Abstract
Purpose
Chronic kidney disease – mineral and bone disorder (CKD-MBD) is a complex disorder of bone and mineral metabolism that results in excess risk of fractures, cardiovascular events and mortality. Management of the bone disorder of CKD-MBD may require bone biopsy to determine appropriate treatment strategies. However, it is unclear when biopsy may be necessary and whether or not state-of-the art imaging and serologic testing can supplant the bone biopsy as a tool to assist with management decisions.
Recent Findings
Advances in imaging methods now permit the non-invasive assessment of structural aspects of bone quality. Furthermore, common bone imaging tools, such as dual energy X-ray absorptiometry can be used to stratify for fracture risk. Circulating markers of bone turnover can be used to assess risk of bone loss and fracture, but they are less useful to diagnose type of renal osteodystrophy.
Summary
Although advances in imaging now permit assessment of fracture risk more accurately in CKD patients, assessment of type of renal osteodystrophy remains poor without bone biopsy. The virtual bone biopsy will be possible only when we are able to non-invasively assess turnover with good accuracy. A bone biopsy is needed in settings of unclear bone turnover.
Keywords: Renal osteodystrophy, CKD-MBD, bone biopsy, bone imaging, bone turnover markers
Introduction
Renal osteodystrophy (ROD) is a bone disorder that occurs in chronic kidney disease (CKD) patients and is associated with increased fracture risk. Tetracycline double-labeled transiliac crest bone biopsy with histomorphometry is the gold standard for the diagnosis and classification of ROD. However, bone biopsy is not practical to obtain in all patients all of the time. Thus, there is great interest in developing non-invasive approaches that can be used in the clinic to assess ROD in CKD populations. Here we review non-invasive methods and their utility in assessing ROD.
Chronic Kidney Disease – Mineral and Bone Disorder
ROD is a complex disorder of bone due to the individual and combined actions of metabolic and hormonal abnormalities that occur with CKD: hyperphosphatemia, hypocalcemia, hyperparathyroidism (HPT), deficiency of 25(OH)D and decreased renal synthesis of 1,25(OH)2D, chronic metabolic acidosis, and premature hypogonadism. These abnormalities impair bone remodeling and mineralization, and result in cortical and trabecular defects. The term CKD-mineral and bone disorders (CKD-MBD) has been implemented by the Kidney Disease Improving Global Outcomes (KDIGO) working group to refer more broadly to the systemic disorder of mineral and bone metabolism due to CKD; it is manifested by either one or a combination of (1) abnormalities of calcium, phosphorous, parathyroid hormone (PTH), or vitamin D metabolism; (2) abnormalities of bone turnover, mineralization, volume, linear growth or strength; and (3) vascular or other soft tissue calcification 1. The most important clinical bone related outcome in CKD-MBD is fracture.
Bone strength
Bone strength is dependent on both bone density and quality. Bone density is a measure of bone mass or quantity and is measured by dual energy X-ray absorptiometry (DXA). Bone quality describes bone material properties and includes bone turnover, microarchitecture and mineralization, accumulation of microdamage, and collagen properties. The gold standard to assess bone strength is tetracycline double-labeled transiliac crest bone biopsy with histomorphometry. Analyses performed on bone biopsy samples measure the volume and microarchitecture of cancellous and cortical compartments, the accumulation of microdamage, mineralization, and remodeling. In CKD, these abnormalities may include defects in bone volume and microarchitecture (cortical porosity, thinning and trabecularization, and trabecular thinning and dropout); in mineralization (osteomalacia); and in remodeling (adynamic bone disease, osteitis fibrosa cystica or mixed osteodystrophy). Unfortunately, the routine use of bone biopsy to evaluate ROD is not always practical. Bone biopsy is invasive, expensive, not widely available, and physicians performing this procedure require specialized training 1–3. Furthermore, once obtained the biopsy only provides information about the type of bone disorder at one site (the anterior iliac crest) and at one point in time. Thus, non-invasive approaches that can be used in the clinic to diagnose bone disease and monitor treatment responses would be helpful.
