Abstract
Objective
To characterize biomarkers of bone turnover and their relation with bone mineral mass in a cross-sectional cohort of females with Rett syndrome (RTT) and to examine the role of dietary, biochemical, hormonal, and inflammatory factors on bone mineral mass and bone biomarkers in this disorder.
Methods
Total body bone mineral content (BMC) and density (BMD) were determined by dual-energy x-ray absorptiometry. Dietary nutrient intakes were determined from 3-day food records. Biomarkers of bone turnover, bone metabolites, vitamin D metabolites, hormones, and inflammatory markers were measured by standard clinical laboratory methods.
Results
Serum osteocalcin, bone alkaline phosphatase, and C-telopeptide showed significant inverse relations with age in the RTT cohort. Mean osteocalcin concentrations were significantly lower and mean bone alkaline phosphatase concentrations were significantly higher for individual age groups in the RTT cohort than mean values for their respective age ranges in the reference population. Significant inverse associations were identified between urinary calcium losses, expressed as calcium:creatinine ratios, and total body BMC and BMD z-scores. Dietary protein, calcium, and phosphorus intakes, expressed as a proportion of Dietary Reference Intakes for age and gender, showed significant positive associations with total body BMD z-scores.
Conclusion
This study suggests decreased bone formation rather than increased bone resorption may explain in part the deficits in bone mineral mass in RTT and that attention to the adequacy of dietary protein, calcium and phosphorus intakes may offer an opportunity to improve bone health in RTT.
Keywords: bone density, osteocalcin, bone alkaline phosphatase, C-telopeptide, calcium
Rett syndrome (RTT) is an X-linked neurodevelopmental disorder caused by mutations in the gene encoding methyl-CpG-binding protein 2 (MECP2) (1). The hallmark features of RTT are apparently normal development until 6 to 18 months of life, followed by a period of rapid developmental regression, loss of acquired speech, the replacement of purposeful hand use with hand stereotypies, and deceleration in head growth (2). Low bone mineral mass and increased fracture risks are common features of the growth abnormalities of RTT (3). Low bone mineral content (BMC) and bone mineral density (BMD) are reported in at least 45% of females with RTT and are associated with fractures in 30% of affected individuals (3). Although causality has not been established, early deficits in bone mineral mass impede the attainment of peak bone mass, an important determinant of lifelong bone health, in RTT.
Altered bone turnover is the consequence of decreased bone formation, measured by osteocalcin and bone alkaline phosphatase, or increased bone resorption, measured by C-telopeptide (4). In the few studies that characterized the pattern of bone turnover in RTT, bone biomarkers including osteocalcin, bone alkaline phosphatase, and type I procollagen carboxyterminal propeptide did not differ between affected and unaffected individuals (5-7). In contrast, reduced bone volume, determined by histomorphometry, and low bone formation rates, measured by double tetracycline labeling, have been documented in three females with RTT (8). Reduced bone volume and osteoid deposition, cortical bone thinning, abnormalities in bone growth plates, and decreased mineral apposition rates of trabecular and calvareal bone have been described in a MECP2 null mouse model, findings consistent with osteoblast, rather than osteoclast, dysfunction (9).
In this study, we characterized biomarkers of bone turnover and their relation with bone mineral mass and examined the role of dietary, biochemical, hormonal, and inflammatory factors on bone mineral mass and bone biomarkers in females with RTT. This study extends our efforts to describe the natural history of bone mineral status in RTT and provides additional information about risk factors associated with poor bone mineralization in this disorder (3).
Subjects and Methods
Subjects
Fifty females who met the clinical criteria for RTT were enrolled (3). Their age range categories included 16 prepubertal, 6 peripubertal and 28 postpubertal females. Individuals were excluded if they received calcium supplements within 6 months before study; had previous bis-phosphonate therapy; had the clinical features of hypercalcemia, hypoparathyroidism, or vitamin D deficiency; or had spinal rod placement for scoliosis. Parental permission for each individual's participation was obtained in writing; each participant's assent was waived because of her cognitive impairment. The study protocol was approved by the Institutional Review Board for Human Subject Research at Baylor College of Medicine.
Procedures
All females with RTT were admitted to the General Clinical Research Center, Texas Children's Hospital, for 1) assessment of growth, body composition, and bone mineral status; 2) determination of dietary nutrient intakes; and 3) laboratory analysis of the biomarkers of bone mineral metabolism and related bone metabolites, hormones, and inflammatory markers.
Height was measured using a fixed stadiometer or horizontal length board with a moveable foot piece (Harpendon, Crymech, Great Britain). Weight was measured using an electronic balance (Scale-Tronix, Inc., Wheaton, IL). Body mass index (BMI) was calculated as weight divided by height squared. Height, weight, and BMI were converted to z-scores based on standard reference data (10). Total body BMC and BMD, lean body mass, and body fat were determined by dual-energy x-ray absorptiometry (Delphi-A systems, Version 11.2, Hologic, Inc., Bedford, MA) (3). Midazolam was administered intravenously immediately before the scan to prevent involuntary movements that could invalidate the analysis. To correct for bone size as a function of age and body size, total body BMC and BMD were converted to z-scores based on values measured in a reference population comprised of females (11). The reference database included bone scans for more than 2200 healthy children for whom ethnic, racial, and gender distributions were nearly equal. These data were used to develop a predictive algorithm for normative total body BMC and BMD based on age, gender, ethnicity, race, and height. For individuals whose ages exceeded 20 y, adult female references provided with the instrument were used. A significant bone mineral deficit was defined as total body BMD z-score <-2 SD. Lean body mass and body fat were expressed as a proportion of body weight.
