinating often; the control visit was the same duration. Total retrieved fluoride was highest for this child.
TABLE 1.
The Absorption and Excretion of Topically Applied 5% Sodium Fluoride Varnish to the Teeth of 6 12- to 15-Month-Old Children
| Patient | Gender | Age (mo) | Weight (kg) | EPA + RfD + NOAEL (mg) | Fapplied (mg) | Time (min) | volurine (mL) | Furine (μg) | Mean[F]plasmaa (μg/L) | Peak[F]plasmaa (μg/L) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | M | 12 | 11.0 | 0.66 | GGG | 299 | tx | 167 | 106 | 30 | 82 |
| ctl | 266 | 96 | 27 | ||||||||
| B | M | 15 | 9.5 | 0.57 | 0.29 | 444 | tx | 315 | 120 | 26 | 72 |
| ctl | 369 | 38 | 8.2 | ||||||||
| C | M | 14 | 10.1 | 0.61 | 0.22 | 318 | tx | 72 | 100 | 29 | 79 |
| ctl | 129 | 20 | 5.7 | ||||||||
| D | F | 13 | 8.6 | 0.52 | 0.21 | 225 | tx | 245 | 20 | 9.4 | 26 |
| ctl | 162 | 6 | 2.8 | ||||||||
| E | F | 15 | 9.9 | 0.59 | 0.18 | 280 | tx | 48 | 40 | 13 | 37 |
| ctl | 87 | 65 | 22 | ||||||||
| F | F | 15 | 9.6 | 0.58 | 0.22 | 250 | tx | 171 | 54 | 21 | 57 |
| ctl | 225 | 30 | 11 | ||||||||
| Mean + (SD) | 9.8 + (0.8) | 0.59 + (0.05) | 0.22 + (0.04) | 303 + (77) | tx | 170 + (101) | 73 + (41) | 21 + (8) | 59 + (23) | ||
| ctl | 206 + (102) | 43 + (33) | 13 + (9) | ||||||||
ctl, control; EPA, Environmental Protection Agency; NOAEL, no observable adverse effect level; RfD, EPA oral reference dose; tx, treatment.
Saliva contamination obfuscated measurement of Fapplied for the first participant.
Estimated from urine fluoride using the previous study7 as in Fig 1.
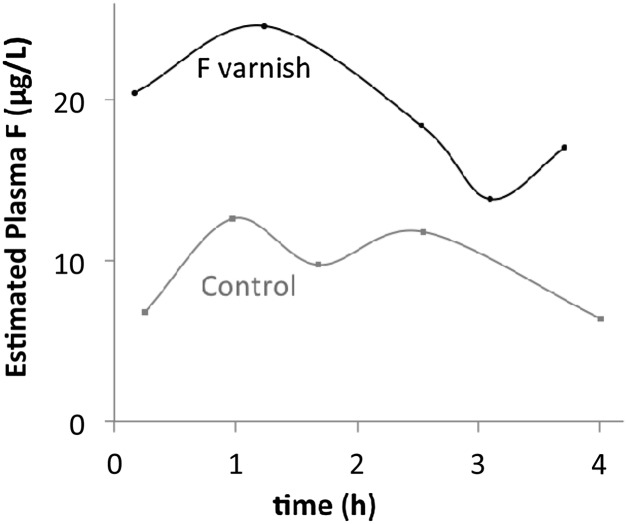
Plasma fluoride is estimated from total urine fluoride to a mean of 21 μg/L (range, 9.4 to 30 μg/L) after application of fluoride varnish and 13 μg/L (range, 2.8 to 27 μg/L) during the control visit.22 One child urinated often enough both days to enable plotting of estimated plasma fluoride levels across time (Fig 1, Supplemental Table 1). Rapid absorption and excretion of fluoride was observed, with peak concentration before 2 hours, and concentrations approaching the range of control levels by the third urination, less than 3 hours after exposure. Adverse effects were neither observed nor reported by parents.
FIGURE 1.
