Abstract
Objectives
To determine the degree to which hyperglycemia predicts the development of frailty and/or lower extremity mobility limitations.
Design
Secondary data analysis of longitudinal data collected in a prospective cohort study.
Setting
Baltimore, Maryland
Participants
We examined 329 women from the Women’s Health and Aging Studies II aged 70–79 years at baseline who had all variables needed for analysis.
Methods
Hemoglobin A1c [HbA1c] at baseline was the independent variable and categorized as: <5.5%, 5.5 to 5.9%, 6.0–6.4%, 6.5–7.9%, ≥8%. The incidence of frailty and lower extremity mobility limitations (based on self-reported walking difficulty, walking speed, and short performance physical battery [SPPB] score) was determined (follow-up≈9 years). Frailty was assessed using the Cardiovascular Health Study criteria. Covariates included demographics, body mass index, interleukin-6, and clinical history of comorbidities. Statistical analyses included Kaplan-Meier survival curves and Cox regression models adjusting for key covariates.
Results
In time-to-event analyses, HbA1c category was associated with incidence of walking difficulty (p=0.049) and low physical performance (p=0.001); association with incidence of frailty and low walking speed had a trend towards significance (both p=0.10). In demographics-adjusted regression models, HbA1c≥8% (versus<5.5%) was associated with an approximately three-times increased risk of incident frailty and three-to-five times increased risk of lower extremity mobility limitations (all p<0.05). In fully adjusted models, HbA1c≥8% (versus<5.5%) was associated with incident frailty (hazard ratio[HR]=3.33, 95% confidence interval=1.24–8.93), walking difficulty (HR=3.47,1.26–9.55), low walking speed (HR=2.82,1.19–6.71), and low physical performance (HR=3.60,1.52–8.53).
Conclusions
Hyperglycemia is associated with the development of frailty and lower extremity mobility limitations in older women; future studies should identify mediators of these relationships.
Keywords: Hyperglycemia, Elderly, Frailty, Mobility, Disability
INTRODUCTION
Diabetes in the elderly is a growing public health concern with almost two-thirds of older U.S. adults having either diabetes or pre-diabetes (1). Further, the numbers of persons with diabetes will almost double by the year 2030 (2). In the geriatric population, diabetes can have a significant impact on physical functioning and has been associated with lower extremity mobility limitations (3,4). Diabetes has also been associated with frailty, a geriatric condition of physiological vulnerability to stressors associated with adverse outcomes such as disability and mortality (5–8). However, whether hyperglycemia, per se, predicts the development of frailty and/or lower extremity mobility limitations has not been fully described.
Our group has previously demonstrated that hyperglycemia is associated with frailty in cross-sectional studies (9). We have also reported altered glucose-insulin dynamics in frail women compared to their counterparts with 2-hour post oral glucose tolerance test (OGTT) levels better discriminating frailty status compared to fasting values (8). However, the direction of the association between frailty status and abnormalities in glucose metabolism remains unclear. In the Cardiovascular Health Study (CHS), longitudinal associations between insulin resistance and incident frailty have been described (10). Nonetheless, these studies are limited by the use of short-term fasting measures of glycemia while biomarkers such as hemoglobin A1c, which reflect exposure to glucose over the past three months and are influenced by both fasting and postprandial hyperglycemia, may be less variable. Hyperglycemia has also been found to be cross-sectionally associated with lower extremity disability (4,9) but, to our knowledge, associations of hyperglycemia with declines in lower extremity mobility function over time have not been previously explored.
In the present study, we sought to describe the association of hyperglycemia with incident frailty and lower extremity mobility limitations in a longitudinal cohort of older, community-dwelling women. Our hypotheses were: 1) hyperglycemia (assessed by hemoglobin A1c) predicts the development of frailty and lower extremity limitations; 2) the association of hyperglycemia with frailty and lower extremity limitations is independent of potential confounders; and 3) the association of hyperglycemia with frailty and lower extremity limitations is non-linear.
