Abstract
Worldwide, HIV disproportionately affects women who are often unable to negotiate traditional HIV preventive strategies such as condoms. In the absence of an effective vaccine or cure, chemoprophylaxis may be a valuable self-initiated alternative. Topical microbicides have been investigated as one such option. The first generation topical microbicides were non-specific, broad-spectrum antimicrobial agents, including surfactants, polyanions, and acid buffering gels, that generally exhibited contraceptive properties. After extensive clinical study, none prevented HIV infection, and their development was abandoned. Second generation topical microbicides include agents with selective mechanisms of antiviral activity. Most are currently being used for, or have previously been explored as, drugs for treatment of HIV. The most advanced of these is tenofovir 1% gel: the first topical agent shown to significantly reduce HIV infection by 39% compared to placebo. This review summarizes the evolution of topical microbicides for HIV chemoprophylaxis, highlights important concepts learned, and offers current and future considerations for this area of research.
Keywords: Microbicide, HIV prevention, female genital tract, tenofovir gel, pre-exposure prophylaxis
1 Introduction
Once considered a terminal disease, HIV infection was reclassified into a manageable chronic illness through the use of potent combination antiretroviral therapy. Despite these therapeutic advances, the HIV field has struggled to implement effective prevention strategies. Current recommendations include high adherence to male condoms, sexual abstinence, and male circumcision.1 Of these recommendations available to women, those at high risk of HIV are often unable to negotiate condom use and sexual abstinence.2 Since women represent 50% of HIV infected individuals globally, and over 60% of HIV infected individuals in sub-Saharan Africa3, developing preventative agents this population can control is essential to lowering the global burden of HIV.
Chemoprophylaxis strategies under investigation include systemic and topical antiviral agents, which may be applied topically to the genital and lower gastrointestinal tracts. Truvada®, a fixed dose combination of the 2 nucleoside reverse transcriptase inhibitors(NRTI) (tenofovir disoproxil fumarate and emtricitabine), is the only compound currently approved by the FDA for use as chemoprophylaxis in uninfected individuals. High adherence to a once daily regimen of Truvada® is the only oral dosing strategy shown to protect women in stable serodiscordant relationships (>60% efficacy when compared to placebo).4,5 Clinical trials evaluating Truvada® in high-risk women not involved in stable relationships have failed to demonstrate protection owing to a lack of adherence to study drug.6,7 Additionally, long-term systemic exposure to antiretrovirals in healthy individuals is not without risk; as these agents have been associated with decreased bone mineral density and proximal renal tubulopathy.8 Therefore, topical agents may offer significant advantages over systemically administered therapies by maximizing local mucosal tissue concentrations and limiting systemic exposure. Topical microbicides have been a focus of the HIV prevention field for over two decades. Although a topical product for HIV protection has not yet received regulatory approval, much has been learned about product development and women’s acceptance of product use. Here, we review the history of the development of topical microbicides and current and future directions in the field.
2 First Generation Topical Microbicides
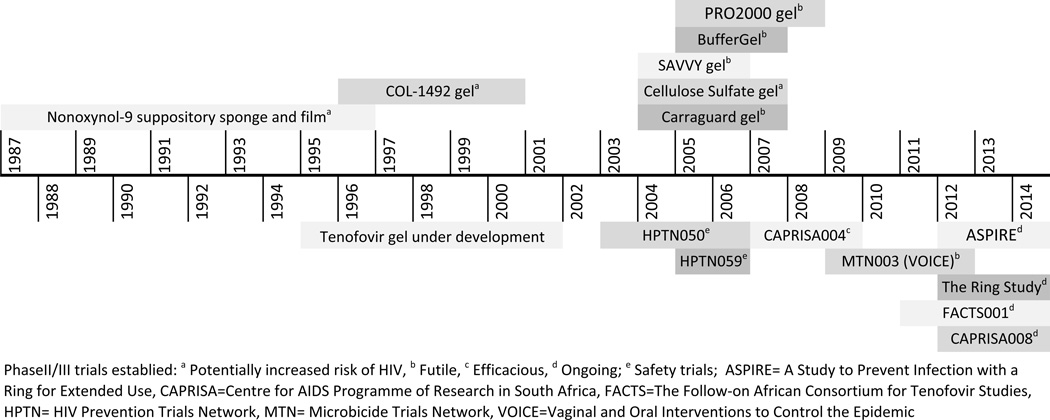
A number of topical agents with markedly different mechanisms of antiviral activity (Figure 1) have been explored for chemoprophylaxis and can be categorized as first- and second-generation agents. In general, first generation agents are non-specific, with a broad spectrum of antimicrobial activity as well as contraceptive properties.9 This class includes three groups of agents: surfactants, polyanions and acid buffering agents. The preclinical and clinical investigation of these agents has been reviewed in detail elsewhere.9 Figure 2 provides a timeline of the clinical development of these agents and a brief synopsis follows.
Figure 1. Mechanism of Action of Microbicide Agents.
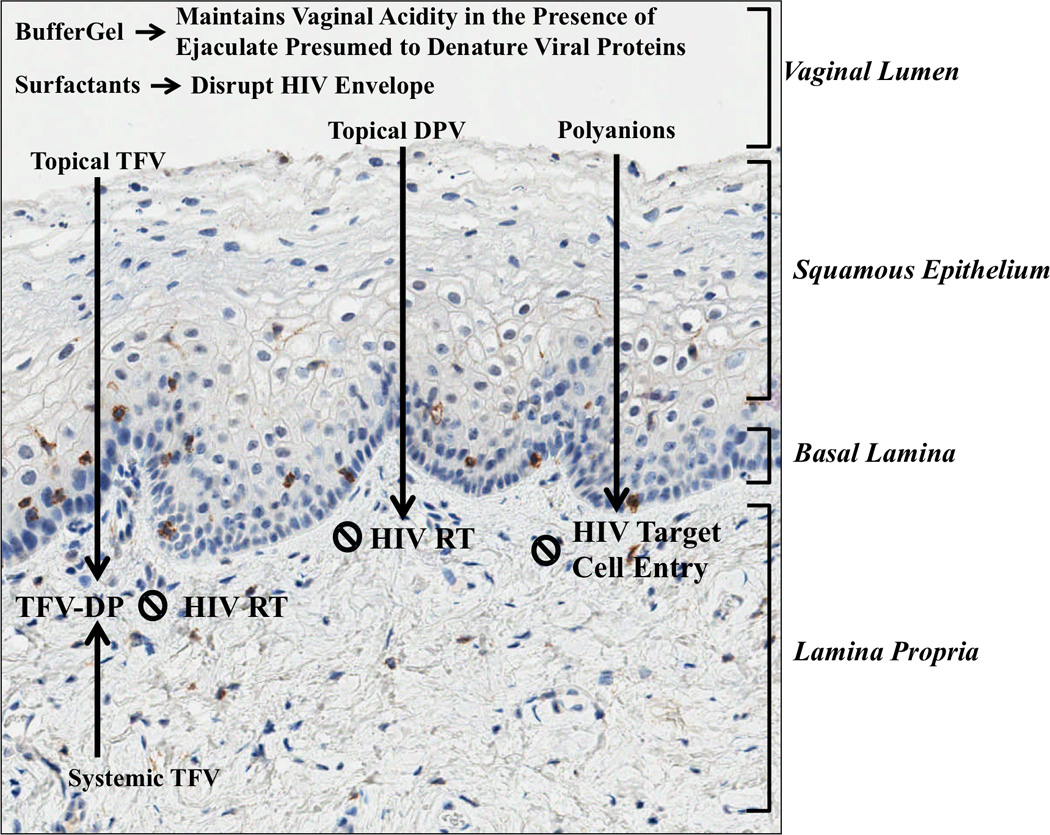
Figure 1 depicts the proposed mechanism antiviral activity of microbicides overlaid on an image of vaginal wall histology. Blue staining represents cell nuclei and red staining represents CD4+ cells. Immunohistochemistry was performed by the Translational Pathology Laboratory at the University of North Carolina at Chapel Hill. DPV=Dapivirine, HIV RT= HIV reverse transcriptase, TFV=Tenofovir, TFV-DP=Tenofovir-diphosphate.
Figure 2. Developmental Timeline of Topical Microbicides.
Figure 2 provides a timeline for the clinical efficacy trials of first generation topical microbicides (superior placement) and the safety and efficacy trials for second generation agents (inferior placement).
2.I Surfactants
Surfactants were the first topical microbicides investigated for their ability to prevent HIV infection. Nonoxynol-9(N-9), a commercially available spermicide, was clinically investigated before extensive animal efficacy data and human safety data were available.10 An observational study and a randomized controlled trial(RCT) conducted simultaneously in two groups of high risk African women reported contradictory results.11,12 The observational study found an inverse relationship between N-9 use and HIV infection, with a relative risk (RR) of 0.2 (95% CI, 0.1–0.7) when frequency of N-9 use with sexual activity was ≥67%.12 The RCT reported no benefit of N-9, but 47% of women in the treatment arm complained of vulvular irritation, burning and ulceration (Table 1). A subsequent study found N-9 suppositories administered twice and four times daily produced 2.5 and 5 times more epithelial disruption than placebo.13
Table 1.
