Abstract
Humans with stress-related anxiety disorders exhibit increases in arousal and alcohol drinking, as well as altered pain processing. Our lab has developed a predator odor stress model that produces reliable and lasting increases in alcohol drinking. Here, we utilize this predator odor stress model to examine stress-induced increases in arousal, nociceptive processing, and alcohol self-administration by rats, and also to determine the effects of corticotropin-releasing factor-1 receptors (CRF1Rs) in mediating these behavioral changes. In a series of separate experiments, rats were exposed to predator odor stress, then tested over subsequent days for thermal nociception in the Hargreaves test, acoustic startle reactivity, or operant alcohol self-administration. In each experiment, rats were systemically injected with R121919, a CRF1R antagonist, and/or vehicle. Predator odor stress increased thermal nociception (i.e., hyperalgesia) and acoustic startle reactivity. Systemic administration of R121919 reduced thermal nociception and hyperarousal in stressed rats but not unstressed controls, and reduced operant alcohol responding over days. Stressed rats exhibited increased sensitivity to the behavioral effects of R121919 in all three tests, suggesting up-regulation of brain CRF1Rs number and/or function in stressed rats. These results suggest that post-stress alcohol drinking may be driven by a high-nociception high-arousal state, and that brain CRF1R signaling mediates these stress effects.
Keywords: Stress, Predator Odor, Hargreaves, Operant, Self-administration, Escalation, Startle, Hyperalgesia, Hyperarousal, CRF, CRF1R, Alcohol Use Disorder
1. INTRODUCTION
Humans with stress-related anxiety disorders (e.g., post-traumatic stress disorder [PTSD]), exhibit increases in arousal and alcohol drinking, as well as altered pain processing (Engdahl et al., 1998; Norrholm et al., 2011; Roy et al., 2012; Sartor et al., 2010). In animals, a single intense stress or chronic stress produces hyperalgesia (Boccalon et al., 2006; da Silva Torres et al., 2003; Geerse et al., 2006; Quintero et al., 2000; Zhang et al., 2012), increases anxiety-like behaviour, and produces hyperarousal in rats (Campos et al., 2013; Kinn Rød et al., 2012; Serova et al., 2013). Alcohol dependence also promotes hyperarousal and hyperalgesia (i.e., increased nociception), each of which may promote compulsive alcohol drinking (Edwards et al., 2012; Egli et al., 2012; Koob, 1999). Our lab utilizes a predator odor stress model that produces lasting increases in alcohol consumption by rats (Edwards et al., 2013), allowing for the hypothesis that hyperarousal and hyperalgesia may be important motivational factors in stress-induced escalation of alcohol drinking.
CRF is important for emotional regulation (Heilig et al., 1994; Pisu et al., 2013; Regev et al., 2011; Regev et al., 2012) and is dysregulated in individuals with anxiety, depression, and drug abuse disorders (Arborelius et al., 1999; Bale and Vale, 2004; Koob, 2010; Risbrough, 2006). Exogenously administered CRF increases anxiety-like behaviour in rodents (Arborelius et al., 1999; Swerdlow et al., 1986a). Alcohol- and drug-dependent rats exhibit increases in anxiety-like behavior and escalated alcohol and drug self-administration thought to be driven by CRF hyper-function in the extended amygdala (e.g., George et al., 2007; Gilpin, 2012; Koob, 2008). Brain CRF signalling is also attributed a role in nociceptive processing (Greenwood-Van Meerveld et al., 2006; Lariviere and Melzack, 2000; McNally and Akil, 2002), and CRF-1 receptors (CRF1Rs) mediate increased nociception in rats exposed to stress or made dependent on drugs or alcohol (Edwards et al., 2012; Ji and Neugebauer, 2007, 2008).
Hyperarousal is a hallmark behavioural effect of stress and also of alcohol dependence (Chester et al., 2008; Chester et al., 2005; Davis et al., 2010; Koob, 1999). The acoustic startle response (ASR) is a reflexive reaction to an auditory stimulus that is mediated by the caudal pontine reticular nucleus (Koch, 1999). Importantly, descending projections from limbic regions (e.g., central amygdala and bed nucleus of stria terminalis) modulate the startle response and CRF signalling in these pathways is particularly important for mediating the startle response under various experimental parameters (Davis et al., 2010; Walker et al., 2009). For example, CRF enhances startle in rats (Liang et al., 1992) and antagonism of CRF1Rs attenuates CRF- and fear-potentiated ASR (Fujiwara et al., 2011; Risbrough et al., 2003; Swerdlow et al., 1986b; Walker et al., 2009).
Stress exposure is an important determinant for alcohol consumption in humans (Keyes et al., 2011). Various stressors increase alcohol consumption in rodent models (Chester et al., 2004; Croft et al., 2005; Logrip and Zorrilla, 2012; Lowery et al., 2008; Meyer et al., 2013), but these effects are transient, inconsistent across stressors and labs, and often highly dependent on experimental parameters (Becker et al., 2011). That said, stress and alcohol dependence each produce increases in extracellular CRF in the central amygdala (CeA), and CRF is a key modulator of behavioral and physiological responses to stress as well as alcohol-related behaviors (for reviews, see Gilpin, 2012; Koob, 2008). In fact, antagonism of CRF1Rs reduces escalated alcohol consumption by alcohol-dependent and stressed rodents (Funk et al., 2007; Lowery et al., 2008; Molander et al., 2012).
Our lab recently described a stress model in which predator odor exposure produces lasting (≥19 days) increases in alcohol self-administration (Edwards et al., 2013). The purpose of this study was to characterize arousal, nociception and alcohol self-administration in rats exposed to predator odor stress and to examine the role of CRF1Rs in those behavioral effects. Because CRF1Rs mediate arousal, nociception, and alcohol consumption, we hypothesized that peripheral administration of R121919, a selective CRF1R antagonist, would attenuate predator odor stress-induced increases in startle reactivity, thermal nociception, and alcohol self-administration.
