Abstract
Background
Neutrophil gelatinase-associated lipocalin (NGAL) is a biomarker of acute kidney injury (AKI). Recently, elevated NGAL levels have also been reported in heart failure, coronary heart disease, and stroke. Other studies demonstrate that NGAL is upregulated in failing myocardium and in atherosclerotic plaque. Our aim was to synthesize the current evidence on NGAL and cardiovascular disease (CVD), and to clarify the prognostic significance of systemic NGAL levels in CVD.
Methods
We performed a systematic review to identify experimental and human studies on NGAL and CVD. We excluded articles which specifically dealt with AKI or renal endpoints.
Results
We identified 22 studies, including both animal and human data. NGAL is highly expressed in the heart, both in failing myocardium and myocarditis, and is also expressed in atherosclerotic plaques. Areas of co-localization of NGAL and matrix metalloproteinase (MMP)-9 exhibited increased MMP-9 proteolytic activity. Systemic NGAL levels correlated with renal function and severity of CVD in several, but not all, studies. An association between elevated systemic NGAL levels and clinical outcomes (e.g., death, hospital readmissions) were reported in six CVD studies, but these had limited adjustment for potential confounders.
Conclusions
There is ample literature to support a putative role of NGAL in the pathophysiology of CVD, but at present there is insufficient data regarding the clinical utility of systemic NGAL levels in the management of CVD. Available evidence regarding NGAL as a predictor of outcomes in CVD is very limited.
Keywords: atherosclerosis, biomarkers, brain natriuretic peptide (BNP), cardiovascular disease, cerebrovascular disease, coronary artery disease, heart failure, matrix metalloproteinase, myocarditis, neutrophil gelatinase associated lipocalin (NGAL), stroke
Introduction
Neutrophil gelatinase-associated lipocalin (NGAL) is a protein belonging to the lipocalin family, expressed by neutrophils and various epithelial cells (1). It represents one of the earliest kidney markers of ischemic and nephrotoxic kidney injury in animals models and is detected in blood and urine of humans soon after acute kidney injury (AKI). It is emerging as a promising troponin-like biomarker for the early diagnosis of AKI, and for the prediction of clinical outcomes, such as dialysis requirement and mortality in cardiac surgery, critical care, and contrast nephropathy (2–5). In addition, NGAL measurements have potential relevance in chronic kidney diseases (6).
Beyond the kidney, recent evidence suggests that NGAL plays a crucial role in vascular remodeling and plaque instability in atherosclerosis (7, 8). Other studies demonstrate that NGAL is upregulated in cardiomyocytes in failing myocardium (9). Collectively, these findings provide biological plausibility for the potential role of NGAL as a biomarker in cardiovascular disease (CVD). Elevated NGAL levels have been reported in various cardiovascular conditions, including both acute and chronic heart failure (AHF, CHF), coronary heart disease (CHD), and stroke (9–12).
To date, the overall relationship between NGAL and CVD remains unclear. We therefore performed a systematic review to synthesize the evidence on the pathophysiological relationship between NGAL and myocardial remodeling and atherosclerotic plaque formation. Second, we sought to clarify the prognostic significance, if any, of systemic NGAL levels in CVD.
Concise methods
Detailed methods are described in ESM File 1 which accompanies the article at http://www.degruyter.com/view/j/cclm.2012.50.issue-9/issue-files/cclm.2012.50.issue-9.xml. We performed a systematic search to identify animal and human studies that assessed the relationship between NGAL and CVD. The literature search (MEDLINE, PubMed) was based on three search themes using Boolean operators, without language, restrictions. Search terms included keyword/MESH headings describing heart failure, CHD and other heart diseases, cerebrovascular disease, peripheral vascular disease, and NGAL (ESM File 1).
We also utilized the “Related citations” tool of PubMed, hand-searched bibliographies of relevant articles and consulted with experts to find additional pertinent studies.
Study selection
Two reviewers (DC, SG) independently performed an initial eligibility screen. Studies reporting original data that specifically mentioned NGAL and any CVD were selected for further review for the following eligibility criteria: 1) reporting of NGAL expression or protein in any CVD animal model; 2) NGAL expression or protein in animal or human tissue from subjects with CVD; 3) blood or urine NGAL levels in CVD patients. Since our objective was to examine the link between NGAL and CVD itself, we excluded articles which specifically dealt with AKI or renal endpoints.
Data extraction and synthesis
Two authors (DC, SG) extracted the data independently using standardized case report forms. For pre-clinical studies, we extracted data on the animal model, specimen retrieval, techniques and findings regarding NGAL expression or protein. For clinical studies, outcomes of interest included association of blood or urine NGAL levels with severity of CVD, other biomarkers, death and major adverse CV events. The heterogeneous reporting on mortality did not permit a quantitative pooled analysis; a tabular summary of the relevant results is provided.
Results
Study selection and characteristics
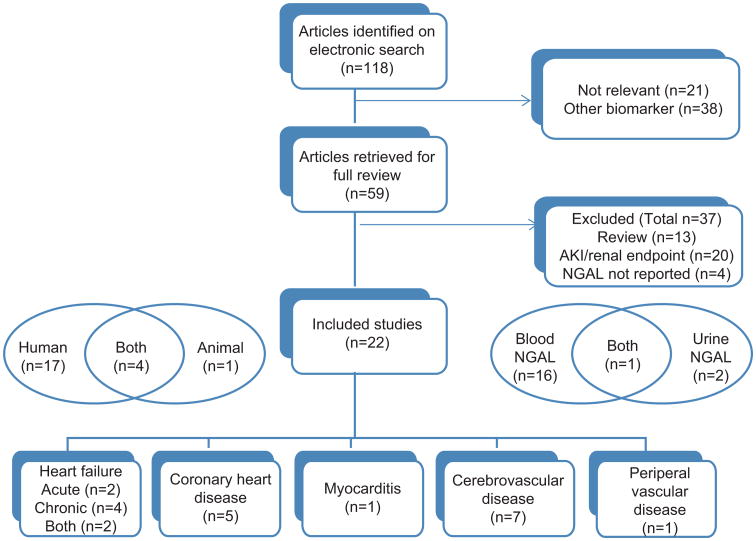
One hundred and eighteen citations were identified; 22 studies were included in the analysis (Figure 1). Four studies reported both human and animal data, one, animal data alone, and 17, human data. The studies were related to AHF and CHF (n=8), CHD (n=5), myocarditis (n=1), cerebrovascular disease (n=7) and peripheral vascular disease (n=1). The last was a study of tissue from abdominal aortic aneursyms (AAA).
Figure 1.
Study selection diagram.
Systemic NGAL levels (blood or urine) were measured in 18 of the included studies.
Systemic NGAL levels were measured in 19 clinical studies (Figure 1); some had control subjects for comparison. In CHF, six studies reported either blood or urine NGAL. Blood NGAL levels were measured in four studies on AHF, four on CHD and five on cerebrovascular disease. Six studies examined associations between NGAL levels and clinical outcomes, such as death, myocardial infarction (MI), stroke, transplant and hospital readmissions; follow-up ranged from 30 days to 4 years.