First Generation Topical Microbicides Clinical Trials Summary.
| Microbicide | Study Summary | Site(s) | Treatment Arm Number |
Results Summary |
|---|---|---|---|---|
| Nonoxynol-9 | Observational trial to determine the relationship between 100mg N-9 suppositories use and HIV incidence12 | Cameroon | 273 | Frequency of use inversely associated with HIV incidence [HIV RR 0.2 (95% CI, 0.1–0.7) if N-9 frequency of use ≥67% vs <67%] |
| Phase II RCT to determine if 1000mg N-9 sponge gel prevents HIV11 | Nairobi | N-9 Sponge=74 Placebo Sponge=64 |
No benefit, possible harm [HR=1.7 (95% CI, 0.9 – 3.0)] |
|
| Phase II RCT to determine if 70mg N-9 film prevents HIV14 | Cameroon | N-9 Film=479 Placebo Film=463 |
No benefit, possible harm [N-9 film vs placebo ERa=6.6 vs 6.7 (Rate ratio=1.0 (95% CI, 0.7 – 1.5)] [N-9 film vs placebo ER ratioa =1.3 (95% CI, 1.0 – 1.6)] |
|
| Phase II/III RCT to determine if 52.5mg COL-1492 3.5% gel prevents HIV17 | Benin, Côte d’Ivoire, South Africa, Thailand | COL-1492 gel=376 Polycarbophil gel =389 |
Frequency of use positively associatedwith HIV incidence [HR=1.5 (95% CI, 1.0–2.2); p=0.047] [High gel use HR=1.8 (95% CI, 1.0–3.2); Low gel use HR=1.1 (0.54–2.4)] |
|
| SAVVY | Phase III RCT to determine if 1% SAVVY gel prevents HIV24 | Ghana | SAVVY gel=1026 HEC gel=1012 |
Discontinued early because of a lower than anticipated HIV incidence rate [Kaplan-Meier cumulative probability of HIV infection =0.01 for SAVVY and = 0.011 for placebo (p=0.731)] |
| Phase III RCT to determine if 1% SAVVY gel prevents HIV25 | Nigeria | SAVVY gel=1041 HEC gel=1040 |
Discontinued early because of a lower than anticipated HIV incidence rate [HR 1.7 (95% CI, 0.9–3.5); p=0.127] |
|
| Carraguard | Phase II RCT to determine if Carraguard prevents HIV27 | South Africa | Carraguard gel=3103 Methylcellulose gel=3099 |
No Benefit [CarraguardIRa=3.3 (95% CI, 2.8–3.9) vs placebo IR =3.8 (95% CI, 3.2–4.4); p=0.3] |
| Cellulose sulfate | Phase III RCT to Phase III RCT to determine if cellulose sulfate 6% gel prevents HIV37 | Nigeria | Cellulose sulfate gel=820 HEC gel=824 |
Discontinued early for anticipated futility [HR-0.8 (95% CI, 0.3–1.8) |
| Phase III RCT to determine if cellulose sulfate 6% gel prevents HIV38 | Benin, India, South Africa, Uganda | Cellulose sulfate gel=717 HEC gel=708 |
Discontinued early for possible harm [ITT HR=1.61 (95% CI, 0.86 – 3.01); p=0.13] |
|
| PRO2000 | PhaseIIb RCT to determine if 0.5% PRO2000 prevents HIV40 | Malawi, South Africa, Zambia, Zimbabwe, and United States | PRO2000 gel=764 HEC gel=760 |
Non-statistical trend towards benefit [PRO2000 vs placebo gel HR=0.7 (p=0.1); PRO2000 vs no gel HR=0.67 (p=0.06)] |
| Phase III RCT to determine if 0.5% PRO2000, 2% PRO2000 prevents HIV41 | South Africa, Tanzania, Uganda, Zambia | 0.5% PRO2000 gel=3326 2% PRO2000 gel=2734 Control=3325 |
2% PRO2000 arm stopped early for futility [HR=1.21 (95% CI, 0.88 – 1.68; p=0.24)] No benefit with 0.5% PRO2000 [HR=1.05 (95% CI, 0.82 – 1.34); p=0.71] |
|
| BufferGel | PhaseIIb RCT to determine if BufferGel prevents HIV40 | Malawi, South Africa, Zambia, Zimbabwe, and United States | BufferGel=764 HEC gel=760 No gel=762 |
No Benefit [BufferGelIRa = 4.1, placebo gel IR=3.9, no gel IR=4.0] |
per 100 women years; CI= confidence interval, ER=event rate, HR= hazard ratio, HEC= hydroxyethylcellulose, IR= incidence rate, ITT= intention to treat, N-9= Nonoxynol-9, RCT=randomized controlled trial, RR=relative risk
Investigators hypothesized that the formulation was the source of N-9’s deleterious effects, and two reformulations were explored: a vaginal film and a 3.5% gel (COL-1492). The vaginal film demonstrated no HIV protection with a 30% increased rate of vaginal irritation (Table 1).14 Following two small safety studies, COL-1492 was studied in a RCT15,16 which demonstrated a higher rate of HIV acquisition in the treatment arm (14.7 vs 10.3; Table 1).17 The increased acquisition positively correlated with the frequency of N-9 use (HR=3.5 with >3.5 applications per day; P<0.0001) and epithelial disruption (HR=2.2 with one episode of a genital lesion with epithelial disruption; P=0.0003).
A number of reports revealed the irritant effects of N-9 in the female genital tract.13,18,19 Yet, safety studies preceding the clinical trials failed to anticipate (and mimic) the extensive product use found in these trials. While Kreiss et. al. reported an average use of 14 times per week for 14 months11, the highest average use achieved by a safety evaluation was 1.2 times a day for only 3 months.16 This made detecting N-9’s deleterious effects unlikely.
Following the disappointing N-9 results, a contraceptive surfactant named C31G (SAVVY) was investigated. Three concentrations of SAVVY (0.5%, 1.0%, and 1.7%) were exhaustively studied in a series of four safety trials. These concluded that while SAVVY gel exhibited a similar adverse event profile with N-9, its safety profile was acceptable for phase II/III trials.20–23 Two large-scale trials subsequently evaluated the efficacy of 1% SAVVY in reducing HIV transmission in women (Table 1). Both studies’ independent data monitoring committees(DMC) recommended early termination when the interim analyses revealed the original sample size would need to be more than doubled to meet their power requirements.24,25 Although one study did note a non-statistical trend towards a higher HIV incidence [hazard ratio (HR)=1.7] in the SAVVY group, these limited results allow few definitive conclusions.25
The study authors of the SAVVY efficacy trials cited an overestimation of anticipated HIV acquisition rates, as local HIV incidence rates were estimated based on previous experience rather than directly measured at the study sites.24,25 An alternative explanation for the low acquisition rates was an undervalued effect size of co-interventions such as condoms and safe sex education during the trial. Despite the potential of co-intervention to dilute the effect size, these are necessary components for pre-exposure prophylaxis(PrEP) trials to comply with ethical practices. These experiences illustrated the importance of a careful examination of study feasibility considering local incidence rates and co-intervention effects when designing HIV prevention trials. Additionally, these series of studies illustrated the importance of genital irritation investigations during product development.
2.II Polyanions
The first agent explored within the polyanion class was Carrageenan, a product derived from seaweed. After several variations of the carrageenan product were assessed in a number of phase I trials, a 1:1 κ-, λ-carraggenan 3% gel (Carraguard) was employed in an efficacy trial.26,27 Once again, this product showed no benefit for HIV prevention (Table 1).27 Although self-reported usage was high in this study (approximately 96% for both active and control arms), analysis of vaginal proteins on reportedly used applicators revealed actual usage was probably only 41–43%. However, secondary analyses of women who used the study product with every sex act (9% of the total study sample) as well as above average users (43% of the total study sample) also failed to discern a difference between the treatment and control arms with a HR of 1.03 (95% CI, 0.49–2.14) and 0.73 (95% CI, 0.51–1.04) respectively. Rather than poor adherence alone, an alternative hypothesis for Carraguard’s failure is that the placebo gel, methylcellulose, offered physical protection against HIV transmission due to its negatively charged polycarbophil base and high buffering capacity.28 Subsequently, hydroxyethylcellulose(HEC) has been widely adopted as a universal placebo for topical microbicide trials. It’s advantages include: being an uncharged linear polymer gelling agent designed to display negligible buffering or barrier protection; having no in vitro or in vivo anti-HIV or herpes simplex virus(HSV) activity; and no acid buffering capacity in seminal plasma.29
Cellulose sulfate was the next polyanion explored in efficacy trials following an extensive safety and acceptability evaluation in the female genital tract30–34 and on male genitalia.35,36 Two trials (single site37 and multisite38) were simultaneously conducted to compare cellulose sulfate 6% gel (Ushercell) with the newly defined universal placebo gel, HEC. Both trials were stopped early by their DMCs after an interim analysis of the multisite dataset revealed a HR of 2.23 (p=0.02) for the treatment arm (Table 1).38 While a final analysis of the multisite trial results failed to reach statistical significance [HR of 1.61 (95% CI, 0.86–3.01)] the committee recommended termination of the single site trial despite a slightly lower event rate (1.7 vs 2.1 per 100 women years) in the treatment arm. In vitro results later revealed clinically relevant concentrations of cellulose sulfate reduced epithelial barrier function and activated the pro-inflammatory signaling pathway nuclear factorκB(NFκB).39 These findings demonstrate the importance of careful in vitro investigation as a decision making tool prior to clinical evaluation.