METHODS
Subjects
The study population consisted of women ages 70–79 years at baseline enrolled in the Women’s Health and Aging Studies II who represented the two-thirds least disabled women living in the community (11). 436 women enrolled with seven study visits from 1994–2008. 382 had hemoglobin A1c (HbA1c) levels available at baseline. After excluding participants with missing covariates (n=11), stroke or Parkinson’s disease (more likely to have lower extremity limitations due to primary disease; n=5), or HbA1c<4.5% (n=2), 364 women remained. The excluded women did not differ significantly from those included in our study.
For incident frailty analysis, we further excluded women who had the outcome (frailty) at baseline (n=11), missing outcome (frailty) status at baseline (n=2), or no follow-up (n=22). This resulted in 329 women available for this analysis. The total number of participants who completed study visits was as follows: two visits (n=25), three visits (n=58); four visits (n=22); five visits (n=26); six visits (n=74); and all seven visits (n=124).
For lower extremity outcomes, similar exclusion criteria were used. This resulted in the following analytic samples to examine incidence of: self-reported walking difficulty (n=329); low walking speed defined as a level in the lowest quartile (<0.82 m/s) for the study population (n=259); and low physical performance defined as a short physical performance battery (SPPB) score in the lowest quartile (<9) for the study population (n=267).
Variables
The main outcome was frailty as described by Fried et al. (5,6). Five criteria were used: shrinking (body mass index [BMI] <18.5 kg/m2 or 5% annual weight loss), weakness (decreased grip strength), poor endurance (exhaustion), slowness (low walking speed), and physical inactivity. Those with 0 criteria were categorized as nonfrail, 1–2 criteria as prefrail, and 3+ criteria as frail.
Lower extremity mobility outcomes included subjective and objective measures. Participants self-reported any difficulty walking a quarter mile at all visits. Walking speed was calculated based on a 4 meter measured walk test at usual pace at all visits. Assessment of the SPPB consisting of chair stands, walk test, and tandem stands for balance was available for all visits except visit 4, and scores calculated using criteria defined by Guralnik et al. and adapted for clinical use (12–14).
The main exposure of interest was HbA1c. Nonfasting blood samples were obtained and HbA1c measured using a BioRad assay from frozen whole blood (9).
Demographic information was obtained from a standardized questionnaire. Height and weight were measured to calculate BMI. BMI was categorized according to World Health Organization criteria as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30 kg/m2) (15). For the analysis of incident walking difficulty only, underweight was categorized as <20 kg/m2 since all women with BMI <18.5 kg/m2 developed walking difficulty. IL-6 was measured from frozen serum using a commercial enzyme-linked immunosorbent assay (Quantikine Human, R&D Systems, Minneapolis, MN). History of diabetes, coronary artery disease (CAD), osteoarthritis (OA), and chronic obstructive pulmonary disease (COPD) was self-reported. CAD included congestive heart failure, myocardial infarction, or angina.
Peripheral artery disease (PAD) was defined as an ankle-brachial index<0.9 (3). Peripheral neuropathy (PN) was defined by physician report or inability to feel the complete vibration of a 128 Hz tuning fork in either great toe.
Statistical Analysis
Baseline characteristics were compared by incident frailty status using either the chi-squared test or student’s t test. Incidence was defined as first occurrence of each event in all analyses. Kaplan-Meier survival curves explored the association of HbA1c (categorized to account for potential non-linear relationships into <5.5%, 5.5–5.9%, 6.0–6.4%, 6.5–7.9%, ≥8%) with the time-to-event for frailty and lower extremity outcomes. HbA1c categories were chosen based on previous studies suggesting increased risk of mortality at both the highest and lowest thresholds (16,17), with cut-offs for the intermediate categories based on diagnostic criteria for diabetes (18). Cox regression models for discrete time outcomes were constructed to characterize the independent association of HbA1c at baseline with outcomes in the following sequential models: Model 1: adjusted for demographics (age, race, education); Model 2: adjusted for model 1+BMI; Model 3: adjusted for model 2+IL-6; Model 4: adjusted for model 3+ comorbidities (CAD, COPD, OA, PAD, PN). For the frailty model, we performed sensitivity analyses adjusting for prefrailty status at baseline. Potential quadratic associations of HbA1c with incident frailty and other outcomes were explored in regression models but since none were found, the lowest HbA1c category (<5.5%) was chosen as the reference for all analyses. The statistical program used was SAS 9.2 version (SAS institute, Inc., Cary, NC).
