Abstract
There are many active drugs to treat metastatic renal cell carcinoma (mRCC) patients who progress through their first-line vascular endothelial growth factor (VEGF) inhibitor. Many clinicians choose a second-line VEGF inhibitor based on the type of response to first-line VEGF inhibitor, without data supporting this practice. This study was conducted to determine the association of response to second-line VEGF inhibitor with response to first-line VEGF inhibitor. All mRCC patients in participating centers of the International mRCC Database Consortium who were treated from January 2004 through June 2011 with a second-line VEGF inhibitor after failure of a different first-line VEGF inhibitor were retrospectively identified. The primary outcome is objective response rate (ORR) and the secondary outcome is progression-free survival (PFS) in each line of therapy. Of 1,602 total database patients, 464 patients received a first- and second-line VEGF inhibitor. The ORR to first-line therapy was 22 %, and the ORR to second-line therapy was 11 %. The ORR to second-line therapy was not different among patients achieving partial response versus stable disease versus progressive disease to first-line therapy (14 % vs. 10 % vs. 11 %, respectively; chi-squared trend test p=0.17). The median PFS on first-line VEGF-targeted therapy was 7.5 months (95 % CI, 6.6–8.1), and the median PFS on second-line VEGF inhibitor was 3.9 months (95 % CI, 3.6–4.5). There was no correlation between first-line and second-line PFS (Pearson correlation coefficient 0.025; p=0.59). The clinical response to a second-line VEGF inhibitor is not dependent on response to the first-line VEGF-inhibitor. Further studies are needed to define clinical parameters that predict response to second-line therapy to optimize the sequence of VEGF-targeted therapy in metastatic RCC patients.
Keywords: Association of TKIs, First-line and second-line VEGF inhibitors, Renal cell cancer, Tyrosine kinase inhibitors, VEGF-targeted therapy
Introduction
Recent advances in the management of metastatic renal cell carcinoma (RCC) have included agents targeting circulating ligand for vascular endothelial growth factor (VEGF), bevacizumab [1, 2], or small molecule VEGF receptor inhibitors including pazopanib [3, 4], sorafenib [5, 6], sunitinib [7, 8], and axitinib [9–11]. Currently, the standard treatment of metastatic RCC entails empiric sequential use of single agents. The data that influence selecting both initial therapy and subsequent therapy of the available agents are incomplete [12].
VEGF-targeted therapy remains the most active treatment for most patients with metastatic RCC, but the initial duration of disease control is approximately 11 months [7], and all patients eventually develop resistance. With little available sequencing data to direct the most effective second-line agent, the clinician is challenged whether to continue with another VEGF-targeted therapy based on good response to the first-line agent or whether to change to a drug with a different mechanism of action. Many physicians presume that patients who achieve response to initial VEGF blockade will respond better to second-line VEGF-targeted therapy, while non-responding patients should switch to an alternative mechanism (e.g., mammalian target of rapamycin (mTOR) inhibition) [13–15]. However, not enough data exist to support this practice. Therefore, a retrospective review of an international database of metastatic RCC patients was undertaken in order to identify patients who received a VEGF-targeting agent in the first- and second-line setting in order to investigate the association of clinical outcome between the two lines of therapy.
Patients and methods
Study design
Patients identified for this analysis were treated in participating Cancer Centers of the International mRCC Database Consortium in the USA, Canada, Denmark, South Korea, and Singapore during the time period from January 2004 through June 2011. A retrospective review was performed in all patients who received first-line VEGF-targeted therapy during this time frame. The decision about the subsequent anticancer therapy was then made at the discretion of the treating physicians. Patients who received a second-line VEGF-targeted therapy were identified for this analysis. Patients who received mTOR inhibitors as second-line agents were excluded. Patients who were re-challenged with the same first-line agent were also excluded.
Endpoints and assessments
The primary endpoint was the association of the objective response to second-line therapy versus the objective response to first-line therapy of mRCC. The objective response was calculated for both first-line and second-line therapies and defined based on the Response Evaluation Criteria in Solid Tumors (RECIST) 1.0 criteria as per investigator assessment. Best response was categorized as either a complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD).