Animal studies
Major findings are summarized in Table 1. In a model for atherosclerotic heart disease, atherosclerotic and control mice were exposed to hypoxic stress, and NGAL-homolog 24p3 and matrix metalloproteinase (MMP)-9 were analyzed 48 h later (7). Aortas from atherosclerotic mice exhibited higher levels of NGAL mRNA than normal mice. Hypoxic stress increased NGAL mRNA expression in the aorta only in atherosclerotic mice that developed MI, suggesting that NGAL was upregulated as a result of infarction or possibly that NGAL expression was necessary for MI development. Abundant NGAL protein was seen in the lipid core and border regions of aortic plaques of atherosclerotic mice, and co-localized with MMP-9. These regions of co-localization also displayed high MMP-9 activity. Normal aortas of control mice displayed neither NGAL nor MMP-9 protein. Hypoxic stress also significantly increased NGAL mRNA in the myocardial tissue of both normal and atherosclerotic mice; the highest increase was observed in atherosclerotic mice developing MI (7). Similarly, NGAL and MMP-9 also co-localized in the cardiomyocytes of atherosclerotic mice.
Table 1.
Summary of animal studies on NGAL and cardiovascular disease.
| Reference | Animal model | Specimen | Analysis | Molecule | Major findings |
|---|---|---|---|---|---|
| Hemdahl (7) | apoE-/- × LDLR-/-(atherosclerotic mice) and C57BL/6J (control mice) exposed to hypoxic stress | Aortic plaque | mRNA expression | NGAL/24p3 | Baseline: Atherosclerotic mice (Ath) > control mice (Con). After hypoxic stress: Ath who suffered MI (Ath-MI) > Ath-no MI. |
| MMP-9 | After hypoxic stress: Ath-MI > Ath-no MI. | ||||
| Immunohistochemistry | NGAL/24p3 and MMP-9 | Abundant NGAL protein co-localized with MMP-9 in Ath. No NGAL or MMP-9 demonstrated in Con. |
|||
| Myocardium | mRNA expression | NGAL/24p3 | Baseline: Ath=Con. After hypoxic stress: ↑ in both Ath and Con; greatest ↑ seen in Ath-MI. |
||
| MMP-9 | Baseline: Ath > Con. After hypoxic stress: ↑ only in Con; no change in Ath. |
||||
| Immunohistochemistry | NGAL/24p3 and MMP-9 | NGAL protein co-localized with MMP-9 in cardiomyocytes of Ath. No NGAL or MMP-9 demonstrated in Con. |
|||
| Yndestadt (9) | Wistar rats undergoing ligation of left coronary artery or sham operation | Heart ventricle | mRNA expression | NGAL/24p3 | Rats with HF had ↑NGAL expression in the non-ischemic area of the ventricle 2, 7, 28, and 64 days after the induction of MI. This upregulation was demonstrated in cardiomyocytes, but not in fibroblasts or endothelial cells. |
| Ding (13) | Lewis rats immunized with porcine cardiac myosin (EAM) and control rats | Heart ventricle | mRNA expression | Lcn2/NGAL | In EAM rat hearts, NGAL expression started to rise after 9 days, peaked on days 12–18, and returned to near-baseline levels at day 60. This upregulation was demonstrated in cardiomyocytes, endothelial cells, pericytes, smooth muscle cells and fibroblasts. This increase was not seen in control rats. |
| Immunohistochemistry | Lcn2/NGAL | In EAM rat hearts, NGAL immunostaining was seen in cardiomyocytes, vascular smooth muscle cells, fibroblastoid cells, and leukocytes. This was hardly detected in control rat hearts. |
|||
| Plasma | ELISA | Lcn2/NGAL, IL-1β, BNP | In EAM rats, NGAL and IL-1β levels increased at day 13, remained elevated at day 33, and returned to near-baseline levels after day 45. | ||
| Bu (14) | Sprague-Dawley rats subjected to balloon injury of the left common carotid artery | Carotid artery | mRNA expression | NGAL, MMP-9 | Angioplastic injury to the artery induced high expression of NGAL mRNA at day 14 but not at day 3. MMP-9 was markedly upregulated in the vessels shortly after injury. Blocking of NF-κB resulted in virtually complete suppression of NGAL and MMP-9 expression. |
| te Boekhorst (15) | ApoE2/2/eNOS2/2 mice were injected with NGAL/24p3-targeted micelles | Abdominal aorta | Histology and MRI | NGAL/24p3 | NGAL can be detected in murine atherosclerotic arteries using targeted high-resolution MRI. |
EAM, experimental autoimmune myocarditis.
In a rat model for AHF post-MI, the left coronary artery was ligated (9), resulting in a transmural infarction of the left ventricular (LV) free wall. Myocardial NGAL/lipocalin-2 expression was investigated; rats with HF had significantly raised NGAL expression in the non-ischemic area of the LV after the induction of MI, representing development from acute to a chronic stage of HF in this model. This upregulation of NGAL was restricted to cardiomyocytes. The investigators further examined the effect of various stimuli relevant to HF, e.g., cytokines, neurohormones and microbial antigens, on NGAL expression in rat cardiomyocytes. Agonists of toll-like receptor (TLR)-2 and TLR-4, leukemia inhibitory factor (LIF) and interleukin (IL)-1β promoted a marked increase in NGAL mRNA expression, indicating a role for innate immune responses in the regulation of myocardial NGAL levels.
In a rat model for autoimmune myocarditis, NGAL was strongly expressed in cardiomyocytes, vascular wall cells and fibroblasts (13). Elevation of both the cardiac expression of NGAL and plasma NGAL levels was more pronounced during the active stages of myocarditis, and closely paralleled cardiac IL-1β and plasma IL-1β levels.
In terms of cerebrovascular disease, NGAL was highly induced in the intima after balloon injury to the common carotid artery in rats, as was MMP-9 (14). Blocking of NF-κB by adenovirus-mediated expression of a dominant-negative mutant of inhibitor of NF-κB kinase β (dnIKKβ) resulted in virtually complete suppression of NGAL and MMP-9 expression, implying a pivotal role for NF-κB signaling in their transcriptional regulation. Expression of NGAL mRNA and protein was also upregulated in an NF-κB -dependent manner in vascular smooth muscle cells in response to IL-1β stimulation. Finally, ApoE2/2 /eNOS 2/2 mice were injected with NGAL/24p3-targeted micelles then subjected to magnetic resonance imaging (MRI) (15). Enhancement on MRI of the abdominal aorta was observed at 72 h. The specificity of these results for atherosclerotic plaque was validated by histology, and co-localization of micelles, macrophages, and NGAL/24p3 was observed.
Studies on human tissue
Human tissue that has been studied for NGAL expression includes heart tissue, myocardial biopsy, carotid and internal mammary arteries, and human AAA (Table 2).