Contemporary fracture epidemiology in chronic kidney disease
Fracture is an important clinical outcome in ROD, and its prevention motivates our efforts to devise disease assessment strategies. In CKD fractures are common 4–9, increase in proportion to CKD severity 6,10,11 and are associated with excess morbidity, mortality and health economic costs 12–14. Several investigations have provided 21st century updates on CKD-associated fracture epidemiology 10,15–18. Naylor et al. 10 reported 3-year combined incidence rates of hip, forearm, pelvis, and proximal humerus fractures from a Canadian population of men and women age ≥40 years from across the CKD spectrum. In women older than 65 years fracture incidence per 1000 person-years was 15.0, 20.5, 24.2, 31.2, and 46.3 for patients with an eGFR ≥60 mL/min, 45–59 mL/min, 30–44 mL/min, 15–29 mL/min and <15 mL/min respectively. Similarly, corresponding estimates for men older than 65 years were 5.7, 7.3, 10.1, 15.3, and 24.3 respectively. Nair et al. 15 used the United States Renal Data Systems (USRDS) to investigate trends in fracture epidemiology from 1996 to 2009 in patients with incident ESRD. Compared to incident ESRD patients in 1996, hip fracture rates were increased by 43% in 2004. It is noteworthy that in 2009 hip fracture rates had decreased from 2004 levels but remained increased by 27% compared to 1996. Arneson et al. 18 used Medicare data from 1993 to 2010 to investigate hip fracture incidence in ESRD patients compared to the general population and reported similar results. Potential explanations for decreasing hip fracture incidence rates in ESRD patients may include patient-specific characteristics and changes in CKD-MBD management. Kidney transplant recipients are also susceptible to fracture 5,8,19,20. Over the first 3 years of transplantation hip fracture risk is 34% higher than patients on dialysis 5, and hip and spine fracture risk are more than 4- and 23- fold higher 20 than the general population, and about one-quarter of recipients will fracture within the first 5 years of transplantation 19.
These recent epidemiologic data confirm that fractures remain a serious and alarming CKD-MBD complication, in particular since they are associated with high morbidity, mortality and cost. In one study, a third to almost half of hemodialysis patients admitted with a fracture were discharged to a skilled-nursing facility after the incident hospitalization. During the year after discharge, patients had an adjusted mean of 3.8–5.2 additional hospitalizations, comprising on average an extra 33–52 inpatient days compared to patients without fracture 15. Moreover, after hip fracture mortality risk was reported to increase by 16%15 to 60%13, and were associated with high economic costs 21.
Mechanisms of decreased bone strength in CKD from bone histology
Recent bone biopsy studies have enlightened our understanding of how ROD effects bone strength, and suggest that a non-invasive assessment of bone disease must measure the underlying microstructural and dynamic defects in bone quality that drive decreases in skeletal fragility. 22,23. Malluche et al. 22 reported findings from 630 biopsies obtained from patients in the United Stated and Europe. Their mean age was 55±1 years, 48% were women, 14% were blacks, 5% were on peritoneal dialysis, and mean dialysis vintage was 51±1.8 months. There were notable race differences in bone quality. Low turnover predominated in whites (62%) and normal turnover predominated in blacks (68%). Mineralization defects were rare (3% of patients). Trabecular bone volume was low in one-third of whites and high in two-thirds of blacks; for both races low trabecular volume was associated with thinned rather than lost trabeculae. The majority of blacks had normal cortical thickness but high porosity, whereas there was approximately the same number of whites with low or normal cortical thickness, and normal or high cortical porosity. Abnormal microarchitecture was associated with abnormal turnover. High turnover was associated with greater severity of cortical porosity, and low turnover was associated with lower trabecular volume and thinner cortices. Malluche et al. 23 also reported on relationships between bone quality and strength in 35 CKD-5D patients. Low turnover was associated with lower trabecular volume than either normal or high turnover. High turnover was associated with lower relative mineral content. Similar to their larger publication of 630 patients, low trabecular volume was associated with thinned trabeculae. Regarding bone mechanical competence (i.e. strength), bone with high turnover had lower stiffness and failure load than bone with normal or low turnover.
Imaging bone disease in CKD
DXA, QCT and HR-pQCT are imaging methods that quantify bone mass and structural aspects of bone quality. They do not measure turnover or mineralization. Therefore, they cannot be used alone to determine ROD type or assess completely disease severity. Furthermore, monitoring disease activity with imaging methods may be difficult because there is no data correlating changes in the parameters measured by these tools with changes in fracture risk.