2. MATERIALS AND METHODS
2.1 General Methods
2.1.1 Animals
Male Wistar rats (Charles River) weighing 200–300 g, and aged 6–8 weeks at start of experiment, were housed in groups of two in a humidity- and temperature-controlled (22°C) vivarium on a reverse 12-h light/dark cycle (lights off at 8 a.m.). Behavioral tests were conducted during the dark period. Animals had ad libitum access to food and water.
2.1.2 Drugs
The CRF1R antagonist R121919 (generously supplied by Neurocrine, Inc.) was solubilized first in 1M HCl (10% final volume), then diluted into 2-hydroxypropyl-β-cyclodextrin (HBC; Sigma-Aldrich, 20% wt/vol final concentration in distilled water) and back-titrated with NaOH to pH 4.5. In Experiments 2 and 3, rats were administered four R121919 doses (0, 5, 10, 20 mg/2 ml/kg s.c.) in a within-subject Latin-square design 60 minutes prior to behavioral tests. In Experiment 4, rats were repeatedly injected with a single dose of R121919 (10 mg/kg) or equivalent volume of vehicle 60 min prior to drinking sessions, as previously described by our group (Roberto et al., 2010).
2.1.3 Stress Exposure
Rats were transferred from the home cage to a clean cage and exposed to predator odor (bobcat urine; stressed) or ambient air (control) for a period of 15 minutes. Urine was added to a sponge that was placed beside the cage where stressed rats were not able to come in contact with the sponge or urine.
2.1.4 Hargreaves test procedure
Hind paws were individually stimulated from below using a halogen heat source from an IITC model 309 Hargreaves apparatus (IITC Life Sciences, Inc., Woodland Hills, CA). A 20-second cut-off was always employed to prevent tissue damage in non-responsive subjects, although preliminary experiments allowed selection of a light intensity that produced much shorter hind paw withdrawal latencies (~8 s; see Table 1). On test days, rats were placed in the examination room for 5 minutes to allow for acclimation to the light and testing environment and were then placed in Plexiglas® enclosures with glass floors suspended 30 cm from the table top and allowed to habituate for an additional 5 min prior to testing. On each test day, each hind paw was targeted twice (i.e. left paw, then right paw, 1 min break, then left and right paw again), producing 4 scores that were averaged into one score. The average latency to produce a nocifensive withdrawal response represented an index of thermal nociception (i.e., lower scores indicative hyperalgesia) that was analyzed as described below.
Table 1. Hargreaves Intensity Response and Baseline.
Table depicts the mean paw withdrawal latency of rats (n=21; top panel) during light intensity- response tests to determine appropriate light intensity for pharmacology tests using the Hargreaves test of thermal nociception. A separate group of rats (n=31; bottom panel) were tested for baseline nociception with and without vehicle injection 60 minutes prior to testing over four days. There was no effect of vehicle injection on paw withdrawal latencies in unstressed rats.
| Intensity Response | |||
| 25 AI | 50 AI | 75 AI | 99 AI |
| 18.77 ± 0.38 | 15.08 ± 0.43 | 8.79 ± 0.25 | 5.95 ± 0.21 |
| Baseline (75 AI) | |||
| No Injection | No Injection | Vehicle Injection | Vehicle Injection |
| 7.81 ± 0.30 | 8.18 ± 0.34 | 8.04 ± 0.24 | 8.20 ± 0.14 |
2.1.5 Acoustic startle response testing
Acoustic startle response (ASR) testing was conducted with a commercial startle reflex system (S-R Lab; San Diego Instruments, San Diego, CA). The sound-attenuated test chamber includes an exhaust fan, a sound source, and an internal light that is off during testing. Inside the test chamber, a single Plexiglas rodent cylinder (8.7 cm internal diameter) sits on a 12.5 × 25.5 cm Plexiglas stand. The acoustic startle response was transduced by a piezoelectric accelerometer mounted below the Plexiglas stand and then converted into arbitrary units by a personal computer program. Prior to testing, an S-R calibrator tube was used to calibrate the chambers. Each test session was preceded by a 5-min habituation period during which 70 dB of background white noise is present. This background white noise was present throughout the test session. The test session consists of 31 trials with startle stimuli of three different decibel levels. During each of the 31 trials, a 750-ms burst of 95 dB, 105 dB, or 115 dB white noise was presented. The startle response of the rat was recorded for each of the first 100 ms of each trial. The main dependent variable, average ASR, is an average of these 100 (one per ms) response outputs. The 95 dB and 115 dB stimuli were each presented 8 times and the 105 dB stimulus presented 9 times, each separated by a 30-s fixed intertrial interval.
2.1.6 Operant Alcohol Oral Self-Administration
Rats were trained to orally self-administer ethanol or water in a concurrent, two-lever, free-choice contingency that did not incorporate a sweet fading procedure. Prior to placement in operant boxes, rats were given a single 24-hr period of access to 10% w/v ethanol vs. water in the home cage. Intakes were not measured during this 24-hr home cage ethanol access, the purpose of which was to prevent neophobia upon presentation of ethanol in operant boxes. Rats were then given a single 15-hr operant session to learn to press a single lever for water (right lever; FR1) in the presence of ad libitum food on floor of operant chamber. Rats were then allowed one 3-hr two-lever operant session for 10% w/v ethanol (right lever; FR1) vs. water (left lever; FR-1), one 2-hr session, then one 1-hr session, followed by daily 30-min sessions on the two-lever contingency until reaching stable intake rates. All operant sessions after the initial 15-hr session occurred in the absence of food. Rats underwent daily 30-min sessions for ~15 days, at which point ethanol response rates were stable for individual rats across days.
2.2 Experimental Procedures
2.2.1 Experiment 1
One week after arrival into colony rooms, rats (n=21) were subjected to an intensity response curve to determine a light intensity that produces moderate hind paw withdrawal latencies. The selected intensity (75 A.I.) produced an average baseline threshold of approximately 8 seconds (see Table 1). Five days following predator odor stress, control (n=5) and stressed (n=16) rats were tested for stress-induced changes in hind paw withdrawal latency (i.e., thermal nociception) at light intensity = 75 A.I.
