Abstract
Background
Cigarette smoking in adults is associated with abnormalities in brain neurobiology. Smoking-induced central nervous system oxidative stress (OxS) is a potential mechanism associated with these abnormalities. The goal of this study was to compare cognitively-normal elders on cerebrospinal fluid (CSF) levels of F2-isoprostane biomarkers of OxS.
Methods
Elders with a lifetime history of smoking (smokers; n=50; 75±5 years of age; 34±28 pack-years; approximately 12% were actively smoking at the time of study) were compared to never-smokers (n=61; 76±6 years of age) on CSF iPF2α-III and 8,12, iso-iPF2α-VI F2-isoprostanes levels. F2-isoprostanes levels were quantitated with HPLC-atmospheric pressure chemical ionization-tandem mass spectrometry. Associations between F2-isoprostanes levels, hippocampal volumes, and cigarette exposure measures were also evaluated.
Results
Smokers showed higher iPF2α-III level than never-smokers. An age x smoking status interaction was observed for 8,12, iso-iPF2α-VI, where smokers demonstrate a significantly greater concentration with increasing age than non-smokers. In smokers only, higher 8,12, iso-iPF2α-VI concentration was associated with smaller hippocampal volume, and greater iPF2α-III level was related to greater pack years.
Conclusions
This is the first study to demonstrate that a history of cigarette smoking in cognitively-normal elders was associated with significantly elevated CSF F2-isoprostane levels and greater age-related increases in F2-isoprostane levels, and that higher F2-isoprostane levels in smokers were related to smaller hippocampal volume. These findings provide additional novel evidence that a history of chronic smoking during adulthood is associated with adverse effects on the human brain that are potentially persistent even with extended smoking cessation.
Keywords: cigarette smoking, oxidative stress, isoprostanes, hippocampus, cerebrospinal fluid, elder adults
1. INTRODUCTION
It is now apparent that cigarette smoking-related morbidity extends well beyond cardiovascular disease, chronic obstructive pulmonary diseases, and various cancers, and includes neurobiological and neurocognitive abnormalities, some of which are progressive over time, and are not directly attributable to the foregoing biomedical conditions (Azizian et al., 2009; Durazzo et al., 2010; Sharma and Brody, 2009; Swan and Lessov-Schlaggar, 2007). Specifically, chronic cigarette smoking in young-to-elder adults, without a history of clinically significant biomedical or psychiatric disorders, is associated with abnormalities in brain morphology, biochemistry, microstructural integrity, and neurocognition (Durazzo et al., 2012, 2013; Kuhn et al., 2012, 2010; Sabia et al., 2012; Wagner et al., 2013); see Durazzo et al., 2010 for review). Additionally, former-smokers show neurobiological and neurocognitive abnormalities that are intermediate to active-smokers and never-smokers (Durazzo et al., 2010). Smoking is also associated with significantly increased risk factor for Alzheimer’s disease (AD) and other diseases that promote dementia (Cataldo et al., 2010; Giunta et al., 2012; Peters et al., 2008). Cigarette smoke is a complex mixture of approximately 5000 combustion products that contains a high number of toxic and carcinogenic compounds (Talhout et al., 2011). Chronic cerebral oxidative stress (OxS) is induced by cigarette smoking, and OxS has been suggested as a mechanism promoting the neurobiological and neurocognitive deficits observed in smokers (Durazzo and Meyerhoff, 2007; Fowles et al., 2000; Haustein, 1999; Swan and Lessov-Schlaggar, 2007; Yang and Liu, 2003). Cerebral OxS is operationalized as the detection of damage to brain tissue (e.g., lipid peroxidation, proteolysis) that is caused by reactive oxygen species (ROS), or more broadly by damage from ROS, reactive nitrogen species (RNS), and other oxidizing agents (Seet et al., 2011; Sutherland et al., 2013; Wang and Michaelis, 2010). Increased levels of free radicals and oxidants results from an imbalance between the generation of these compounds by endogenous (i.e., normal cellular metabolism) and/or exogenous sources (e.g., smoking), and their chemical reduction by antioxidants/radical scavengers (Schulz et al., 2000; Valavanidis et al., 2009; Wang and Michaelis, 2010). Cigarette smoke contains extremely high concentrations of short-and-long-lived free radicals (Ambrose and Barua, 2004; Valavanidis et al., 2009), and smoking inhibits synthesis of essential endogenous intracellular anti-oxidants, such as glutathione (Bloomer, 2007; Moriarty et al., 2003). The brain is highly susceptible to OxS damage due to its high metabolism and susceptibility of membrane phospholipids to radical attack (Anbarasi et al., 2006; Chalela et al., 2001; Kovacic, 2005; Mueller et al., 2001), and the hippocampi are particularly vulnerable to OxS (Wang and Michaelis, 2010). Central nervous system (CNS) OxS may trigger inflammation via increased proinflammatory cytokine release (Voloboueva and Giffard, 2011), which, in turn, amplifies OxS through generation of additional ROS and other inflammatory mediators (Crews et al., 2006; Guerri and Pascual, 2010; Perricone et al., 2009). Correspondingly, CNS OxS and inflammation tend to occur in tandem in many diseases/disorders, including AD, atherosclerosis, alcohol/substance use disorders, and cigarette smoking (Butterfield et al., 2013; Crews and Nixon, 2009; Durazzo et al., 2010; Enciu et al., 2013; Gill et al., 2010; Khandelwal et al., 2011; Khanna et al., 2013). Animal models and human post-mortem studies indicate that cigarette smoking-induced OxS promotes brain tissue damage via lipid peroxidation and proteolysis (Anbarasi et al., 2005, 2006; Ho et al., 2012; Khanna et al., 2013; Rueff-Barroso et al., 2010; Sonnen et al., 2009; Tyas et al., 2003). Since smoking is a risk factor for AD, it is possible that smoking-induced OxS increases the risk for AD-like neuropathological changes; however, there are no published in vivo studies that have specifically compared human smokers and non-smokers on CNS-derived biomarkers of cerebral OxS.