Parents recorded the type and quantity of foods, beverages, and supplements consumed during two weekdays and one weekend day. Energy, protein, calcium, phosphorus, and vitamin D content of foods and beverages consumed were estimated using a nutrient database (The Minnesota Nutrition Data System, Version 4.02, Minneapolis, MN). The nutrient composition of supplements was ascertained from the manufacturer's label. The estimates of dietary energy and nutrient intakes were standardized by converting absolute values to proportions (%) of basal energy expenditure or the Dietary Reference Intake for age and gender, respectively (12,13).
After a 10-hr overnight fast, a 50 ml venous blood sample was obtained at 0630 using aseptic techniques for biomarkers of bone turnover (osteocalcin, bone alkaline phosphatase, C-telopeptide), bone metabolites (whole blood ionized calcium, serum calcium, phosphorus), vitamin D metabolites (25-hydroxyvitamin D, 1,25-dihydroxyvitamin D), hormones (intact parathyroid hormone [PTH], estradiol, leptin), and inflammatory markers (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], interleukin-6 [IL-6]). A spot urine sample was obtained between 0600 and 1000, using a cotton ball technique, for urinary calcium and creatinine concentrations. Urinary calcium losses were expressed as calcium:creatinine ratios.
Osteocalcin was measured by electrochemiluminescent immunoassay and bone alkaline phosphatase and 25-hydroxyvitamin D were measured by chemiluminescent immunoassay (ARUP Laboratories, Salt Lake City, UT). C-telopeptide was measured by electrochemiluminescent immunoassay, intact PTH was measured by immunochemiluminometric assay, and leptin was measured by radioimmunoassay (Quest Diagnostics, San Juan Capistrano, CA). 1,25-Dihydroxyvitamin D was measured by high pressure liquid chromatography with tandem mass spectrometry (Esoterix, Inc., Calabasas Hills, CA). ESR and CRP were measured by standard clinical laboratory methods. Serum IL-6 was measured by enzyme-linked immunoassay (R&D Systems, Inc., Minneapolis, MN). Ionized calcium was measured by an ion sensitive electrode method. Serum calcium and phosphorus and urinary calcium and creatinine were measured by an automated clinical laboratory technique (Vitros, Johnson & Johnson, Clinical Diagnostics, Rochester, NY).
Statistical Analysis
Descriptive statistics were calculated for individual outcome variables using a computer software program (MiniTab Statistical Software, Version 11.0, State College, PA). Two-sample t-tests were performed to detect differences in the biomarkers of bone turnover between the RTT cohort and female reference standard for each respective age range. For the purpose of comparison, participants were grouped within age ranges specified for each biomarker based on the classification provided by the reference laboratory or reported in the referenced publications. Reference values for osteocalcin and bone alkaline phosphatase at their respective age ranges for females were obtained from published data (14,15). Reference values for C-telopeptide at its respective age ranges for females were provided by Michael Caulfield, PhD, Quest Diagnostics, San Juan Capistrano, CA (personal communication). One sample proportions were performed to determine the proportion of individuals whose values for bone metabolites, urinary calcium:creatinine ratios, vitamin D metabolites, hormones, and inflammatory markers exceeded the normal range for age provided by their respective clinical laboratories or from published data (16,17). Linear regression was applied to detect relations among the biomarkers of bone turnover, total body BMC or BMD, and related bone metabolites, hormones, inflammatory markers, or dietary nutrient intakes after adjusting for age. Significance was determined at p < 0.05.
Results
Growth, Body Composition, and Bone Mineral Status of the RTT Cohort
Mean age of the RTT cohort was 15.5 ± 9.7 y; the age range was 2-38 y (Table 1). Height, weight, and BMI z-scores provided evidence for linear growth stunting. Total body BMC and BMD z-scores were consistent with moderate bone mineral deficits. Moderate (< -2 SD) or severe (< -3 SD) deficits in total body BMC were detected in 43% and 16%, respectively, of the RTT cohort. Moderate or severe deficits in total body BMD were detected in 27% and 18%, respectively.