RESULTS
Seventy-seven of 329 women (23%) developed incident frailty during a mean follow-up of 8.6±3.6 (SD) years. From the baseline non-frail cohort, 70 women (21.3%) died and 57 (17.4%) dropped out before frailty development. The women who did versus did not develop frailty were similar with respect to age, race, and education (Table 1). A significant difference in the distribution of BMI categories was found between groups (p=0.01), with a greater proportion of women being either obese or underweight in the group that developed frailty. Not surprisingly, a greater proportion of women were prefrail at baseline in the incident frailty group versus nonfrail group (55.8% versus 29.4%; p<0.001). There were no significant differences in the clinical history of other comorbidities (all p>0.05). Lastly, inflammatory markers (IL-6) were largely similar whereas mean HbA1c tended to be higher in women who developed incident frailty compared to those that did not (6.17% versus 5.96%, p=0.07).
Table 1.
Selected Baseline Characteristics of the Study Participants, According to the Development of Frailty During Follow-upa
| Incident Frailty During Follow-Up |
||||
|---|---|---|---|---|
| All (N=329) |
Yes (N=77) |
No (N=252) |
p-valueb | |
| Demographics | ||||
| Age (years) | 73.9 ± 2.8 | 74.0 ± 2.9 | 73.9 ± 2.8 | 0.62 |
| Race (% white) | 83.9% | 83.1% | 84.1% | 0.83 |
| Education (years) | 12.6 ± 3.3 | 12.2 ± 2.9 | 12.8 ± 3.4 | 0.17 |
| Body composition | ||||
| BMI categories (%) | 0.01 | |||
| <18.5 kg/m2 | 3.3% | 6.5% | 2.4% | |
| 18.5–24.9 kg/m2 | 36.2% | 29.9% | 38.1% | |
| 25.0–29.9 kg/m2 | 38.9% | 31.2% | 41.3% | |
| ≥ 30 kg/m2 | 21.6% | 32.5% | 18.3% | |
| BMI value (kg/m2)c | 26.6 ± 5.1 | 27.3 ± 5.5 | 26.4 ± 4.9 | 0.16 |
| Clinical history | ||||
| Prefrailty (%) | 35.6% | 55.8% | 29.4% | <0.0001 |
| Osteoarthritis (%) | 66.9% | 66.2% | 67.1% | 0.89 |
| COPD (%)c | 23.4% | 26.0% | 22.6% | 0.54 |
| Coronary artery disease (%) | 13.7% | 16.9% | 12.7% | 0.35 |
| Peripheral arterial disease | 4.6% | 7.8% | 3.6% | 0.12 |
| Peripheral neuropathy | 7.0% | 7.8% | 6.8% | 0.76 |
| Known diabetes (%) | 7.6% | 9.1% | 7.1% | 0.57 |
| Laboratory measures | ||||
| Interleukin-6 (pg/ml) | 3.9 ± 4.8 | 4.2 ±7.0 | 3.8 ± 3.9 | 0.49 |
| HbA1c categories (%)c | 0.24 | |||
| < 5.5% | 19.5% | 15.6% | 20.6% | |
| 5.5–5.9% | 41.0% | 40.3% | 41.3% | |
| 6.0–6.4% | 23.4% | 24.7% | 23.0% | |
| 6.5–7.9% | 11.6% | 10.4% | 11.9% | |
| ≥ 8% | 4.5% | 9.1% | 3.2% | |
| HbA1c value (%) | 6.01±0.89 | 6.17 ± 1.10 | 5.96 ± 0.80 | 0.07 |
Unless otherwise indicated, mean ± standard deviation is shown.
Comparing participants with incident frailty to those without incident frailty.
BMI = body mass index; COPD = chronic obstructive pulmonary disease; HbA1c = hemoglobin A1c.
For lower extremity limitations, 27% of women developed self-reported walking difficulty (mean follow-up 8.4±3.7 years), 63% of women developed low walking speed (mean follow-up 8.8±3.5 years), and 67% developed low physical performance (mean follow-up 8.8±3.5 years).