2.2.2 Experiment 2
One week after arrival into colony rooms, rats (n=31) were tested for baseline nociception. On days 1 and 2, animals were tested for hind paw withdrawal latency at light intensity = 75 A.I. On days 3 and 4, animals were injected with vehicle 60 minutes prior to testing (pre-treatment time based on (Funk et al., 2007)) and again tested for hind paw withdrawal latency at light intensity = 75 A.I. Injections did not affect hind paw withdrawal latency (see Table 1). Rats were divided into two groups counterbalanced for baseline withdrawal latency and five days later, approximately half of these rats (n=15) were exposed to predator odor, and the other half (n=16) were not. On days 2–5 post-stress (first test occurred 48 hrs post-stress), rats were injected with four doses of R121919 (0, 5, 10, and 20 mg/2 ml/kg. s.c.) in a within-subjects Latin-square design and tested 60 min later for hind paw withdrawal latency in the Hargreaves test at light intensity = 75 A.I.
2.2.3 Experiment 3
One week after arrival into colony rooms, rats were divided into two groups counterbalanced for body weight. Approximately half of these animals (n=8) were exposed to predator odor for 15 min while the other half (n=7) were not. At 24 and 48 hours following predator odor stress, rats were injected with two of four R121919 doses (0, 5, 10, and 20 mg/2 ml/kg. s.c.) and tested 60 min later for ASR. After 4 days of no testing or injections (i.e., one week following initial predator odor exposure), rats in the Stress group were re-exposed to predator odor for 15 min. At 24 and 48 hours following the second predator odor stress, rats in both groups were injected with the remaining two R121919 doses (0, 5, 10, and 20 mg/2 ml/kg. s.c.). Because preliminary data from our lab indicate that stress-induced startle differs in rats on day 1 vs. day 4 post-stress, Latin-square drug effects on startle were tested within 24–48 hours of two separate stress exposures (one week apart). Therefore, all drug doses were administered 60 min prior to ASR tests, and all ASR tests occurred 24–48 hours after first or second odor exposure.
2.2.4 Experiment 4
One week after arrival into colony rooms, rats (n=47) began training for operant alcohol self-administration. Once a stable baseline was achieved, animals pressing <10 presses/30 min session were excluded from the study (6 rats). Rats were then divided into two groups counterbalanced for baseline alcohol responding. One group (n=28) was exposed to predator odor for 15 min while the other group (n=13) was not. Each group was divided again into two groups counterbalanced for alcohol intake and two days after predator odor stress, rats were injected with either R121919 (10 mg/2 ml/kg s.c.; stress n=14, control n=7) or vehicle (2 ml/kg, s.c.; stress n=14, control n=6) and 60 minutes later allowed to self-administer alcohol in an operant session. R121919 injections and alcohol self-administration sessions were repeated on days 5, 8, 12, 15 and 19 post predator odor exposure. This R121919 dose was chosen based on data previously published by our group showing that repeated systemic injection of 10 mg/kg R121919 reduces ethanol self-administration in rats transitioning to ethanol dependence (see Figure 5A of Roberto et al., 2010).
2.2.5 Statistical Analysis
Experiment 1 Hargreaves data were analyzed using a t-test where stress history was the factor. Experiment 2 Hargreaves data were normalized to pre-stress baseline and analyzed using a two-way RM ANCOVA where R121919 treatment was the within-subjects factor, stress history was the between-subjects factor and order of R121919 dose administration was the covariate, in part to rule out a placebo effect after repeated drug injections. Experiment 3 ASR data were analyzed using a three-way (Stress history X R121919 dose X Decibel) RM ANCOVA with the order of R121919 dose administration as the covariate. Experiment 3 ASR data were also analyzed using two-way (stress history X R121919 dose) RM ANOVAs for ASR at each decibel level with 0 and 20 mg/kg doses. Experiment 4 self-administration data were analyzed using a three way (Stress history X R121919 dose X day) RM ANOVA. Experiment 4 self-administration data were also analyzed separately for control and predator odor stressed groups using two-way (R121919 dose X day) RM ANOVAs. Post hoc comparisons were conducted using Student-Newman-Keuls test and in all cases statistical significance was set at p<0.05.
3. RESULTS
3.1 Predator Odor Increases Thermal Nociception
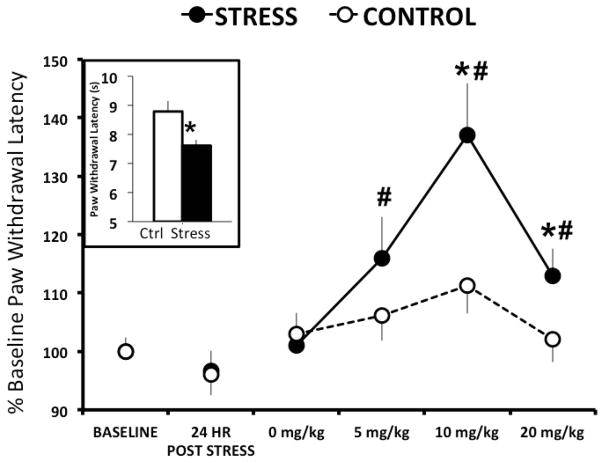
Five days following predator odor stress exposure, rats exposed to stress exhibited significantly lower nociceptive thresholds that controls (t[19]=3.023, P=0.007; Figure 1 inset). These results suggest that predator odor exposure increases thermal nociception in rats up to 5 days following odor exposure.
Figure 1. R121919 dose dependently increases paw withdrawal thresholds 2–5 days following predator odor stress.
Inset depicts mean ± SEM withdrawal latency of control (white; n=5) and predator odor stressed (black; n=16) rats 5 days following stress exposure. Line graph depicts mean ± SEM percent change from baseline paw withdrawal latency for predator odor stressed (solid circles; n=15) and control (open circles; n=16) rats 2–5 days following stress after four doses of systemic R121919. * denotes P<0.05 when stress compared to control. # denotes P<0.05 when dose is compared to vehicle.