DXA is widely available and is the clinical standard to measure fracture risk in patients with healthy kidney function. However, fracture risk screening by DXA in CKD has been controversial. DXA does not have sufficient resolution to discriminate between cortical and trabecular bone, it provides a composite measure of cortical and trabecular compartments, and is unable to determine turnover or mineralization. Furthermore, due to study limitations, results of cross-sectional and prospective studies on fracture discrimination and prediction were inconsistent 20,24–34. However, recent prospective trials in patients with pre-dialysis CKD 35, ESRD on hemodialysis 33 and after kidney transplantation 36 strongly suggest that low areal BMD measured by DXA at the total hip and femoral neck predicts future fracture (Table 1). Indeed, these prospective trials have begun to clarify and validate the use of DXA in CKD and help clinicians use and interpret DXA imaging. They indicate that in CKD, fracture risk screening is possible by imaging the total hip and femoral neck and that the World Health Organization definition of osteoporosis (T-Score ≤ −2.5) is clinically relevant. Unfortunately, there are no prospective data determining whether measurement of areal BMD at the mainly cortical one-third radius (>90% cortical bone) is a better predictor of fracture than measurement of areal BMD at sites composed of mixed cortical and trabecular bone (i.e. total hip).
Table 1.
Summary of prospective fracture trials evaluating the ability of areal BMD by dual energy X-ray absorptiometry to predict incident fracture
| Study | Study population | N | Follow up | Fracture incidence | Fracture risk and areal BMD |
|---|---|---|---|---|---|
| Yenchek et al 2012 (35) | Elderly patients (age 70–79) with and without CKD (GFR under 60ml/min/1.73m2) | 2754 total 587 with CKD |
11 years | Non-CKD: 13.2% CKD: 16.7% |
Non-CKD: FN BMD (T-Score <2.5) HR 1.64 (95% CI 1.20, 2.25) CKD: FN BMD (T-Score <2.5) HR 2.10 (95% 1.24, 3.59)* |
| Iimori et al 2012 (33) | HD dependent | 485 | 5 years | 1.9 fractures per 100 patient years | Total Hip BMD (per SD) HR 0.65 (95% CI 0.49–0.87) |
| Akaberi et al 2008 (36) | Post renal transplant | 238 | 10 years | 19.3% | Total hip BMD (T-Score <2.5) HR 3.5 (CI 1.8–6.4) |
p value for CKD and osteoporosis interaction was not significant
High-resolution imaging methods measure and quantify cortical and trabecular 3-dimensional (volumetric) BMD, geometry, microarchitecture, and strength. Currently, these tools are used for research. QCT has a resolution of 300 μm3 and measures volumetric BMD and geometry of cortical and trabecular compartments. Peripheral QCT has been used to assess skeletal effects of CKD in patients pre-dialysis, on hemodialysis, and after kidney transplantation. Studies reported that in CKD patients cortical deficits predominated 28,37 and cortical abnormalities both discriminated 28 and predicted fracture 38. Similarly, HR-pQCT separately measures cortical and trabecular volumetric BMD and geometry, but its higher nominal resolution of 82 μm3 permits quantification of trabecular number, thickness and separation. Finite element analysis, a computational method to quantify bone strength, can be applied to 3-dimensional HR-pQCT datasets to measure strength either of whole bone or of individual cortical and trabecular compartments. Recently, advanced HR-pQCT processing methods have been developed to characterize cortical porosity 39,40 and trabecular plate and rod structure 41. In patients from across the CKD spectrum, our group and others have demonstrated in cross-sectional and prospective studies that measurement of bone mass, geometry, and microarchitecture by HR-pQCT at the distal radius and tibia discriminated fracture status 24,25,42–44, detected abnormalities in bone quality that negatively impact bone strength 45–47, and elucidated underlying microstructural defects that result in BMD abnormalities measured by DXA 45,46. For example, in a prospective study of 54 patients with moderate to end stage renal disease we found that mean annualized losses of areal BMD at the forearm were 2.9% 45. With HR-pQCT, we identified microstructural mechanisms of forearm bone loss detected by DXA; there was significant loss of cortical area (−2.9%), density (−1.3%) and thickness (−2.8%) and significant increases in cortical porosity (+4.2%). Prospective studies are needed to determine whether measurement of bone mass and microarchitecture by HR-pQCT predicts fracture, and whether therapies that mitigate microarchitectural abnormalities detected by HR-pQCT protect against fracture.