The secondary endpoint was progression-free survival (PFS), defined as the time interval between the date of initiation of therapy to the date of disease progression based on RECIST 1.0 criteria, or cessation of treatment due to intolerance or toxicity. The PFS was calculated separately for each therapy on first-line (PFS1) or second-line therapy (PFS2). Disease assessments were based on radiographic imaging using computed tomography of the chest, abdomen, and pelvis; whole body bone scan; and magnetic resonance imaging of the brain. Assessment was done clinically every 1 to 2 months and radiographically approximately every 3 months based on treating physician practice. Overall survival (OS) was defined as the time interval from first-line drug initiation to death or censored at last follow-up, and for patients who received second-line therapy, the median overall survival (OS) was measured from initiation of first-line therapy.
Statistical methods and analysis
Summary statistics were used to describe the patient population and baseline characteristics. Best responses for treatments were categorized into CR, PR, SD, and PD. Responses for the first drug were compared to responses of the second drug, and a chi-squared trend test was used to detect a significant difference in the proportions of responses.
Progression-free survivals for first- and second-line treatments were calculated for each patient and PFS1 was plotted against PFS2 on a scatter plot. A Pearson correlation coefficient with a p value for significance was calculated comparing PFS1 against PFS2. Kaplan–Meier curves were used for estimating the progression-free survival and overall survival for all patients. Statistical analyses were performed on SAS 9.2 (Cary, NC, USA).
Results
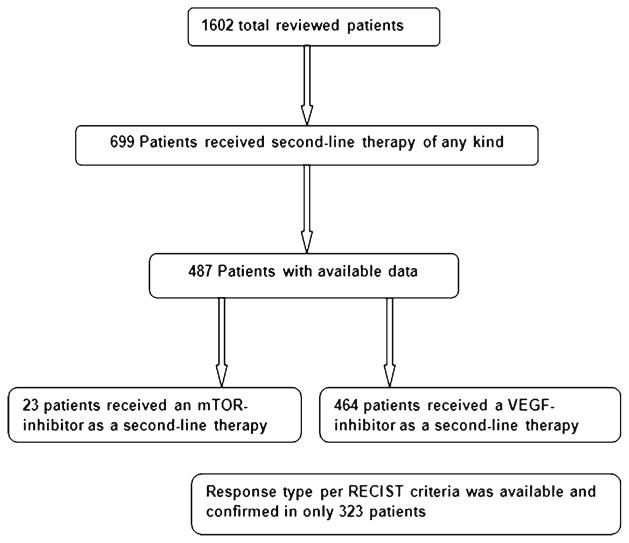
Of 1,602 total database patients, 699 patients received second-line targeted therapy (VEGF inhibitor or mTOR agent). Response data for both first- and second-line were available only in 487 patients, of which 464 patients received VEGF inhibitor as both first- and second-line therapies (Fig. 1). Since there were only 23 patients who received mTOR inhibitors as a second-line therapy, that number was not sufficient to elaborate reliable results about second-line mTOR therapy. Thus, patients that received mTOR inhibitors were excluded from the analysis. RECIST 1.0 criteria response rates were available from 323 patients out of the 464 patients The majority of patients had prior nephrectomy with a median age of 50 years and Karnofsky performance status of more than 80 % (Table 1). Fifty-five percent of patients initiated first-line therapy within a year from diagnosis and less than 10 % had brain metastases. Seventy-nine percent of patients had more than one site of metastasis. Histologically, most patients had a diagnosis of clear cell or predominantly clear cell histology and only 8.2 % had non-clear cell histology. Based on the prognostic criteria of Heng et al. [16], 25 % had favorable risk, 58 % had intermediate, and 17 % had poor risk disease. The first-line VEGF-targeted therapies received were sunitinib (54 %), sorafenib (33 %), and bevacizumab (13 %). The second-line VEGF-targeted therapies were sorafenib (51 %), sunitinib (37 %), bevacizumab (7 %), pazopanib (3 %), and axitinib (2 %).
Fig. 1.
Algorithm of first-line and second-line therapies
Table 1.