3.2 CRF1R Antagonist Reduces Thermal Nociception Following Predator Odor Stress
In Experiment 2, stressed rats did not exhibit lower nociceptive thresholds than controls 24 hrs post-odor exposure (Figure 1), perhaps due to masking of this effect by stress associated with subcutaneous injections prior to Hargreaves testing. A two-way RM ANCOVA of normalized data yielded a significant effect of stress (F[1, 125]=7.158, P=0.011) and treatment (F[5, 125]=10.262, P<0.001; Figure 1) as well as an interaction effect (F[5,125]=2.769, P=0.21) on hind paw withdrawal latency 2–5 days following predator odor exposure. The higher nociceptive thresholds observed in stressed rats relative to controls was attributable to the large drug-induced increase in paw withdrawal latency since stressed rats exhibited reduced nociception at all R121919 doses, but did not differ from controls at the vehicle dose (see Figure 1). R121919 dose-dependently increased the threshold for thermal nociception: rats administered 10 mg/kg R121919 exhibited significantly longer paw withdrawal latencies relative to vehicle (P<0.001), an effect that was driven by increased paw withdrawal latencies in stressed animals. In fact, within the stressed rats, administration of 5 mg/kg, 10mg/kg, and 20mg/kg R121919 significantly increased paw withdrawal latencies from vehicle (P=0.024, P<0.001, and P=0.035, respectively). In contrast, there was no significant difference in paw withdrawal latencies with any drug dose administered to unstressed controls. Additionally, stressed animals exhibited significantly longer paw withdrawal latencies than controls following 10mg/kg and 20 mg/kg R121919 treatments (P<0.001 and P=0.021, respectively). There was no effect of dosing order on paw withdrawal latency.
3.3 CRF1R Antagonist Reduces Predator Stress-Induced Increases in Acoustic Startle Reactivity
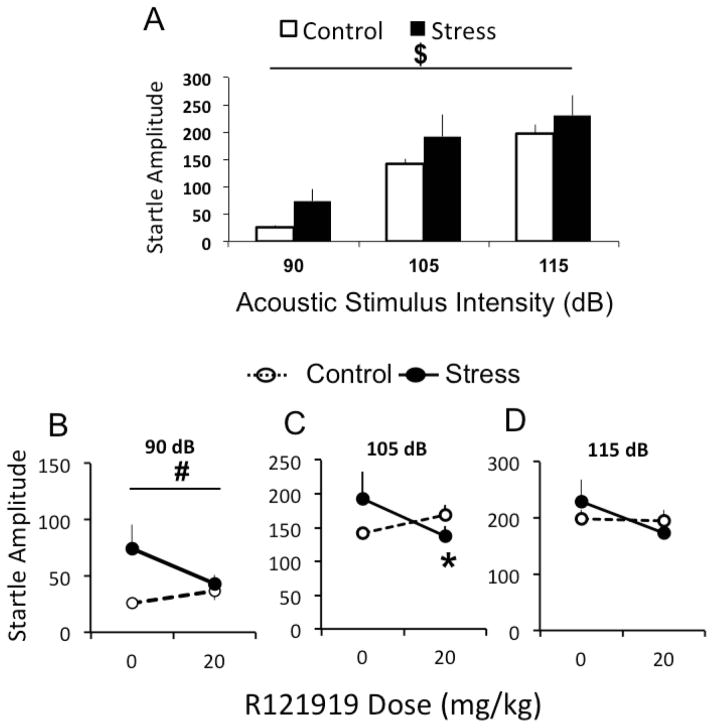
A three-way (stress history X dose R121919 X decibel level) RM ANCOVA yielded a significant effect of decibel level (F[2, 24]= 113.582, P<0.001) on ASR amplitude (Figure 2). Student-Newman-Keuls post hoc analysis revealed that higher decibels produced significantly greater ASR amplitudes, and amplitudes from all decibel levels were significantly different from each other (P<0.001 in all cases). This 3-way ANCOVA yielded a non-significant tendency toward a stress by dose interaction effect (P=0.086; Figure 2). To determine whether the highest R121919 dose affected ASR differently in stressed vs. control rats, ASR data for each decibel were analyzed with a two-way (stress history X R121919 dose) RM ANOVA comparing only 0 mg/kg and 20 mg/kg doses. At 90 dB, stressed rats exhibited significantly higher ASR amplitudes than control rats (F[1, 12]=4.782, P=0.049; Figure 3A) and there was a tendency towards a stress by dose interaction. At 105 dB, there was a significant stress by dose interaction effect (F[1, 12]=7.937, P=0.016) and Student-Newman-Keuls post hoc analysis revealed that R121919 significantly decreased ASR amplitude in stressed rats relative to vehicle (P=0.02) but had no effect in control rats (Figure 3B). At 115 dB, there was a non-significant tendency toward a dose effect and a stress by dose interaction effect (Figure 3C), likely due to a ceiling effect on the startle response at 115 dB. There was no effect of dosing order on ASR amplitude.
Figure 2. R121919 inhibits predator odor stress-induced increase in ASR amplitude 24–48 hours following stress.
Panel A depicts mean ± SEM startle amplitude in response to 90, 105 and 115 dB acoustic startle stimuli of control (white; n=8) and predator odor stressed (black; n=8) rats 24–48 hrs following stress. Panel B depicts mean ± SEM startle amplitude in response to 90, 105 and 115 dB acoustic startle stimuli of control (white; n=8) and predator odor stressed (black; n=8) rats 24–48 hrs following stress after systemic injection with 0 or 20 mg/kg R121919. $ denotes P<0.05 compared between intensities. # denotes P<0.05 main effect of stress. * denotes P<0.05 relative to control.