The utility of combining measures of areal BMD by DXA with measures of bone geometry and microarchitecture by HR-pQCT to enhance fracture prediction above that of either imaging test alone is not established. In a cross-sectional study of CKD patients with and without fracture, Jamal et al. 26 studied the utility of combing measures from DXA with measures from HR-pQCT. Areal BMD was measured by DXA at the mainly trabecular ultradistal radius and total volumetric BMD and cortical thickness were measured by HR-pQCT at the distal radius. When HR-pQCT measures were added to areal BMD by DXA, the AUC was 0.81 (95% CI 0.74 to 0.88). This was not significantly different than areal BMD by DXA at the radius alone (AUC 0.80; 95% CI 0.74 to 0.87; p 0.4). The reasons for this negative finding are likely due to redundancies in parameters measured by these imaging methods. Both DXA and HR-pQCT measure structural aspects of bone quality. Thus, enhancement of assessing bone disease severity in CKD-MBD will most likely be obtained by combining structural with dynamic measures of bone quality.
Assessing bone turnover and osteomalacia
There are no recent bone biopsy studies that update the historical literature on assessing turnover and osteomalacia non-invasively. Nonetheless, their evaluation is an essential component of assessing bone disease. Turnover may range from extremely low (adynamic bone disease) to extremely high (osteitis fibrosa cystica), turnover may change over time, and turnover-type affects treatment. Pharmacologic agents that protect against fracture alter remodeling rates. While anti-resorptive agents may be used in patients with high turnover they are contraindicated in patients with adynamic bone disease. Similarly, while osteoanabolic agents (i.e. Teriparatide) may be used in patients with low turnover they are contraindicated in patients with high turnover. As discussed, bone biopsy is not always possible or practical. Thus, a non-invasive approach to turnover assessment is desirable. Despite assay limitations, non-invasive assessment can be achieved with reasonable accuracy by measuring circulating levels of parathyroid hormone (PTH) and bone turnover markers (BTMs) 48–53. Bone formation markers, such as bone specific alkaline phosphatase (BSAP), osteocalcin, and procollagen type-1 N-terminal propeptide (P1NP) are markers of osteoblast function. Bone resorption markers, such as tartrate-resistant acid phosphatase 5b (Trap-5b) and C-terminal telopeptides of type I collagen (CTX) are markers of osteoclast number and function, respectively. In clinical practice, PTH and BSAP are the most commonly used markers of turnover in CKD-MBD. In general, extremes of PTH predict extremes of bone turnover both in pre-dialysis 52 and dialysis-dependent 53 patients. Unfortunately, prediction of underlying histology is less discriminatory when PTH levels are within the middle range. For BTMs, reference ranges in CKD populations do not exist and some BTMs are renally cleared (osteocalcin, P1NP monomer and CTX). Thus, their use and interpretation in CKD-MBD is challenging. In general, their predictive values are improved at the extremes of BTM levels, and with combining multiple BTMs with or without PTH 48–50. Low vitamin D levels, and low levels of PTH in conjunction with high levels of BSAP have been correlated with osteomalacia, and osteomalacia has been associated with hip fractures.
Measurement of PTH and BTMs may be more helpful in predicting bone loss and fractures than in predicting type of turnover per se 24,33,45,46,54. In a cross sectional study of pre-dialysis CKD patients with and without fracture, we reported that higher levels of PTH and BTMs were associated with lower cortical and trabecular density, and with thinner cortices and trabeculae 24. Moreover, higher levels of P1NP, osteocalcin, CTX, and Trap-5b discriminated fracture 24. In prospective studies of CKD patients both before 45 and after kidney transplantation 46, our group evaluated effects of remodeling measured by circulating levels of PTH and BTMs on changes in bone mass, geometry, microarchitecture, and strength measured by HR-pQCT. Higher concentrations of PTH, BSAP, osteocalcin, P1NP, Trap-5b, and CTX predicted loss of cortical area, density and thickness, increases in cortical porosity, and decreases in bone strength. Regarding the ability of PTH and BTMs to predict fracture, a prospective study of ESRD patients reported that fracture risk was higher in patients with either low (<150 pg/mL) or high (>300 pg/mL) PTH levels, and with higher BSAP levels 33. In kidney transplant recipients PTH levels ≥130 pg/mL at 3-months post-transplantation predicted incident fractures 54. These data indicate that higher remodeling rates, as assessed by PTH and clinically available BTMs, result in bone loss, deterioration of cortical and trabecular microarchitecture, and predict fracture. Prospective fracture studies of PTH and BTMs that include all CKD stages and demographic groups are needed to validate these findings, provide reference ranges that correlate with bone histology, and correlate changes in PTH and BTMs with changes in fracture risk.
Can we non-invasively assess bone disease in CKD?