The final polyanion, naphthalene sulfonate(PRO2000), was studied in two multisite, placebo controlled, RCTs40,41 (Table 1). The first demonstrated a trend towards benefit of PRO2000 0.5% gel with a 33% reduction (p=0.06) in the intent to treat analysis and a 36% reduction (p=0.04) in the per protocol analysis (excluding time off study product during the follow up period, mainly due to pregnancy). Importantly, these benefits were present only when comparing PRO2000 0.5% gel with the no gel arm and not the placebo arm40 The DMC of the larger trial evaluating two concentrations (0.5% and 2%) of PRO2000 gel recommended early cessation of the 2% gel arm for futility and possible harm with an HIV infection HR of 1.2 (95% CI, 0.88–1.68) in the treatment arm.41 While the 0.5% arm was allowed to continue, this formulation ultimately demonstrated futility [HR=1.05 (95% CI, 0.82–1.34)].
In hindsight, the decision to evaluate three microbicides with similar mechanisms and spectrums of activity42,43 in nearly simultaneous clinical trials may have been a misstep. However, until recently, microbicide development was largely conducted by individual researchers, rather than organized entities. Collaborative networks like the International Partnership for Microbicides(IPM) [established in 2002] and the Microbicide Trials Network(MTN) [established in 2006] have been formed to increase communication between these research entities and streamline product development.
Additionally, few animal studies examining clinically relevant viral challenges were conducted prior to designing the polyanion efficacy trials.42 At the time, however, animal models for HIV prevention largely relied on the non-human primate(NHP) models, which posed many challenges for characterizing a concentration-response relationship to inform clinical trials. These include differing viral species and inoculum concentrations required for infection as well as anatomical, histological, and physiological differences.44 To address some of these differences, human explant and humanized mouse models have been used.45 These also exhibit challenges for extrapolating efficacy data. The benefits and challenges of these preclinical efficacy models have been reviewed in detail elsewhere.46 Despite the lack of robustness in the animal models, a comparison of within class agents may still have assisted in determining which of these would have the greatest potential for protective effect prior to the investment of resources in clinical trials.
The final first generation agent was BufferGel: an acid buffering gel. The development of BufferGel as a preventative agent concluded after an efficacy trial demonstrated a non-statistical trend towards an increase in HIV incidence rate(IR) per 100 women years of BufferGel over both the placebo gel arm (HR=1.1, p=0.63), and the no-gel arm (HR=1.05, p=0.78) (Table 1).40
After more than 10 phase II/III clinical trials, exploring 6 possible first generation prevention agents, in nearly 30,000 study participants, the resulting data perhaps posed more questions than they answered but also assisted in streamlining the development of the next generation of microbicides. These data informed the field on the importance of epithelial integrity, identifying a suitable universal placebo, forming collaborative research networks, and developing more robust preclinical efficacy models.
3. Second Generation Topical Microbicides
The second generation of microbicides focuses on antiretrovirals. Although these agents have yielded conflicting results in clinical prevention trials47,48, their ongoing development is an important focus of the HIV prevention field.
3.I Tenofovir Gel
In 2006, Gilead Sciences granted CONRAD and the IPM co-exclusive license to develop tenofovir into a vaginal gel to be used for HIV prevention.49 Following this licensing, the gel was formulated with a viscosity enhancing agent (HEC), a humectant (glycerin), preservatives (methyl and proplyl parabens), sodium edetate, and cirtic acid and adjusted to a pH of 4.5 in order to maintain physiological vaginal pH. Tenofovir’s active intracellular metabolite, tenofovir diphosphate(-DP), is a chain-terminating agent that competes with deoxyadenosine triphosphate(dATP) for incorporation by reverse transcriptase into the proviral DNA strand. The unique features that make tenofovir an attractive microbicide candidate are as follows: 1) HIV inhibition prior to integration into the host genome; 2) fewer activation steps required for conversion into tenofovir-DP when compared to nucleoside analogs; 3) long intracellular half-life of tenofovir-DP; 4) relatively high barrier to resistance; and 5) extensive clinical experience and safety data from using tenofovir for HIV treatment.50,51
Tenofovir gel concentrations of 1% through 10% have been studied in 9 different macaque or humanized mouse models with various times of administration proceeding or following viral challenge with varying results (Table 2).46,52 Pharmacokinetic evaluation of plasma tenofovir concentrations indicated mean concentrations of < 30ng/ml (0.03% of the applied dose) were detectable within 30 minutes of application suggesting rapid but low uptake of the gel from the vaginal lumen. A linear concentration-effect relationship between infectibility and tenofovir-DP was found in an ex vivo model of infection using rectal tissue. Additionally, topical administration of tenofovir resulted in a higher degree of ex vivo protection when compared with oral dosing54. Most recently, this relationship has been described in a human vaginal tissue explant model, with a tenofovir-DP 50% effective concentration(EC50) of 909 fmol/mg.55 Importantly, this study described a poor relationship between tenofovir concentration and HIV protection due to the variability in intracellular conversion of tenofovir to tenofovir-DP. The limited antiretroviral PK/PD data available for HIV prevention provides an important knowledge gap that must be addressed to optimize the drug development process.
Table 2.
Intravaginal Tenofovir Gel Efficacy in Animal Studies.
| Animal Model |
Intravaginal Inoculate |
Treatment Time of Administration |
Treatment Arm | Protection | |
|---|---|---|---|---|---|
| N (Infected /Total) |
% | ||||
| NHP | Multiple SIVmac251 | −24h, 0h, 24h, 48h | 10% TFV gel | 0/4 | 100 |
| 1 ml placebo | 2/2 | 0 | |||
| NHP | Single SIVmac251 | −24h, −15m, −24h | 10% TFV gel | 1/5 | 80 |
| 1% TFV gel | 1/5 | 80 | |||
| −15m | 1% TFV gel | 2/5 | 60 | ||
| N/A | Untreated | 5/5 | 0 | ||
| NHP | Single SIVmac251 | −15m | 1% TFV gel | 1/5 | 80 |
| −2h | 1% TFV gel | 3/5 | 40 | ||
| −8h | 1% TFV gel | 1/5 | 80 | ||
| −15m | Vehicle | 1/5 | 80 | ||
| N/A | Untreated | 2/5 | 60 | ||
| NHP | Single SIVmac251 | −15m | 1% TFV gel | 1/5 | 80 |
| −2h | 1% TFV gel | 1/5 | 80 | ||
| −8h | 1% TFV gel | 2/5 | 60 | ||
| −15m | Vehicle | 2/5 | 60 | ||
| N/A | Untreated | 4/5 | 20 | ||
| NHP | Single SIVmac251 | −2h | 1% TFV gel | 0/5 | 100 |
| −2h | Vehicle | 2/5 | 60 | ||
| N/A | Untreated | 2/5 | 60 | ||
| NHP | Single SIVmac251 | −12h | 1% TFV gel | 5/8 | 38 |
| −24h | 1% TFV gel | 8/8 | 0 | ||
| −72h, −48h, −24h | 1% TFV gel | 6/8 | 25 | ||
| −12h | Vehicle | 8/8 | 0 | ||
| −24h | Vehicle | 8/8 | 0 | ||
| N/A | Untreated | 8/8 | 0 | ||
| NHP | Multiple intravaginal SHIVSF162p3 | −30m | 5%FTC and 1%TFV gel | 0/6 | 100 |
| Vehicle | 5/6 | 17 | |||
| Untreated | 2/2 | 0 | |||
| 1% TFV gel | 0/6 | 100 | |||
| Vehicle | 3/3 | 0 | |||
| NHP | Single SIVmac251 | −24h, 0h, 24h, 48h | 10% TFV gel | 0/5 | 100 |
| Vehicle | 5/5 | 0 | |||
| NHP | Multiple SHIVSF162P3 | −30m, −72h | 1% TFV gel | 4/6 | 74 |
| 2% HEC gel | 9/10 | 10 | |||
| BLT mice | #1 HIV-1JR-CSF | −4h, 4h | 1% TFV gel | 7/8 | 88 |
Adapted from:Gengiah TN, Baxter C, Mansoor LE, Kharsany AB, Abdool Karim SS: A drug evaluation of 1% tenofovir gel and tenofovir disoproxil fumarate tablets for the prevention of HIV infection. Expert Opin Investig Drugs. 2012;21(5):695–715 and Garcia-Lerma JG and Heneine W: Animal models of antiretroviral prophylaxis for HIV prevention. CurrOpin HIV AIDS 2012;7(6):505–513; BLT=bone/liver/thymus, FTC=emtricitabine, HEC= hydroxyethylcellulose, NHP=non-human primate, and TFV=tenofovir
A safety investigation demonstrated high tolerability and acceptability of both a 0.3% and 1% tenofovir gel in the female genital tract56 and on male genitalia.57 This was not found to be the case following rectal exposure.54 Anton et. al. demonstrated a 5.1 fold increase in the frequency of adverse event reports (including abdominal bloating, pain, and cramps; defecation urgency; diarrhea; flatulence; mucosal red spots; colon polyps; and nausea) following 7 daily rectal applications of TFV 1% gel when compared to HEC placebo gel. Additionally, two participants in the treatment group experienced severe (Grade 3) adverse events of abdominal bloating, pain, and cramps; defecation urgency; and tenesmus.54 The authors hypothesized the high incidence of adverse event reporting was secondary to the high osmolality (3111 mOsmol/kg) of the gel. Subsequent reformulation of tenofovir gel with decreased glycerin content (5% vs the 20% in the original formulation) reduced osmolality (836 mOsmol/kg)58 and recently yielded a safety and acceptability profile comparable to placebo.59