The goal of this study was to compare cognitively-normal elders with a history of cigarette smoking during lifetime (smokers) to never-smokers on cerebrospinal fluid (CSF) levels of F2-isoprostanes, and test the associations between these biomarkers of OxS and hippocampal volume, a structure that typically shows marked atrophy in those with mild cognitive impairment and AD (Jack et al., 2013). F2-isoprostanes are prostaglandin-like compounds formed from free radical-mediated peroxidation of arachidonic acid, a highly abundant polyunsaturated fatty acid in the brain (Korecka et al., 2010; Milne et al., 2005). F2-isoprostanes are established biomarkers of radical-induced OxS (Rokach et al., 2004) that have been employed to assess OxS-related tissue damage in neurodegenerative diseases, atherosclerosis, pulmonary diseases, and chronic smoking (Galasko and Montine, 2010; Korecka et al., 2010; Milne et al., 2005; Pratico, 2008, 2010; Yao et al., 2003), and levels increase with advancing age (Montine et al., 2011). In this report we adhere to the F2-isoprostane nomenclature proposed by Rokach and colleagues (1997). iPF2α-III (alternate nomenclatures: 8-iso-PGF2α; 15-F2t-IsoP) and 8,12, iso-iPF2α-VI (alternate nomenclature: 5-F2c-IsoP) are among the most studied of the F2-isoprostanes, with the majority of research focused on iPF2α-III (Cracowski et al., 2002; Haschke et al., 2007; Korecka et al., 2010). In active and former adult smokers, urine and plasma iPF2α-III levels were significantly elevated relative to never-smokers (Harman et al., 2003; Helmersson et al., 2005; Milne et al., 2005; Yan et al., 2007). CSF F2-isoprostane levels may more accurately characterize lipid peroxidation of brain tissue than those obtained from peripheral levels because blood and urine-based F2-isoprostanes concentrations will also reflect radical-mediated peroxidation of plasma lipids and tissue comprising peripheral organ systems (Milne et al., 2005; Montine et al., 2011). We posit that a history of cigarette smoking serves as a source of chronic OxS that places a significant burden on the integrity of brain morphology, which is exacerbated by advancing age. Accordingly, we tested the following hypotheses:
Smokers demonstrate significantly higher CSF iPF2α-III and 8,12, iso-iPF2α-VI and (iso-iPF2α-VI) concentrations than never-smokers, and a smoking status (smoker vs. non-smoker) x age interaction is observed, where smokers show significantly higher iPF2α-III and iso-iPF2α-VI levels with increasing age than never-smokers.
In smokers and never-smokers, higher CSF iPF2α-III and iso-iPF2α-VI concentrations are associated with smaller hippocampal volumes.
In smokers, higher cigarette pack-years are related to higher CSF iPF2α-III and iso-iPF2α-VI concentrations.
2. METHODS
2.1. Participants and study design
Participants were 111 cognitively-normal elder controls (75.5 ± 5.1 years of age) from Alzheimer’s disease Neuroimaging Initiative (ADNI) project, Phase 1. Phase 1 of ADNI (ADNI1) was a multisite study supported by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the FDA, private pharmaceutical companies, and non-profit organizations, as a 5-year public-private partnership. The primary goal of ADNI1 was to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biomarkers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD (Mueller et al., 2005a, 2005b). Written informed consent was obtained from all participants before procedures were performed. The study was conducted according to the Declaration of Helsinki, and U.S. 21 CFR Part 50 – Protection of Human Subjects, and Part 56 – Institutional Review Boards.
Participants who reported they never smoked cigarettes during lifetime were assigned to the non-smoker group (n = 61), and those who reported any history of cigarette smoking during lifetime were designated as smokers (n = 50). Twenty-five of 50 smokers had sufficiently detailed smoking history information to calculate pack-years (34 ± 28); three participants were actively smoking at the time of study, and 22 were former-smokers with 35 ± 14 years of smoking cessation. Antioxidant (vitamin E), anti-hypertensive, and statin/cholesterol absorption blocking agents (statin/CAB) usage was recorded (binary variables – yes, no) and body mass index (BMI) calculated for all participants. See Table 1 for group demographic and clinical information.
Table 1.
Participant demographics and clinical variables
| Variable | Never-smokers (n = 61) | Smokers (n = 50) |
|---|---|---|
| Age | 76.1 ± 5.5 | 74.9 ± 4.7 |
| Education | 15.9 ± 2.9 | 15.5 ± 2.8 |
| Mini Mental Status Exam (total score) |
29.3 ± 0.8 | 28.9 ± 1.2 |
| Caucasian (%) | 95 | 98 |
| Male (%)a | 40 | 66 |
| Pack-years | NA | 34 ± 28 |
| APOE ε4 carriers (%) | 25 | 22 |
| Body mass indexb | 25.7 ± 4.1 | 27.6 ± 4.7 |
| Statin/cholesterol absorption inhibitor use (%) |
38 | 42 |
| Vitamin E use (%) | 22 | 22 |
| Antihypertensive use (%) | 43 | 48 |
| Cholesterol (mg/dl) | 199 ± 36.2 | 187 ± 41.1 |
| Triglycerides (mg/dl) | 134 ± 90.2 | 149 ± 95.5 |
| Hippocampal volume (cm3)c |
7.35 ± 0.77 | 7.23 ± 0.78 |
| White matter hyperintensity volume (cm3)c |
0.94 ± 2.3 | 0.54 ± 0.90 |
Note:
never-smokers < smokers (p < .05);
volumes adjusted for intracranial volume;
NA = not applicable.
2.2. CSF iPF2α-III and iso-iPF2α-VI acquisition and quantitation
ADNI procedures for CSF sampling via lumbar puncture, transport, and storage were previously described in detail (Shaw et al., 2009). Quantitation of iPF2α-III and iso-iPF2α-VI was achieved with a HPLC-atmospheric pressure chemical ionization-tandem mass spectrometry method that demonstrates high sensitivity and selectivity for these F2-isoprostanes (Korecka et al., 2010).
2.3. MRI image acquisition and processing
Participants completed a 1.5 Tesla magnetic resonance scan. T1-weighted MRI scans using 3D volumetric magnetization prepared rapid gradient echo (MPRAGE) and 3D T2-weighted sequences were acquired for morphological analyses (see Jack et al., 2008 for acquisition parameters). All images were calibrated with phantom-based geometric corrections to ensure consistency among different study sites (Gunter et al., 2006). The publicly available Freesurfer (v4.5) volumetric segmentation and cortical surface reconstruction methods (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2004, 1999) were used to obtain regional cortical and subcortical brain volumes (mm3) from T1-weighted images, including the hippocampus. Volumes for left and right hippocampi were summed because we had no a priori predictions about laterality for associations between iPF2α-III and iso-iPF2α-VI concentrations and hippocampal volumes. Total white matter (WM) hyperintensity volume was calculated from T2-weighted images (Haight et al., 2013).
2.4. Statistical Analyses
2.4.1 Primary analyses
Comparisons of smokers and never-smokers on demographic and clinical variables were conducted with independent sample t-tests and Fisher’s exact tests where appropriate. Group comparisons on iPF2α-III and iso-iPF2α-VI concentrations were conducted with generalized linear modeling (GENLIN). Age, sex, vitamin E and statin/CAB use, BMI, smoking status (smoker vs. non-smoker), and smoking status x age interaction were predictors. BMI, vitamin E use, and statin/CAB use were used as predictors because they are associated with F2-isoprostanes levels (Cracowski et al., 2002; Milne et al., 2005; Pratico, 2008). Follow-up t-tests (two-tailed) comparing smokers to never-smokers (controlled for age, sex, BMI, and vitamin E and statin/CAB use) were conducted when smoking status was a significant predictor of isoprostane concentration; effect sizes for mean F2-isoprostane differences between smokers and never-smokers were calculated with Cohen’s d. Associations between iPF2α-III and iso-iPF2α-VI concentrations, hippocampal volumes were examined with linear regression (semi-partial coefficients are reported) in the combined sample (i.e., smokers + never-smokers) and separately for each group. Relationships between smoking exposure measures (pack-years and number of years of smoking cessation for former-smokers) and isoprostane concentrations in smokers were also assessed with linear regression. Covariates for these analyses were age, sex, and vitamin E and statin/CAB use. Intracranial volume and APOE ε4 (APOE4) carrier status were also included as a covariate for analyses involving the hippocampus; APOE genotype was previously related to hippocampal volume (Chiang et al., 2010; O’Dwyer et al., 2012). P-values < .05 were considered statistically significant in all analyses.