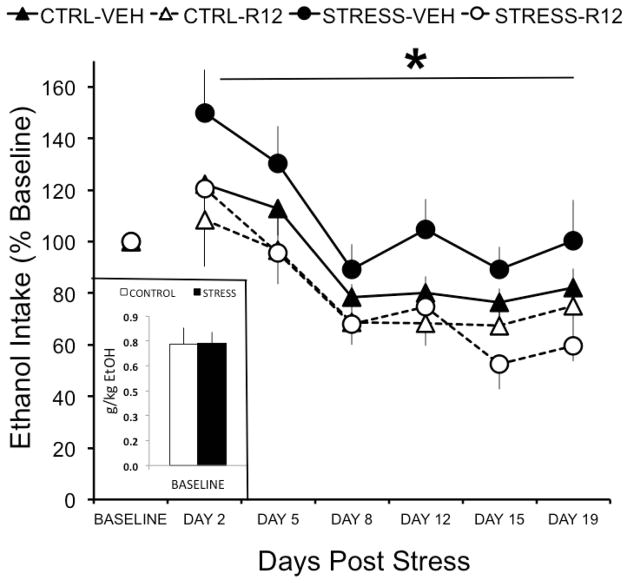
Figure 3. R121919 reduces alcohol self-administration following predator odor stress.
Inset depicts mean ± SEM baseline alcohol intake (g/kg) for predator odor stressed (black) and control (white) rats. Line graph depicts mean ± SEM percent change from baseline alcohol intake (g/kg) in predator odor exposed (circles) and control (triangles) rats chronically administered systemic R121919 (10 mg/kg; solid symbols; stress n=14, control n=7) or vehicle (open symbols; stress n=14, control n=6) on days 2, 5, 8, 12, 15, and 18 post stress. * denotes P<0.05 when drug is compare to vehicle in stressed animals.
3.4 CRF1R Antagonist Reduces Alcohol Self-Administration Following Predator Odor Stress
A three-way (Stress history X R121919 dose X day) RM ANOVA yielded a significant effect of drug (F[1, 32]= 6.249, P=0.018) on alcohol self-administration (Figure 3), but no main effect of stress and no interaction effect. Based on the results of Experiments 2 & 3, we hypothesized a priori that R121919 would more effectively reduce operant alcohol self-administration in stressed rats than in unstressed controls. Therefore, self-administration data for each stress group were analyzed with a two-way (Drug X Day) RM ANOVA. R121919 significantly reduced alcohol self-administration over days in stressed rats (F[1,23]=10.398, P=0.004), but not in unstressed controls (Figure 3). To assess whether time produced non-specific decreases in alcohol self-administration regardless of drug exposure, a t-test compared operant alcohol responding at baseline vs. day 19 in vehicle-injected unstressed controls and revealed no effect of day (p>0.05).
4. DISCUSSION
Here, we report that predator odor stress (Adamec et al., 2006, 2012; Campos et al., 2013; Cohen & Zohar, 2004) increases nociceptive processing and arousal in rats. A selective CRF1R antagonist reduced thermal nociception and hyperarousal following predator odor stress. CRF1R antagonism also reduced operant alcohol self-administration across groups, and this effect appeared to be driven by the effects of the drug in stressed animals. Importantly, following repeated drug administration, there were no placebo effects of vehicle injection on behavior, suggesting that behavioral effects of R121919 were attributable to its pharmacological actions at CRF1Rs.
Here, we report that predator odor stress produced hyperalgesia in rats, confirming previous reports of stress-induced hyperalgesia in humans (Imbe et al., 2006) and rodents (Imbe et al., 2010; Quintero et al., 2000), although stress-induced analgesia has also been reported (for review, see Butler and Finn, 2009). Interestingly, it has been suggested that the degree of control an animal exerts over stressful stimuli may be the major determinant in the effect of that stress on nociceptive processing (Vidal and Jacob, 1986). For example, rats subjected to inescapable stress exhibit analgesia whereas those subjected to the same escapable stress do not develop analgesia (Maier et al., 1982). Another potentially critical factor is the predictability of stress events, such that more predictability leads to stress-induced analgesia and less predictability leads to stress-induced hyperalgesia (Beecher, 1969; Vidal and Jacob, 1986).
Our findings suggest that stress-induced hyperalgesia is driven by increased CRF-CRF1R signaling, similar to the mechanical allodynia exhibited by alcohol- and heroin-dependent rats (Edwards et al., 2012). Systemic antagonism of CRF1Rs attenuates stress-induced hyperalgesia associated with inflammatory and neuropathic pain in mice (Hummel et al., 2010), and intra-CeA antagonism of CRF1Rs attenuates the visceromotor response to noxious levels of colorectal distension (Johnson et al., 2012). The CeA sends dense and highly organized projections to the periaqueductal gray (PAG; Oka et al., 2008; Rizvi et al., 1992) that co-localize CRF and substance P (Gray and Magnuson, 1992), and are important for gating the anti-nociceptive pain response mediated by opioids in the PAG (Oliveira and Prado, 2001; Xu et al., 2003). Data from fear conditioning studies show that the PAG and another amygdaloid nucleus (lateral amygdala) are each activated by a painful stimulus, and this activation is dampened by signals predictive of the painful stimulus (Johansen et al., 2010). Collectively, these findings support the notion that CRF1Rs in the amygdala, where CRF is abundantly expressed (Swanson et al., 1983), is critical for modulation of pain processing regardless of whether or not it is related to prior stress or drug dependence.
We report that predator odor produced hyperarousal 24–48 hrs post odor-exposure, in agreement with previous reports that predator stress produces lasting increases in startle reactivity in rats (Adamec et al., 2010) and mice (Clay et al., 2011). Other modalities of stress (e.g., footshock or restraint) also produce increases in arousal in rodents as measured by the acoustic startle test (Chester et al., 2008; Jiang et al., 2011; Rasmussen et al., 2008). Similar to our results, others have reported stress-induced increases in startle amplitude that last up to ten days post stress exposure (Jiang et al., 2011; Rasmussen et al., 2008).