While development of rectal microbicides in the context of men who have sex with men (MSM) has been reviewed elsewhere60, these findings underscore an important consideration for HIV prevention trials in the heterosexual population: Heterosexual women engage in receptive anal intercourse. A systematic review of publications from 2002 forward revealed that 3.5% to 40% of female sex workers(FSW) in Africa reported engaging in receptive anal intercourse in the previous 3 months.61 These estimations likely underrepresent the actual prevalence of anal intercourse in the heterosexual population.62–64
The first clinical study of tenofovir 1% gel was designed by the Centre for the AIDS Program of Research in South Africa(CAPRISA). CAPRISA004 was a phase IIa, double blind, RCT designed to assess the efficacy of 1% tenofovir gel in preventing HIV in urban and rural settings in South Africa. CAPRISA004 used a coitally-dependent “BAT24” dosing scheme: one dose of gel within 12 hours Before sex and one dose of gel within 12 hours After sex, not exceeding Two doses in 24 hours. This was based on the dosing strategy used for nevirapine prophylaxis to prevent mother-to-child transmission of HIV in the HIVNET012 study.65 An overall 39% reduction of HIV acquisition was seen over 18 months of use. This efficacy went up to 54% in high adherers (> 80% gel adherence)47(Table 3). Post-hoc analysis indicated that women who achieved vaginal lumen concentrations of >1000ng/ml of tenofovir exhibited a higher degree of protection than those with lumen concentration of <1000ng/ml and the placebo group (incidence rate per 100 women years =2.4, 7.8, and 9.1 respectively).66 CAPRISA004 also demonstrated 51% protection against HSV-2 in the tenofovir gel arm (p=0.003). FACTS 001 is a second, larger, regulatory study than CAPRISA 004. CAPRISA008 is an ongoing follow up to CAPRISA004, which is primarily assessing the feasibility of dispensing tenofovir gel through family planning services in Africa.
Table 3.
Second Generation Topical Microbicides Clinical Trials Summary.
| Study Summary | Treatment Arm Number |
Results Summary | |
|---|---|---|---|
| Tenofovir 1% Gel | HPTN050: Phase I randomized trial to evaluate the safety of 0.3% and TFV 1% gel administered once and twice daily for 14 days in HIV negative and positive women56 |
Once daily TFV 0.3% gel=12 TFV1% gel=12 Twice daily TFV 0.3% gel=12 TFV 1% gel=48 |
Twice daily 1% TFV gel was as well tolerated as once daily 0.3% TFV gel when used in HIV positive or negative women. |
| HPTN059: Phase I randomized trial to evaluate the safety and acceptability of TFV 1% gel vs placebo with daily or coitally dependent dosing107 | Daily TFV 1% gel=50 Coital TFV 1% gel=50 Daily HEC gel=50 Coital HEC gel=50 |
No difference in safety or acceptability between dosing groups | |
| Phase I RCT to evaluate the safety of TFV 1% gel applied to the genitalia of circumcised and uncircumcised men for 6–10 hours daily for 7 days57 | TFV 1% gel=36 K-Y Jelly=12 |
1% TFV gel was well tolerated by circumcised and uncircumcised men. 13% of men in the treatment arm vs 18% in control arm reported symptoms of mild pruritus, pain, dysuria, and/or warm feeling. | |
| CAPRISA004: PhaseII/III RCT to determine if BAT24 administration of TFV 1% gel prevents HIV in South African womem47 | TFV 1% gel=445 HEC gel=444 |
BAT24 administration of 1% TFV gel decreased HIV incidence [Tenofovir gel vs placebo gel IRa= 5.6 (CI:4.0–7.7) vs 9.1 (CI:6.9–11.7), p=0.017] |
|
| VOICE: Phase III RCT to determine if once daily administration of Viread®, Truvada®, and TFV 1% gel prevent HIV in Ugandan, South African and Zimbabwean women7. | All Arms=5029 | All arms stopped early for futility [Tenofovir gel vs placebo gel IR= 6.0 vs 6.1] |
|
| FACTS001: A phase III RCT to determine if BAT24 administration of TFV 1% gel prevents HIV and HSV-2 in South African women108 | Anticipated=2900 | Ongoing | |
| Dapirivine | IPM001 and IPM008: Two phase I RCT to assess the safety of a 25mg and 200mg reservoir-type intravaginal ring containing DPV after a 7 day exposure93 | 25mg matrix ring=10 200mg reservoir ring=12 Silicone elastomer ring=15 |
Both rings were well tolerated and exhibited low systemic and sustained vaginal exposure [Adverse events reported in 80%, 75% and 74% in the 25mg, 200mg and placebo ring arms respectively] [Matrix vs reservoir plasma AUC0–24h (pg*h/mL)=21,870 vs 411; CVF AUC0–24h(µg*hr/g)=15,430 vs 72.5] |
| IPM018: A phase I RCT to assess the safety and pharmacokinetics of matrix-type and reservoir-type intravaginal ring containing 25mg of DPV after a 7 day exposure92 | 25mg matrix ring=8 25mg reservoir ring=8 Silicone elastomer ring=8 |
Both rings were well tolerated and exhibited low systemic and sustained vaginal exposure [Adverse events reported in 50%, 62.5% and 62.5% in the matrix, reservoir, and placebo ring arms respectively] [Matrix vs reservoir plasma AUC0–24h (pg*h/mL)=21,870 vs 411; CVF AUC0–24h(µg*hr/g)=15,430 vs 72.5] |
|
| IPM015: A phase I RCT to assess the safety, acceptability and pharmacokinetics of DPV 25mg matrix-type ring inserted every 4 weeks for 12 weeks in healthy African women94. | 25mg matrix ring=140 Silicone elastomer matrix ring=140 |
The DPV ring was well tolerated with no difference in adverse event reporting between treatment and placebo arm The DPV ring exhibited low systemic with DPV plasma concentrations <1ng/mL) |
|
| MTN020 (ASPIRE): A phase III RCT to determine if the 25mg DPV intravaginal ring inserted once every 4 weeks prevents HIV infection in African women109 | Anticipated=3476 | Ongoing | |
| IPM027 (The Ring Study): A phase III RCT to determine if the 25mg DPV intravaginal ring inserted once every 4 weeks prevents HIV infection in African women96 | Anticipated=1650 | Ongoing | |
per 100 women years; ASPIRE= A Study to Prevent Infection with a Ring for Extended Use, AUC=area under the curve, BAT24=one dose 12 hours Before sex and a second dose within 12 hours After sex and no more than Two doses in 24 hours, CAPRISA=Centre for AIDS Programme of Research in South Africa, CI= confidence interval, CVF=cervical vaginal fluid, DPV=dapivirine, FACTS=The Follow-on African Consortium for TenofovirStudies, HEC= hydroxyethylcellulose, HPTN= HIV Prevention Trials Network, HR= hazard ratio, HSV-2= Herpes simplex virus type2, IR= incidence rate, MTN= Microbicide Trials Network, RCT=randomized controlled trial, TFV=tenofovir, VOICE=Vaginal and Oral Interventions to Control the Epidemic
Soon after the results of CAPRISA004 were published in 2010, the MTN released a statement announcing the early termination of the 1% tenofovir gel arm of the VOICE prevention trial secondary to futility.48 VOICE was a five-arm, 15 site investigation of daily tenofovir gel or tablet, with or without emtricitabine, and matched placebos that assessed HIV prevention efficacy (Table 3). Despite the high self-reported adherence and participant retention, a post hoc analysis of plasma samples in the active arms revealed only approximately 30% of women were using products frequently enough to detect drug in their plasma.7 These adherence results parallel other PrEP studies evaluating daily regimens. In FEM-PrEP and iPrEX, only 24% and 51% of participants in the treatment arms providing samples for PK analysis had detectable drug concentrations. These data suggested less than daily use for the majority of participants.6,67 Excluding TDF2 (>30% attrition rate) and the Tenofovir Bangkok Study (directly observed dosing),5,68 only Partners PrEP, demonstrated high adherence rates with detectable tenofovir concentrations in 82% of participants providing samples for PK analysis. In this study, participants knew that their partners were infected with HIV.69 Therefore, it is possible that daily administration of a product for chemoprophylaxis against non-daily risky behavior resulted in more study procedure fatigue than the BAT24 regimen used in CAPRISA004.70
The lack of adherence in this at-risk population suggests that more research is needed to understand motivation and willingness to use a product daily for protection against HIV infection. High adherence has been strongly linked to the ability of any chemoprophylaxis strategy to prevent infection.6,47,67 Over 18 product acceptability studies have investigated both oral and vaginal chemoprophylactic agents.71 In general, acceptability studies evaluating possible topical drug delivery devices like gels, intravaginal rings, and diaphragms indicate a high degree of acceptability for product-related characteristics such as “ease of use”.72–75 Additionally, a high percentage of participants (51–76%) testing various intravaginal gels rate themselves as “likely to use the product”.72,75 All women (n=151; 95%CI 98–100%) testing an intravaginal ring for up to 12 weeks reported they would use the product if it were found to be effective in HIV prevention.73