Table 1. Characteristics of females (n=50) with Rett syndrome.
| Characteristic | Value |
|---|---|
| Age (y) | 15.5 ± 9.7 |
| Race/Ethnicity (%) | |
| Caucasian | 64 |
| African American | 16 |
| Hispanic | 14 |
| Asian | 6 |
| MECP2 mutation (%)* | |
| Missense | 40 |
| Early truncation | 33 |
| Late truncation | 20 |
| Deletion | 7 |
| Height (cm) | 128.0 ± 20.6 |
| (z-score) | -2.2 ± 1.6 |
| Weight (kg) | 31.1 ± 15.0 |
| (z-score) | -2.4 ± -2.5 |
| Body mass index (kg/m2) | 17.7 ± 4.7 |
| (z-score) | -1.1 ± 2.1 |
| Lean body mass (kg) | 19.6 ± 8.4 |
| (% body weight) | 66 ± 7 |
| Body fat (kg) | 10.1 ± 7.1 |
| (% body weight) | 31 ± 7 |
| Total body bone mineral content (g) | 850 ± 466 |
| (% body weight) | 3 ± 1 |
| (z-score) | -2.2 ± 1.0 |
| Total body bone mineral density (g/cm2) | 0.792 ± 0.140 |
| (z-score) | -1.7 ± 1.2 |
| Seizure disorder (%) | 66 |
| Anticonvulsant use (%) | 53 |
| Ambulates independently (%) | 74 |
Individuals with positive MECP2 mutation (n=45)
Biomarkers of Bone Turnover and Associations with Bone Mass
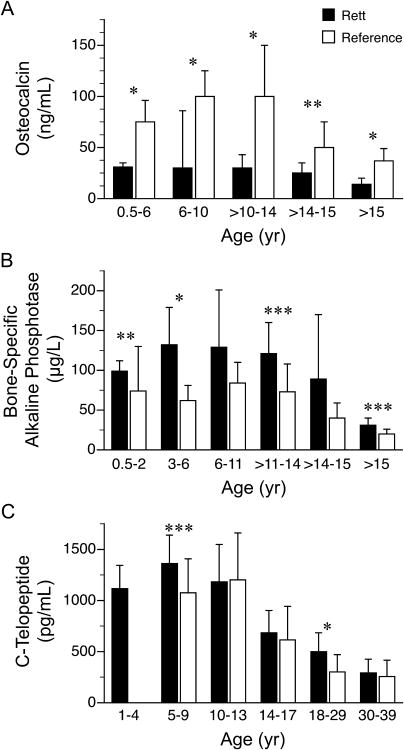
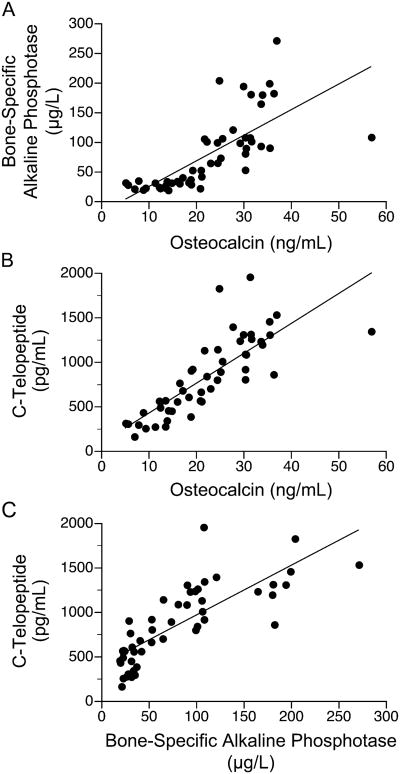
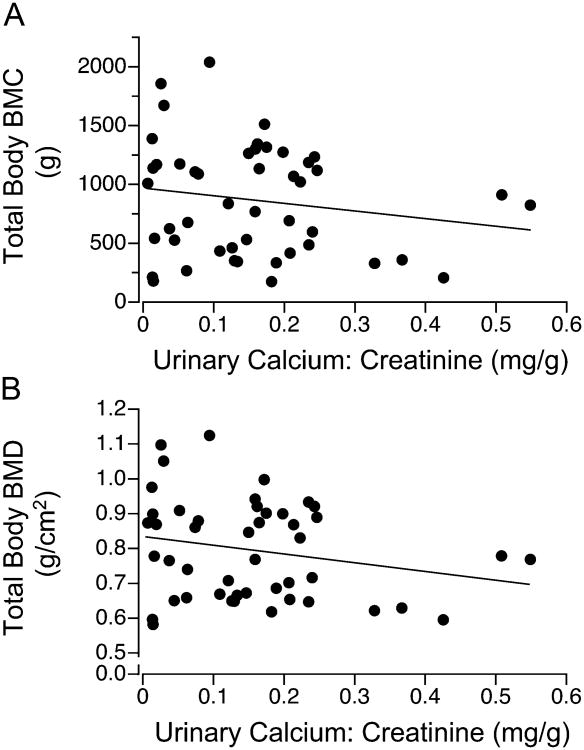
Osteocalcin (β1 = -0.8, p < 0.001, r = -0.78), bone alkaline phosphatase (β1 = -4.3, p < 0.001, r = -0.67), and C-telopeptide (β1 = -35.4, p < 0.001, r = -0.75) concentrations, but not urinary calcium losses, showed an inverse relation with age. Osteocalcin concentrations for all age groups were significantly lower, whereas the concentrations of bone alkaline phosphatase for age groups ≤ 6 y, 12-14 y, and ≥15 y and C-telopeptide for age groups 5-9 y and 18-29 y were significantly higher in the RTT cohort than their respective values for designated age groups in the reference population provided by the reference laboratories (Fig. 1, Supplemental Table 1a.). Osteocalcin concentrations were associated with bone alkaline phosphatase (β1 = 2.9, p < 0.01, r = 0.74) and C-telopeptide (β1 = 20, p < 0.001, r = 0.84) concentrations, and bone alkaline phosphatase levels were associated with C-telopeptide levels (β1 = 3.4, p < 0.001, r = 0.85) when adjusted for age (Fig. 2). Urinary calcium losses, but not serum osteocalcin, bone alkaline phosphatase, or C-telopeptide, were associated inversely with total body BMC (β1 = -0.73, p < 0.01, r = -0.85) and BMD (β1 = -0.0003, p < 0.01, r = -0.84) when adjusted for age (Fig. 3).