Estimated plasma fluoride normalizes in a few hours after application of fluoride varnish. Plasma levels are estimated from urinary fluoride collected in each diaper from the same toddler (participant F) on treatment and control days, using the relationship between urine and plasma fluoride in 17 toddlers measured by Ekstrand and colleagues.21
Discussion
We aimed to assess the pharmacokinetics of fluoride varnish application in toddlers using urine fluoride as a proxy for serum. The delivered dose was well within the safety limits for daily exposure set by the Environmental Protection Agency. Fluoride was rapidly excreted and returned to control levels within 3 to 4 hours. In older children, using a different varnish, others found both the time to peak concentration and time to return to baseline each about 1 hour longer. Peak levels were also somewhat higher.22
We found 1 brushful was sufficient to cover the teeth. Others have observed that pediatric providers generally applied the full packet (0.4 mL) regardless of size or number of teeth.24 Today, packets of 0.25 mL are available, but to decrease the probability of acute toxicity further we recommend the manufacturers market packets of less volume.
The National Academy of Sciences established a maximum daily intake of 0.06 mg/kg to avoid severe dental mottling, the earliest clinical expression of systemic fluorosis.25 The mean acute applied dose is under this level by a factor of 3.0 and the highest dose was under by a factor of 2.7. Although these levels are under the no observable adverse effect level, fluoride from other dietary sources must be considered. Even with mean retention of 84% of applied fluoride after 5 hours of voiding, the estimated serum concentrations present a safety factor of 45. Estimated peak serum fluoride presents a safety factor of 16. Whether it is possible to extrapolate from these data on 1 product to other varnish products is unknown. Different inactive ingredients and varying viscosities may affect absorption kinetics.
Conclusions
These data suggest exposures from fluoride varnish are below the level of known toxicity and do not exceed the National Academy of Sciences limits for dental mottling. The margin of safety is likely without detriment.
Supplementary Material
Footnotes
Dr Milgrom was the principal author of the design of the study and reviewed and revised the manuscript; Dr Taves participated in the design of the study, did the fluoride analyses, and drafted the initial manuscript; Dr Kim participated in the design of the study, carried out the clinical procedures and data collection, and contributed to the manuscript; Dr Watson participated in the design of the study and contributed to the manuscript; Dr Horst participated in the analyses of the data and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT0175483). The study was conducted under US Food and Drug Administration IND 110869.
FINANCIAL DISCLOSURE: Dr Milgrom is a principal in ADP Silver Dental Arrest, LLC, that is developing other fluoride products; he has received compensation for this work. The remaining authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grant 1U54DE019346 from the National Institute of Dental and Craniofacial Research and by an unrestricted gift from the Premier Dental Products Company, Plymouth Meeting, PA. Funded, in part, by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bader JD, Rozier RG, Lohr KN, Frame PS. Physicians’ roles in preventing dental caries in preschool children: a summary of the evidence for the U.S. Preventive Services Task Force. Am J Prev Med. 2004;26(4):315–325 [DOI] [PubMed] [Google Scholar]
- 2.American Dental Association Council on Scientific Affairs . Professionally applied topical fluoride: evidence-based clinical recommendations. J Am Dent Assoc. 2006;137(8):1151–1159 [DOI] [PubMed] [Google Scholar]
- 3.AAPD Guideline on Fluoride Therapy. Revised 2008. Available at: www.aapd.org/media/Policies_Guidelines/G_FluorideTherapy.pdf. Accessed April 10, 2013