Table 2.
Summary of studies on NGAL and cardiovascular disease using human tissue.
| Reference | Population | Specimen | Analysis | Mediator | Major findings |
|---|---|---|---|---|---|
| Yndestadt (9) | Endstage HF patients (NYHA class III-IV, LVEF <35%) undergoing cardiac transplantation (n=2) | Heart tissue retrieved immediately on explantation | Immunohistochemistry | Lcn2/NGAL | NGAL immunostaining was strongest in cardiomyocytes within the failing myocardium. |
| Ding (13) | Patients with posthumous diagnosis of myocarditis (n=3) | Heart tissue retrieved on autopsy | Immunohistochemistry, Western blot | Lcn2/NGAL | NGAL was found in cardiomyocytes, vascular wall cells, fibroblastoid cells and leukocytes. |
| Patients with (n=7) and without (n= 19) histological diagnosis of myocarditis | Myocardium retrieved by biopsy | mRNA expression | Lcn2/NGAL | NGAL mRNA levels in human hearts with myocarditis were significantly greater than in those without myocarditis. | |
| Hemdahl (7) | Patients undergoing endarterectomy for symptomatic carotid stenosis (n=4) or coronary artery bypass surgery (n=6) | Carotid artery, internal mammary artery | Immunohistochemistry | NGAL and MMP-9 | MMP-9 and NGAL co-localized with macrophages in the lipid core of atherosclerotic plaques. NGAL was also present in the normal human internal mammary artery, whereas MMP-9 was not detected. |
| te Boekhorst (15) | Patients undergoing carotid endarterectomy (n=122) | Carotid artery | Immunohistochemistry, gel zymography, ELISA | NGAL, MMP, and NGAL/MMP-9 complex | Plaque levels of NGAL tended to be higher when intra-plaque hemorrhage or luminal thrombus was present. Plaque NGAL concentration correlates with plaque activity of MMP-9 and MMP-8, but not MMP-2. NGAL concentrations in local plaque-related blood tended to be higher than in peripheral blood (p=0.06). NGAL/MMP-9 concentrations was significantly higher in local plaque-related blood than peripheral blood (p=0.007). Activity of the NGAL/MMP-9 complex was significantly higher in carotid artery plaques compared with the control mammary arteries.a |
| Folkesson (8) | Patients undergoing elective surgery for infrarenal AAA (n=27) | Abdominal aorta | Western blot, immunohistochemistry, gel zymography, mRNA expression | NGAL, MMP, and NGAL/MMP-9 complex | NGAL is expressed in the intraluminal thrombus and the interface between the thrombus and the underlying AAA wall. Active NGAL/MMP-9 complexes are present in aortic wall segments and in the thrombus. |
Source of internal mammary arteries not described in reference (14).
In terms of CHF, tissue aliquots from failing myocardium (explantation of hearts from endstage HF patients undergoing cardiac transplantation) were compared to control (non-failing) human LV tissue (obtained from sex- and age-matched potential cardiac donors). The strongest NGAL immuno-staining was observed in cardiomyocytes within failing myocardium, with some immunoreactivity also seen in vascular smooth muscle and endothelial cells (9).
There is also evidence for the potential role of NGAL in the pathogenesis of human myocarditis. NGAL was detected in cardiomyocytes, vascular wall cells and fibroblastoid cells and leukocytes in human hearts with myocarditis (13). The authors propose that NGAL's role in the heart may involve acting in an autocrine fashion to clean up iron and other potential free radical generating molecules that may leak into the extracellular space following cardiomyocyte injury.
Atherosclerotic plaque in both carotid artery and AAA demonstrate co-localization of NGAL and MMP-9, particularly in the lipid core of the plaque (7, 8, 15). NGAL was also detected in AAA thrombus, the interface between the thrombus and the underlying aneurysm wall, and in the wall itself (8). Double staining showed that neutrophils are the major source of NGAL expression. Similar to the findings in carotid artery, NGAL and complexes of NGAL and active MMP-9 were seen in the thrombus and aneurysm wall (15). Plaque levels of NGAL itself tended to be higher when intra-plaque hemorrhage or luminal thrombus was present.
Clinical studies
Chronic heart failure (CHF)
The major findings of clinical studies on heart disease are summarized in Table 3. CHF patients have been found to have significantly higher levels of both serum and urine NGAL when compared with control subjects, despite having only modest reductions in estimated glomerular filtration rates (eGFR) (9, 10, 17, 18). Furthermore, both serum and urine NGAL levels correlated with various indices of renal function, such as creatinine (range, r=0.22–0.55, p<0.0001–<0.05), eGFR (r=−0.53 to −0.29, p<0.001–<0.01), cystatin C (r=0.20–0.60, p<0.0001), blood urea nitrogen (r=0.42, p<0.0001) and urinary albumin (r=0.33, p=0.001) (10, 17, 19) (Tables 3, 4). However, in other studies, serum and urine NGAL did not have significant correlations with these renal indices (16, 18).
Table 3.
Summary of human studies on NGAL and cardiac disease.