Figure 1.
Serum (A) osteocalcin, (B) bone-specific alkaline phosphatase, and (C) C-telopeptide concentrations (mean ± SD) in females (n=50) with Rett syndrome and reference standards. Data for reference standards obtained from Wyness et al., 2013; Huang et al., 2011; *p<0.001, **p<0.01, ***p<0.05.
Figure 2.
Relations among biomarkers in females (n=50) with Rett syndrome: (A) bone alkaline phosphatase(ug/L) = 44 + 2.9 osteocalcin(ng/mL) + 2.0 age (y), p<0.01, r=0.74; (B) C-telopeptide(pg/mL) = 697 + 20 osteocalcin(ng/mL) + 19 age (y), p<0.001, r=0.84; (C) C-telopeptide(pg/mL) = 908 + 3.4 bone alkaline phosphatase(ug/L) + 21 age (y), p<0.001, r=0.85.
Figure 3.
Relation between total body BMC or BMD and urinary calcium:creatinine ratios in females (n=50) with Rett syndrome: (A) total body BMC(g) = 342 − 0.73 urinary calcium:creatinine(mg/g) + 40 age (y), p<0.01, r = -0.85; (B) total body BMD(g/cm2) = 0.65 − 0.0003 urinary calcium:creatinine(mg/g) + 0.01 age (y), p<0.01, r = -0.84.
Biomarkers of Bone Turnover and Associations with Bone Metabolites, Hormones, Inflammatory Markers
Median values for bone metabolites, hormones, and inflammatory markers in the RTT cohort were within normal ranges (Table 2); however, 22% of serum calcium (p<0.001), 20% of ionized calcium (p<0.001), 17% of urinary calcium (p<0.01), 26% of 25-hydroxyvitamin D (p<0.001); 30% of 1,25-dihydroxyvitamin D (p<0.001), 18% of PTH (p<0.001), 27% of leptin (p<0.001), and 32% of ESR (p<0.001) samples exceeded values for their respective reference ranges. Of these abnormal samples, 100% of ionized calcium, 93% of 1,25-dihydroxyvitamin D, 92% of leptin, and 100% of ESR values were above, whereas 82% of serum calcium, 62% of urinary calcium, 100% of 25-hydroxyvitamin D, and 78% of PTH values were below their reference ranges.
Table 2. Bone metabolites, hormones, and inflammatory markers in females with Rett syndrome.
| Biomarker | Number of Subjects | Values (median, minimum, maximum) | Reference Range (95% CI) |
|---|---|---|---|
| Calcium (mg/dL) | 50 | 9.4 (8.3, 10.9) | 8.7 – 10.7 |
| Ionized calcium (mmol/L) | 50 | 1.27 (1.15, 1.36) | 1.10 – 1.30 |
| Phosphorous (mg/dL) | 50 | 4.5 (3.0, 6.5) | 2.5 – 6.8 |
| Urinary calcium:creatinine ratio (mg/g) | |||
| 1 –2 y | 4 | 98 (13, 425) | 20 – 560 |
| 3 –4 y | 5 | 189 (61, 367) | 20 – 410 |
| 5 –6 y | 4 | 119 (16, 328) | 10 – 300 |
| 7 –9 y | 3 | 44 (37, 207) | 11 – 457 |
| 10 – 12 y | 6 | 123 (14, 234) | 12 – 309 |
| 13 – 15 y | 3 | 190 (78, 239) | 8 - 313 |
| 16 – 17 y | 3 | 52 (29, 159) | 20 -- 271 |
| ≥18 y | 20 | 168 (6, 548) | 20 – 300 |
| 25-Hydroxyvitamin D (nmol/L) | 50 | 64 (19, 117) | > 50 nmol/L |
| 1,25-Dihydroxyvitamin D (pg/mL) | |||
| 1 – 17 y | 30 | 62 (12, 171) | 15 – 90 |
| ≥18 y | 20 | 54 (23, 82) | 21 – 65 |
| Parathyroid hormone (pg/mL) | 50 | 29 (9, 73) | 10 – 65 |
| Estradiol (ng/dL)* | |||
| Prepubertal | 16 | 0.5 ( - ) | < 1.5 |
| Peripubertal | 6 | 0.8 (0.5, 1.6) | -- |
| Postpubertal | 28 | 4.4 (0.5, 40) | 3 – 30 |
| Leptin (ng/mL) | |||
| 0 –4 y | 9 | 8 (2, 34) | -- |
| 5 –9 y | 7 | 8 (4, 19) | 1 – 17 |
| 10 – 14 y | 7 | 9 (3, 57) | 1 – 16 |
| 15 – 17 y | 5 | 34 (1, 52) | 1 – 25 |