The overall incidence rate for frailty and lower extremity limitations during follow-up was: 2.26 (1.78–2.83) per 100 person-years for frailty, 2.78 (2.24–3.42) per 100 person-years for walking difficulty, 8.46 (7.20–9.88) per 100 person-years for low walking speed, and 8.61 (7.40–9.97) per 100 person-years for low physical performance.
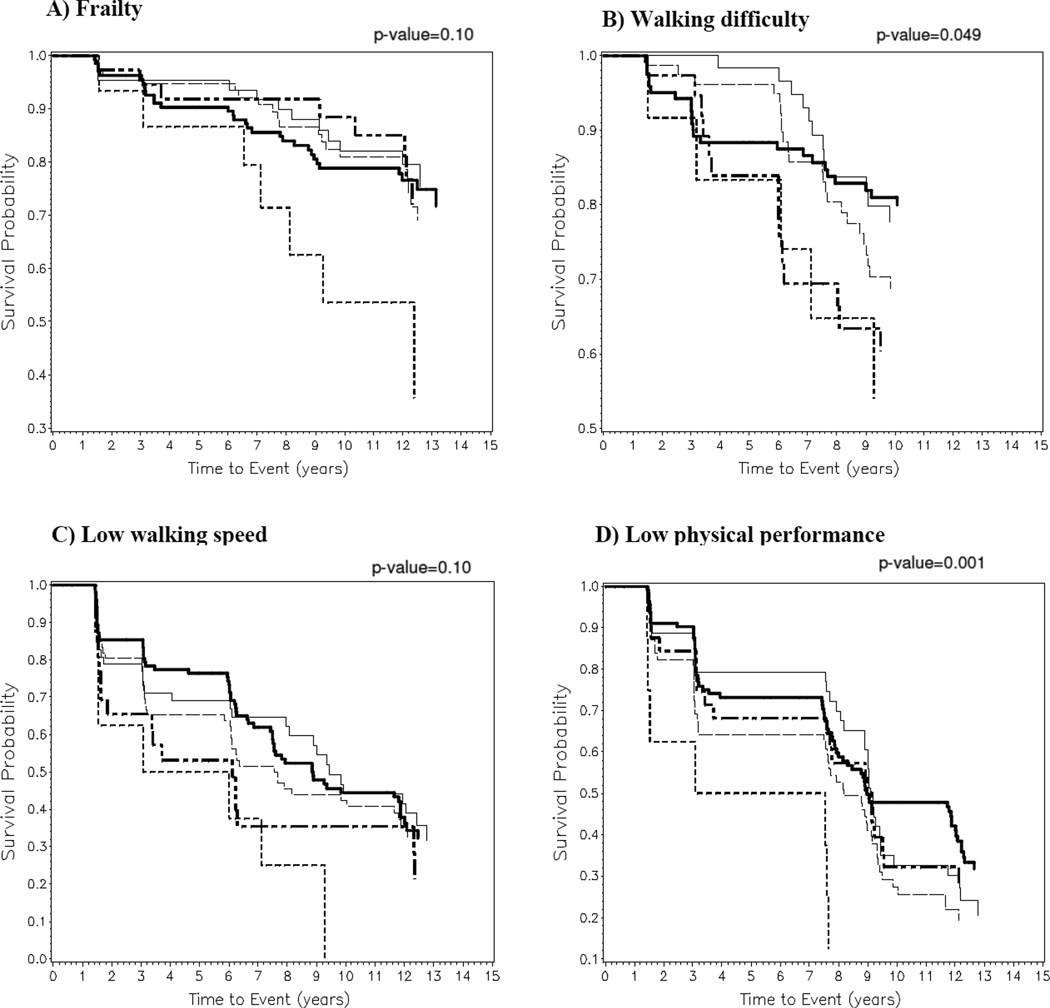
We next examined Kaplan-Meier survival curves for time-to-event analyses (Figures 1A–D). The women were divided into the following HbA1c categories for incident frailty analysis: <5.5% (n=64), 5.5–5.9% (n=135), 6–6.4% (n=77), 6.4–7.9% (n=38), ≥8% (n=15). Similar HbA1c categories were used for lower extremity outcomes. HbA1c category was associated with the probability of participants developing frailty (p=0.10) and low walking speed (p=0.10), although results were not statistically significant based on the log-rank test (Figures 1A and 1C). However, HbA1c category was significantly associated with the probability of developing incident walking difficulty (Figure 1B, p=0.049) and low physical performance (Figure 1D, p=0.001).