| Reference | Population | Renal function | Specimen(s) | NGAL levels: Diseased vs. control subjects | Other major findings |
|---|---|---|---|---|---|
| Chronic heart failure | |||||
| Damman (10) | Clinically stable CHF outpatients (LVEF <45%) (n=90) | Mean eGFR 64 mL/min/1.73 m2 | Urine | Diseased > controls | sCr, eGFR, urinary albumin excretion and NT-pro-BNP were significantly correlated with urinary NGAL levels, combining CHF patients and controls. |
| Damman (16) | Clinically stable CHF outpatients (LVEF <45%) (n=90) | Mean measured GFR 78 mL/min/1.73 m2 | Urine | Diseased > controls | sCr, measured GFR, urinary albumin excretion and NT-pro-BNP did not correlate with urinary NGAL levels among CHF patients. NGAL levels did not differ between patients with CHF with or without CKD. |
| Poniatowski (17) | CHF due to CAD (n=150) | Mean eGFR 74.6–85.8 mL/min/1.73 m2 | Serum and urine | No controls | In multiple regression analysis, predictors of serum NGAL were NYHA class, cystatin C, and eGFR. |
| Bolignano (18) | CHF (elderly, age >65 years) (n=46) | Mean eGFR 71.5–73.6 mL/min/1.73 m2 | Serum | Diseased > controls | NGAL correlated with NYHA class. CHF patients with a serum NGAL value >783 ng/mL had a significantly higher mortality (HR 4.08, 95% CI 1.29–12.96) over a 2-year follow-up. |
| Yndestad (9) | CHF (n=150) | Mean eGFR not stated but 120/150 pts had sCr <1.4 mg/dL | Serum | Diseased > controls | Highest NGAL levels observed in NYHA class III and IV NGAL correlated with Nt-pro-BNP, but not with LVEF, cardiac index, and LV dimensions. NGAL also correlated with leukocyte count and CRP. |
| Shrestha (19) | CHF (LVEF ≤35%) (n=130) | Mean eGFR 72 mL/min/1.73 m2 | Plasma | No controls | Patients with ischemic etiology for CHF demonstrated higher NGAL levels compared with non-ischemic etiology [84 (IQR 64,129) vs. 72 (IQR 52, 102) ng/mL, respectively; p=0.024]. |
| Acute heart failure | |||||
| Yndestad (9) | Acute post-MI HF (n=236) | Mean eGFR not stated | Serum | No controls | Elevated NGAL levels were found in patients with NYHA class III (vs. NYHA I/II), both at baseline and on follow-up. NGAL correlated weakly with Nt-pro-BNP and leukocyte count. Patients with higher baseline serum NGAL levels (above the median) tended to have more adverse CV events. |
| Shrestha (19) | AHF admitted to ICU (LVEF ≤35%) (n=69) | Mean eGFR 71 mL/min/1.73 m2 | Plasma | No controls | NGAL levels were modestly associated with indices of diastolic dysfunction, but not after adjustment for renal function. There was no association between plasma NGAL and hemodynamic indices of intracardiac pressures. |
| Alvelos (20) | AHF admitted to hospital (n =121) | Mean eGFR 40 mL/min/1.73 m2 | Serum | No controls | NGAL and BNP were independent predictors of death and rehospitalization at 3 months. |
| Maisel (21) | AHF admitted to hospital (n=186) | Median eGFR 55–57 mL/min/1.73 m2 at discharge | Plasma | No controls | NGAL and BNP at time of hospital discharge were independent predictors of death and AHF rehospitalization at 30 days. |
| Coronary heart disease | |||||
| Haapio (22) | Suspected CHD who underwent nuclear stress perfusion testing (n=34) | Mean sCr 0.9–1.0 mg/dL | Plasma | No controls | NGAL levels were below limits of detection in 11/34 subjects. Patients with detectable plasma NGAL had more segmental perfusion defects at rest, and lower LVEF at rest and with stress, and lower end systolic volume with stress. |
| Choi (23) | Angiographically confirmed CHD (n=49) | NS | Serum | Diseased > controls | On multivariable analysis, NGAL level, systolic blood pressure and insulin resistance were independently associated with the presence of CHD. NGAL correlated positively with body weight, fasting insulin levels and insulin resistance, and negatively with HDL levels. |
| Zografos (11) | Suspected CHD who underwent angiography (n=73) | All pts had “normal sCr” | Serum | No controls | NGAL levels were higher in patients with angiographically confirmed CHD compared to those with normal coronary arteries. NGAL levels correlated with the number of diseased vessels and the severity of the CHD. |
| Astrom Olsson (24) | Patients with first AMI (n=49) | NS | Serum | No controls | There was no change in NGAL levels following reperfusion of AMI by percutaneous coronary intervention. |
NS, not stated.
Table 4.
Correlation between systemic NGAL levels and CVD markers, CVD severity and major adverse cardiovascular events in humans.
| Reference | Blood and/or urine NGAL levels correlate with | ||||
|---|---|---|---|---|---|
|
|
|||||
| Renal function | Natriuretic peptides | Biomarkers of inflammation | Clinical severity of CVD | Mortality/major adverse CV events | |
| Chronic heart failure | |||||
| Damman (10) | Yes | Yes | NS | NS | NS |
| Damman (16) | No | No | NS | NS | NS |
| Poniatowski (17) | Yes | NS | NS | Yes | NS |
| Bolignano (18) | No | NS | NS | Yes | Yes (univariate analysis) |
| Yndestad (9) | NS | Yes | Yes | Yes | NS |
| Shrestha (19) | Yes | Yes | NS | Yes | Yes (univariate), but no longer significant on multivariate analysis |
| Acute heart failure | |||||
| Yndestad (9) | NS | Yes | Yes | Yes | No (univariate analysis) |
| Shrestha (19) | Yes | Yes | NS | No | NS |
| Alvelos (20) | NS | NS | NS | NS | Yes (uni- and multivariate analysis) |
| Maisel (21) | Yes | Yes | NS | NS | Yes (uni- and multivariate analysis) |
| Coronary heart disease | |||||
| Zografos (11) | NS | NS | NS | Yes | NS |
| Cerebrovascular disease | |||||
| Elneihoum (25) | NS | – | NS | No | NS |
| Giaginis (26) | Yes | – | NS | No | NS |
| Elneihoum (12) | NS | – | Yes | NS | NS |
| Falke (27) | NS | – | NS | NS | Yes (uni- and multivariate analysis) |
NS, not stated.
Other than renal function, NGAL values also correlated with the clinical severity of CHF (e.g., New York Heart Association, NYHA class) (9, 17, 18) and as well as N terminal pro-brain natriuretic peptide (Nt-pro-BNP, r=0.24–0.37, p<0.001–0.008) (9, 10, 19). However, some investigators did not observe this after reanalyzing the same cohort (10, 16). There were also divergent results with regards to ventricular structure and function. Serum NGAL was weakly correlated with left ventricular ejection fraction (LVEF, r=0.19, p=0.05) in one small study (18), while other studies found no correlation between plasma or urine NGAL and echocardiographic indices of LV cardiac structure or LV or RV systolic function (9, 19). In another study, plasma NGAL levels were modestly associated with indices of diastolic dysfunction, but not after adjustment for renal function (19). Other divergent results include higher serum NGAL levels observed in ischemic, compared with non-ischemic, etiology for CHF in one study (19), but not in others (9, 10).
In terms of NGAL's prognostic value, in a small study of 46 elderly CHF patients, those with a serum NGAL >783 ng/mL, a value selected by receiver operator characteristic (ROC) curve analysis, had a significantly higher mortality over 2-years [unadjusted hazard ratio (HR) 4.08, 95% CI 1.29–12.96] (18). However, the results of such univariate analysis should be interpreted in light of the small sample size and the collinearity of NGAL with other factors which affect long-term outcomes in HF, including renal function. Indeed, in another study, plasma NGAL predicted cardiac death or transplantation after adjustment for age, gender, LVEF, and mitral E/Ea, but not after adjustment for renal function (19).
Acute heart failure (AHF)
Similar to the results seen in CHF, serum NGAL also appeared to correlate with renal function, like eGFR (r=−0.60 to −0.42, p<0.0001–<0.001), serum creatinine (sCr, r=0.44–0.57, p<0.0001–<0.001) and cystatin C (r=0.65, p<0.0001) (19, 21, 28, 29). Significantly elevated serum NGAL levels were found in patients with NYHA class III (vs. NYHA I/II), both at baseline and on follow-up (9). Moreover, NGAL correlated weakly, but significantly, with Nt-pro-BNP (r=0.15–0.38, p=0.004–0.03) and BNP (r=0.22, p=0.002) (9, 19, 21) (Tables 3 and 4).