2.4.2. Secondary analyses
Some investigations of OxS in neurodegenerative diseases and aging employed F2-isoprostanes measures that represent the concentrations of multiple F2-isoprostanes or a composite of all 64 constitutional isomers (see Galasko and Montine, 2010; Milne et al., 2005; Montine et al., 2011). In order to determine if a composite isoprostane measure could be used to characterize the level of OxS in smokers and non-smokers, iPF2α-III and iso-iPF2α-VI concentrations were standardized to never-smokers to form z-scores, and the z-scores were then summed for each F2-isoprostane for all participants to form a single measure. We employed standardized scores to form a single isoprostane measure because the concentration of iso-iPF2α-VI was approximately 3.6-fold greater than iPF2α-III [see (Korecka et al., 2010) for details] across the sample, and the use of standardized scores mitigated any potential influence of these concentration differences on the formation of the composite F2-isoprostane measure. GENLIN and follow-up t-tests, as described for iPF2α-III and iso-iPF2α-VI, were used to compare smokers and never-smokers on the standardized composite isoprostane level. Associations between the composite isoprostane concentration, hippocampal volumes, and smoking exposure variables were tested as described for iPF2α-III and iso-iPF2α-VI. All analyses were completed with SPSS (v22) and R (v3.0.2).
3. RESULTS
3.1. Demographic and clinical variables
There were no significant differences between smokers and never-smokers on age, education, WM hyperintensity and hippocampal volumes (adjusted for intracranial volume), and triglyceride and total cholesterol levels. Groups were equivalent on non-exclusionary self-reported medical conditions (e.g., hypertension, hyperlipidemia) that may influence OxS and brain morphology, as well as on the frequency of statin/CAB, vitamin E, and antihypertensive use. Smokers had a significantly higher percentage of males (p = .008) and higher BMI (p = .02) than never-smokers (see Table 1).
3.2. Primary analyses
3.2.1. Group comparisons on iPF2α-III and iso-iPF2α-VI concentrations
iPF2α-III: Main effects were observed for smoking status [χ2 (1) = 10.98, p = .001], BMI [χ2 (1) = 10.28, p = .001], and vitamin E use [χ2 (1) = 6.01, p = .014]. Smokers demonstrated a higher iPF2α-III concentration than never-smokers (p = .001; effect size = 0.64; see Figure 1). Higher BMI was associated with higher iPF2α-III concentration, while vitamin E use was associated with lower iPF2α-III level. No significant effects were found for age, sex, statin/CAB use, or the smoking status x age interaction (all p > .30).
Figure 1.
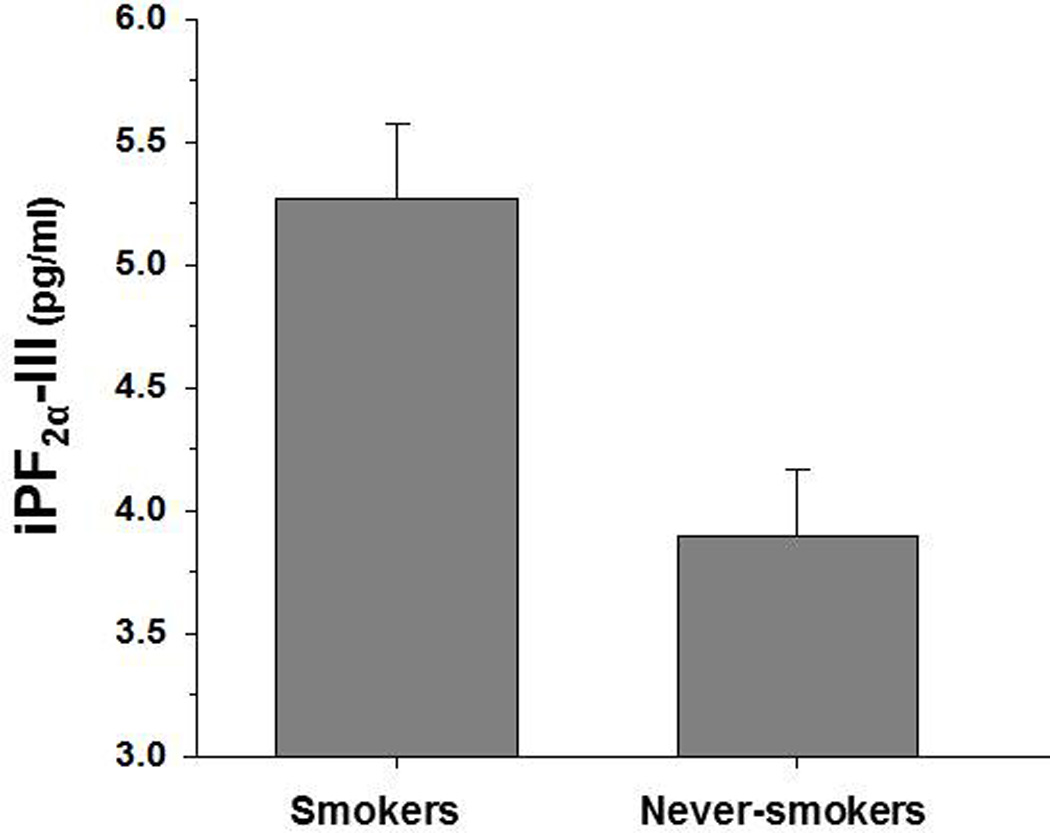
Group concentrations for iPF2α-III.
iso-iPF2α-VI: A smoking status x age interaction was observed [χ2 (1) = 5.85, p = .016], where smokers demonstrated a significantly greater increase of iso-iPF2α-VI level with increasing age than never-smokers (see Figure 2). Main effects were observed for age [χ2 (1) = 8.20, p = .004], BMI [χ2 (1) = 9.18, p = .002], and vitamin E use [χ2 (1) = 3.87, p = .049]. Higher age and BMI were associated with higher iso-iPF2α-VI, whereas vitamin E use was related to lower iso-iPF2α-VI concentration. No main effects were apparent for sex (p = .36), statin/CAB use (p = .12), and smoking status (p = .16; smokers showed numerically higher levels).