| ≥18 y | 20 | 16 (4, 56) | 5 – 24 |
| Erythrocyte sedimentation rate (mm/h) | 49 | 15 (2, 50) | 0 – 20 |
| C-reactive protein (mg/dL) | 50 | 0.3 (0.3, 1.8) | < 1.0 |
| Interleukin-6 (pg/mL) | 50 | 1.8 (0.2, 10.5) | 0.4 – 9.6 |
Prepubertal, Tanner I; peripubertal, Tanner II, III; postpubertal, Tanner IV, V
Leptin was associated inversely with osteocalcin (β1 = -0.13, p < 0.05. r = -0.77) and C-telopeptide (β1 = -5.6, p < 0.05, r = -0.80), but not bone alkaline phosphatase or urinary calcium losses when adjusted for age. Serum phosphorus was associated with C-telopeptide (β1 = 220, p < 0.01, r = 0.82), but not osteocalcin, bone alkaline phosphatase, or urinary calcium losses when adjusted for age. ESR was associated inversely with bone alkaline phosphatase (β1 = -1.5, p < 0.01, r = -0.72), but not osteocalcin, C-telopeptide, or urinary calcium losses when adjusted for age. None of the other bone or vitamin D metabolites, hormones, or inflammatory markers was associated with the biomarkers of bone metabolism or urinary calcium losses.
Bone Mass and Associations with Dietary Intakes
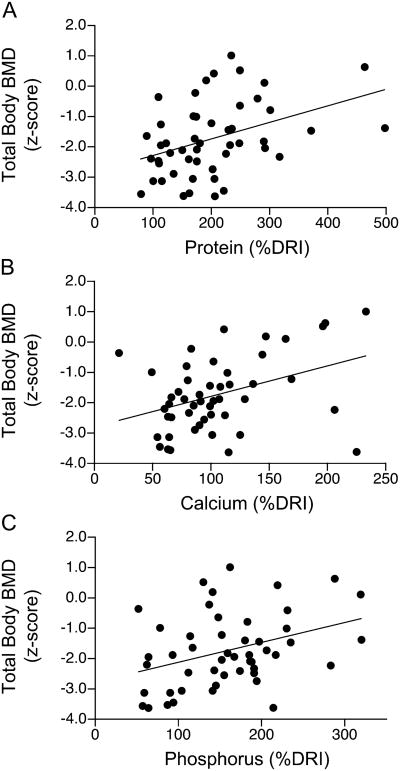
Dietary protein, calcium, phosphorus, and vitamin D intakes averaged 200 ± 98%, 107 ± 48%, 158 ± 67%, and 63 ± 37%, respectively, of the Dietary Reference Intakes for age and gender; 40%, 18%, and 84% of the RTT cohort did not achieve 90% or more of the Dietary Reference Intakes for calcium, phosphorous, and vitamin D, respectively. Dietary energy intakes averaged 140 ± 46% of basal energy expenditure for age and gender. Dietary protein (β1 = 0.01, p < 0.001, r = 0.54) and phosphorus (β1 = 0.01, p < 0.001, r = 0.43), but not energy or vitamin D, intakes were associated with total body BMC z-scores; calcium intakes tended to show an association with total body BMC z-scores (β1 = 0.01, p < 0.07, r = 0.22). Dietary protein (β1 = 0.01, p < 0.01, r = 0.38), calcium (β1 = 0.01, p < 0.01, r = 0.37), and phosphorus (β1 = 0.01, p < 0.01, r = 0.34), but not energy or vitamin D, intakes were associated with total body BMD z-scores (Fig. 4). Protein intakes correlated with calcium (p < 0.01), phosphorus (p < 0.001), and energy (p < 0.001), but not vitamin D intakes. Calcium intakes correlated with phosphorus (p < 0.001) and vitamin D (p < 0.01), but not energy intakes.
Figure 4.
Relations between total body BMD and dietary protein, calcium, or phosphorus in females (n=50) with Rett syndrome: (A) total body BMD(z-score) = -2.8 + 0.005 protein(% DRI), p<0.01, r=0.38; (B) total body BMD(z-score) = -2.8 + 0.01 calcium(%DRI), p<0.01, r=0.37; (C) phosphorous(%DRI) = -2.8 + 0.007 phosphorus(%DRI), p<0.01, r=0.34.
Bone Mass and Associations with Bone Metabolites, Hormones, Inflammatory Markers
Serum phosphorus was associated with total body BMD (β1 = 0.8, p < 0.01, r = 0.43), but not BMC, z-scores when adjusted for age. Ionized calcium tended to show an inverse association with total body BMC (β1 = -1360, p < 0.08, r = -0.84) and BMD (β1 = -0.4, p < 0.08, r = -0.82) z-scores when adjusted for age. Serum calcium, vitamin D metabolites, hormones, or inflammatory markers were not associated with total body BMC and BMD z-scores.