In terms of prognosis, higher baseline serum NGAL levels have been associated with all-cause death and readmissions at 3 months (20), and a trend to higher incidence of the composite endpoint of non-fatal MI, CV death, all-cause death, and stroke at median follow-up of 27 months (9). The GALLANT study (n=186) instead looked at the prognostic value of plasma NGAL at the time of hospital discharge (21). Interestingly, discharge plasma NGAL level was a stronger predictor of 30-day outcome (all-cause death and HF readmissions) than BNP (adjusted HR for NGAL, 19.91, 95% CI 3.47–114.19 vs. adjusted HR for BNP, 2.33, 95% CI 0.93–5.79). The investigators speculate that NGAL is not only a risk predictor for renal injury but is an overall strong risk marker for cardiac events in the setting of AHF.
In addition, although not the primary focus of this review (not included in Table 3), there is preliminary evidence elevated admission serum NGAL levels predicts worsening renal function in patients with acute decompensated HF, with area under the ROC curve ranging from 0.70 to 0.93 (28, 29). Of note, recent evidence suggests that the monomeric form of NGAL is associated more closely with AKI (30, 31). Furthermore, blood NGAL values observed in HF and CHD patients (21, 22) are relatively lower compared to those seen in AKI, particularly in cardiac surgery and intensive care units (2, 32). Therefore, a more specific assay targeting the monomeric form, with an extended range, may be more appropriate for diagnosing renal injury, particularly in CVD patients (33, 34).
Coronary heart disease (CHD)
Four studies measured NGAL levels in patients with CHD (Table 3). In 34 adults who underwent elective nuclear stress perfusion testing, only 23 had detectable plasma NGAL levels; the rest had values below the lower limit of the assay (60 ng/mL). Those with detectable plasma NGAL had more segmental perfusion defects at rest, and lower LVEF at rest and with stress, and lower end systolic volume with stress (22). In a second study, serum NGAL was significantly higher in 49 patients with angiographically confirmed CHD when compared to 42 age-, gender-, and BMI-matched controls (82.6 ng/mL in CHD vs. 43.8 ng/mL in controls, p <0.001) (23). Furthermore, they observed a positive correlation between serum NGAL and body weight, fasting insulin levels and insulin resistance, and a negative correlation with high density lipoprotein (HDL) levels. On multivariable analysis, NGAL level was independently associated with the presence of CHD, along with systolic blood pressure and insulin resistance. In another study, serum NGAL levels were measured in 73 patients who underwent first-time angiography for suspected CHD (11). Marginally higher NGAL levels were noted in patients with angiographically confirmed CHD compared to those with normal coronary arteries (29 vs. 22.4 ng/mL, respectively, p =0.004). Furthermore, they observed statistically significant correlations between NGAL levels and the number of diseased vessels (r = 0.39, p =0.01) and the severity of CHD, as indicated by the modified Gensini score (r = 0.356, p =0.002). The last study investigated markers of neutrophil activation, including serum NGAL, following reperfusion of acute myocardial infarction (AMI) by percutaneous coronary intervention (PCI) (24). No significant changes in NGAL were seen within 24 h of PCI, in contrast to an increase in cytokines and C-reactive protein (CRP), and a decrease in plasma myeloperoxidase and malondialhyde.
Cerebrovascular disease
Key results are summarized in Table 5. Numerous abnormalities of leukocyte function have been described in patients with acute and chronic ischemic vascular diseases, including cerebrovascular disease (36). In subjects with asymptomatic early atherosclerosis, plasma levels of markers of systemic leukocyte activation, including NGAL, neutrophil proteinase-4 (NP4), tumor necrosis factor (TNF)-α, soluble TNF receptor protein-1 (sTNFR-1), were correlated with age, blood pressure, and smoking (25). No significant correlations were found between these markers and the degree of atherosclerosis. Similarly, in patients with advanced carotid atherosclerosis, plasma NGAL was associated with hypertension, increased age and homocysteine, but not with degree of carotid stenosis (26). As in the HF studies, NGAL correlated with renal function (sCr, r=0.46; eGFR, r=−0.49, p<0.0001 for both) in this study.
Table 5.
Summary of human studies on NGAL and cerebrovascular disease.
| Reference | Population | Renal function | Specimen(s) | NGAL levels: Diseased vs. control subjects | Other major findings |
|---|---|---|---|---|---|
| Elneihoum (25) | Asymptomatic carotid artery plaque (n=156) | NS | Plasma | No controls | Plasma levels of markers of systemic leukocyte activation, including NGAL, were correlated with age, blood pressure, and smoking. No significant correlations were found between these markers and the plaque score. |
| Giaginis (26) | Carotid endarterectomy (n=141) | NS | Plasma | No controls | NGAL correlated with age, sCr, eGFR and homocysteine, but not with ≥ 90% carotid stenosis. |
| Elneihoum (12) | Acute ischemic stroke (n=72) and TIA (n=48) | NS | Plasma | Diseased > controls | NGAL correlated with other markers of leukocyte activation: fibrinogen, ESR, leukocyte counts, sTNFR-1, and NP4. NGAL did not correlate with lipid levels nor with indicators of insulin resistance. |
| Anwaar (35) | Acute cerebral infarction (n=45) and TIA (n=14) | NS | Plasma | No controls | NGAL and sTNFR-1 and intraplatelet AMP were higher 1 year after the acute ischemic event. |
| Falke (27) | Acute ischemic stroke (n=90) and TIA (n=54) | NS | Plasma | No controls | Higher plasma levels of sTNFR1 and NGAL were significant independent predictors of cardiovascular mortality at 4 years follow-up. |
NS, not stated.
In patients with acute ischemic cerebrovascular insult [72 with stroke, 48 with transient ischemic attack (TIA)], leukocyte activation markers were measured 1–3 days after the cerebrovascular event (12). Plasma NGAL was significantly higher in the stroke group (median 122 μg/L, p<0.0001 vs. age- and sex-matched controls) and the TIA group (median 110 μg/L, p<0.01 vs. control) than in the control group (median 97 μg/L). They also noted significant correlations between plasma NGAL and other markers of leukocyte activation: fibrinogen (r=0.40), erythrocyte sedimentation rate (ESR, r=0.35), leukocyte counts (r=0.3), sTNFR-1 (r=0.45), and NP4 (r=0.45). These results suggest that activated leukocytes and their secretory inflammatory mediators are involved in acute cerebrovascular ischemia and its consequences. No correlation was observed between NGAL and the degree of carotid artery atherosclerotic changes. In a follow-up study by the same investigators on 59 patients with acute ischemic stroke or TIA, they found that levels of plasma NGAL, sTNFR-1 and cyclic 3′, 5′ -adenosine monophosphate (cAMP) were higher 1 year following the acute event of cerebral ischemia, indicating persistent and increasing leukocyte activation even after the acute stage of ischemic cerebrovascular disease (35). On 4-year follow-up of these same patients, high levels of NGAL and sTNFR-1 at the time of ischemic event were associated with higher cardiovascular mortality (odds ratio 3.6 and 2.0, respectively) after adjustment for age, sex and diagnosis (27) (Tables 4 and 5).