Non-invasive assessment of bone disease needs to detect abnormalities in bone structure, remodeling and mineralization, predict clinical outcomes, inform treatment decisions, and predict changes in clinical outcomes in response to treatment. In figure 1 we demonstrate a prototype approach to stratify patients into those who do and do not require bone biopsy. While this approach requires validation in prospective fracture trials and against bone histology, our group explored a modified version 24. We investigated whether severity of disease and fracture status could be determined by combining imaging of bone structure by DXA and HR-pQCT with biochemical assessment of turnover by PTH and BTMs. This cross sectional study included 82 patients with stage 3–5 CKD, 23 with and 59 without fractures, we reported that higher levels of PTH and BTMs were associated with more severely deteriorated microarchitecture and that fracture discrimination was significantly improved by combing a markers of formation (P1NP, osteocalcin) and resorption (Trap-5b) with areal BMD at the femoral neck. The rationale for our approach is supported by new evidence indicating that DXA and high resolution imaging methods measure bone mass and microarchitecture, uncover structural abnormalities that impact fragility, and risk-stratify patients for fracture. However, several important limitations to our approach remain to be resolved. We lack non-invasive tools to detect osteomalacia. PTH and BTMs are inconsistent markers of underlying histology, reference ranges for BTMs that correlate with histology across the CKD spectrum do not exist, changes in BTMs over time have not been correlated against changes in bone histology, and correlations between changes in BTMs and changes in fracture risk need to be determined. Furthermore, changes in bone mass and microarchitecture over time have not been correlated with changes in either disease severity or fracture risk, and the optimal time-interval for disease monitoring has not been established. Thus, the decision to treat and to monitor treatment response in a CKD patient managed with vitamin D analogs, or an anti-resorptive or osteoanabolic agent may not be completely possible without a bone biopsy.
Figure 1.
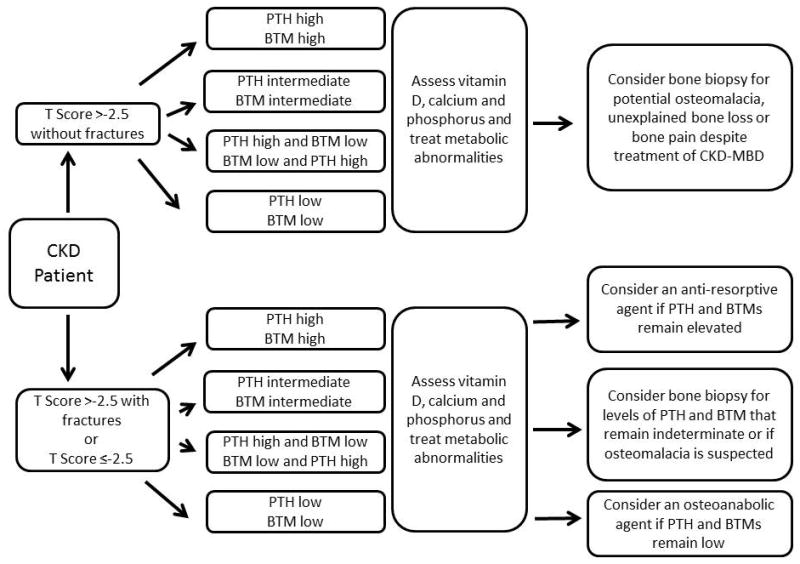
Prototype algorithm of a non-invasive approach to assess bone disease in CKD
Conclusion
Bone disease is CKD is common and potentially life threatening. The current gold standard to assess ROD is invasive and suboptimal for disease screening and management. Advances in imaging methods enable non-invasive measurement of structural aspects of bone disease that drive increased skeletal fragility; however, before the “virtual bone biopsy” is ready for prime time more accurate methods of non-invasively assessing turnover and osteomalacia, validated against bone histology, are needed. Finally, prospective fracture trials are necessary to demonstrate that clinical bone outcomes are improved by a non-invasive approach to ROD management.
Key points.
Renal osteodystrophy is a complex bone disorder that is associated with high risk of fractures, morbidity and mortality
New data from prospective fracture studies in CKD populations indicate that low areal bone mineral density measured by dual energy X-ray absorptiometry predicts future fractures
Advances in imaging methods now provide the ability to non-invasively assess microarchitectural aspects of bone quality that correlate with decreases in bone strength
Non-invasive assessment of bone turnover and mineralization is still suboptimal
Future research needs to focus on developing non-invasive methods to measure turnover and mineralization and to study whether combining measures of bone structure by imaging with measures of turnover by serum biochemistries quantifies disease severity, predicts fracture and informs treatment decisions
Acknowledgments
This research was supported by grants from the National Institutes of Health: K23 DK080139 (T.L.N.), and the National Center for Advancing Translational Sciences through Grant Number UL1 TR000040, and a Columbia University Herbert Irving Award (T.L.N.).