Patient characteristics prior to first-line VEGF-targeted therapy
| Percent | No. of patientsa | |
|---|---|---|
| Median age (range) | 50 (43.8–77.7) | 464 |
| Diagnosis to treatment > 1 year | 55.5 | 463 |
| Karnofsky performance status < 80 % | 14.3 | 427 |
| Non clear cell histology | 8.2 | 429 |
| Sarcomatoid histology | 6.6 | 411 |
| Thrombocytosis (>ULN) | 14.0 | 422 |
| Anemia (<LLN) | 52.0 | 427 |
| Neutrophilia (>ULN) | 9.5 | 410 |
| Hypercalcemia (>ULN) | 6.0 | 402 |
| Lactate dehydrogenase elevated (>ULN) | 31.6 | 339 |
| Heng et al. prognostic group | 382 | |
| Favorable | 25 | |
| Intermediate | 58 | |
| Poor | 17 | |
| Prior nephrectomy | 89.7 | 464 |
| Greater than 1 site of metastasis | 78.6 | 462 |
| Brain metastases | 8.9 | 462 |
| First-line therapy | 464 | |
| Sunitinib | 54 | |
| Sorafenib | 33 | |
| Bevacizumab | 13 | |
| Second-line therapy | 464 | |
| Sunitinib | 37 | |
| Sorafenib | 51 | |
| Axitinib | 2 | |
| Bevacizumab | 7 | |
| Pazopanib | 3 |
ULN upper limit of normal, LLN lower limit of normal
Varies due to missing data
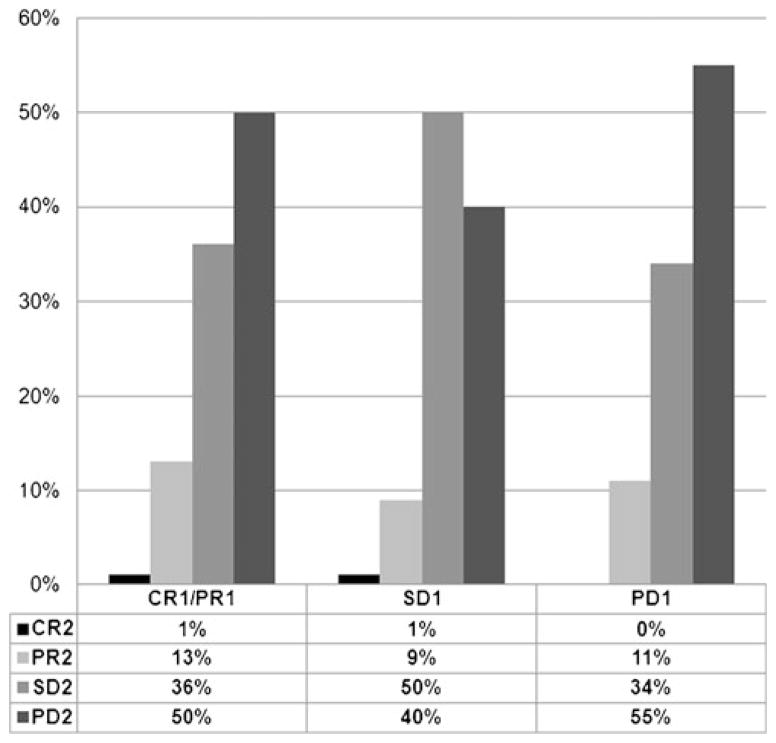
The RECIST-defined objective response rate (ORR; complete plus partial responses) was available for both first-line and second-line therapies in 323 patients. The ORR to first-line therapy was 22 %. The ORR to second-line therapy was 11 %, with an ORR of 14, 10, and 11 % in patients who achieved a CR/PR, SD, or PD as the best response to first-line VEGF-targeted therapy, respectively. There was no significant association between first-line ORR and second-line ORR (chi-squared trend test p=0.17; Fig. 2). In addition, there was no association when considering a specific sequence of agents (e.g., sunitinib followed by sorafenib or vice versa). Response rates for individual VEGF-targeted agent are found in Table 2.
Fig. 2.
Best response to second-line targeted therapy grouped by initial best response to first-line targeted therapy. Abbreviations: CR1 complete response to first-line VEGF-targeted therapy, PR1 partial response to first-line VEGF-targeted therapy, SD1 stable disease to first-line VEGF-targeted therapy, PD1 progressive disease to first-line VEGF-targeted therapy, CR2 complete response to second-line VEGF-targeted therapy, PR2 partial response to second-line VEGF-targeted therapy, SD2 stable disease to second-line VEGF-targeted therapy, PD2 progressive disease to second-line VEGF-targeted therapy
Table 2.