- 4.Section on Pediatric Dentistry and Oral Health . Preventive oral health intervention for pediatricians. Pediatrics. 2008;122(6):1387–1394 [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Gomez FJ, Crall J, Gansky SA, Slayton RL, Featherstone JDB. Caries risk assessment appropriate for the age 1 visit (infants and toddlers). J Calif Dent Assoc. 2007;35(10):687–702 [PubMed] [Google Scholar]
- 6.Whitford GM. Acute and chronic fluoride toxicity. J Dent Res. 1992;71(5):1249–1254 [DOI] [PubMed] [Google Scholar]
- 7.Newbrun E. Systemic benefits of fluoride and fluoridation. J Public Health Dent. 2004;64(1):35–39
- 8.Higuchi H, Sumikura H, Sumita S, et al. Renal function in patients with high serum fluoride concentrations after prolonged sevoflurane anesthesia. Anesthesiology. 1995;83(3):449–458 [DOI] [PubMed] [Google Scholar]
- 9.Goldberg ME, Cantillo J, Larijani GE, Torjman M, Vekeman D, Schieren H. Sevoflurane versus isoflurane for maintenance of anesthesia: are serum inorganic fluoride ion concentrations of concern? Anesth Analg. 1996;82(6):1268–1272 [DOI] [PubMed] [Google Scholar]
- 10.Frink EJ, Jr, Malan TP, Jr, Isner RJ, Brown EA, Morgan SE, Brown BR, Jr. Renal concentrating function with prolonged sevoflurane or enflurane anesthesia in volunteers. Anesthesiology. 1994;80(5):1019–1025 [DOI] [PubMed] [Google Scholar]
- 11.DeSouza GJ, Gold MI. There is no evidence of sevoflurane nephrotoxicity. Anesth Analg. 1997;84(3):700. [DOI] [PubMed] [Google Scholar]
- 12.Angmar-Månsson B, Lindh U, Whitford GM. Enamel and dentin fluoride levels and fluorosis following single fluoride doses: a nuclear microprobe study. Caries Res. 1990;24(4):258–262 [DOI] [PubMed] [Google Scholar]
- 13.Fejerskov O. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res. 2004;38(3):182–191 [DOI] [PubMed] [Google Scholar]
- 14.Hellwig E, Lennon AM. Systemic versus topical fluoride. Caries Res. 2004;38(3):258–262 [DOI] [PubMed] [Google Scholar]
- 15.Bayless JM, Tinanoff N. Diagnosis and treatment of acute fluoride toxicity. J Am Dent Assoc. 1985;110(2):209–211 [DOI] [PubMed] [Google Scholar]
- 16.Milgrom P, Zero DT, Tanzer JM. An examination of the advances in science and technology of prevention of tooth decay in young children since the Surgeon General’s Report on Oral Health. Acad Pediatr. 2009;9(6):404–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fell JM, Thakkar H, Newman DJ, Price CP. Measurement of albumin and low molecular weight proteins in the urine of newborn infants using a cotton wool ball collection method. Acta Paediatr. 1997;86(5):518–522 [DOI] [PubMed] [Google Scholar]
- 18.Taves DR. Separation of fluoride by rapid diffusion using hexamethyldisiloxane. Talanta. 1968;15(9):969–974 [DOI] [PubMed] [Google Scholar]
- 19.Sara R, Wänninen E. Separation and determination of fluoride by diffusion with hexamethyldisiloxane and use of a fluoride-sensitive electrode. Talanta. 1975;22(12):1033–1036 [DOI] [PubMed] [Google Scholar]
- 20.Martínez-Mier EA, Cury JA, Heilman JR, et al. Development of gold standard ion-selective electrode-based methods for fluoride analysis. Caries Res. 2011;45(1):3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekstrand J, Fomon SJ, Ziegler EE, Nelson SE. Fluoride pharmacokinetics in infancy. Pediatr Res. 1994;35(2):157–163 [DOI] [PubMed] [Google Scholar]
- 22.Ekstrand J, Koch G, Petersson LG. Plasma fluoride concentration and urinary fluoride excretion in children following application of the fluoride-containing varnish Duraphat. Caries Res. 1980;14(4):185–189 [DOI] [PubMed] [Google Scholar]
- 23.US Environmental Protection Agency, Integrated Risk Information System: fluorine (soluble fluoride) (CASRN 7782-41-4); 1989. Available at: www.epa.gov/iris/subst/0053.htm. Accessed April 12, 2013
- 24.Roberts JF, Longhurst P. A clinical estimation of the fluoride used during application of a fluoride varnish. Br Dent J. 1987;162(12):463–466 [DOI] [PubMed] [Google Scholar]
- 25.National Academy of Sciences. Fluoride in drinking water: a scientific review of EPA's standards. March 2006. Available at: http://dels.nas.edu/resources/static-assets/materials-based-on-reports/reports-in-brief/fluoride_brief_final.pdf. Accessed April 12, 2013
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.