Figure 2.
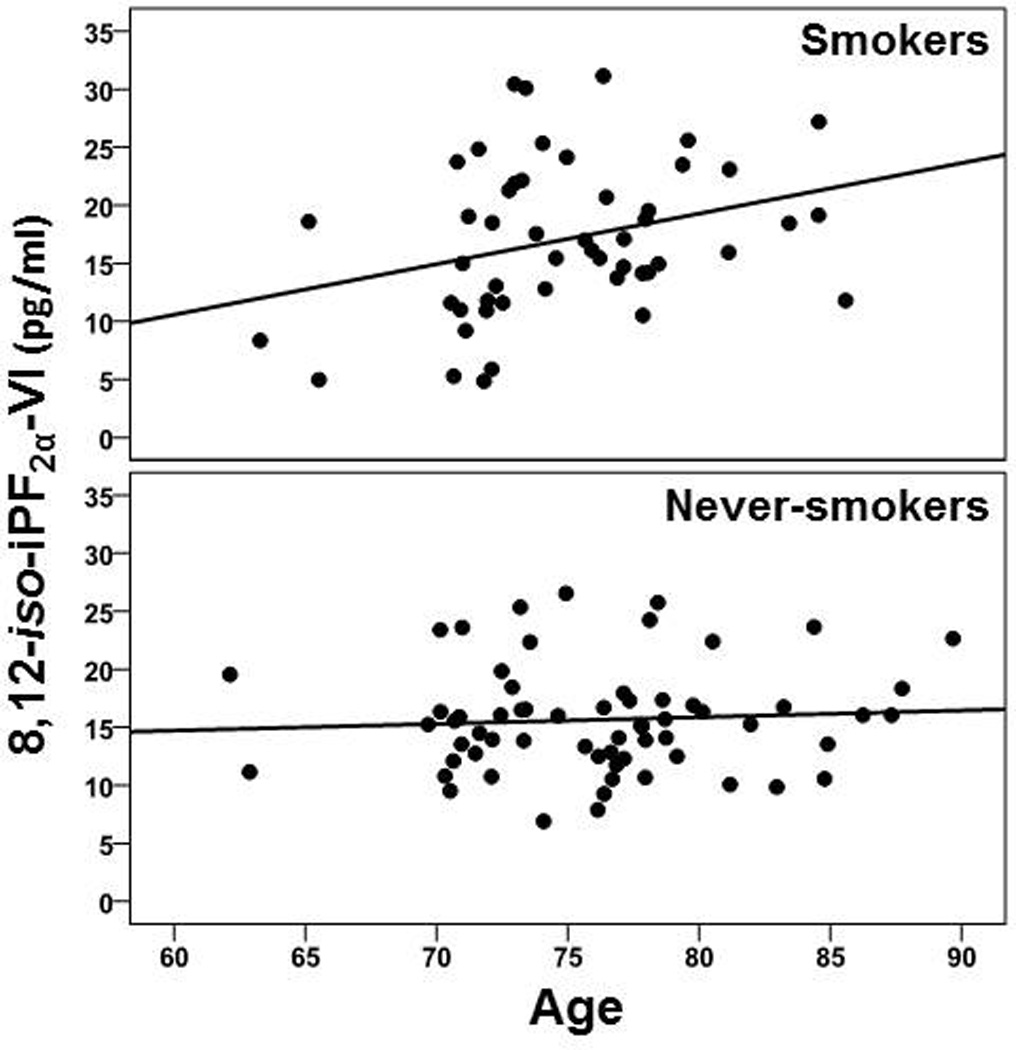
Group concentrations for 8,12-iso-iPF2α-VI across age
3.2.2. Associations of iPF2α-III and iso-iPF2α-VI concentrations with hippocampal volume and smoking exposure variables
iPF2α-III: No significant associations between iPF2α-III level and hippocampal volume were observed in the combined sample (i.e., smokers + never-smokers) or individually for smokers and never-smokers (all r < −0.23, p > .10). In smokers, higher iPF2α-III level was associated with greater pack-years (r = 0.47, p = .018), and greater years of smoking cessation showed a trend for association with lower iPF2α-III level (r = −0.39, p = .08).
iso-iPF2α-VI: There were no significant associations between iso-iPF2α-VI level and hippocampal volume in the combined sample (r = −0.08, p > .42) or never-smokers alone (r = 0.21, p = .12). However, in smokers, greater iso-iPF2α-VI level was associated with smaller hippocampal volume (r = −0.34, p = .024; see Figure 3), and showed a trend for a positive association with pack-years (r = 0.38, p = .061). Years of smoking cessation was not related to iso-iPF2α-VI level (r = −0.13, p = .58).
Figure 3.
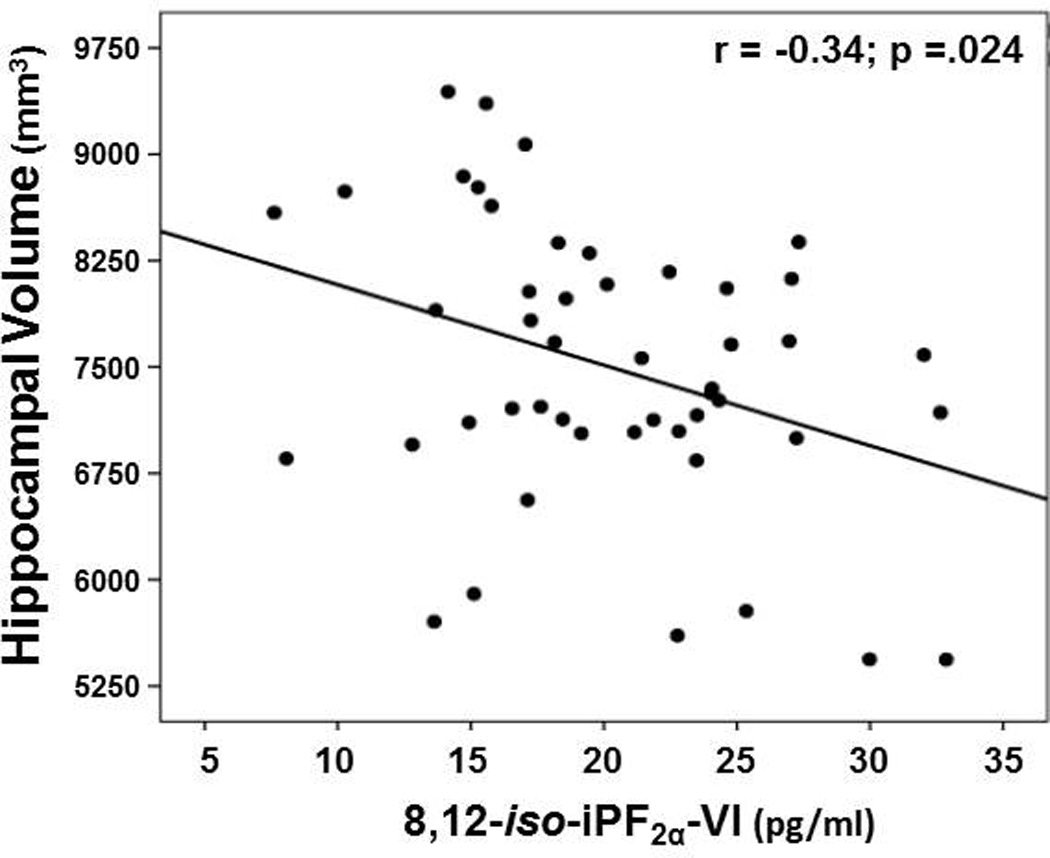
Association between hippocampal volume and 8,12-iso-iPF2α-VI concentration in smokers
3.3. Secondary analyses
3.3.1. Group comparisons on the standardized composite F2-isoprostane concentration
A smoking status x age interaction was observed [χ2 (1) = 8.20, p = .042], where smokers demonstrated a significantly greater increase of composite isoprostane level with increasing age than never-smokers. Main effects were observed for age [χ2 (1) = 7.01, p = .007], BMI [χ2 (1) = 13.32, p < .001], and vitamin E use [χ2 (1) = 5.31, p = .021], and smoking status [χ2 (1) = 7.17, p = .007]. Smokers demonstrated a greater composite level than never-smokers (p = .007; effect size = 0.51). Higher age and BMI were associated with a higher composite level, whereas vitamin E use was related to a lower composite concentration. Sex (p = .55) and statin/CAB use (p = .26) were not significant predictors.