First- and second-line overall response rates (RR) for each targeted therapy
| Targeted therapy | First-line RR | Second-line RR |
|---|---|---|
| Sunitinib | 31 % (69/226) | 18 % (22/125) |
| Sorafenib | 14 % (19/139) | 6 % (11/171) |
| Bevacizumab | 10 % (6/59) | 0 % (0/20) |
| Axitinib | N/A | 36 % (4/11) |
| Pazopanib | N/A | 0 % (0/2) |
Patient numbers are small and comparisons should not be made. Denominators are based on the availability of response information for each patient
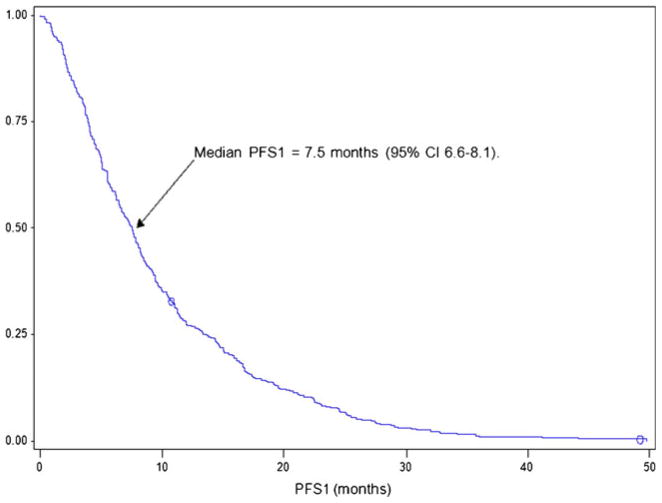
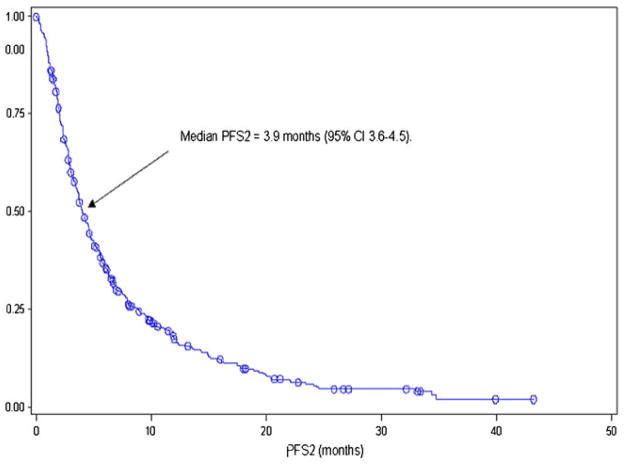
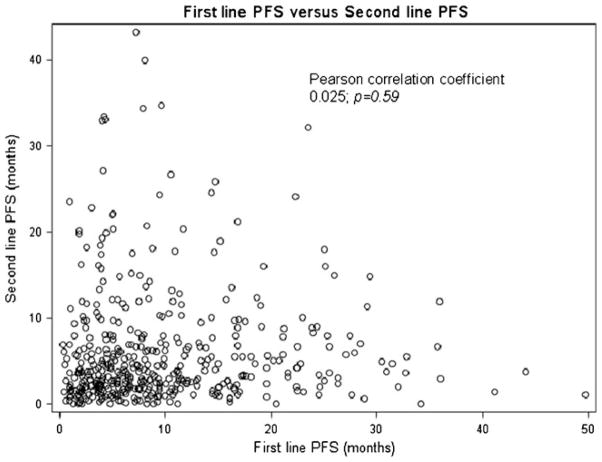
The median PFS on first-line VEGF-targeted therapy (PFS1) was 7.5 months (95 % CI, 6.6–8.1, Fig. 3), and the median PFS associated with second-line VEGF-targeted therapy (PFS2) was 3.9 months (95 % CI, 3.6–4.5, Fig. 4). There was no association between PFS1 and PFS2 (Pearson correlation coefficient 0.025; p=0.59, Fig. 5). The median OS from initiation of first-line therapy was 26.5 months, with 20 % exceeding 60 months at the time of data analysis.
Fig. 3.
First-line PFS of VEGF-targeted therapy in patients who eventually receive second-line VEGF-targeted therapy
Fig. 4.
Second-line PFS of VEGF-targeted therapy in patients who eventually receive second-line VEGF-targeted therapy
Fig. 5.
Correlation between progression-free survival on first-line VEGF-targeted therapy and progression-free survival on second-line VEGF-targeted therapy
Discussion
The literature concerning the choice of second-line systemic therapy in RCC is currently incomplete. The available trial data do not account for all treatment choices or patient scenarios and it becomes increasingly complex as the treatment armentarium continues to grow. As such, many practitioners choose second-line VEGF-targeted therapy based on response to first-line therapy but with little data to support this practice. The present analysis did not find any association between clinical response to first-line VEGF-targeted therapy and clinical response to second-line VEGF-targeted therapy as measured by ORR or PFS.