In the current study, systemic administration of a CRF1R antagonist normalized startle amplitude in stressed animals, consistent with previous reports that CRF1R antagonism reduces startle reactivity in mice, while administration of CRF to CRF1R knockout mice does not affect startle amplitude (Risbrough et al., 2003). In rats, descending projections from limbic circuitry (e.g., CeA and bed nucleus of stria terminalis) to the caudal pontine reticular nucleus (Koch, 1999) modulate the startle response under specific testing conditions, and CRF signaling is thought to be particularly important in these descending projections (Davis et al., 2010; Walker et al., 2009). Because alcohol-dependent and alcohol-preferring rodents consume high quantities of alcohol (e.g., Bell et al., 2006; Gilpin et al., 2009) and exhibit increased startle reactivity (Chester et al., 2005; Chester et al., 2004), while acute alcohol reduces startle reactivity (Brunell and Spear, 2006; Pian et al., 2008), our results suggest that stress-induced escalation of alcohol drinking may be driven by the ability of alcohol to dampen stress-induced hyperarousal.
Previous results from our lab (Edwards et al., 2013) show that predator odor stress produces lasting increases in alcohol drinking by a subset of stressed rats. Those results contrast with a large literature that describes transient and inconsistent mean group effects of stress on alcohol conusmption by rodents (Becker et al., 2011). The critical distinction between studies that do and do not show stress-induced escalation of alcohol drinking may be the biological or behavioral identification of at-risk subpopulations of alcohol drinkers. Indeed, we previously showed robust and lasting escalation of operant alcohol responding in subsets of rats that exhibit high stress reactivity (Edwards et al., 2013). The present study failed to produce a significant main effect of stress on operant alcohol responding when data from all stressed rats were analyzed as a single group, although there did appear to be a trend toward increased alcohol responding by vehicle-injected stressed rats at days 2 and 5 post-stress (Figure 3).
Although predator odor stress did not produce increases in alcohol drinking in the current study, systemic administration of a CRF1 receptor antagonist significantly reduced alcohol drinking in stressed rats while having no effect in control rats. Systemic antagonism of CRF receptors attenuates both stress-induced reinstatement of extinguished alcohol-seeking behavior (Liu and Weiss, 2002), as well as stress-induced increases in anxiety-like behavior in post-dependent rats (Valdez et al., 2003). Stress and alcohol dependence each increase CRF expression and CRF signaling in the CeA (Merali et al., 1998; Merlo Pich et al., 1995; Roberto et al., 2010), and CRF-CRF1R signaling in the CeA is attributed a central role in driving escalated alcohol consumption (Koob, 2008). In fact, antagonism of CRF1Rs in the CeA (but not in other limbic regions) reduces escalated alcohol consumption by alcohol-dependent rats (Funk et al., 2007) and binge alcohol-drinking mice (Lowery-Gionta et al., 2012). Future studies will localize the effects of CRF1R antagonism on stress-induced escalation of alcohol drinking in this model.
Across multiple behavioral tests in Experiments 1–3, stressed rats exhibited heightened sensitivity to the behavioral effects of R121919, a CRF1R antagonist. This pattern of results suggests lasting up-regulation of CRF1R number and/or function in the brains of rats exposed to predator odor stress. This up-regulation of CRF/CRF1R signaling resembles that seen in the brains of alcohol-dependent rats (Koob, 2008), which is especially intriguing since withdrawal from chronic alcohol or high-dose bolus alcohol is characterized in part by increased startle reactivity (Chester et al., 2005; Chester et al., 2004) and increased nociceptive processing (Edwards et al., 2012). Furthermore, systemic administration of a CRF1R antagonist reduces allodynia in alcohol-dependent rats (Edwards et al., 2012). Upregulation of CRF/CRF1R signaling in alcohol-dependent rats is particularly pronounced in the central amygdala, and CRF signaling in this brain region is attributed a central role in mediating the negative affective state associated with alcohol withdrawal (Gilpin, 2012; Koob, 2008). Related to above discussions of the neurobiology of stress-induced hyperalgesia and hyperarousal, CRF-containing neurons project from CeA to both the PAG, important for descending control of pain processing (Helmstetter et al., 1998; Oliveira and Prado, 2001), and also to the pons circuitry responsible for mediating the acoustic startle response (Davis et al., 2010; Gray and Magnuson, 1992; Walker et al., 2009; Wallace et al., 1992). Collectively, these results allow for the hypothesis that stress-induced upregulation of CRF/CRF1R signaling in CeA produces a negative affective state via descending projections to effector regions (e.g., pontine nucleus; PAG), and that this negative affective state drives subsequent escalation of alcohol drinking in these animals.
In summary, the present study reports that predator stress increases nociceptive processing and acoustic startle reactivity. Thermal nociception and hyperarousal were each attenuated in stressed rats by CRF1R blockade with systemically administered R121919. Additionally, chronic administration of R121919 reduced alcohol self-administration following exposure to predator odor stress. Future studies will localize the effects of CRF1R antagonism on post-stress alcohol self-administration, arousal, and nociception, and will characterize the role of CRF1Rs in these behaviors in animals that exhibit high vs. low reactivity to predator odor stress, as previously described by our lab.
Highlights.
Predator odor stress increases thermal nociception and startle reactivity in rats
CRF1 receptor antagonism reduces thermal nociception in stressed rats
CRF1 receptor antagonism reverses stress-induced hyperarousal
CRF1 receptor antagonism reduces alcohol drinking in stressed rats
Stressed rats are more sensitive to the behavioral effects of a CRF1R antagonist
Acknowledgments
This work was funded by NIH grant AA018400 (NWG) and by LSUHSC Start-Up funds (NWG), as well as T32 training grant AA007577.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Emily A. Roltsch, Email: eroltsch@lsuhsc.edu.
Brittni B. Baynes, Email: bbayne@lsuhsc.edu.
Jacques P. Mayeux, Email: jmaye2@lsuhsc.edu.
Annie M. Whitaker, Email: awhita@lsuhsc.edu.
Brandon A. Baiamonte, Email: bbaiam@lsuhsc.edu.
Nicholas W. Gilpin, Email: ngilpi@lsuhsc.edu.