Figure 1. Kaplan-Meier Curves for Incident Frailty and Lower Extremity Mobility Limitations, by Category of Baseline Hemoglobin A1c.
Kaplan-Meier curves demonstrating the time-to-event of outcomes during follow-up for older women categorized by level of baseline hemoglobin A1c (HbA1c) including <5.5% (thin solid line  ), 5.5–5.9% (thick solid line
), 5.5–5.9% (thick solid line  ), 6.0–6.4% (thin dashed line
), 6.0–6.4% (thin dashed line  ), 6.5–7.9% (thick short and long dashed line
), 6.5–7.9% (thick short and long dashed line  ), and ≥8% (thick short dashed line
), and ≥8% (thick short dashed line  ). The association between HbA1c category and probability of survival for the outcomes are as follows: A) frailty (p=0.10); B) self-reported walking difficulty (p=0.049); C) low walking speed (p=0.10); and D) low physical performance battery score (p=0.001). The x-axis shows the time to event in years. The y-axis shows the probability of survival.
). The association between HbA1c category and probability of survival for the outcomes are as follows: A) frailty (p=0.10); B) self-reported walking difficulty (p=0.049); C) low walking speed (p=0.10); and D) low physical performance battery score (p=0.001). The x-axis shows the time to event in years. The y-axis shows the probability of survival.
The association between HbA1c category and incident frailty was further explored in regression models (Table 2). HbA1c≥8% was significantly associated with incident frailty after adjusting for demographics (HR=3.63, 95% CI 1.41–9.33; Model 1) compared to the reference category (HbA1c<5.5%). This association was slightly attenuated after further adjustment for BMI and IL-6 but essentially unchanged. After adjustment for comorbidities, the association remained significant (HR=3.33, 1.24–8.93; Model 4). In sensitivity analyses, we further adjusted for prefrailty in the fully-adjusted model to explore its contributions as a potential confounder (9); the association was attenuated such that it was no longer significant (HR=2.64, 0.95–7.34). However, prefrailty may also contribute as a mediator of the association between HbA1c and incident frailty among women who are nonfrail at baseline.
Table 2.
Adjusted Hazard Ratios and 95% Confidence Intervals for Frailty and Lower Extremity Mobility Limitations in WHAS II, According to the HbA1c Category at Baselinea
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Outcomes | ||||
| Frailty | ||||
| HbA1c categoryd | ||||
| <5.5% (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| 5.5–5.9% | 1.28 (0.65–2.49) | 1.35 (0.69–2.65) | 1.30 (0.66–2.55) | 1.29 (0.65–2.55) |
| 6.0–6.4% | 1.18 (0.57–2.45) | 1.26 (0.59–2.67) | 1.25 (0.59–2.65) | 1.25 (0.58–2.69) |
| 6.5–7.9% | 1.17 (0.44–2.81) | 1.05 (0.41–2.7) | 1.01 (0.39–2.61) | 1.04 (0.40–2.70) |
| ≥8% | 3.63 (1.41–9.33) | 3.16 (1.19–8.35) | 3.12 (1.18–8.27) | 3.33 (1.24–8.93) |
| Difficulty walking quarter mile | ||||
| HbA1c categoryd | ||||
| <5.5% (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| 5.5–5.9% | 1.10 (0.59–2.05) | 1.18 (0.63–2.21) | 1.17 (0.62–2.20) | 1.14 (0.60–2.15) |
| 6.0–6.4% | 1.36 (0.71–2.61) | 1.25 (0.64–2.43) | 1.24 (0.64–2.41) | 1.28 (0.65–2.51) |
| 6.5–7.9% | 2.26 (1.05–4.87) | 1.70 (0.77–3.75) | 1.70 (0.77–3.75) | 1.77 (0.79–3.97) |
| ≥8% | 5.03 (1.91–13.27) | 2.93 (1.08–7.96) | 2.91 (1.07–7.91) | 3.47 (1.26–9.55) |
| Low walking speedb | ||||
| HbA1c categoryd | ||||