3.3.2. Associations of standardized composite F2-isoprostane concentration with hippocampal volume and smoking exposure measures
There were no significant associations between the composite isoprostane concentration and hippocampal volume in the combined sample (i.e., smokers + never-smokers) or never-smokers alone (all r < −0.19, p > .10). For smokers, higher composite concentration showed a trend for association with smaller hippocampal volume (r = −0.24, p = .10). Higher composite isoprostane level was related to greater pack-years (r = 0.49, p = .014), but composite concentration was not significantly associated with years of smoking cessation (r = −0.29, p = .20).
4. DISCUSSION
The major findings from this cohort of cognitively-normal elders were as follows: (1) individuals with a history of cigarette smoking (i.e., smokers), relative to never-smokers, demonstrated: higher CSF iPF2α-III and composite isoprostane level (i.e., sum of standardized iPF2α-III and iso-iPF2α-VI), as well as greater iso-iPF2α-VI and composite F2-isoprostane concentration with increasing age. (2) Across groups, vitamin E use was associated with lower isoprostane levels, and higher BMI was related to higher isoprostane levels; sex and use of statin/cholesterol absorption blocking agents were not significantly related to iPF2α-III, iso-iPF2α-VI, or composite F2-isoprostane concentrations. (3) In smokers only, higher iso-iPF2α-VI concentration was associated with smaller hippocampal volume; greater iPF2α-III and composite isoprostane levels were related to greater pack years.
This is the first study to demonstrate that a history of cigarette smoking in cognitively normal elders is associated with significantly elevated CSF F2-isoprostanes, greater age-related increases in isoprostane levels, as well as that higher F2-isoprostane levels in smokers was related to smaller hippocampal volume. The higher CSF iPF2α-III and composite F2-isoprostane concentrations observed in these elder smokers are consistent with previous reports of elevated urine and plasma F2-isoprostane levels in both active and former-smokers (Harman et al., 2003; Helmersson et al., 2005; Milne et al., 2005; Yan et al., 2007), while the greater age-related increases in F2-isoprostane levels observed in smokers are novel for F2-isoprostanes measured in any biological tissue. The positive associations between BMI and F2-isoprostanes, and inverse relationship between vitamin E use and F2-isoprostane levels across groups in this study was consistent with previous reports (Galasko and Montine, 2010; Milne et al., 2005). The equivalence of smokers and non-smokers on cerebrovascular risk factors (e.g., triglyceride and cholesterol levels, WM hyperintensity volume, antihypertensive use), and the robust group differences and age-related effects on F2-isoprostane levels after adjusting for BMI and use of statin/cholesterol absorption blocking agents, argues against clinically significant cerebrovascular disease in smokers as a primary explanation for the smoking-related findings. Three of the 25 smokers with detailed smoking history information were actively smoking at the time of assessment. This is consistent with the 10% prevalence of active-smokers in those ≥ 60 years-of-age in the US (Dube et al., 2010), and with the frequency of cognitively-normal active-smokers we observed in larger sample of participants (n = 263) from ADNI GO/ADNI 2 study phases (Durazzo et al., in press). Therefore, most of the cognitively-normal elders for which we did not have detailed smoking history were likely former-smokers with 30–35 years of smoking cessation.
Although groups were statistically equivalent on hippocampal volume, an association between higher iso-iPF2α-VI level and smaller hippocampal volume was observed in smokers only; the lack of a significant relationship between iso-iPF2α-VI concentration and hippocampal volume in never-smokers was not due to a restriction of range in this group because smokers and never-smokers showed statistically equivalent variances on iso-iPF2α-VI concentration and hippocampal volume (data not shown). This indicates that CSF iso-iPF2α-VI level in smokers may serve as a unique biomarker of the effects of radical-mediated lipid peroxidation on the morphological integrity of brain regions known to be vulnerable to OxS. The significant positive relationship of iPF2α-III level and standardized composite isoprostane concentration with pack-years, and the trend for association of lower iPF2α-III level with greater years of smoking cessation, suggests the dose/duration of cigarettes exposure is linearly related to level of OxS in this group of primarily former-smokers. The greater age-related increases in iso-iPF2α-VI and composite isoprostane levels in this group of elder smokers are congruent with our cross-sectional studies that demonstrated chronic smoking is associated with greater age-related hippocampal atrophy in cognitively-normal middle-aged adults (Durazzo et al., 2013), and greater age-related cortical atrophy in middle-aged adults with an alcohol use disorder (Durazzo et al., 2014). It is not surprising that smokers failed to exhibit greater age-related increases in iPF2a-III since age was not a significant predictor of iPF2a-III concentration across groups.