However, the dichotomous measure of “willingness to use” and high Likert-scale scores for product characteristics fail to account for the multidimensional nature of acceptability and adherence. For instance, low HIV risk perception, a marital status of single, and being under 25 years of age have all been shown to be important predictors of low adherence in PrEP trials.76 Additionally, nationality has been previously demonstrated as a covariate of product acceptability with vaginal gels74,77, and regionally constrained acceptability studies likely fail to depict the global acceptability of any given product. Finally, unknown product related factors (ie cost, efficacy, regulation) might impact the acceptability of these agents. Galea et. al. reported a 40% reduction in mean acceptability when participants were given a scenario of a chemoprophylactic agent costing US$10 vs US$250 a month and a 35% reduction when efficacy was reduced from 95% to 75%.78
The results of VOICE C, a qualitative study among randomly selected VOICE participants from the Johannesburg site, revealed womens’ decision to participate in the study or use study product was largely influenced by three prevailing themes: personal health (with participation being perceived as both risky and healthy), social relationships, and ambivalence towards the research.79 Women typically acknowledged missing or skipping doses in the context of “forgetting, being bored or lazy, and being busy or on the go”. A representative interview from a non-adherent study participant illustrates the complexity of adherence in clinical trials. In this interview the study participant indicated she decided to participate because she wanted to protect herself but was demotivated to use study product because, having experienced no side effects, she believed her product was placebo and by rumors that other participants had experienced severe toxicities. Payment for study visits and free health checks, however, strongly motivated her to continue participating despite her lack of compliance.80
Upon licensure and the removal of these intrinsic incentives for participating in clinical trials, the motivation to seek (and purchase) chemoprophylactic agents may indicate 1) high perception of risk and 2) knowledge of tenofovir’s protective potential on the part of the end user. Therefore, it is possible individuals using chemoprophylaxis in the post-marketing phase may exhibit higher rates of adherence than women in phase II/III clinical trials. Given the complexity of the covariates impacting adherence in prevention trials, it is hard to predict acceptability as measured by adherence. Regardless, the failure, to date, of clinical trials evaluating daily regimens in heterosexual women6,48 provide evidence for the need to simplify chemoprophylactic dosing regimens. Furthermore, while poor adherence confounds both oral and topical dosing strategies6,7, the 100-fold increased tissue concentrations of tenofovir-DP following topical administration and CAPRISA004 data suggest that in a setting of inconsistent dosing, protection is more likely with topical administration.70,81
Yet local toxicity with local administration of antiretroviral is an important consideration. No in vivo mutagenicity or carcinogenicity evaluations for topical tenofovir exposure have been reported. In oral carcinogenicity studies, liver adenomas were exhibited in female CD1 mice given doses of 600mg/kg/day.82,83 While these findings are an unlikely concern for topical tenofovir given its low systemic exposure, doses up to 300 mg/kg/day in female Sprague-Dawley rats did reveal a slight increase in uterine polyps/endometrial stroma.82 However, this increase was considered to be toxicologically insignificant due to a lack of a treatment-effect relationship. Additionally, oral zidovudine, a chemically similar compound, has been shown to increase the incidence of vaginal epithelial neoplasms at doses of greater than 30mg/kg/day in mice and 300mg/kg/day in rats.84 In light of the 100-fold increased concentrations achieved in the female genital tract by topical administration these findings warrant investigation into the long-term safety of topical tenofovir.
3.II Alternatives to Tenofovir Gel
Alternative drug delivery systems designed to lessen traditional gel dosing burden may improve participant acceptability and adherence. Contraceptive intravaginal rings(IVR) have been used clinically since the approval of NuvaRing® (MERCK, Whitehouse Station, NJ) by the US FDA in 2001, which was implemented to overcome imperfect adherence to once daily oral hormonal therapy. An IVR formulation containing tenofovir disoproxil fumarate has been shown to offer complete protection against up to 16 SHIV vaginal challenges over 4 months in macaques.85 Additionally, Smith et. al. noted vaginal lumen tenofovir concentrations within 24 hours of insertion that were 100,000ng/mL: 100-times higher than the 1,000ng/mL reported to be effective in CAPRISA004.86 However, correlating pharmacokinetic animal data with human data should be done with caution, given inter-species differences in pharmacokinetics. As of December 2013, a phase I study examining the safety and pharmacokinetics of a 14-day exposure to the tenofovir disoproxil fumarate ring in healthy volunteers is being conducted.87
An antiretroviral alternative to tenofovir is dapivirine, a non-nucleoside reverse transcriptase inhibitor(NNRTI). Dapivirine’s development as an antiretroviral for HIV treatment was abandoned by Janssen (Tibotec) due to poor bioavailability, and the compound was subsequently licensed to IPM for further chemoprophylaxis development. Since dapivirine will never be licensed for HIV treatment, it offers advantages over tenofovir, one of the most widely used antiretrovirals for therapy, including attenuating theoretical concerns that: (1) transmitted resistance to antiretrovirals used clinically may mitigate the antiviral potency of chemoprophylactic agents, and (2) antiretroviral resistance may develop in subjects with breakthrough infections on chemoprophylaxis and reduce effective treatment options for those who become infected. This latter concern has not been demonstrated with clinical trials thus far, however this may be due to low adherence in the majority of studies not permitting enough drug exposure to promote the development of resistance. Although in vitro data suggest dapivirine resistance may select for cross-resistance to all NNRTIs, this has not yet been demonstrated clinically.88
Dapivirine has been formulated into a diaphragm56, lyophilized tablet for vaginal delivery89, intravaginal film90, and intravaginal ring.91 While most of these are still in the preclinical stages of development, an intravaginal ring containing 25mg or 200mg of dapivirine has been shown to be safe and acceptable for participants in phase I studies.92,93 Additionally, a large, RCT to evaluate the safety, acceptability and pharmacokinetics of dapivirine 25mg matrix-type ring in healthy African women demonstrated an acceptable safety profile with low systemic absorption of dapivirine following 12 weeks of once monthly ring insertions. The mean plasma dapivirine concentrations in this study were < 1ng/ml (more than 100 fold-lower systemic exposure compared to oral administration of dapivirine 100mg twice daily).94,95 Currently, the Ring Study(IPM027) will assess the efficacy of a 25mg dapivirine ring in 5 African sites.96 Enrolling concurrently, the ASPIRE(MTN020) RCT is being conducted across 15 African sites to evaluate the efficacy of the dapivirine intravaginal ring in preventing HIV transmission.97
3.III Combination Products in Development
Recently, co-formulation products have been of great interest in HIV prevention research. Combination therapy with multiple drugs with different mechanisms of action is standard of care for HIV treatment. Co-formulation of microbicide agents into a single device may exhibit antiviral additivity or synergy and possibly reduce opportunity for breakthrough infection. Thus far, an intravaginal ring containing dapirivine and maraviroc has been developed and tested in safety trials.98 Tenofovir has also been co-formulated in a vaginal ring with acyclovir (an anti-HSV-2 agent)99 but is not yet in clinical development. Several phase II/III trials evaluating the efficacy of long acting injections for HIV prevention are ongoing but outside of the scope of this review.