Discussion
Low bone mineral mass and increased fracture risks are common features of RTT (3). In the present study, the reduction in osteocalcin concentrations in the presence of expected C-telopeptide concentrations supports the notion that decreased bone formation rather than increased bone resorption may explain in part the finding of low bone mineral mass in RTT. Increased urinary calcium loss, rather than bone biomarkers, served as the primary variable associated with low BMC and BMD. Dietary factors, but not hormonal or inflammatory markers, were associated with altered bone mineral status. Attention to the adequacy of dietary protein, calcium, and phosphorus intakes may offer an opportunity to improve bone health in RTT.
Bone growth in childhood involves a process of epiphyseal (linear) bone growth, bone modeling (changes in circumference and thickness), and remodeling. The use of bone biomarkers represents the aggregate effect of these processes by providing a dynamic picture of bone turnover. Osteocalcin, a protein synthesized by osteoblasts, and bone alkaline phosphatase, a glycoprotein found in the cellular membrane of osteoblasts are associated with bone matrix formation, whereas C-telopeptide, a pyridinoline-containing crosslinking peptide formed during the post-translational modification of collagen, represents bone matrix resorption (4). Bone biomarkers vary throughout childhood and increase during the pubertal growth spurt, indicating that bone formation and resorption are accelerated during this time (18).
In this study, the low osteocalcin levels in the RTT cohort showed a distinct pattern that differed from reference values, suggesting that the rate of bone formation was depressed, not only throughout childhood, but especially during the adolescent growth spurt. Although bone alkaline phosphatase levels in the RTT cohort were higher at selected age groups, the function of this biomarker in bone matrix formation is unknown. Whether slower growth in conjunction with their short stature explains the lower osteocalcin levels or whether bone formation is reduced in a broader sense is unclear. Osteocalcin is reduced in low bone turnover states such as hypoparathyroidism and malnutrition (6). In this study, osteocalcin was not associated with PTH levels or other bone metabolites. In addition, individuals in the RTT cohort were adequately nourished, measured by their BMI and body fat stores. Thus, low osteocalcin levels, in the presence of normal C-telopeptide levels, suggest an alteration in bone turnover in RTT and reflect the aggregate effects of low bone mass with fewer bone cells and reduced bone modeling, rather than increased remodeling activity. Our findings are consistent with those described in the MECP2 null mouse model and in females with RTT studied with tetracycline labeling (8,9). Taken together, these studies support a cautious approach to the use of bisphosphonate drugs because altered bone formation, rather than resorption, appears to be the primary cause of poor bone mineralization in RTT.
Although biomarkers of bone formation and resorption correlate with each other, no bone marker is specific for longitudinal bone growth or bone mineral accretion in healthy children (18). Bone biomarkers generally reflect longitudinal bone growth, rather than mineral accretion, because of their correlation with height velocity and growth hormone secretion and their lack of correlation with BMD (19,20). In this study, we observed the characteristic interrelationships among bone biomarkers in the RTT cohort. However, we were unable to assess linear bone growth because of the cross-sectional design of our study and we did not measure growth hormone dynamics in the RTT cohort. As in healthy children, none of the biomarkers of bone turnover correlated with bone mineralization in the RTT cohort. The absence of a relation between bone biomarkers and measures of bone mineralization has been reported in children with other neurological disabilities (21).
The pattern of bone growth and mineral accretion in RTT has been characterized, but the factors associated with altered bone health are poorly understood (3,6,22-24). Total body BMC and BMD, in absolute terms, increase with age in RTT; however, the rate of bone growth and mineral deposition is relatively slower compared with unaffected individuals, leading to lower BMC and BMD z-scores over time (3,22,23). In this study, BMC and BMD were associated inversely with urinary calcium loss, the primary marker of bone mineral status, and tended to be associated indirectly with ionized calcium concentrations in the RTT cohort. This observation is relevant because hypercalcuria has been associated with a history of fractures in healthy children (25). We did not demonstrate an association between PTH and BMC, BMD, or C-telopeptide, even though PTH is the primary hormonal mediator of bone resorption. In this study, we attributed lower PTH concentrations to increased ionized calcium concentrations. We noted that 30% of the RTT cohort had 1,25-dihydroxyvitamin D concentrations above the normal limit, suggesting a compensatory increase in intestinal calcium absorption. This observation is consistent with our finding of increased fractional absorption of calcium in females with RTT (5).
Although treatment strategies to improve bone health in RTT are limited, the positive associations between bone mineral mass and dietary factors favor attention to the quality of the diet. In this study, total body BMC and BMD z-scores showed positive associations with dietary protein, calcium, and phosphorus intakes. Others have shown that calcium-enriched foods or calcium carbonate supplements lead to increased total body BMC or BMD in healthy, pre- and post-pubertal adolescents (26,27). Dietary calcium absorption also is enhanced by the adequacy of vitamin D in the diet. Vitamin D deficiency has been reported in 20% of a large cohort of females with RTT (28). In this study, 25-hydroxyvitamin D levels were < 50 nmol/L in 26% of the RTT cohort, reflecting in part lower dietary intake relative to current guidelines (13).