These data imply that response to first-line VEGF-targeting therapy is not a valid criterion upon which to choose subsequent therapy. The current data demonstrates that patients who are primary refractory to first-line VEGF therapy still achieve an 11 % objective response rate to second-line VEGF therapy. Further, PFS of the front-line agents is not indicative of PFS to second-line VEGF therapy. Additional investigation is needed to define the clinical parameters that do predict response to subsequent therapy in order to tailor individual treatment sequences.
The limitations of this study include its retrospective nature and the inherent issues of missing data and selection bias. For example, only a limited subset of patients had sufficient second-line response data to include in this analysis. Further, response and PFS were determined by treating physician assessment rather than independent radiologic assessment. However, this may make the study more generalizable as it is reflective of global, real-world clinical practice. A further limitation is that only a small percentage of patients received axitinib or pazopanib as a second-line therapy. It is possible that newer agents may have differing activity in the second-line setting than earlier established agents. Finally, less than 50 % of the initially enrolled patients received second-line therapy due to patient ineligibility such as poor performance status or differing availability of drugs in different regions.
Since VEGF-targeted therapies can induce tumor necrosis and minimal tumor shrinkage, RECIST may not be optimal for predicting clinical outcome, raising a debate about the best way to assess the response in mRCC [17, 18]. In our study, we used RECIST criteria out of convention but we also demonstrated no correlation with PFS. A recent retrospective study added further methods of assessing the best response to VEGF-targeted agents by systematically evaluating the optimal early post-therapy imaging changes to determine responders and non-responders at the first posttreatment follow-up computed tomography (CT). That study evaluated the objective response according to RECIST 1.0, Choi criteria, tumor shrinkage of ≥10 % decrease in sum of the longest diameter (SLD), and 15 or 20 % decrease in mean CT tumor density. The conclusion was that a 10 % reduction in the SLD on the first follow-up CT was an optimal early predictor of outcome [18].
There are now two agents with level 1 evidence for the treatment of metastatic RCC after failure of initial targeted therapy. Everolimus, an oral mTOR inhibitor, is an approved treatment after failure on one or more VEGF-targeted therapies. In the phase III trial, everolimus demonstrated a 3-month improvement in PFS over placebo (4.9 vs. 1.9 months) after first-line sunitinib, sorafenib, or both [13, 19]. Axitinib, a potent VEGFR inhibitor, also demonstrated an improvement in PFS over sorafenib in patients previously treated with one prior regimen, a VEGF inhibitor, an mTOR inhibitor, or immunotherapy [9–11]. The current data do not lend insight into the important choice of selection of a second-line mechanism [14, 15]. A clinical trial investigating second-line sorafenib versus temsirolimus after progression on first-line sunitinib therapy (NCT00474786) has recently been reported, showing no difference in PFS but an OS advantage to sorafenib in this setting. This is another piece of evidence that suggests that second-line VEGF inhibitors remain a reasonable choice.
Current considerations in selecting therapy include the potential drug toxicities, patient comorbidities, the accessibility to each drug, and the feasibility of oral versus intravenous route of drug administration. For example, patients with significant lung disease on oxygen or poorly controlled diabetes mellitus may not be optimal candidates for an mTOR inhibitor as there is a risk of non-infectious pneumonitis and hyperglycemia with this class of agents. Similarly, patients with refractory hypertension on several antihypertensive agents or significant cardiovascular (CV) morbidities may not initially choose a VEGF inhibitor as these are known to increase the risk of hypertension and CV events. The results of this study indicate that prior response to VEGF-targeted therapy should not influence the decision regarding the choice of second-line targeted therapy.
Despite the inherent bias that a good response to front-line VEGF-targeted therapy should be associated with a good response to a similar second-line therapy, no such association was found in this large, international dataset. As such, the optimal sequence of drug therapy for the treatment of an advanced RCC patient remains empiric and based on physician and patient preferences and toxicity profiles.
Footnotes
This study was presented at ASCO on June 7th, 2011 in Chicago, IL, USA.
Conflict of interest This study received no direct or indirect industry or pharmaceutical company support. The following authors have indicated a financial interest that is relevant to this study or to the subject matter under consideration in this article (consultant or advisory role (C), Honoraria (H), research funding (R))—Brian I. Rini: Bayer/Onyx, Roche (C) and GSK, Pfizer (C, R). Lori Wood: Pfizer and Novartis (C, R). Georg A. Bjarnason: Pfizer Canada (C, H, and R), Novartis (C, H), GSK (C, H). Lauren C. Harshman: Novartis, Pfizer (C on advisory board); BMS, Genentech, Novartis (R). Ulka Vaishampayan: Pfizer, Novartis and GSK (H & R). Jennifer J. Knox: AVEO (C), Novartis (R), Pfizer (C, R), Bayer (R). Daniel Heng: Pfizer, Novartis, Bayer (C, H). All remaining authors have declared no conflicts of interest.