Footnotes
Conflicts of Interest
Dr. Babayev: none
Dr. Nickolas: Columbia University has licensed patents to Abbot Diagnostics for NGAL as a marker of AKI
References
- 1.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–53. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.Parfitt AM. A structural approach to renal bone disease. J Bone Miner Res. 1998;13:1213–20. doi: 10.1359/jbmr.1998.13.8.1213. [DOI] [PubMed] [Google Scholar]
- 3.Jee WS. The past, present, and future of bone morphometry: its contribution to an improved understanding of bone biology. Journal of bone and mineral metabolism. 2005;23 (Suppl):1–10. doi: 10.1007/BF03026316. [DOI] [PubMed] [Google Scholar]
- 4.Nickolas TL, McMahon DJ, Shane E. Relationship between Moderate to Severe Kidney Disease and Hip Fracture in the United States. J AmSocNephrol. 2006;17:3223–32. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 5.Ball AM, Gillen DL, Sherrard D, et al. Risk of Hip Fracture Among Dialysis and Renal Transplant Recipients. JAMA: The Journal of the American Medical Association. 2002;288:3014–8. doi: 10.1001/jama.288.23.3014. [DOI] [PubMed] [Google Scholar]
- 6.Dooley AC, Weiss NS, Kestenbaum B. Increased risk of hip fracture among men with CKD. Am J Kidney Dis. 2008;51:38–44. doi: 10.1053/j.ajkd.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J AmSocNephrol. 2007;18:282–6. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 8.Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney International. 2000;58:396–9. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonds DE, Larson JC, Schwartz AV, et al. Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study. The Journal of clinical endocrinology and metabolism. 2006;91:3404–10. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 10*.Naylor KL, McArthur E, Leslie WD, et al. The three-year incidence of fracture in chronic kidney disease. Kidney Int. 2014 doi: 10.1038/ki.2013.547. Update of fracture epidemioloy. [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–5. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 12.Mittalhenkle A, Gillen DL, Stehman-Breen CO. Increased risk of mortality associated with hip fracture in the dialysis population. AmJ Kidney Dis. 2004;44:672–9. [PubMed] [Google Scholar]
- 13.Abbott KC, Oglesby RJ, Hypolite IO, et al. Hospitalizations for fractures after renal transplantation in the United States. Ann Epidemiol. 2001;11:450–7. doi: 10.1016/s1047-2797(01)00226-5. [DOI] [PubMed] [Google Scholar]
- 14**.Tentori F, McCullough K, Kilpatrick RD, et al. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014;85:166–73. doi: 10.1038/ki.2013.279. Update of clinical outcomes following fracture events in CKD patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Nair SS, Mitani AA, Goldstein BA, Chertow GM, Lowenberg DW, Winkelmayer WC. Temporal trends in the incidence, treatment, and outcomes of hip fracture in older patients initiating dialysis in the United States. Clin J Am Soc Nephrol. 2013;8:1336–42. doi: 10.2215/CJN.10901012. Update of fracture epidemiology in CKD patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Wagner J, Jhaveri KD, Rosen L, Sunday S, Mathew AT, Fishbane S. Increased bone fractures among elderly United States hemodialysis patients. Nephrol Dial Transplant. 2014;29:146–51. doi: 10.1093/ndt/gft352. Update of fracture epidemiology in CKD patients. [DOI] [PubMed] [Google Scholar]
- 17.Beaubrun AC, Kilpatrick RD, Freburger JK, Bradbury BD, Wang L, Brookhart MA. Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol. 2013;24:1461–9. doi: 10.1681/ASN.2012090916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arneson TJ, Li S, Liu J, Kilpatrick RD, Newsome BB, St Peter WL. Trends in hip fracture rates in US hemodialysis patients, 1993–2010. Am J Kidney Dis. 2013;62:747–54. doi: 10.1053/j.ajkd.2013.02.368. [DOI] [PubMed] [Google Scholar]
- 19.Nikkel LE, Hollenbeak CS, Fox EJ, Uemura T, Ghahramani N. Risk of fractures after renal transplantation in the United States. Transplantation. 2009;87:1846–51. doi: 10.1097/TP.0b013e3181a6bbda. [DOI] [PubMed] [Google Scholar]