| <5.5% (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| 5.5–5.9% | 1.04 (0.67–1.61) | 1.06 (0.68–1.66) | 1.04 (0.67–1.63) | 1.02 (0.65–1.59) |
| 6.0–6.4% | 0.95 (0.59–1.53) | 0.98 (0.60–1.59) | 0.91 (0.56–1.49) | 0.93 (0.56–1.52) |
| 6.5–7.9% | 1.25 (0.69–2.28) | 1.24 (0.67–2.29) | 1.12 (0.60–2.09) | 0.97 (0.52–1.82) |
| ≥8% | 3.14 (1.35–7.34) | 2.89 (1.23–6.81) | 2.70 (1.15–6.33) | 2.82 (1.19–6.71) |
| Low physical performancec | ||||
| HbA1c categoryd | ||||
| <5.5% (reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| 5.5–5.9% | 0.99 (0.66–1.50) | 1.01 (0.67–1.54) | 1.00 (0.66–1.53) | 0.97 (0.63–1.49) |
| 6.0–6.4% | 1.23 (0.78–1.93) | 1.24 (0.79–1.96) | 1.19 (0.75–1.89) | 1.19 (0.75–1.88) |
| 6.5–7.9% | 1.14 (0.64–2.02) | 1.13 (0.63–2.02) | 1.05 (0.58–1.88) | 1.06 (0.58–1.92) |
| ≥8% | 3.31 (1.43–7.69) | 3.25 (1.38–7.66) | 3.09 (1.31–7.28) | 3.60 (1.52–8.53) |
Model 1: adjusted for age, race, education. Model 2: adjusted for variables in model 1 and BMI category. Model 3: adjusted for variables in model 2 and interleukin-6. Model 4: adjusted for variables in model 3 and clinical history of comorbidities including osteoarthritis, chronic obstructive pulmonary disease, coronary artery disease, peripheral arterial disease, and peripheral neuropathy.
Walking speed in the lowest quartile (<0.82 m/s).
Short performance physical battery (SPPB) score in the lowest quartile (<9).
HbA1c = hemoglobin A1c.
HbA1c≥8% was also associated with significantly increased difficulty walking after adjustment for demographics (HR=5.03, 95% CI 1.91–13.27; Model 1) compared to reference (Table 2). This association was moderately attenuated but remained significant after adjustment for BMI (HR=2.93, 1.08–7.96; Model 2), IL-6 (HR=2.91,1.07–7.91; Model 3), and comorbidities (HR=3.47,1.26–9.55; Model 4). Further, HbA1c 6.5–7.9% versus reference category was also significantly associated with incident walking difficulty in demographics-adjusted models (HR=2.26,1.05–4.87; Model 1) suggesting a possible graded association between HbA1c and incident walking difficulty, although this was no longer statistically significant in fully adjusted models (Model 4).
Similarly, HbA1c≥8% compared to reference category was associated with development of low walking speed after adjustment for demographics (HR=3.14,1.35–7.34; Model 1), BMI (HR=2.89,1.23–6.81; Model 2), IL-6 (HR=2.70,1.15–6.33; Model 3), and comorbidities (HR=2.82,1.19–6.71).
Lastly, HbA1c≥8% versus reference was also associated with development of low physical performance after adjustment for demographics (HR=3.31,1.43–7.69), BMI (HR=3.25,1.38–7.66), IL-6 (HR=3.09,1.31–7.28), and comorbidities (HR=3.60,1.52–8.53).
DISCUSSION
In the present study, participants in the highest HbA1c category (≥8%) compared to lowest (<5.5%) had a significant three-fold increased risk for the development of frailty and three-to-five-fold increased risk for development of lower extremity mobility limitations after adjustment for demographics. The association of HbA1c with incident frailty and lower extremity mobility limitations remained independent of potential confounders and was non-linear, with most events occurring in the highest HbA1c category (≥8%). These findings suggest that hyperglycemia, particularly in the diabetic range, can predict the onset of incident frailty and lower extremity mobility limitations less than a decade later.