4 Adherence Measures
Recently, two behavioral adherence measures, audio computer-assisted self-interviewing(ACASI) and clinic product counts(CPC), were found to be less than 50% accurate at predicting non adherence in the VOICE trial.100 This poor predictive ability warrants consideration of objective measures for adherence monitoring in prevention studies. Applicator dyes, plasma drug concentrations, upper layer packed cells(ULPC), occipital hair, vaginal swabs and electronic monitoring containers are minimally invasive measures of adherence that do not rely on subject recall or report.101–104 Two clinical PrEP trials, MTN017 and MTN020, are currently employing regular plasma PK analysis to monitor and motivate participant adherence.105,106 These trials will provide valuable information concerning the utility of planned PK analysis for adherence monitoring in clinical trials. Of the above objective adherence measures, only electronic monitoring offers real-time adherence monitoring and the ability to implement intervention at the moment of lapsed adherence. Each option poses unique challenges for implementation and the optimal measure for PrEP trials is yet to be determined.
5 Final Comments
In the absence of an effective HIV vaccine, antiretroviral therapies for HIV treatment are being extended to HIV prevention. Although lifelong drug therapy for prevention is less than ideal, topical microbicides provide advantages over oral therapy in that they limit systemic exposure and may offer more flexible dosing. With the exciting pipeline of prevention agents and delivery devices currently in phase II/III clinical investigation, the HIV microbicide field is on the cusp of offering efficacious, tolerable, and easy-to-use choices to women seeking a prevention option they can control. The lessons learned from twenty years of microbicide development are being carefully considered in next generation PrEP development. Agents, vehicles, and devices safety evaluation should realistically mimic anticipated use prior to phase II/III trials. Lead compounds within a class of microbicides should be selected on the basis of developed pharmacokinetic-pharmacodynamic relationships, with superior efficacy and minimal toxicity both in vitro and in animal models before being investigated in clinical trials. Finally, measures of adherence that do not rely on subject report (such as biomarkers) should be incorporated into study designs. Optimally, real-time feedback could lead to real-time adherence interventions to both support clinical trial participants and to select optimal interventions during community-based roll-out of PrEP.
Acknowledgement
This work was supported by the Centers for AIDS Research [grant number CFAR P30 AI50410], the National Institutes of Allergy and Infectious Diseases [grant number U01 AU095031], and the National Institute of General Medical Sciences [grant number 5T32GM086330]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the supporting agencies listed above.
Footnotes
Declaration of Competing Interests and Financial Disclosure: Angela Kashuba and her laboratory are part of the study teams for CAPRISA 004 and 008, FACTS 001, MTN 006, HPTN 066, FemPrEP, and CONRAD 113, 114, and 117
References
- 1.Centers for Disease Control and Prevention. HIV prevention. [Updated 2014. Accessed January 14, 2014]; http://www.cdc.gov/hiv/basics/prevention.html.
- 2.Langen TT. Gender power imbalance on women's capacity to negotiate self-protection against HIV/AIDS in Botswana and South Africa. Afr Health Sci. 2005;5(3):188–197. doi: 10.5555/afhs.2005.5.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Gender women and health: Gender inequalities and HIV. [Updated 2014. Accessed January 4, 2014]; http://www.who.int/gender/hiv_aids/en/. [Google Scholar]
- 4.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo J, Ramjee G, Nair G, et al. Pre-exposure prophylaxis for HIV in women: Daily oral tenofovir, oral tenofovir/emtricitabine or vaginal tenofovir gel in the VOICE study (MTN 003). 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. March 3–6; 2013. Abstract 26LB. [Google Scholar]
- 8.Truvada full prescribing information. Foster City, CA 94404: Gilead Sciences, Inc; 2012. [Google Scholar]
- 9.Romano JW, Robbiani M, Doncel GF, Moench T. Non-specific microbicide product development: Then and now. Curr HIV Res. 2012;10(1):9–18. doi: 10.2174/157016212799304625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller CJ, Alexander NJ, Gettie A, Hendrickx AG, Marx PA. The effect of contraceptives containing nonoxynol-9 on the genital transmission of simian immunodeficiency virus in rhesus macaques. Fertil Steril. 1992;57(5):1126–1128. [PubMed] [Google Scholar]
- 11.Kreiss J, Ngugi E, Holmes K, et al. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268(4):477–482. [PubMed] [Google Scholar]
- 12.Zekeng L, Feldblum PJ, Oliver RM, Kaptue L. Barrier contraceptive use and HIV infection among high-risk women in Cameroon. AIDS. 1993;7(5):725–731. doi: 10.1097/00002030-199305000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Roddy RE, Cordero M, Cordero C, Fortney JA. A dosing study of nonoxynol-9 and genital irritation. Int J STD AIDS. 1993;4(3):165–170. doi: 10.1177/095646249300400308. [DOI] [PubMed] [Google Scholar]
- 14.Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. N Engl J Med. 1998;339(8):504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 15.Van Damme L, Niruthisard S, Atisook R, et al. Safety evaluation of nonoxynol-9 gel in women at low risk of HIV infection. AIDS. 1998;12(4):433–437. doi: 10.1097/00002030-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Van Damme L, Chandeying V, Ramjee G, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 phase II study group. AIDS. 2000;14(1):85–88. doi: 10.1097/00002030-200001070-00010. [DOI] [PubMed] [Google Scholar]
- 17.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: A randomised controlled trial. Lancet. 2002;360(9338):971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 18.Chvapil M, Droegemueller W, Owen JA, Eskelson CD, Betts K. Studies of nonoxynol-9. I. The effect on the vaginas of rabbits and rats. Fertil Steril. 1980;33(4):445–450. [PubMed] [Google Scholar]
- 19.Niruthisard S, Roddy RE, Chutivongse S. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex Transm Dis. 1991;18(3):176–179. doi: 10.1097/00007435-199107000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Mauck CK, Weiner DH, Creinin MD, Barnhart KT, Callahan MM, Bax R. A randomized phase I vaginal safety study of three concentrations of C31G vs. extra strength gynol II. Contraception. 2004;70(3):233–240. doi: 10.1016/j.contraception.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Mauck CK, Frezieres RG, Walsh TL, Schmitz SW, Callahan MM, Bax R. Male tolerance study of 1% C31G. Contraception. 2004;70(3):221–225. doi: 10.1016/j.contraception.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Mauck CK, Creinin MD, Barnhart KT, et al. A phase I comparative postcoital testing study of three concentrations of C31G. Contraception. 2004;70(3):227–231. doi: 10.1016/j.contraception.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Ballagh SA, Baker JM, Henry DM, Archer DF. Safety of single daily use for one week of C31G HEC gel in women. Contraception. 2002;66(5):369–375. doi: 10.1016/s0010-7824(02)00433-x. [DOI] [PubMed] [Google Scholar]
- 24.Peterson L, Nanda K, Opoku BK, et al. SAVVY (C31G) gel for prevention of HIV infection in women: A phase 3, double-blind, randomized, placebo-controlled trial in Ghana. PLoS One. 2007;2(12):e1312. doi: 10.1371/journal.pone.0001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feldblum PJ, Adeiga A, Bakare R, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: A randomized controlled trial in Nigeria. PLoS One. 2008;3(1):e1474. doi: 10.1371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirrone V, Wigdahl B, Krebs FC. The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antiviral Res. 2011;90(3):168–182. doi: 10.1016/j.antiviral.2011.03.176. [DOI] [PubMed] [Google Scholar]
- 27.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9654):1977–1987. doi: 10.1016/S0140-6736(08)61842-5. [DOI] [PubMed] [Google Scholar]
- 28.Kilmarx PH, Paxton L. Need for a true placebo for vaginal microbicide efficacy trials. Lancet. 2003;361(9359):785–786. doi: 10.1016/S0140-6736(03)12645-1. author reply 786. [DOI] [PubMed] [Google Scholar]
- 29.Tien D, Schnaare RL, Kang F, et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21(10):845–853. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- 30.Doh AS, Ngoh N, Roddy R, Lai JJ, Linton K, Mauck C. Safety and acceptability of 6% cellulose sulfate vaginal gel applied four times per day for 14 days. Contraception. 2007;76(3):245–249. doi: 10.1016/j.contraception.2007.05.083. [DOI] [PubMed] [Google Scholar]
- 31.Mauck C, Weiner DH, Ballagh S, et al. Single and multiple exposure tolerance study of cellulose sulfate gel: A phase I safety and colposcopy study. Contraception. 2001;64(6):383–391. doi: 10.1016/s0010-7824(01)00271-2. [DOI] [PubMed] [Google Scholar]
- 32.Malonza IM, Mirembe F, Nakabiito C, et al. Expanded phase I safety and acceptability study of 6% cellulose sulfate vaginal gel. AIDS. 2005;19(18):2157–2163. doi: 10.1097/01.aids.0000194797.59046.8f. [DOI] [PubMed] [Google Scholar]
- 33.El-Sadr WM, Mayer KH, Maslankowski L, et al. Safety and acceptability of cellulose sulfate as a vaginal microbicide in HIV-infected women. AIDS. 2006;20(8):1109–1116. doi: 10.1097/01.aids.0000226950.72223.5f. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz JL, Mauck C, Lai JJ, et al. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: A randomized double-blind phase I safety study. Contraception. 2006;74(2):133–140. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Mauck C, Frezieres R, Walsh T, Robergeau K, Callahan M. Cellulose sulfate: Tolerance and acceptability of penile application. Contraception. 2001;64(6):377–381. doi: 10.1016/s0010-7824(01)00270-0. [DOI] [PubMed] [Google Scholar]