Large sample, population-based studies found that former-smokers, with variable durations of smoking cessation (e.g., 1 to >28 years), showed higher urine F2-isoprostane levels than never-smokers. It was proposed that former-smokers, despite extended periods of smoking cessation, may continue to manifest cytokine-mediated inflammation and OxS and/or incomplete recovery/repair of various cell types in multiple organ systems (Harman et al., 2003; Helmersson et al., 2005). Other large population studies reported former-smokers demonstrated significantly elevated blood-based biomarkers of inflammation (e.g., C-reactive protein) after 10–20 years of smoking cessation; the magnitude of biomarker elevation was related to number of cigarettes smoked per day (Wannamethee et al., 2005; Yanbaeva et al., 2007). Therefore, the CSF F2-isoprostane findings for these primarily former-smokers may be related to persistent chronic inflammatory processes promoting chronic OxS, and/or CNS tissue damage that did not fully resolve with smoking cessation. While speculative, this is supported by the positive association between isoprostane levels pack-years, and the trend for an inverse relationship between iPF2a-III concentration and years of smoking cessation. Additionally, we recently demonstrated that elder former smoker controls from ADNI-GO/ADNI-2 phases demonstrated significantly higher cortical fibrillar amyloid beta (Aβ) levels than elder never-smoker controls, as measured with florbetapir positron emission tomography (Durazzo et al., in press). The significantly higher cortical Aβ level in these former elder smokers is highly relevant because widespread elevations of cortical Aβ is hallmark neuropathological feature of MCI and AD, and chronic OxS is proposed as a potential mechanism contributing to the inception and progression of pathological Aβ accumulation during the preclinical stages of AD (Giunta et al., 2012; Sutherland et al., 2013; Swerdlow, 2012). Overall, the findings from the present study and previous research on OxS biomarkers indicate a history of smoking serves as a source of chronic OxS that may impose enduring adverse effects on the integrity of brain morphology, particularly with greater cigarette smoke exposure and increasing age. The association between F2-isoprostane biomarkers of OxS and hippocampal volume in smokers suggests that chronic OxS may be a mechanism by which cigarette smoking increases the risk for late-life AD (Durazzo et al., in press). However, it is possible the isoprostane findings for these smokers may be, at least partially, related to unrecorded differences between smokers and never-smokers in brain reserve (Stern, 2012), diet and physical activity (Barnes and Yaffe, 2011; Durazzo et al., 2010), subclinical pulmonary, cardiovascular, and/or cerebrovascular diseases, other genetic factors (e.g., polymorphisms brain derived neurotrophic factor), and level of current/past exposure to environmental tobacco smoke (Durazzo et al., in press).
This study has other limitations that may influence the generalizability of the findings. The participants were predominately well-educated elder Caucasians. Detailed smoking information was available for 50% of the participants with a history of smoking. Cigarette smoking in the US promotes at least a 10-year reduction in life expectancy (Jha et al., 2013), which may create a survivor bias due to premature death. In other words, the study of the effects of smoking in elders will be inescapably biased toward the healthiest smokers - those individuals who survived or did not experience significant smoking-related morbidity (Chang et al., 2012; Kukull, 2001). Therefore, the effects of smoking on F2-isoprostane levels in this elder sample may be underestimated due to survivor bias. Finally, the accuracy of the automated segmentation methods used for the hippocampal volumetry in this study may be influenced normal and/or pathological variations of shape and tissue contrast (Mueller and Weiner, 2009).
In conclusion, a history of cigarette smoking in this cognitively-normal elder sample was associated with higher CSF F2-isoprostane concentrations, as well as significantly greater levels with increasing age. These findings provide additional evidence that chronic smoking during adulthood is associated with adverse effects on the brain that are potentially persistent, even with long-term cessation. iPF2α-III and iso-iPF2α-VI appeared to show differential associations with smoking status and hippocampal volume; therefore, the formation of a composite measure of these isoprostanes may conceal possible singular relationships of iPF2α-III and iso-iPF2α-VI with smoking status, brain morphology or other AD biomarkers in future studies. Since smoking-related OxS is proposed as a potential mechanism promoting AD neuropathology (see Durazzo et al., in press), additional longitudinal research is needed on the potential unique characteristics of CSF iPF2a-III and iso-iPF2a-VI as biomarkers of smoking-related OxS and their association with brain morphology and other biomarkers of AD-related neuropathology (e.g., CSF or PET measurements of Aβ and tau) in cognitively-normal controls, and those with MCI and AD.
Acknowledgments
This work was supported by the National Institutes of Health (NIH DA24136 to TCD) and by the use of resources and facilities at the San Francisco Veterans Administration Medical Center. All data collection and sharing for this project was supported by ADNI. ADNI is funded by the National Institute on Aging (U01 AG024904; PI MWW), the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co.,Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., and Wyeth, as well as non-profit partners the Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org; http://www.fnih.org; http://www.fnih.org; http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University Southern California. This research was also supported by NIH grants P30 AG010129, K01 AG030514, R01 AG010897, R01 AG012435, and The Dana Foundation and by resources. The above funding agencies had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript. Original data used in preparation of this article were obtained from the ADNI database (www.loni.usc.edu/ADNI). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Authorship_List.pdf.
Role of funding sources. The study sponsors had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosures
Author contribution. Dr. Durazzo was responsible for the concept and design of the study, completed all statistical analyses, and wrote the manuscript. Drs. Mattsson, Weiner, Korecka, Trojanowski, and Shaw were involved in manuscript editing and data interpretation. Drs. Korecka, Trojanowski, and Shaw designed the HPLC-atmospheric pressure chemical ionization-tandem mass spectrometry method for isoprostane quantitation. Dr. Durazzo had full access to all the original ADNI data presented study and takes responsibility for the accuracy of the data analyses. All authors approved the final manuscript.
Conflict of Interest. The Authors have no conflicts of interest to disclose.