- 20.Vautour LM, Melton LJ, 3rd, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT. Long-term fracture risk following renal transplantation: a population-based study. Osteoporos Int. 2004;15:160–7. doi: 10.1007/s00198-003-1532-y. [DOI] [PubMed] [Google Scholar]
- 21.Wiktorowicz ME, Goeree R, Papaioannou A, Adachi JD, Papadimitropoulos E. Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteoporos Int. 2001;12:271–8. doi: 10.1007/s001980170116. [DOI] [PubMed] [Google Scholar]
- 22.Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011;26:1368–76. doi: 10.1002/jbmr.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Malluche HH, Porter DS, Monier-Faugere MC, Mawad H, Pienkowski D. Differences in bone quality in low- and high-turnover renal osteodystrophy. J Am Soc Nephrol. 2012;23:525–32. doi: 10.1681/ASN.2010121253. Evaluates the effect of bone quality on bone strength in ROD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nickolas TL, Cremers S, Zhang A, et al. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol. 2011;22:1560–72. doi: 10.1681/ASN.2010121275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickolas TL, Stein E, Cohen A, et al. Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol. 2010;21:1371–80. doi: 10.1681/ASN.2009121208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamal SA, Cheung AM, West SL, Lok CE. Bone mineral density by DXA and HR pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporos Int. 2012 doi: 10.1007/s00198-012-1908-y. [DOI] [PubMed] [Google Scholar]
- 27.Jamal SA, Hayden JA, Beyene J. Low bone mineral density and fractures in long-term hemodialysis patients: a meta-analysis. Am J Kidney Dis. 2007;49:674–81. doi: 10.1053/j.ajkd.2007.02.264. [DOI] [PubMed] [Google Scholar]
- 28.Jamal SA, Gilbert J, Gordon C, Bauer DC. Cortical pQCT measures are associated with fractures in dialysis patients. J Bone MinerRes. 2006;21:543–8. doi: 10.1359/jbmr.060105. [DOI] [PubMed] [Google Scholar]
- 29.Jamal SA, Chase C, Goh YI, Richardson R, Hawker GA. Bone density and heel ultrasound testing do not identify patients with dialysis-dependent renal failure who have had fractures. AmJ Kidney Dis. 2002;39:843–9. doi: 10.1053/ajkd.2002.32006. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi T, Kanno E, Tsubota J, Shiomi T, Nakai M, Hattori S. Retrospective study on the usefulness of radius and lumbar bone density in the separation of hemodialysis patients with fractures from those without fractures. Bone. 1996;19:549–55. doi: 10.1016/s8756-3282(96)00246-3. [DOI] [PubMed] [Google Scholar]
- 31.Piraino B, Chen T, Cooperstein L, Segre G, Puschett J. Fractures and vertebral bone mineral density in patients with renal osteodystrophy. ClinNephrol. 1988;30:57–62. [PubMed] [Google Scholar]
- 32.Jassal SK, von Muhlen D, Barrett-Connor E. Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res. 2007;22:203–10. doi: 10.1359/jbmr.061014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Iimori S, Mori Y, Akita W, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients--a single-center cohort study. Nephrol Dial Transplant. 2012;27:345–51. doi: 10.1093/ndt/gfr317. Prospective study of DXA in ESRD patients. [DOI] [PubMed] [Google Scholar]
- 34.McCarthy JT, Rule AD, Achenbach SJ, Bergstralh EJ, Khosla S, Melton LJ., 3rd Use of renal function measurements for assessing fracture risk in postmenopausal women. Mayo Clin Proc. 2008;83:1231–9. doi: 10.4065/83.11.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Yenchek RH, Ix JH, Shlipak MG, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7:1130–6. doi: 10.2215/CJN.12871211. Prospective study of DXA in pre-dialysis CKD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akaberi S, Simonsen O, Lindergard B, Nyberg G. Can DXA predict fractures in renal transplant patients? Am J Transplant. 2008;8:2647–51. doi: 10.1111/j.1600-6143.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- 37.Leonard MB. A structural approach to skeletal fragility in chronic kidney disease. Seminars in Nephrology. 2009;29:133–43. doi: 10.1016/j.semnephrol.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denburg MR, Tsampalieros AK, de Boer IH, et al. Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. The Journal of clinical endocrinology and metabolism. 2013;98:1930–8. doi: 10.1210/jc.2012-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010;25:882–90. doi: 10.1359/jbmr.091020. [DOI] [PubMed] [Google Scholar]