- 36.Jespers V, Buve A, Van Damme L. Safety trial of the vaginal microbicide cellulose sulfate gel in HIV-positive men. Sex Transm Dis. 2007;34(7):519–522. doi: 10.1097/01.olq.0000253340.76118.89. [DOI] [PubMed] [Google Scholar]
- 37.Halpern V, Ogunsola F, Obunge O, et al. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: Results of a phase III trial in Nigeria. PLoS One. 2008;3(11):e3784. doi: 10.1371/journal.pone.0003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359(5):463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 39.Mesquita PM, Cheshenko N, Wilson SS, et al. Disruption of tight junctions by cellulose sulfate facilitates HIV infection: Model of microbicide safety. J Infect Dis. 2009;200(4):599–608. doi: 10.1086/600867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdool Karim SS, Richardson BA, Ramjee G, et al. Safety and effectiveness of BufferGel and 0.5% PRO2000 gel for the prevention of HIV infection in women. AIDS. 2011;25(7):957–966. doi: 10.1097/QAD.0b013e32834541d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCormack S, Ramjee G, Kamali A, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (microbicides development programme 301): A phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;376(9749):1329–1337. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shattock RJ, Doms RW. AIDS models: Microbicides could learn from vaccines. Nat Med. 2002;8(5):425. doi: 10.1038/nm0502-425. [DOI] [PubMed] [Google Scholar]
- 43.Grant RM, Hamer D, Hope T, et al. Whither or wither microbicides? Science. 2008;321(5888):532–534. doi: 10.1126/science.1160355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Rompay KK. The use of nonhuman primate models of HIV infection for the evaluation of antiviral strategies. AIDS Res Hum Retroviruses. 2012;28(1):16–35. doi: 10.1089/aid.2011.0234. [DOI] [PubMed] [Google Scholar]
- 45.Denton PW, Garcia JV. Mucosal HIV-1 transmission and prevention strategies in BLT humanized mice. Trends Microbiol. 2012;20(6):268–274. doi: 10.1016/j.tim.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romano J, Kashuba A, Becker S, et al. Pharmacokinetics and pharmacodynamics in HIV prevention; current status and future directions: A summary of the DAIDS and BMGF sponsored think tank on pharmacokinetics (PK)/pharmacodynamics (PD) in HIV prevention. AIDS Res Hum Retroviruses. 2013;29(11):1418–1427. doi: 10.1089/aid.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Micorbicide Trial Network (MTN) MTN statement on decision to discontinue use of tenofovir gel in VOICE, a major HIV prevention study in women. [Updated 2011. Accessed December 10, 2013];2011 Nov 25; http://www.mtnstopshiv.org/node/3909 Web site..
- 49.CONRAD. Tenofovir gel overview. [Updated 2013. Accessed December 11, 2013]; http://www.conrad.org/tenofovir.html. [Google Scholar]
- 50.Shattock RJ, Rosenberg Z. Microbicides: Topical prevention against HIV. Cold Spring Harb Perspect Med. 2012;2(2):a007385. doi: 10.1101/cshperspect.a007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wainberg M. Microbicides 2004 Conference. London, England: 2004. The prospect for RT inhibitors as topical microbicides. Abstract MMM-03. [Google Scholar]
- 52.Gengiah TN, Baxter C, Mansoor LE, Kharsany AB, Abdool Karim SS. A drug evaluation of 1% tenofovir gel and tenofovir disoproxil fumarate tablets for the prevention of HIV infection. Expert Opin Investig Drugs. 2012;21(5):695–715. doi: 10.1517/13543784.2012.667072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobard C, Sharma S, Martin A, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol. 2012;86(2):718–725. doi: 10.1128/JVI.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anton PA, Cranston RD, Kashuba A, et al. RMP-02/MTN-006: A phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2012;28(11):1412–1421. doi: 10.1089/aid.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicol M, Emerson C, Nelson J, et al. 21st Conference on Retroviruses and Opportunistic Infections. Boston, MA: 2014. Tenofovir diphosphate concentration-response relationship for HIV prevention in vaginal tissue. Abstract 512. [Google Scholar]
- 56.Mayer KH, Maslankowski LA, Gai F, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20(4):543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz JL, Poindexter A, Wheeless A, Mauck CK, Callahan MM. Safety evaluation of 1% tenofovir gel in healthy men. Int J STD AIDS. 2009;20(6):384–386. doi: 10.1258/ijsa.2008.008309. [DOI] [PubMed] [Google Scholar]
- 58.Dezzutti CS, Rohan LC, Wang L, et al. Reformulated tenofovir gel for use as a dual compartment microbicide. J Antimicrob Chemother. 2012;67(9):2139–2142. doi: 10.1093/jac/dks173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGowan I, Hoesley C, Cranston RD, et al. A phase 1 randomized, double blind, placebo controlled rectal safety and acceptability study of tenofovir 1% gel (MTN-007) PLoS One. 2013;8(4):e60147. doi: 10.1371/journal.pone.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGowan I. The development of rectal microbicides for HIV prevention. Expert Opin Drug Deliv. 2014;11(1):69–82. doi: 10.1517/17425247.2013.860132. [DOI] [PubMed] [Google Scholar]
- 61.Baggaley RF, Dimitrov D, Owen BN, et al. Heterosexual anal intercourse: A neglected risk factor for HIV? Am J Reprod Immunol. 2013;69(Suppl 1):95–105. doi: 10.1111/aji.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minani I, Alary M, Lowndes C, et al. 18th International Society of Sexually Transmitted Disease Research. London, England: 2009. Higher levels of HIV-related risky behavior in polling booth surveys compared to face-to-face interviews in a general population survey in Cotonou. Abstract OS1.8.03. [Google Scholar]
- 63.Gorbach PM, Mensch BS, Husnik M, et al. Effect of computer-assisted interviewing on self-reported sexual behavior data in a microbicide clinical trial. AIDS Behav. 2013;17(2):790–800. doi: 10.1007/s10461-012-0302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hanck SE, Blankenship KM, Irwin KS, West BS, Kershaw T. Assessment of self-reported sexual behavior and condom use among female sex workers in India using a polling box approach: A preliminary report. Sex Transm Dis. 2008;35(5):489–494. doi: 10.1097/OLQ.0b013e3181653433. [DOI] [PubMed] [Google Scholar]
- 65.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362(9387):859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 66.Karim SS, Kashuba AD, Werner L, Karim QA. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: Implications for HIV prevention in women. Lancet. 2011;378(9787):279–281. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok tenofovir study): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 69.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karim SS, Karim QA. XVIII International AIDS Conference. Vienna, Austria: 2010. Effectiveness and safety of vaginal microbicide 1% tenofovir gel for prevention of HIV infection in women. Abstract TUSS0204 and oral presentation. [Google Scholar]
- 71.Mensch BS, van der Straten A, Katzen LL. Acceptability in microbicide and PrEP trials: Current status and a reconceptualization. Curr Opin HIV AIDS. 2012;7(6):534–541. doi: 10.1097/COH.0b013e3283590632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frezieres RG, Walsh T, Kilbourne-Brook M, Coffey PS. Couples' acceptability of the SILCS diaphragm for microbicide delivery. Contraception. 2012;85(1):99–107. doi: 10.1016/j.contraception.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 73.van der Straten A, Montgomery ET, Cheng H, et al. High acceptability of a vaginal ring intended as a microbicide delivery method for HIV prevention in African women. AIDS Behav. 2012;16(7):1775–1786. doi: 10.1007/s10461-012-0215-0. [DOI] [PubMed] [Google Scholar]
- 74.Giguere R, Carballo-Dieguez A, Ventuneac A, et al. Variations in microbicide gel acceptability among young women in the USA and Puerto Rico. Cult Health Sex. 2012;14(2):151–166. doi: 10.1080/13691058.2011.630099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carballo-Dieguez A, Giguere R, Dolezal C, et al. "Tell juliana": Acceptability of the candidate microbicide VivaGel(R) and two placebo gels among ethnically diverse, sexually active young women participating in a phase 1 microbicide study. AIDS Behav. 2012;16(7):1761–1774. doi: 10.1007/s10461-011-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gengiah TN, Moosa A, Naidoo A, Mansoor LE. Adherence challenges with drugs for pre-exposure prophylaxis to prevent HIV infection. Int J Clin Pharm. 2014;36(1):70–85. doi: 10.1007/s11096-013-9861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Minnis AM, Gandham S, Richardson BA, et al. Adherence and acceptability in MTN 001: A randomized cross-over trial of daily oral and topical tenofovir for HIV prevention in women. AIDS Behav. 2013;17(2):737–747. doi: 10.1007/s10461-012-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Galea JT, Kinsler JJ, Salazar X, et al. Acceptability of pre-exposure prophylaxis as an HIV prevention strategy: Barriers and facilitators to pre-exposure prophylaxis uptake among at-risk Peruvian populations. Int J STD AIDS. 2011;22(5):256–262. doi: 10.1258/ijsa.2009.009255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Straten A, Stadler J, Montgomery E, et al. Women's experiences with oral and vaginal pre-exposure prophylaxis: The VOICE-C qualitative study in Johannesburg, South Africa. PLoS One. 2014;9(2):e89118. doi: 10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hillier S. 21st Conference on Retroviruses and Opportunistic Infections. Boston, MA: Oral presentation; 2014. HIV prevention research: Progress and challenges. [Google Scholar]
- 81.Hendrix CW, Chen BA, Guddera V, et al. MTN-001: Randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013;8(1):e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.European Medicines Agency. Scientific discussion for the approval of viread. [Updated 2004. Accessed Febuary 20, 2014]; http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000419/WC500051732.pdf. [Google Scholar]
- 83.US Food and Drug Administration. Viread (tenofovir disoproxil fumarate) label update. [Updated 2009. Accessed Febuary 20, 2014]; http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm125021.htm.