Discussion
Neutrophil gelatinase-associated lipocalin has generated great interest as a novel biomarker for the timely detection of AKI, and for the prediction of clinical outcomes, such as dialysis requirement and mortality in several common clinical scenarios (1–6).
Although best known for its properties as a renal biomarker, NGAL has multiple functions, depending on the context of its primary ligand in specific tissue (1). For instance, when NGAL is complexed with MMP-9, there is enhancement of the active MMP-9 pool with resultant upregulation of MMP-9's well-known pro-angiogenic and pro-invasive properties. This interaction between NGAL and MMP-9 may be especially pertinent to the pathophysiology of atherosclerotic plaques and acute coronary syndromes. MMP expression has been shown to be induced by activated macrophages both directly through the oxidative stress that comes with the intracellular accumulation of lipid and indirectly by cytokine release (37). Excessive MMP activity can weaken vessels and, more importantly, destabilize plaques leading to an increased risk of rupture. Evidence suggests a critical role for MMP-9 in acute coronary syndrome (38). Degradation of MMP-9 has been shown to be significantly inhibited in the presence of NGAL and results in the preservation of MMP-9 enzymatic activity (39). This particular aspect of NGAL biology has important cardiovascular implications, and served as an important stimulus for this systematic review.
To our knowledge, this is the first systematic review on NGAL and cardiovascular disease, specifically focusing on its “non-renal” aspects. Multiple animal and human studies were identified. Data from animal studies and human tissue demonstrate that NGAL is highly expressed in the heart, both in failing myocardium and myocarditis, and it is also expressed in atherosclerotic plaques (Tables 1 and 2). Furthermore, NGAL and MMP-9 co-localized in these tissues, and these areas of co-localization exhibited increased MMP proteolytic activity. Collectively, these data provide biological plausibility for an active role of NGAL in CVD, particularly with regards to atherosclerotic plaques and myocardial remodeling.
Do systemic NGAL levels reflect the severity of CVD (Table 4)? In both AHF and CHF, NGAL correlated with clinical and neurohormonal measures of disease severity in some but not all, studies. In an analogous manner, blood NGAL levels showed an association with the presence and severity of coronary lesions. However, in both carotid and aortic atherosclerotic disease, these levels did not appear to correlate with degree of stenosis in the studied vessels. It is worthy of note that plasma levels of markers of NGAL correlated with other markers of systemic leukocyte activation, such as leukocyte counts, CRP, fibrinogen, ESR, sTNFR-1, NP4, cAMP in CVD, such as HF and cerebrovascular disease. In stroke patients, these plasma markers remained elevated 1 year following an acute event of cerebral ischemia, indicating persistent and increasing leukocyte activation even after the acute stage of ischemic cerebrovascular disease (35). These results support the hypothesized relationship between the level of systemic leukocyte activation and CVD.
It is likewise important to note that studies showing a relationship between levels of NGAL and inflammatory markers only measured NGAL in the blood (9, 12). Recent evidence has shown that blood NGAL, but not urine NGAL, correlates with leukocytosis (40). Conversely, the presence of urinary tract infection and leukocyturia has been observed to affect urine NGAL levels (41). It is the dimeric form which is predominantly secreted by neutrophils (30, 31).
In general, NGAL appeared to correlate with various indices of renal function, especially in HF. Elevated urine NGAL was felt to represent tubular damage (10). Investigators have also speculated that both serum and urine NGAL may be potential early and sensitive markers of kidney impairment/injury in CHF patients. This was, however, not a universal finding. It is interesting that one set of investigators published their data on the same 90 CHF patients in two papers. In the first, they concluded that urine NGAL correlated with renal indices and Nt-pro-BNP (10); in the second, NGAL did not correlate with measured GFR, estimated renal plasma flow, urinary albumin excretion or Nt-pro-BNP (16). This difference could potentially be attributed to inclusion of data from control subjects in the first, but not the second, paper.
Some investigators have suggested that measurement of NGAL may be of prognostic value. Such an association has been observed in CHF, AHF and cerebrovascular disease (9, 18–21, 27) (Table 4). In contrast, others argue that systemic NGAL levels are largely determined by underlying impairment of renal rather than myocardial function, and that prognostic significance does not persist after adjustment for underlying renal function (19). Indeed, in most of these studies, there has been minimal to no adjustment for potential confounders, such as CKD. It is relevant therefore, that in AHF, discharge plasma NGAL level was a stronger predictor of 30-day outcome than BNP, the guideline-supported standard for risk prediction (42), even after adjustment for renal function. If reproduced in other studies, one could speculate that a high NGAL at the time of discharge for AHF might, at a minimum, be a signal for early follow-up. It is also conceivable that the prognostic significance is related to NGAL being a marker of leukocyte activation.
Ours is the first comprehensive review on the cardiovascular aspects of NGAL, and have important implications for clinicians and future research strategies. Nonetheless, we acknowledge certain limitations in this systematic review. Likely there is inherent publication bias, as negative biomarker studies often go unpublished. The studies which reported on prognosis were of modest sample sizes (range, 46–236), with limited adjustment for confounding variables and cannot be considered definitive.
Conclusions
In summary, we performed a novel systematic review on NGAL and cardiovascular disease, focusing on its “non-renal” aspects. Data from animal and human tissue indicate the NGAL is highly expressed in cardiomyocytes and other heart tissue, and co-localized with MMP-9 in atherosclerotic plaques. Modest elevations of systematic NGAL levels have been reported in a variety of CVDs, including heart failure, coronary disease and stroke. A prognostic value for NGAL has been suggested in few studies, in part limited by modest sample size and minimal adjustment for potential confounders. There appears to be ample evidence to support a putative role of NGAL in the pathophysiology of CVD, but there is insufficient data regarding the clinical use of urine or plasma NGAL levels in the current management of CVD. Available evidence regarding NGAL as a predictor of outcomes in CVD is very limited and remains inconclusive. While research on NGAL in CVD is still in the early stages, on the basis of current data we believe it merits further study to clarify its potential role, if any, in the arsenal of cardiac biomarkers available to scientists and clinicians.
Acknowledgments
The authors would like to thank Jeong Chul Kim and Manish Kaushik for their assistance with retrieving articles.
Research funding: There was no funding for this article. A. Maisel has received research support from Abbott Diagnostics for other work.
Biographies

Dinna N. Cruz, MD, MPH, is Director for Research at the Department of Nephrology, Dialysis and Transplantation at San Bortolo Hospital and the International Renal Research Institute Vicenza (IRRIV) in Vicenza, Italy. As a nephrologist and epidemiologist, her research interests and expertise include extra-corporeal blood purification techniques, acute kidney injury and the cardiorenal syndromes. She has authored over several peer-reviewed publications on various aspects of renal and cardiorenal disease in prestigious international journals and has contributed chapters to key international nephrology textbooks. She serves as Associate Editor for Blood Purification and Clinical Kidney Journal and as a reviewer for a number of high ranking Nephrology, ICU, and Internal Medicine journals.