References
- Adamec R, Fougere D, Risbrough V. CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. Int J Neuropsychopharmacol. 2010;13:747–757. doi: 10.1017/S1461145709990496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec R, Strasser K, Blundell J, Burton P, McKay DW. Protein synthesis and the mechanisms of lasting change in anxiety induced by severe stress. Behav Brain Res. 2006;167:270–286. doi: 10.1016/j.bbr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Adamec R, Toth M, Haller J, Halasz J, Blundell J. A comparison of activation patterns of cells in selected prefrontal cortical and amygdala areas of rats which are more or less anxious in response to predator exposure or submersion stress. Physiol Behav. 2012;105:628–638. doi: 10.1016/j.physbeh.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens M, Plotsky P, Nemeroff C. The role of corticotropin-releasing factor in depression and anxiety disorders. 1999 doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher HK. Anxiety and pain. JAMA. 1969;209:1080. [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Boccalon S, Scaggiante B, Perissin L. Anxiety stress and nociceptive responses in mice. Life Sci. 2006;78:1225–1230. doi: 10.1016/j.lfs.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effects of acute ethanol or amphetamine administration on the acoustic startle response and prepulse inhibition in adolescent and adult rats. Psychopharmacology (Berl) 2006;186:579–586. doi: 10.1007/s00213-006-0380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RK, Finn DP. Stress-induced analgesia. Prog Neurobiol. 2009;88:184–202. doi: 10.1016/j.pneurobio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Campos AC, Ferreira FR, da Silva WA, Guimarães FS. Predator threat stress promotes long lasting anxiety-like behaviors and modulates synaptophysin and CB1 receptors expression in brain areas associated with PTSD symptoms. Neurosci Lett. 2013;533:34–38. doi: 10.1016/j.neulet.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Chester JA, Barrenha GD, Hughes ML, Keuneke KJ. Age- and sex-dependent effects of footshock stress on subsequent alcohol drinking and acoustic startle behavior in mice selectively bred for high-alcohol preference. Alcohol Clin Exp Res. 2008;32:1782–1794. doi: 10.1111/j.1530-0277.2008.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Effects of chronic alcohol treatment on acoustic startle reactivity during withdrawal and subsequent alcohol intake in high and low alcohol drinking rats. Alcohol Alcohol. 2005;40:379–387. doi: 10.1093/alcalc/agh172. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res. 2004;28:385–393. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Clay R, Hebert M, Gill G, Stapleton LA, Pridham A, Coady M, Bishop J, Adamec RE, Blundell JJ. Glucocorticoids are required for extinction of predator stress-induced hyperarousal. Neurobiol Learn Mem. 2011;96:367–377. doi: 10.1016/j.nlm.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J. An animal model of posttraumatic stress disorder: the use of cut-off behavioral criteria. Ann N Y Acad Sci. 2004;1032:167–178. doi: 10.1196/annals.1314.014. [DOI] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- da Silva Torres IL, Cucco SN, Bassani M, Duarte MS, Silveira PP, Vasconcellos AP, Tabajara AS, Dantas G, Fontella FU, Dalmaz C, Ferreira MB. Long-lasting delayed hyperalgesia after chronic restraint stress in rats-effect of morphine administration. Neurosci Res. 2003;45:277–283. doi: 10.1016/s0168-0102(02)00232-8. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF1 receptor antagonism. Neuropharmacology. 2012;62:1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl B, Dikel TN, Eberly R, Blank A. Comorbidity and course of psychiatric disorders in a community sample of former prisoners of war. Am J Psychiatry. 1998;155:1740–1745. doi: 10.1176/ajp.155.12.1740. [DOI] [PubMed] [Google Scholar]
- Fujiwara K, Asakura M, Yanagida T, Nakano M, Kanai S, Tanaka D, Sasuga Y, Osada K. The delayed sensitization of CRH response developed after chronic variable stress on the acoustic startle reflex. Nihon Shinkei Seishin Yakurigaku Zasshi. 2011;31:17–22. [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerse GJ, van Gurp LC, Wiegant VM, Stam R. Individual reactivity to the open-field predicts the expression of cardiovascular and behavioural sensitisation to novel stress. Behav Brain Res. 2006;175:9–17. doi: 10.1016/j.bbr.2006.07.011. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol. 2012;46:329–337. doi: 10.1016/j.alcohol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN. Operant behavior and alcohol levels in blood and brain of alcohol-dependent rats. Alcohol Clin Exp Res. 2009;33:2113–2123. doi: 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992;13:451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Johnson AC, Schulkin J, Myers DA. Long-term expression of corticotropin-releasing factor (CRF) in the paraventricular nucleus of the hypothalamus in response to an acute colonic inflammation. Brain Res. 2006;1071:91–96. doi: 10.1016/j.brainres.2005.11.071. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Tershner SA, Poore LH, Bellgowan PS. Antinociception following opioid stimulation of the basolateral amygdala is expressed through the periaqueductal gray and rostral ventromedial medulla. Brain Res. 1998;779:104–118. doi: 10.1016/s0006-8993(97)01104-9. [DOI] [PubMed] [Google Scholar]
- Hummel M, Cummons T, Lu P, Mark L, Harrison JE, Kennedy JD, Whiteside GT. Pain is a salient “stressor” that is mediated by corticotropin-releasing factor-1 receptors. Neuropharmacology. 2010;59:160–166. doi: 10.1016/j.neuropharm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Imbe H, Iwai-Liao Y, Senba E. Stress-induced hyperalgesia: animal models and putative mechanisms. Front Biosci. 2006;11:2179–2192. doi: 10.2741/1960. [DOI] [PubMed] [Google Scholar]
- Imbe H, Okamoto K, Donishi T, Senba E, Kimura A. Involvement of descending facilitation from the rostral ventromedial medulla in the enhancement of formalin-evoked nocifensive behavior following repeated forced swim stress. Brain Res. 2010;1329:103–112. doi: 10.1016/j.brainres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J Neurophysiol. 2007;97:3893–3904. doi: 10.1152/jn.00135.2007. [DOI] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. J Neurophysiol. 2008;99:1201–1212. doi: 10.1152/jn.01148.2007. [DOI] [PubMed] [Google Scholar]