This study was limited by the absence of a control group to compare the features of bone mass deficits and the factors that influence bone mineral accretion. An observational study such as ours identifies factors associated with low bone mineral mass, but they may not be causally related. Small body size, deficits in lean body mass, anticonvulsant use, and nonambulatory status contribute to poor bone mineral status (3,24,29,30). Furthermore, the determination of BMD by dual energy x-ray absorptiometry should be made cautiously in individuals with short stature insofar as smaller, adequately mineralized bones may appear less dense by this method. The changing geometry and size of bone, the ratio of cortical to trabecular bone, and the thickness of soft tissues enveloping bone also can influence tissue density. We used z-scores, adjusted for height, weight, gender, and racial differences, to correct for these pitfalls, but they do not address possible changes associated with MECP2 mutations. Although others have noted associations between BMC and specific MECP2 mutations, we did not observe correlations between bone biomarkers or bone mass and classes of MECP2 mutations (3,22-24). The use of 3-day food records is subject to error insofar as food consumption may be over- or underestimated. This error is reduced in those individuals who receive gastrostomy feedings with known volumes of formula administration.
In summary, low osteocalcin concentrations provided supportive evidence for reduced bone formation, rather than enhanced bone resorption, and may explain in part the finding of low bone mass in RTT. Urinary calcium loss, rather than bone biomarkers, served as a marker of low bone mineral status. Reduced dietary intakes, particularly protein, calcium, and phosphorus, paralleled the deficits in bone mass and bone mineralization. Attention to the quality of the diet may offer an opportunity to improve bone health in RTT.
Supplementary Material
Acknowledgments
The authors thank the families and their daughters who are affected with RTT for their participation in the study, Maryse Laurent and Roman Shypailo for their technical support, the Staff of the GCRC for their nursing support, Ann McMeans, R.D., L.D., for dietary analyses, Gay Horelica for administrative support, Adam Gillum for graphic design, and E. O'Brien Smith, Ph.D. for statistical consultation.
Sources of Funding: This study was supported in part with funds from the International Rett Syndrome Foundation, the Rett Syndrome Association of Illinois, The Blue Bird Circle, and the National Institutes of Health, MO1-RR00188 General Clinical Research Center. This work is a publication of the USDA/ARS Children's Nutrition Research Center, Baylor College of Medicine, Houston, TX, and has been funded in part with federal funds from the US Department of Agriculture, Agricultural Research Service (Cooperative Agreement Number 58-6250-1-003). The content of this publication does not necessarily reflect the views or policies of the US Department of Agriculture, nor does mention of trade names commercial products, or organizations imply endorsement by this agency.
Abbreviations
- BMC
bone mineral content
- BMD
bone mineral density
- BMI
body mass index
- CRP
C-reactive protein
- IL-6
interleukin-6
- ESR
erythrocyte sedimentation rate
- MECP2
methyl-CpG-binding protein 2
- PTH
parathyroid hormone
- RTT
Rett syndrome
- SD
standard deviation
Footnotes
Financial disclosures: The authors do not have any financial disclosures to report.
References
- 1.Amir RE, Van den Veyver IB, Schultz R, et al. Influence of mutation type and X chromosome inactivation on Rett syndrome phenotypes. Ann Neurol. 2000;47:670–79. [PubMed] [Google Scholar]
- 2.Neul JL, Kaufmann WE, Glaze DG, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68:944–50. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motil KJ, Ellis KJ, Barrish JO, et al. Bone mineral content and bone mineral density are lower in older than in younger females with Rett syndrome. Pediatr Res. 2008;4:435–43. doi: 10.1203/PDR.0b013e318180ebcd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szulc P, Seeman E, Delmas PD. Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int. 2000;11:281–94. doi: 10.1007/s001980070116. [DOI] [PubMed] [Google Scholar]
- 5.Motil KJ, Schultz RJ, Abrams S, et al. Fractional calcium absorption is increased in girls with Rett syndrome. J Pediatr Gastroenterol Nutr. 2006;42:419–26. doi: 10.1097/01.mpg.0000189370.22288.0c. [DOI] [PubMed] [Google Scholar]
- 6.Cepollaro C, Gonnelli S, Bruni D, et al. Dual x-ray absorptiometry and bone ultrasonography in patients with Rett syndrome. Calcif Tissue Int. 2001;69:259–62. doi: 10.1007/s002230010027. [DOI] [PubMed] [Google Scholar]
- 7.Gonelli S, Caffarelli C, Hayek J, et al. Bone ultrasonography at phalanxes in patients with Rett syndrome: a 3-year long study. Bone. 2008;42:737–42. doi: 10.1016/j.bone.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Budden SS, Gunness ME. Bone histomorphometry in three females with Rett syndrome. Brain Dev. 2001;23 Suppl 1:S133–7. doi: 10.1016/s0387-7604(01)00338-2. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor RD, Zayzafoon M, Farach-Carson MC, et al. Mecp2 deficiency decreases bone formation and reduces bone volume in a rodent model of Rett syndrome. Bone. 2009;45:346–56. doi: 10.1016/j.bone.2009.04.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention, National Center for Health Statistics. [Accessed March 1, 2013];CDC growth charts: United States. Accessed at: http://www.cdc.gov/growthcharts.