Contributor Information
Mhd Y. Al-Marrawi, Taussig Cancer Institute, Cleveland Clinic Foundation, Cleveland, OH, USA
Brian I. Rini, Taussig Cancer Institute, Cleveland Clinic Foundation, Cleveland, OH, USA
Lauren C. Harshman, Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute/Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Georg Bjarnason, Sunnybrook Odette Cancer Centre, Toronto, ON, Canada.
Lori Wood, Queen Elizabeth II Health Sciences Center, Halifax, NS, Canada.
Ulka Vaishampayan, Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA.
Mary MacKenzie, London Health Sciences Center, London, ON, Canada.
Jennifer J. Knox, Princess Margaret Hospital, University of Toronto, Toronto, ON, Canada
Neeraj Agarwal, Division of Medical Oncology, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Hulayel Al-Harbi, University of Calgary, Calgary, AB, Canada.
Christian Kollmannsberger, Vancouver Cancer Center, British Columbia Cancer Agency, Vancouver, BC, Canada.
Min-Han Tan, Institute of Bioengineering and Nanotechnology, National Cancer Centre Singapore, Singapore, Singapore.
Sun Young Rha, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, South Korea.
Frede N. Donskov, Department of Oncology, Aarhus University Hospital, Aarhus, Denmark
Scott North, Cross Cancer Institute, University of Alberta, Edmonton, AB, Canada.
Toni K. Choueiri, Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute/Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Daniel Y. Heng, Email: daniel.heng@albertahealthservices.ca, Tom Baker Cancer Centre, University of Calgary, 1331 29th St NW, Calgary, AB T2N 4N2, Canada.
References
- 1.Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28(13):2144–2150. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Halabi S, Rosenberg JE, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28(13):2137–2143. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Marrawi MY, Rini BI. Pazopanib for the treatment of renal cancer. Expert Opin Pharmacother. 2011;12(7):1171–1189. doi: 10.1517/14656566.2011.571206. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Szczylik C, Hutson TE, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(8):1280–1289. doi: 10.1200/JCO.2008.19.3342. Erratum in J Clin Oncol. 2009 May 1; 27(13):2305. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27(20):3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27 (22):3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rini BI, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol. 2009;27(27):4462–4468. doi: 10.1200/JCO.2008.21.7034. [DOI] [PubMed] [Google Scholar]
- 10.Rini BI, Wilding GT, Hudes G, et al. Axitinib (AG-013736; AG) in patients (pts) with metastatic renal cell cancer (RCC) refractory to sorafenib [abstract] J Clin Oncol. 2007;25(Suppl 1):Abstract 5032. [Google Scholar]
- 11.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. doi: 10.1016/S0140-6736(11)61613-9. Epub 2011 Nov 4. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell cancer. J Clin Oncol. 2004;22(3):454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomized, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. Epub 2008 Jul 22. [DOI] [PubMed] [Google Scholar]
- 14.Porta C, Paglino C, Imarisio I. Sequencing tyrosine kinase inhibitors or immediately switching to mTOR inhibitors in advanced kidney cancer: a critical review. Eur J Clin Med Oncol. 2010;2:1–6. [Google Scholar]
- 15.Busch J, Seidel C, Kempkensteffen C, et al. Sequence therapy in patients with metastatic renal cell carcinoma: comparison of common targeted treatment options following failure of receptor tyrosine kinase inhibitors. Eur Urol. 2011;60(6):1163–1170. doi: 10.1016/j.eururo.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 16.Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 17.Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM. Morphology, Attenuation, Size, and Structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol. 2010;194(6):1470–1478. doi: 10.2214/AJR.09.3456. [DOI] [PubMed] [Google Scholar]
- 18.Krajewski KM, Guo M, Van den Abbeele AD, et al. Comparison of four early posttherapy imaging changes (EPTIC; RECIST 1.0, tumor shrinkage, computed tomography tumor density, Choi criteria) in assessing outcome to vascular endothelial growth factor-targeted therapy in patients with advanced renal cell carcinoma. Eur Urol. 2011;59(5):856–862. doi: 10.1016/j.eururo.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–4265. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]