To our knowledge, the association of hyperglycemia with incident frailty has only been explored in one other study to date (11). In this study, homeostasis model-insulin resistance (HOMA-IR) was calculated based on fasting glucose and insulin. For every SD increment in HOMA-IR, the adjusted HR for frailty was 1.15 (95% CI 1.02–1.31). In comparison, we found that the association of HbA1c with incident frailty was non-linear. An advantage of HbA1c is that levels are also influenced by differences in postprandial hyperglycemia, which may be more greatly related to frailty status than fasting levels alone (8). The other advantages of HbA1c are that it can be obtained without fasting and may be less variable with repeat testing (19). Thus, the results of our study provide a new perspective on a previously described relationship between hyperglycemia and incident frailty.
We have formerly demonstrated that higher HbA1c levels are cross-sectionally associated with greater walking difficulties (9). In the present study, we report for the first time that the development of difficulty in self-reported walking or performance based measures of lower extremity function is greater in persons with HbA1c≥8% versus <5.5% at baseline, independent of confounders. Objective measures may also detect preclinical limitations that predict future disability (12, 14).
We did not find evidence for a J-shaped association of HbA1c with incident frailty or lower extremity outcomes, although such associations have been described between HbA1c and mortality (16,17,20). Explanations for this discordance include the potential relationship of hypoglycemia to sudden death (21), unrelated to the presence of frailty. Further, frailty events occurring right before death may have been undetected in our analyses, yet, when we examined the composite outcome of incident frailty or death, the results were unchanged (data not shown). However, relatively smaller numbers of participants with high HbA1c levels in our study may have limited the ability to detect quadratic associations.
The association of hyperglycemia with incident frailty may be due to several factors. Similar to other studies, we found that frail women on average were more likely to be obese which may be associated with chronic inflammation (22). Inflammation is further associated with decreased leg muscle mass and strength which, in turn, is related to functional impairment, physical disability and frailty (12,23,24). Indeed, lower muscle function is inherent in the definition of frailty syndrome. In addition, chronic hyperglycemia is a risk factor for cardiovascular disease which in turn has been associated with frailty (25). However, we found that hyperglycemia was related to incident frailty status independent of the potential contributions of adiposity, inflammation, and cardiovascular disease. Interestingly, we also found that participants with hyperglycemia were more likely to develop lower extremity mobility limitations independent of confounders. In our study, we further found that associations were independent of potential mediators such as peripheral arterial disease and peripheral neuropathy (3).
A possible implication of these findings is that direct pathways linking hyperglycemia to muscle loss need to be considered. Interestingly, insulin resistance and diabetes have been associated with excessive loss of lean body mass and muscle strength in observational studies (26–27). Insulin resistance is also associated with skeletal muscle mitochondrial dysfunction (28) which may suggest underlying pathways for these epidemiological findings, but further studies are needed.
Limitations of our study include the relatively small sample size of participants in the highest HbA1c category (≥8%). However, we were still able to discern significant associations of HbA1c with incident frailty and lower extremity mobility limitations in adjusted regression models. Our study only included women, limiting generalizability. We also had a relatively high mortality rate likely due to the elderly age of our women; however, these occurred non-systematically [data not shown]. We only explored baseline HbA1c levels, however, changes in HbA1c levels over time may contribute to frailty status as well. Further, the majority of persons with HbA1c ≥8% had known history of diabetes; thus, we were unable to separate the effect of HbA1c from the presence of diabetes, itself. As a result, we cannot exclude the possibility that other aspects of the diabetes state could contribute to frailty, such as the use of glucose-lowering therapies, or that higher HbA1c among older persons reflects poorer self-care management and increased risk of adverse outcomes. Lastly, while our study focuses on an individual measure (HbA1c), recent studies have suggested that deficits across multiple systems may be most important in frailty development (29). We recognize that complex interactions between dysglycemia and abnormalities in other physiologic systems likely contribute to the pathophysiology of frailty which we hope to explore in a future study. Strengths of our study include the well-characterized WHAS II cohort, use of standardized protocols, inclusion of both self-reported and performance-based measures of lower extremity function, examination of potential non-linear relationships and length of follow-up. Further, use of a relatively long-term glycemic marker (HbA1c) minimized potential variability in the exposure.