REFERENCES
- Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J. Am Coll. Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Anbarasi K, Vani G, Balakrishna K, Devi CS. Effect of bacoside A on membrane-bound ATPases in the brain of rats exposed to cigarette smoke. J. Biochem. Mol. Toxicol. 2005;19:59–65. doi: 10.1002/jbt.20050. [DOI] [PubMed] [Google Scholar]
- Anbarasi K, Vani G, Balakrishna K, Devi CS. Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci. 2006;78:1378–1384. doi: 10.1016/j.lfs.2005.07.030. [DOI] [PubMed] [Google Scholar]
- Azizian A, Monterosso J, O’Neill J, London ED. Magnetic resonance imaging studies of cigarette smoking. In: Henningfield JE, Calvento E, Pogun S, editors. Nicotine Psychopharmacology. Berlin Heidelberg: Springer-Verlag; 2009. pp. 113–143. [Google Scholar]
- Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer RJ. Decreased blood antioxidant capacity and increased lipid peroxidation in young cigarette smokers compared to nonsmokers: impact of dietary intake. Nutr. J. 2007;6:39. doi: 10.1186/1475-2891-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Swomley AM, Sultana R. Amyloid beta-peptide (1–42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013;19:823–835. doi: 10.1089/ars.2012.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer’s Disease: an analysis controlling for tobacco industry affiliation. J. Alzheimers Dis. 2010;19:465–480. doi: 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalela JA, Wolf RL, Maldjian JA, Kasner SE. MRI identification of early white matter injury in anoxic-ischemic encephalopathy. Neurology. 2001;56:481–485. doi: 10.1212/wnl.56.4.481. [DOI] [PubMed] [Google Scholar]
- Chang CC, Zhao Y, Lee CW, Ganguli M. Smoking, death, and Alzheimer disease: a case of competing risks. Alzheimer Dis. Assoc. Disord. 2012;26:300–306. doi: 10.1097/WAD.0b013e3182420b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang GC, Insel PS, Tosun D, Schuff N, Truran-Sacrey D, Raptentsetsang ST, Jack CR, Jr, Aisen PS, Petersen RC, Weiner MW. Hippocampal atrophy rates and CSF biomarkers in elderly APOE2 normal subjects. Neurology. 2010;75:1976–1981. doi: 10.1212/WNL.0b013e3181ffe4d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracowski JL, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol. Sci. 2002;23:360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol. Clin. Exp. Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dube SR, McClave A, James C, Caraballo R, Kaufmann R, Pechacek TF. Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, CDC. 2010. Vital signs: current cigarette smoking among adults aged ≥ 18 years --- United States. MMWR. 2009;59:1135–1140. [Google Scholar]
- Durazzo TC, Insel PS, Weiner MW. The Alzheimer’s Disease Neuroimaging Initiative. Greater regional brain atrophy rate in healthy elders with a history of cigarette smoking. Alzheimers Dement. 2012;8:513–519. doi: 10.1016/j.jalz.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW. The Alzheimer’s Disease Neuroimaging Initiative in press. Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimers Dement. doi: 10.1016/j.jalz.2014.04.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Interactive effects of chronic cigarette smoking and age on hippocampal volumes. Drug Alcohol Depend. 2013;133:704–711. doi: 10.1016/j.drugalcdep.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front. Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int. J. Environ. Res. Public Health. 2010;7:3760–3791. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Pennington D, Abe C, Gazdzinski S, Meyerhoff DJ. Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence. Addict. Biol. 2014;19:132–143. doi: 10.1111/j.1369-1600.2012.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enciu AM, Gherghiceanu M, Popescu BO. Triggers and effectors of oxidative stress at blood-brain barrier level: relevance for brain ageing and neurodegeneration. Oxid. Med. Cell Longev. 2013 doi: 10.1155/2013/297512. Article ID: 297512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatic parcellation of the human cerebral cortex. Cereb. Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fowles J, Bates M, Noiton D. The Chemical Constituents in Cigarettes and Cigarette Smoke: Priorities for Harm Reduction. Porirua, New Zealand: Epidemiology and Toxicology Group, Institute of Environmental Science and Research Ltd; 2000. pp. 1–65. http://www.health.govt.nz/publication/chemical-constituents-cigarettes-and-cigarette-smoke-priorities-harm-reduction. [Google Scholar]
- Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark. Med. 2010;4:27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: toll-like receptors. Free Radic. Biol. Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta B, Deng J, Jin J, Sadic E, Rum S, Zhou H, Sanberg P, Tan J. Evaluation of how cigarette smoke Is a direct risk factor for Alzheimer’s disease. Technol. Innov. 2012;14:39–48. doi: 10.3727/194982412X13378627621752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44:15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Gunter JL, Bernstein MA, Borowski B, Felmlee JP, Blezek D, Mallozzi R. Validation Testing Of The MRI Calibration Phantom For The Alzheimer’s Disease Neuroimaging Initiative Study. ISMRM 14th Scientific Meeting and Exhibition. 2006 [Google Scholar]
- Haight TJ, Landau SM, Carmichael O, Schwarz C, Decarli C, Jagust WJ. Dissociable effects of Alzheimer Disease and white matter hyperintensities on brain metabolism. JAMA Neurol. 2013;70:1039–1045. doi: 10.1001/jamaneurol.2013.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM, Liang L, Tsitouras PD, Gucciardo F, Heward CB, Reaven PD, Ping W, Ahmed A, Cutler RG. Urinary excretion of three nucleic acid oxidation adducts and isoprostane F(2)alpha measured by liquid chromatography-mass spectrometry in smokers, ex-smokers, and nonsmokers. Free Radic. Biol. Med. 2003;35:1301–1309. doi: 10.1016/j.freeradbiomed.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Haschke M, Zhang YL, Kahle C, Klawitter J, Korecka M, Shaw LM, Christians U. HPLC-atmospheric pressure chemical ionization MS/MS for quantification of 15-F2t–isoprostane in human urine and plasma. Clin. Chem. 2007;53:489–497. doi: 10.1373/clinchem.2006.078972. [DOI] [PubMed] [Google Scholar]
- Haustein KO. Smoking tobacco, microcirculatory changes and the role of nicotine. Int. J. Clin. Pharmacol. Ther. 1999;37:76–85. [PubMed] [Google Scholar]
- Helmersson J, Larsson A, Vessby B, Basu S. Active smoking and a history of smoking are associated with enhanced prostaglandin F(2alpha), interleukin-6 and F2-isoprostane formation in elderly men. Atherosclerosis. 2005;181:201–207. doi: 10.1016/j.atherosclerosis.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Ho YS, Yang X, Yeung SC, Chiu K, Lau CF, Tsang AW, Mak JC, Chang RC. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS One. 2012 doi: 10.1371/journal.pone.0036752. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0036752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJL, Whitewell J, Ward C, Dale AM, Felmlee JP, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson. Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the United States. N. Engl. J. Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- Khandelwal PJ, Herman AM, Moussa CE. Inflammation in the early stages of neurodegenerative pathology. J. Neuroimmunol. 2011;238:1–11. doi: 10.1016/j.jneuroim.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Guo M, Mehra M, Royal W., 3rd Inflammation and oxidative stress induced by cigarette smoke in Lewis rat brains. J. Neuroimmunol. 2013;254:69–75. doi: 10.1016/j.jneuroim.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korecka M, Clark CM, Lee VM, Trojanowski JQ, Shaw LM. Simultaneous HPLC-MS-MS quantification of 8-iso-PGF(2alpha) and 8,12-iso-iPF(2alpha) in CSF and brain tissue samples with online cleanup. J .Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2010;878:2209–2216. doi: 10.1016/j.jchromb.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic P. Unifying mechanism for addiction and toxicity of abused drugs with application to dopamine and glutamate mediators: electron transfer and reactive oxygen species. Med. Hypotheses. 2005;65:90–96. doi: 10.1016/j.mehy.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Romanowski A, Schilling C, Mobascher A, Warbrick T, Winterer G, Gallinat J. Brain grey matter deficits in smokers: focus on the cerebellum. Brain Struct. Funct. 2012;217:517–522. doi: 10.1007/s00429-011-0346-5. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Schubert F, Gallinat J. Reduced thickness of medial orbitofrontal cortex in smokers. Biol. Psychiatry. 2010;68:1061–1065. doi: 10.1016/j.biopsych.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kukull WA. The association between smoking and Alzheimer’s disease: effects of study design and bias. Biol. Psychiatry. 2001;49:194–199. doi: 10.1016/s0006-3223(00)01077-5. [DOI] [PubMed] [Google Scholar]
- Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;1(10 Suppl):S10–23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Peskind ER, Quinn JF, Wilson AM, Montine KS, Galasko D. Increased cerebrospinal fluid F2-isoprostanes are associated with aging and latent Alzheimer’s disease as identified by biomarkers. Neuromolecular Med. 2011;13:37–43. doi: 10.1007/s12017-010-8126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic. Biol. Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Trabesinger AH, Boesiger P, Wieser HG. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology. 2001;57:1422–1427. doi: 10.1212/wnl.57.8.1422. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW. Selective effect of age, Apo e4, and Alzheimer’s disease on hippocampal subfields. Hippocampus. 2009;19:558–564. doi: 10.1002/hipo.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, Trojanowski JQ, Toga AW, Beckett L. The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clin. N. Am. 2005a;15:869–877. doi: 10.1016/j.nic.2005.09.008. xi-xii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack CR, Jagust W, Trojanowski JQ, Toga AW, Beckett L. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheimers Dement. 2005b;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dwyer L, Lamberton F, Matura S, Tanner C, Scheibe M, Miller J, Rujescu D, Prvulovic D, Hampel H. Reduced hippocampal volume in healthy young ApoE4 carriers: an MRI study. PLoS One. 2012 doi: 10.1371/journal.pone.0048895. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0048895#pone-0048895-g002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone C, De Carolis C, Perricone R. Glutathione: a key player in autoimmunity. Autoimmun. Rev. 2009;8:697–701. doi: 10.1016/j.autrev.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:36. doi: 10.1186/1471-2318-8-36. http://www.biomedcentral.com/1471-2318/8/36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho RA, Valenca SS, Porto LC. Organ-related cigarette smoke-induced oxidative stress is strain-dependent. Med. Sci. Monit. 2010;16:BR218–BR226. [PubMed] [Google Scholar]
- Pratico D. Prostanoid and isoprostanoid pathways in atherogenesis. Atherosclerosis. 2008;201:8–16. doi: 10.1016/j.atherosclerosis.2008.04.037. [DOI] [PubMed] [Google Scholar]
- Pratico D. The neurobiology of isoprostanes and Alzheimer’s disease. Biochim. Biophys. Acta. 2010;1801:930–933. doi: 10.1016/j.bbalip.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Rokach J, Kim S, Bellone S, Lawson JA, Pratico D, Powell WS, FitzGerald GA. Total synthesis of isoprostanes: discovery and quantitation in biological systems. Chem. Phys. Lipids. 2004;128:35–56. doi: 10.1016/j.chemphyslip.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Rokach J, Khanapure SP, Hwang SW, Adiyaman M, Lawson JA, FitzGerald GA. Nomenclature of isoprostanes: a proposal. Prostaglandins. 1997;54:853–873. doi: 10.1016/s0090-6980(97)00184-6. [DOI] [PubMed] [Google Scholar]
- Rueff-Barroso CR, Trajano ET, Alves JN, Paiva RO, Lanzetti M, Pires KM, Bezerra FS, Sabia S, Elbaz A, Dugravot A, Head J, Shipley M, Hagger-Johnson G, Kivimaki M, Singh-Manoux A. Impact of smoking on cognitive decline in early old age: the Whitehall II cohort study. Arch. Gen. Psychiatry. 2012;69:627–635. doi: 10.1001/archgenpsychiatry.2011.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000;267:4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- Seet RC, Lee CY, Loke WM, Huang SH, Huang H, Looi WF, Chew ES, Quek AM, Lim EC, Halliwell B. Biomarkers of oxidative damage in cigarette smokers: which biomarkers might reflect acute versus chronic oxidative stress? Free Radic. Biol. Med. 2011;50:1787–1793. doi: 10.1016/j.freeradbiomed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Sharma A, Brody A. In vivo brain imaging of human exposure to nicotine and tobacco. In: Henningfield JE, Calvento E, Pogun S, editors. Nicotine Psychopharmacology. Berlin Heidelberg: Springer-Verlag; 2009. pp. 145–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Gray SL, Wilson A, Kohama SG, Crane PK, Breitner JC, Montine TJ. Free radical damage to cerebral cortex in Alzheimer’s disease, microvascular brain injury, and smoking. Ann. Neurol. 2009;65:226–229. doi: 10.1002/ana.21508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GT, Chami B, Youssef P, Witting PK. Oxidative stress in Alzheimer’s disease: primary villain or physiological by-product? Redox. Rep. 2013;18:134–141. doi: 10.1179/1351000213Y.0000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol. Rev. 2007;17:259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH. Alzheimer’s disease pathologic cascades: who comes first, what drives what. Neurotox. Res. 2012;22:182–194. doi: 10.1007/s12640-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health. 2011;8:613–628. doi: 10.3390/ijerph8020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyas SL, White LR, Petrovitch H, Webster Ross G, Foley DJ, Heimovitz HK, Launer LJ. Mid-life smoking and late-life dementia: the Honolulu-Asia Aging Study. Neurobiol. Aging. 2003;24:589–596. doi: 10.1016/s0197-4580(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health. 2009;6:445–462. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Giffard RG. Inflammation, mitochondria, and the inhibition of adult neurogenesis. J. Neurosci. Res. 2011;89:1989–1996. doi: 10.1002/jnr.22768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M, Schulze-Rauschenbach S, Petrovsky N, Brinkmeyer J, von der Goltz C, Grunder G, Spreckelmeyer KN, Wienker T, Diaz-Lacava A, Mobascher A, Dahmen N, Clepce M, et al. Neurocognitive impairments in non-deprived smokers-results from a population-based multicenter study on smoking-related behavior. Addict. Biol. 2013;18:752–761. doi: 10.1111/j.1369-1600.2011.00429.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamethee SG, Lowe GDO, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur. Heart J. 2007;26:1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- Yan W, Byrd GD, Ogden MW. Quantitation of isoprostane isomers in human urine from smokers and nonsmokers by LC-MS/MS. J. Lipid Res. 2007;48:1607–1617. doi: 10.1194/jlr.M700097-JLR200. [DOI] [PubMed] [Google Scholar]
- Yanbaeva DG, Dentener MA, Creutzberg EC, Geertjan Wesseling G, Wouters EFM. Systemic effects of smoking. Chest. 2005;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- Yang YM, Liu GT. Injury of mouse brain mitochondria induced by cigarette smoke extract and effect of vitamin C on it in vitro. Biomed. Environ. Sci. 2003;16:256–266. [PubMed] [Google Scholar]
- Yao Y, Zhukareva V, Sung S, Clark CM, Rokach J, Lee VM, Trojanowski JQ, Pratico D. Enhanced brain levels of 8,12-iso-iPF(2alpha)-VI differentiate AD from frontotemporal dementia. Neurology. 2003;61:475–478. doi: 10.1212/01.wnl.0000070185.02546.5d. [DOI] [PubMed] [Google Scholar]