Sérgio Gaião, MD is a nephrologist and is currently working at the General Intensive Care Unit in Hospital São João, Porto, Portugal. During his nephrology training, he did a research fellowship with the International Renal Research Institute of Vicenza (IRRIV). He also lectures in Critical Care Nephrology at the Faculty of Medicine, University of Porto. He is dedicated to clinical research, specifically biomarkers of acute kidney injury and renal replacement therapy, in part in collaboration with IRRIV.

Alan Maisel, MD, is a Professor of Medicine at the University of California, San Diego. He is the Director of the Coronary Care Unit and Heart Failure Program at the VA San Diego Healthcare System in La Jolla, California. He is an associate editor of the Journal of the American College Cardiology. Dr. Maisel is considered one of the world's experts on cardiac biomarkers and has over 150 scientific publications. He has authored several ground-breaking publications that have paved the way for the development of diagnostic tools for patients with congestive heart failure.

Claudio Ronco, MD, is Director of the Department of Nephrology, Dialysis and Transplantation at San Bortolo Hospital and the International Renal Research Institute Vicenza (IRRIV) in Vicenza, Italy. He is Editor-in-Chief of Blood Purification and the former President of the International Society for Hemodialysis (ISHD). He is a member of the council of several scientific societies. He has co-authored 650 papers, 36 book chapters, 45 books and seven monographic journal issues and has delivered more than 450 lectures at international meetings and universities.

Prasad Devarajan, MD, is the Williams Endowed Chair, Professor of Pediatrics and Developmental Biology, Director of Nephrology and Hypertension, Director of Clinical Laboratories, and CEO of the Dialysis Unit at Cincinnati Children 's Hospital Medical Center and University of Cincinnati. He has authored over 200 peer-reviewed publications. He has been continuously funded by the National Institutes of Health and several other foundations for more than 25 years. His major research interests lie in using proteomic and functional genomic approaches for the discovery of pathogenetic pathways, diagnostic biomarkers, and novel therapies of acute kidney injury and chronic kidney disease. His laboratory has pioneered the discovery, translation, and validation of NGAL as a biomarker of acute kidney injury.
Footnotes
Conflict of interest statement: Authors' conflict of interest disclosure: The authors declare the following conflicts of interest.
Employment or leadership: A. Maisel and C. Ronco are consultants to Alere, C. Ronco is a member of speakers bureau for Abbott Diagnostics. P. Devarajan is a co-inventor on NGAL patents.
Honorarium: D. Cruz has received honoraria for speaking assignments from Alere. P. Devarajan has received honoraria for speaking assignments from Alere and Abbott Diagnostics.
References
- 1.Devarajan P. Neutrophil gelatinase-associated lipocalin – an emerging troponin for kidney injury. Nephrol Dial Transplant. 2008;23:3737–43. doi: 10.1093/ndt/gfn531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz DN, de Cal M, Garzotto F, Perazella MA, Lentini P, Corradi V, et al. Plasma neutrophil gelatinase-associated lipocalin is an early biomarker for acute kidney injury in an adult ICU population. Intensive Care Med. 2010;36:444–51. doi: 10.1007/s00134-009-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz DN, de Geus HR, Bagshaw SM. Biomarker strategies to predict need for renal replacement therapy in acute kidney injury. Semin Dial. 2011;24:124–31. doi: 10.1111/j.1525-139X.2011.00830.x. [DOI] [PubMed] [Google Scholar]
- 4.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–61. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soni SS, Pophale R, Ronco C. New biomarkers for acute renal injury. Clin Chem Lab Med. 2011;49:1257–63. doi: 10.1515/CCLM.2011.664. [DOI] [PubMed] [Google Scholar]
- 6.Soni SS, Cruz D, Bobek I, Chionh CY, Nalesso F, Lentini P, et al. NGAL: a biomarker of acute kidney injury and other systemic conditions. Int Urol Nephrol. 2010;42:141–50. doi: 10.1007/s11255-009-9608-z. [DOI] [PubMed] [Google Scholar]
- 7.Hemdahl AL, Gabrielsen A, Zhu C, Eriksson P, Hedin U, Kastrup J, et al. Expression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26:136–42. doi: 10.1161/01.ATV.0000193567.88685.f4. [DOI] [PubMed] [Google Scholar]
- 8.Folkesson M, Kazi M, Zhu C, Silveira A, Hemdahl AL, Hamsten A, et al. Presence of NGAL/MMP-9 complexes in human abdominal aortic aneurysms. Thromb Haemost. 2007;98:427–33. [PubMed] [Google Scholar]
- 9.Yndestad A, Landro L, Ueland T, Dahl CP, Flo TH, Vinge LE, et al. Increased systemic and myocardial expression of neutrophil gelatinase-associated lipocalin in clinical and experimental heart failure. Eur Heart J. 2009;30:1229–36. doi: 10.1093/eurheartj/ehp088. [DOI] [PubMed] [Google Scholar]
- 10.Damman K, van Veldhuisen DJ, Navis G, Voors AA, Hillege HL. Urinary neutrophil gelatinase associated lipocalin (NGAL), a marker of tubular damage, is increased in patients with chronic heart failure. Eur J Heart Fail. 2008;10:997–1000. doi: 10.1016/j.ejheart.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Zografos T, Haliassos A, Korovesis S, Giazitzoglou E, Voridis E, Katritsis D. Association of neutrophil gelatinase-associated lipocalin with the severity of coronary artery disease. Am J Cardiol. 2009;104:917–20. doi: 10.1016/j.amjcard.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Elneihoum AM, Falke P, Axelsson L, Lundberg E, Lindgarde F, Ohlsson K. Leukocyte activation detected by increased plasma levels of inflammatory mediators in patients with ischemic cerebrovascular diseases. Stroke. 1996;27:1734–8. doi: 10.1161/01.str.27.10.1734. [DOI] [PubMed] [Google Scholar]
- 13.Ding L, Hanawa H, Ota Y, Hasegawa G, Hao K, Asami F, et al. Lipocalin-2/neutrophil gelatinase-B associated lipocalin is strongly induced in hearts of rats with autoimmune myocarditis and in human myocarditis. Circ J. 2010;74:523–30. doi: 10.1253/circj.cj-09-0485. [DOI] [PubMed] [Google Scholar]
- 14.Bu DX, Hemdahl AL, Gabrielsen A, Fuxe J, Zhu C, Eriksson P, et al. Induction of neutrophil gelatinase-associated lipocalin in vascular injury via activation of nuclear factor-kappaB. Am J Pathol. 2006;169:2245–53. doi: 10.2353/ajpath.2006.050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.te Boekhorst BC, Bovens SM, Hellings WE, van der Kraak PH, van de Kolk KW, Vink A, et al. Molecular MRI of murine atherosclerotic plaque targeting NGAL: a protein associated with unstable human plaque characteristics. Cardiovasc Res. 2011;89:680–8. doi: 10.1093/cvr/cvq340. [DOI] [PubMed] [Google Scholar]