- 40.Nishiyama KK, Macdonald HM, Hanley DA, Boyd SK. Women with previous fragility fractures can be classified based on bone microarchitecture and finite element analysis measured with HR-pQCT. Osteoporos Int. 2013;24:1733–40. doi: 10.1007/s00198-012-2160-1. [DOI] [PubMed] [Google Scholar]
- 41.Liu XS, Stein EM, Zhou B, et al. Individual trabecula segmentation (ITS)-based morphological analyses and microfinite element analysis of HR-pQCT images discriminate postmenopausal fragility fractures independent of DXA measurements. J Bone Miner Res. 2012;27:263–72. doi: 10.1002/jbmr.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamal S, Cheung AM, West S, Lok C. Bone mineral density by DXA and HR pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporos Int. 2012;23:2805–13. doi: 10.1007/s00198-012-1908-y. [DOI] [PubMed] [Google Scholar]
- 43.Cejka D, Patsch JM, Weber M, et al. Bone microarchitecture in hemodialysis patients assessed by HR-pQCT. Clin J Am Soc Nephrol. 2011;6:2264–71. doi: 10.2215/CJN.09711010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trombetti A, Stoermann C, Chevalley T, et al. Alterations of bone microstructure and strength in end-stage renal failure. Osteoporos Int. 2012 doi: 10.1007/s00198-012-2133-4. [DOI] [PubMed] [Google Scholar]
- 45.Nickolas TL, Stein EM, Dworakowski E, et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res. 2013;28:1811–20. doi: 10.1002/jbmr.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iyer SNL, Nishiyama K, Dworakowski E, Cremers S, Zhang A, McMahon DJ, Boutroy S, Liu XS, Ratner L, Cohen D, Guo XE, Shane E, Nickolas TL. Kidney transplantation with early corticosteroid withdrawal: paradoxical effects at the central and peripheral skeleton. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013080851. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boutroy SNT, Stein EM, Cohen A, Shane E. Impaired cortical bone in predialysis CKD patients is even more marked in those with fragility fracture. ASN; San Diego, CA: 2010. [Google Scholar]
- 48.Couttenye MM, D’Haese PC, Van Hoof VO, et al. Low serum levels of alkaline phosphatase of bone origin: a good marker of adynamic bone disease in haemodialysis patients. Nephrol Dial Transplant. 1996;11:1065–72. [PubMed] [Google Scholar]
- 49.Bervoets AR, Spasovski GB, Behets GJ, et al. Useful biochemical markers for diagnosing renal osteodystrophy in predialysis end-stage renal failure patients. AmJ Kidney Dis. 2003;41:997–1007. doi: 10.1016/s0272-6386(03)00197-5. [DOI] [PubMed] [Google Scholar]
- 50.Coen G, Ballanti P, Bonucci E, et al. Bone markers in the diagnosis of low turnover osteodystrophy in haemodialysis patients. Nephrol Dial Transplant. 1998;13:2294–302. doi: 10.1093/ndt/13.9.2294. [DOI] [PubMed] [Google Scholar]
- 51.Lehmann G, Ott U, Kaemmerer D, Schuetze J, Wolf G. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease Stages 3 – 5. Clin Nephrol. 2008;70:296–305. doi: 10.5414/cnp70296. [DOI] [PubMed] [Google Scholar]
- 52.Lehmann G, Stein G, Huller M, Schemer R, Ramakrishnan K, Goodman WG. Specific measurement of PTH (1–84) in various forms of renal osteodystrophy (ROD) as assessed by bone histomorphometry. Kidney Int. 2005;68:1206–14. doi: 10.1111/j.1523-1755.2005.00513.x. [DOI] [PubMed] [Google Scholar]
- 53.Herberth J, Branscum AJ, Mawad H, Cantor T, Monier-Faugere MC, Malluche HH. Intact PTH combined with the PTH ratio for diagnosis of bone turnover in dialysis patients: a diagnostic test study. Am J Kidney Dis. 2010;55:897–906. doi: 10.1053/j.ajkd.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perrin P, Caillard S, Javier RM, et al. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am J Transplant. 2013;13:2653–63. doi: 10.1111/ajt.12425. [DOI] [PubMed] [Google Scholar]