- 84.Ayers KM, Clive D, Tucker WE, Jr, Hajian G, de Miranda P. Nonclinical toxicology studies with zidovudine: Genetic toxicity tests and carcinogenicity bioassays in mice and rats. Fundam Appl Toxicol. 1996;32(2):148–158. doi: 10.1006/faat.1996.0118. [DOI] [PubMed] [Google Scholar]
- 85.Smith J, Rastogi R, Teller R, et al. 20th Conference on Retroviruses and Opportunistic Infections. Atlanta, Georgia: 2013. A tenofovir disoproxil fumarate intravaginal ring completely protects against repeated SHIV vaginal challenge in nonhuman primates. [Google Scholar]
- 86.Smith J, Rastogi R, Teller R, et al. 20th Conference on Retroviruses and Opportunistic Infections. Atlanta, GA: 2013. A novel intravaginal ring design releasing tenofovir delivers comparable tenofovir diphosphate levels in vaginal target cells in macaques to gel dosing. [Google Scholar]
- 87. ClinicalTrials.gov. Safety and pharmacokinetics (PK) of a polyurethane tenofovir disoproxil fumarate (TDF) vaginal ring (TDF IVR-001) [Updated 2013. Accessed December 11, 2013];2013 http://clinicaltrials.gov/ct2/show/NCT02006264?term=tenofovir+ring&rank=1.
- 88.Schader SM, Oliveira M, Ibanescu RI, Moisi D, Colby-Germinario SP, Wainberg MA. In vitro resistance profile of the candidate HIV-1 microbicide drug dapivirine. Antimicrob Agents Chemother. 2012;56(2):751–756. doi: 10.1128/AAC.05821-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woolfson AD, Umrethia ML, Kett VL, Malcolm RK. Freeze-dried, mucoadhesive system for vaginal delivery of the HIV microbicide, dapivirine: Optimisation by an artificial neural network. Int J Pharm. 2010;388(1–2):136–143. doi: 10.1016/j.ijpharm.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 90.Akil A, Parniak MA, Dezzuitti CS, et al. Development and characterization of a vaginal film containing dapivirine, a non- nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res. 2011;1(3):209–222. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malcolm RK, Woolfson AD, Toner CF, Morrow RJ, McCullagh SD. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. J Antimicrob Chemother. 2005;56(5):954–956. doi: 10.1093/jac/dki326. [DOI] [PubMed] [Google Scholar]
- 92.Nel A, Smythe S, Young K, et al. Safety and pharmacokinetics of dapivirine delivery from matrix and reservoir intravaginal rings to HIV-negative women. J Acquir Immune Defic Syndr. 2009;51(4):416–423. doi: 10.1097/qai.0b013e3181acb536. [DOI] [PubMed] [Google Scholar]
- 93.Romano J, Variano B, Coplan P, et al. Safety and availability of dapivirine (TMC120) delivered from an intravaginal ring. AIDS Res Hum Retroviruses. 2009;25(5):483–488. doi: 10.1089/aid.2008.0184. [DOI] [PubMed] [Google Scholar]
- 94.Nel A, Kamupira M, Woodsong C, et al. 19th Conference on Retrovirus and Opportunistic Infections. Seattle, WA: 2012. Safety, acceptability and pharmacokinetic assessment (adherence) of monthly dapivirine vaginal microbicide rings (ring-004) for HIV prevention. [Google Scholar]
- 95.van 't Klooster G, van der Geest R, Gille D. Pharmacokinetics, tolerability and safety of multiple oral doses of TMC120 (R147681) in 32 healthy subjects. [April 9, 2004];Tibotec Pharmaceuticals Ltd Trial No TIBO-0001-104. TMC120- C104-CRR. [Google Scholar]
- 96. ClinicalTrials.gov. Safety and efficacy trial of a dapivirine vaginal matrix ring in healthy HIV-negative women. [Updated 2013. Accessed January 5, 2014]; http://clinicaltrials.gov/ct2/show/NCT01539226?term=The+Ring+Study+AND+dapivirine&rank=12.
- 97. ClinicalTrials.gov. Phase 3 safety and effectiveness trial of dapivirine vaginal ring for prevention of HIV-1 in women (ASPIRE) [Updated 2012. Accessed January 10, 2014]; http://clinicaltrials.gov/ct2/show/NCT01617096?term=01617096&rank=1.
- 98. ClinicalTrials.gov. Safety and pharmacokinetics of dapirivine/maraviroc vaginal ring. [Updated 2013. Accessed December 11, 2013]; http://clinicaltrials.gov/ct2/show/NCT01363037?term=NCT01363037&rank=1.
- 99.Moss JA, Malone AM, Smith TJ, et al. Simultaneous delivery of tenofovir and acyclovir via an intravaginal ring. Antimicrob Agents Chemother. 2012;56(2):875–882. doi: 10.1128/AAC.05662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Straten A, Brown E, Marrazzo J, et al. 21st Conference on Retroviruses and Opportunistic Infections. Boston, MA: Oral presentation; 2014. Divergent adherence estimates with pharmacokinetic and behavioral measures in VOICE (MTN003) [Google Scholar]
- 101.Adams JL, Sykes C, Menezes P, et al. Tenofovir diphosphate and emtricitabine triphosphate concentrations in blood cells compared with isolated peripheral blood mononuclear cells: A new measure of antiretroviral adherence? J Acquir Immune Defic Syndr. 2013;62(3):260–266. doi: 10.1097/QAI.0b013e3182794723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu AY, Yang Q, Huang Y, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: Hair as a potential adherence measure for pre-exposure prophylaxis (PrEP) PLoS One. 2014;9(1):e83736. doi: 10.1371/journal.pone.0083736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kibengo FM, Ruzagira E, Katende D, et al. Safety, adherence and acceptability of intermittent tenofovir/emtricitabine as HIV pre-exposure prophylaxis (PrEP) among HIV-uninfected Ugandan volunteers living in HIV-serodiscordant relationships: A randomized, clinical trial. PLoS One. 2013;8(9):e74314. doi: 10.1371/journal.pone.0074314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bangsberg DR. PReP adherence measurements. [Updated 2011. Accessed March 5, 2014];Microbicide Trials Network (MTN) Web site. http://www.mtnstopshiv.org/sites/default/files/attachments/BangsbergREVISEDMicrobicideAdherenceMonitoring3-2011.pdf.
- 105.Microbicide Trials Network (MTN) Backgrounder ASPIRE: A study to prevent infection with a ring for extended use. [Updated 2013. Accessed March 11, 2014]; http://www.mtnstopshiv.org/news/studies/mtn020/backgrounder. [Google Scholar]
- 106.Microbicide Trials Network (MTN) Backgrounder MTN-017: Phase II safety and acceptability study of tenofovir gel reformulated for rectal use <br/>. [Updated 2013. Accessed March 11, 2014]; http://www.mtnstopshiv.org/news/studies/mtn017/backgrounder. [Google Scholar]
- 107.Hillier SL. Microbicides 2008 Conference. New Delhi, India: 2008. Safety and acceptability of coitally dependent use of 1% tenofovir over six months of use. Abstract No. 655. [Google Scholar]
- 108.FACTS Consortium. The follow-on african consortium for tenofovir studies (FACTS001) study. [Updated 2013. Accessed December 10, 2013]; http://www.facts-consortium.co.za/?page_id=83. [Google Scholar]
- 109.MTN020 Study Protocol. A multi-center, randomized, double-blind, placebo-controlled phase 3 safety and effectiveness trial of a vaginal matrix ring containing dapivirine for the prevention of HIV-1 infection in women. [Updated 2011. Accessed December 11, 2013]; http://www.mtnstopshiv.org/sites/default/files/attachments/MTN-020%20Version1%200_28September2011_CLEAN.pdf. [Google Scholar]