- Jiang X, Zhang ZJ, Zhang S, Gamble EH, Jia M, Ursano RJ, Li H. 5-HT2A receptor antagonism by MDL 11,939 during inescapable stress prevents subsequent exaggeration of acoustic startle response and reduced body weight in rats. J Psychopharmacol. 2011;25:289–297. doi: 10.1177/0269881109106911. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci. 2010;13:979–986. doi: 10.1038/nn.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AC, Tran L, Schulkin J, Greenwood-Van Meerveld B. Importance of stress receptor-mediated mechanisms in the amygdala on visceral pain perception in an intrinsically anxious rat. Neurogastroenterol Motil. 2012;24:479–486. e219. doi: 10.1111/j.1365-2982.2012.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Hasin DS. Stressful life experiences, alcohol consumption, and alcohol use disorders: the epidemiologic evidence for four main types of stressors. Psychopharmacology (Berl) 2011;218:1–17. doi: 10.1007/s00213-011-2236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinn Rød AM, Milde AM, Grønli J, Jellestad FK, Sundberg H, Murison R. Long-term effects of footshock and social defeat on anxiety-like behaviours in rats: relationships to pre-stressor plasma corticosterone concentration. Stress. 2012;15:658–670. doi: 10.3109/10253890.2012.663836. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere WR, Melzack R. The role of corticotropin-releasing factor in pain and analgesia. Pain. 2000;84:1–12. doi: 10.1016/S0304-3959(99)00193-1. [DOI] [PubMed] [Google Scholar]
- Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992;12:2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP. Stress history increases alcohol intake in relapse: relation to phosphodiesterase 10A. Addict Biol. 2012;17:920–933. doi: 10.1111/j.1369-1600.2012.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Sparrow AM, Breese GR, Knapp DJ, Thiele TE. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 2008;32:240–248. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Drugan RC, Grau JW. Controllability, coping behavior, and stress-induced analgesia in the rat. Pain. 1982;12:47–56. doi: 10.1016/0304-3959(82)90169-5. [DOI] [PubMed] [Google Scholar]
- McNally GP, Akil H. Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neuroscience. 2002;112:605–617. doi: 10.1016/s0306-4522(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I. Stress Increases Voluntary Alcohol Intake, but Does not Alter Established Drinking Habits in a Rat Model of Posttraumatic Stress Disorder. Alcohol Clin Exp Res. 2013;37:566–574. doi: 10.1111/acer.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R. Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology. 2012;37:1047–1056. doi: 10.1038/npp.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Tsumori T, Yokota S, Yasui Y. Neuroanatomical and neurochemical organization of projections from the central amygdaloid nucleus to the nucleus retroambiguus via the periaqueductal gray in the rat. Neurosci Res. 2008;62:286–298. doi: 10.1016/j.neures.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Oliveira MA, Prado WA. Role of PAG in the antinociception evoked from the medial or central amygdala in rats. Brain Res Bull. 2001;54:55–63. doi: 10.1016/s0361-9230(00)00420-2. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Ehlers CL. Differential effects of acute alcohol on prepulse inhibition and event-related potentials in adolescent and adult Wistar rats. Alcohol Clin Exp Res. 2008;32:2062–2073. doi: 10.1111/j.1530-0277.2008.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisu MG, Garau A, Olla P, Biggio F, Utzeri C, Dore R, Serra M. Altered stress responsiveness and hypothalamic-pituitary-adrenal axis function in male rat offspring of socially isolated parents. J Neurochem. 2013 doi: 10.1111/jnc.12273. [DOI] [PubMed] [Google Scholar]
- Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H. Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacol Biochem Behav. 2000;67:449–458. doi: 10.1016/s0091-3057(00)00374-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Crites NJ, Burke BL. Acoustic startle amplitude predicts vulnerability to develop post-traumatic stress hyper-responsivity and associated plasma corticosterone changes in rats. Psychoneuroendocrinology. 2008;33:282–291. doi: 10.1016/j.psyneuen.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, Chen A. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2011;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- Regev L, Tsoory M, Gil S, Chen A. Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry. 2012;71:317–326. doi: 10.1016/j.biopsych.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Risbrough V. Role of corticotropin releasing factor in anxiety disorders: A translational research perspective. 2006;50:550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology (Berl) 2003;170:178–187. doi: 10.1007/s00213-003-1535-6. [DOI] [PubMed] [Google Scholar]
- Rizvi TA, Ennis M, Shipley MT. Reciprocal connections between the medial preoptic area and the midbrain periaqueductal gray in rat: a WGA-HRP and PHA-L study. J Comp Neurol. 1992;315:1–15. doi: 10.1002/cne.903150102. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MJ, Costanzo M, Leaman S. Psychophysiologic identification of subthreshold PTSD in combat veterans. Stud Health Technol Inform. 2012;181:149–155. [PubMed] [Google Scholar]
- Sartor CE, McCutcheon VV, Pommer NE, Nelson EC, Duncan AE, Waldron M, Bucholz KK, Madden PA, Heath AC. Posttraumatic stress disorder and alcohol dependence in young women. J Stud Alcohol Drugs. 2010;71:810–818. doi: 10.15288/jsad.2010.71.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by. Psychopharmacology (Berl) 1986a;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology (Berl) 1986b;88:147–152. doi: 10.1007/BF00652231. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vidal C, Jacob J. Hyperalgesia induced by emotional stress in the rat: an experimental animal model of human anxiogenic hyperalgesia. Ann N Y Acad Sci. 1986;467:73–81. doi: 10.1111/j.1749-6632.1986.tb14619.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DM, Magnuson DJ, Gray TS. Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic, and adrenergic cell groups in the rat. Brain Res Bull. 1992;28:447–454. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- Xu W, Lundeberg T, Wang YT, Li Y, Yu LC. Antinociceptive effect of calcitonin gene-related peptide in the central nucleus of amygdala: activating opioid receptors through amygdala-periaqueductal gray pathway. Neuroscience. 2003;118:1015–1022. doi: 10.1016/s0306-4522(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gandhi PR, Standifer KM. Increased nociceptive sensitivity and nociceptin/orphanin FQ levels in a rat model of PTSD. Mol Pain. 2012;8:76. doi: 10.1186/1744-8069-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]