- 11.Ellis KJ, Abrams SA, Wong WW. Body composition of a young, multiethnic female population. Am J Clin Nutr. 1997;65:724–31. doi: 10.1093/ajcn/65.3.724. [DOI] [PubMed] [Google Scholar]
- 12.Food and Nutrition Center, United States Department of Agriculture. [Accessed: March 1, 2013];National Agricultural Library. Accessed at: http://fnic.nal.usda.gov/dietary-guidance/dietary-reference-intakes.
- 13.Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin D and Calcium. The National Academy Press; Washington, DC: [Accessed: March 1, 2013]. pp. 1–999. Accessed at: http://iom.edu/Reports/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D.aspx. [Google Scholar]
- 14.Wyness SP, Roberts WL, Straseski JA. Pediatric reference intervals for four serum bone markers using two automated immunoassays. Clin Chim Acta. 2013;415:169–72. doi: 10.1016/j.cca.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Eapen E, Steele S, et al. Establishment of reference intervals for bone markers in children and adolescents. Clin Biochem. 2011;44:771–8. doi: 10.1016/j.clinbiochem.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Slev PR, Bunker AM, Owen WE, et al. Pediatric reference intervals for random urine calcium, phosphorus and total protein. Pediatr Nephrol. 2010;25:1707–10. doi: 10.1007/s00467-010-1544-8. [DOI] [PubMed] [Google Scholar]
- 17.Matos V, van Melle G, Boulat O, et al. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr. 1997;131:252–7. doi: 10.1016/s0022-3476(97)70162-8. [DOI] [PubMed] [Google Scholar]
- 18.Rauchenzauner M, Schmid A, Heinz-Erian P, et al. Sex- and age-specific reference curves for serum markers of bone turnover in healthy children from 2 months to 18 years. J Clin Endocrinol Metab. 2007;92:443–9. doi: 10.1210/jc.2006-1706. [DOI] [PubMed] [Google Scholar]
- 19.Tobiume H, Kanzaki S, Hida S, et al. Serum bone alkaline phosphatase isoenzyme levels in normal children and children with growth hormone (GH) deficiency: a potential marker for bone formation and response to GH therapy. J Clin Endocrinol Metab. 1997;82:2056–61. doi: 10.1210/jcem.82.7.4081. [DOI] [PubMed] [Google Scholar]
- 20.Kanzaki S, Hosoda K, Moriwake T, et al. Serum propeptide and intact molecular osteocalcin in normal children and children with growth hormone (GH) deficiency: a potential marker of bone growth and response to GH therapy. J Clin Endocrinol Metab. 1992;75:1104–9. doi: 10.1210/jcem.75.4.1400878. [DOI] [PubMed] [Google Scholar]
- 21.Henderson RC, Lark RK, Gurka MJ, et al. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110:e5, 1–10. doi: 10.1542/peds.110.1.e5. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro JR, Bibat G, Hiremath G, et al. Bone mass in Rett syndrome: association with clinical parameters and MECP2 mutations. Pediatr Res. 2010;68:446–51. doi: 10.1203/PDR.0b013e3181f2edd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roende G, Ravn K, Fuglsang K, et al. DXA measurements in Rett syndrome reveal small bones with low bone mass. J Bone Miner Res. 2011;26:2280–6. doi: 10.1002/jbmr.423. [DOI] [PubMed] [Google Scholar]
- 24.Jefferson AL, Woodhead HJ, Fyfe S, et al. Bone mineral content and density in Rett syndrome and their contributing factors. Pediatr Res. 2011;69:293–8. doi: 10.1203/PDR.0b013e31820b937d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olney RC, Mazur JM, Pike LM, et al. Healthy children with frequent fractures: how much evaluation is needed? Pediatrics. 2008;121:890–7. doi: 10.1542/peds.2007-2079. [DOI] [PubMed] [Google Scholar]
- 26.Bonjour JP, Carrie AL, Ferrari S, et al. Calcium-enriched foods and bone mass growth in prepubertal girls: a randomized, double-blind, placebo-controlled trial. J Clin Invest. 1997;99:1287–94. doi: 10.1172/JCI119287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matkovic V, Goel PK, Badenhop-Stevens NE, et al. Calcium supplementation and bone mineral density in females from childhood to young adulthood: a randomized controlled trial. Am J Clin Nutr. 2005;81:175–88. doi: 10.1093/ajcn/81.1.175. [DOI] [PubMed] [Google Scholar]
- 28.Motil KJ, Barrish JO, Lane J, et al. Vitamin D deficiency is prevalent in girls and women with Rett syndrome. J Pediatr Gastroenterol Nutr. 2011;53:569–574. doi: 10.1097/MPG.0b013e3182267a66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crabtree NJ, Kibirige MS, Fordham JN, et al. The relationship between lean body mass and bone mineral content in paediatric health and disease. Bone. 2004;35:965–972. doi: 10.1016/j.bone.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Leonard H, Thomson MR, Glasson EJ, et al. A population-based approach to the investigation of osteopenia in Rett syndrome. Dev Med Child Neurol. 1999;41:323–328. doi: 10.1017/s0012162299000717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.