- 16.Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD, et al. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96:1297–302. doi: 10.1136/hrt.2010.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poniatowski B, Malyszko J, Bachorzewska-Gajewska H, Malyszko JS, Dobrzycki S. Serum neutrophil gelatinase-associated lipocalin as a marker of renal function in patients with chronic heart failure and coronary artery disease. Kidney Blood Press Res. 2009;32:77–80. doi: 10.1159/000208989. [DOI] [PubMed] [Google Scholar]
- 18.Bolignano D, Basile G, Parisi P, Coppolino G, Nicocia G, Buemi M. Increased plasma neutrophil gelatinase-associated lipocalin levels predict mortality in elderly patients with chronic heart failure. Rejuvenation Res. 2009;12:7–14. doi: 10.1089/rej.2008.0803. [DOI] [PubMed] [Google Scholar]
- 19.Shrestha K, Borowski AG, Troughton RW, Thomas JD, Klein AL, Tang WH. Renal dysfunction is a stronger determinant of systemic neutrophil gelatinase-associated lipocalin levels than myocardial dysfunction in systolic heart failure. J Card Fail. 2011;17:472–8. doi: 10.1016/j.cardfail.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvelos M, Lourenco P, Dias C, Amorim M, Rema J, Leite AB, et al. Prognostic value of neutrophil gelatinase-associated lipocalin in acute heart failure. Int J Cardiol. 2011 Aug 26; doi: 10.1016/j.ijcard.2011.07.080. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 21.Maisel AS, Mueller C, Fitzgerald R, Brikhan R, Hiestand BC, Iqbal N, et al. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: the NGAL EvaLuation Along with B-type NaTriuretic Peptide in acutely decompensated heart failure (GALLANT) trial. Eur J Heart Fail. 2011;13:846–51. doi: 10.1093/eurjhf/hfr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haapio M, House AA, de Cal M, Cruz DN, Lentini P, Giavarina D, et al. Heart-kidney biomarkers in patients undergoing cardiac stress testing. Int J Nephrol. 2011;2011:425923. doi: 10.4061/2011/425923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi KM, Lee JS, Kim EJ, Baik SH, Seo HS, Choi DS, et al. Implication of lipocalin-2 and visfatin levels in patients with coronary heart disease. Eur J Endocrinol. 2008;158:203–7. doi: 10.1530/EJE-07-0633. [DOI] [PubMed] [Google Scholar]
- 24.Astrom-Olsson K, Hedstrom E, Hulten LM, Wiklund O, Arheden H, Ohlin AK, et al. Dissociation of the infl ammatory reaction following PCI for acute myocardial infarction. J Invasive Cardiol. 2007;19:452–6. [PubMed] [Google Scholar]
- 25.Elneihoum AM, Falke P, Hedblad B, Lindgarde F, Ohlsson K. Leukocyte activation in atherosclerosis: correlation with risk factors. Atherosclerosis. 1997;131:79–84. doi: 10.1016/s0021-9150(96)06077-7. [DOI] [PubMed] [Google Scholar]
- 26.Giaginis C, Zira A, Katsargyris A, Klonaris C, Theocharis S. Clinical implication of plasma neutrophil gelatinase-associated lipocalin (NGAL) concentrations in patients with advanced carotid atherosclerosis. Clin Chem Lab Med. 2010;48:1035–41. doi: 10.1515/CCLM.2010.211. [DOI] [PubMed] [Google Scholar]
- 27.Falke P, Elneihoum AM, Ohlsson K. Leukocyte activation: relation to cardiovascular mortality after cerebrovascular ischemia. Cerebrovasc Dis. 2000;10:97–101. doi: 10.1159/000016037. [DOI] [PubMed] [Google Scholar]
- 28.Alvelos M, Pimentel R, Pinho E, Gomes A, Lourenco P, Teles MJ, et al. Neutrophil gelatinase-associated lipocalin in the diagnosis of type 1 cardiorenal syndrome in the general ward. Clin J Am Soc Nephrol. 2011;6:476–81. doi: 10.2215/CJN.06140710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aghel A, Shrestha K, Mullens W, Borowski A, Tang WH. Serum neutrophil gelatinase-associated lipocalin (NGAL) in predicting worsening renal function in acute decompensated heart failure. J Card Fail. 2010;16:49–54. doi: 10.1016/j.cardfail.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 2010;5:2229–35. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martensson J, Xu S, Bell M, Martling CR, Venge P. Immunoassays distinguishing between HNL/NGAL released in urine from kidney epithelial cells and neutrophils. Clin Chim Acta. 2012 May 18; doi: 10.1016/j.cca.2012.05.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–57. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronco C, Cruz D, Noland BW. Neutrophil gelatinase-associated lipocalin curve and neutrophil gelatinase-associated lipocalin extended-range assay: a new biomarker approach in the early diagnosis of acute kidney injury and cardio-renal syndrome. Semin Nephrol. 2012;32:121–8. doi: 10.1016/j.semnephrol.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Lippi G, Cervellin G. Neutrophil gelatinase-associated lipocalin: a more specific assay is needed for diagnosing renal injury. Clin Chim Acta. 2012;413:1160–1. doi: 10.1016/j.cca.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Anwaar I, Gottsater A, Ohlsson K, Mattiasson I, Lindgarde F. Increasing levels of leukocyte-derived infl ammatory mediators in plasma and cAMP in platelets during follow-up after acute cerebral ischemia. Cerebrovasc Dis. 1998;8:310–7. doi: 10.1159/000015873. [DOI] [PubMed] [Google Scholar]
- 36.Fisher TC, Meiselmann HJ. Polymorphonuclear leukocytes in ischemic vascular disease. Thromb Res. 1994;74(Suppl 1):S21–34. doi: 10.1016/s0049-3848(10)80004-0. [DOI] [PubMed] [Google Scholar]
- 37.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–62. [PubMed] [Google Scholar]
- 38.Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995;91:2125–31. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- 39.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–65. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 40.Lippi G, Salvagno GL, Banfi G. Serum but not urine concentration of neutrophil gelatinase-associated lipocalin is influenced by acute leukocyte variations. Leuk Lymphoma. 2012 Apr 19; doi: 10.3109/10428194.2012.658390. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Decavele AS, Dhondt L, De Buyzere ML, Delanghe JR. Increased urinary neutrophil gelatinase associated lipocalin in urinary tract infections and leukocyturia. Clin Chem Lab Med. 2011;49:999–1003. doi: 10.1515/CCLM.2011.156. [DOI] [PubMed] [Google Scholar]
- 42.Cruz DN, Goh CY, Palazzuoli A, Slavin L, Calabro A, Ronco C, et al. Laboratory parameters of cardiac and kidney dysfunction in cardio-renal syndromes. Heart Fail Rev. 2011;16:545–51. doi: 10.1007/s10741-011-9231-9. [DOI] [PubMed] [Google Scholar]