In conclusion, our study adds to growing evidence that hyperglycemia is independently associated with the development of frailty and also with incident lower extremity mobility limitations. HbA1c testing may represent a practical method to screen individuals at high risk for the development of adverse geriatric outcomes. However, whether this increased risk for adverse geriatric outcomes is primarily related to HbA1c levels in the moderately uncontrolled diabetic range or higher (i.e. A1c≥8%) needs to be further explored. A better understanding might give insight into whether clinical guidelines proposing less aggressive HbA1c targets in older adults with diabetes are appropriate (30). While the mechanism remains unclear, direct associations of hyperglycemia with muscle loss may contribute and should be investigated in future studies. Intervention studies are ultimately needed to explore if treatment of hyperglycemia may delay or prevent the development of frailty and lower extremity limitations in older adults.
ACKNOWLEDGMENTS
This work was supported by the Johns Hopkins Older Americans Independence Center (P30-AG021334), National Institute on Aging (R37AG19905 and R01AG021493), and the VA Ann Arbor Geriatrics Research, Education and Clinical Center (GRECC).
Sponsor’s Role: None.
Footnotes
Conflict of Interest: No authors have potential conflicts of interest with reference to this paper.
Author Contributions: Drs. Kalyani, Xue, Walston, Cappola, Fried, Brancati and Blaum contributed to the study concept and design, methods, data analysis and interpretation of data, and preparation of the manuscript. Drs. Cappola, Blaum, Fried and Walston contributed to data acquisition. Ms. Tian contributed to study methods, data analysis, and interpretation of data.
REFERENCES
- 1.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 3.Volpato S, Ferrucci L, Blaum C, et al. Progression of lower-extremity disability in older women with diabetes: The Women's Health and Aging Study. Diabetes Care. 2003;26:70–75. doi: 10.2337/diacare.26.1.70. [DOI] [PubMed] [Google Scholar]
- 4.Kalyani RR, Saudek CD, Brancati FL, et al. The Association of Diabetes, Comorbidities, and Hemoglobin A1c with Functional Disability in Older Adults: Results from the National Health and Nutrition Examination Survey (NHANES), 1999–2006. Diabetes Care. 2010;33:1055–1060. doi: 10.2337/dc09-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 6.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 7.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 8.Kalyani RR, Varadhan R, Weiss CO, et al. Frailty Status and Altered Glucose-Insulin Dynamics. J Gerontol A Biol Sci Med Sci. 2011 Aug 26; doi: 10.1093/gerona/glr141. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaum CS, Xue QI, Tian J, et al. Is hyperglycemia associated with frailty status in older women? J Am Geriatr Soc. 2009;57:840–847. doi: 10.1111/j.1532-5415.2009.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Bandeen-Roche K, Chaves PH, et al. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–M52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 13.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 14.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 15.Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation on Obesity. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- 16.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang ES, Liu JY, Moffet HH, et al. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34:1329–1336. doi: 10.2337/dc10-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association. Standards of medical care in diabetes - 2011. Diabetes Care. 2011;34:S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selvin E, Crainiceanu CM, Brancati FL, et al. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167:1545–1551. doi: 10.1001/archinte.167.14.1545. [DOI] [PubMed] [Google Scholar]
- 20.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34:S132–S137. doi: 10.2337/dc11-s220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubbard RE, Lang IA, Llewellyn DJ, et al. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65:377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 23.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 25.Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 26.Park SW, Goodpaster BH, Lee JS, et al. Health, Aging, and Body Composition Study. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SW, Goodpaster BH, Strotmeyer ES, et al. Health, Aging, and Body Composition Study. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 28.Phielix E, Schrauwen-Hinderling VB, Mensink M, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi: 10.2337/db08-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AF, Mangione CM, Saliba D, et al. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51:S265–S280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]