Abstract
Rationale
Indirect acting serotonin (5-HT) receptor agonists (e.g., selective 5-HT reuptake inhibitors [SSRI]) stimulate multiple 5-HT receptors, although the role of particular receptors as well as interaction(s) among different receptors in the therapeutic effects of SSRIs is not fully understood.
Objectives
Relatively few studies have systematically examined direct-acting agonists in combination. This study examined the 5-HT1A receptor agonists 8-hydroxy-2-(di-n-propylamino tetralin hydrochloride (8-OH-DPAT; 0.01–10.0 mg/kg) and 3-chloro-4-fluorophenyl-4-fluoro-4-([(5-methyl-6-methylamino-pyridin-2-ylmethyl)-amino]-methyl)-piperidin-1-yl-methanone (F13714; 0.01–1.0 mg/kg) and the 5-HT2A receptor agonists 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM; 0.32–10.0 mg/kg) and dipropyltryptamine (DPT; 1.0–32.0 mg/kg), alone and in combination, in rats responding under a fixed ratio schedule of food presentation.
Results
When administered alone, each drug decreased the rate of responding in a dose-related manner with the potency order being F13714 > 8-OH-DPAT > DOM > DPT. WAY100635 (5-HT1A receptor antagonist; 0.01–0.1 mg/kg) attenuated the rate-decreasing effects of 8-OH-DPAT and F13714 while MDL100907 (5-HT2A receptor antagonist; 0.01–0.1 mg/kg) attenuated the rate-decreasing effects of DOM and DPT. Dose addition analysis showed that the interaction between 8-OH-DPAT and F13714, as well as the interaction between DOM and DPT, was additive. In contrast, the interaction between 8-OH-DPAT and DOM, as well as the interaction between F13714 and DOM, was infra-additive.
Conclusions
This study shows that, for some dose combinations, agonist actions at one 5-HT receptor subtype attenuate agonist actions at another 5-HT receptor subtype; thus, the combined neuropharmacological actions and therapeutic effects of indirect-acting agonists are not likely to be adequately characterized by examining in isolation activity at particular 5-HT receptor subtypes.
Keywords: Serotonin, receptor, drug interaction, additivity, rat, schedule-controlling responding, agonist, antagonist
Introduction
Selective serotonin (5-HT) reuptake inhibitors (SSRIs) are indirect-acting 5-HT receptor agonists insofar as they block the reuptake of 5-HT that can act at multiple receptor subtypes. Among 5-HT receptors it is thought that 5-HT1A and 5-HT2A receptors are particularly important for the therapeutic (e.g., antidepressant) effects of SSRIs (Celada et al. 2004). However, the actions of indirect acting agonists likely include interactions between activities at different 5-HT receptor subtypes. For example, the 5-HT2A receptor antagonist (+)2,3-dimethoxyphenyl-1-[2-(4-piperidine)-methanol] (MDL100907) enhances the antidepressant-like activity of SSRIs in rats (Marek et al. 2005), suggesting that activation of 5-HT2A receptors might attenuate therapeutic effects of SSRIs that are mediated through other (e.g., 5-HT1A) receptors. The current study administered direct-acting 5-HT receptor agonists in combination to examine interactions that might be relevant to the therapeutic effects of indirect-acting 5-HT receptor agonists.
A number of studies have examined interactions between drugs that are thought to act at different 5-HT receptor subtypes. For example, the 5-HT2A receptor agonist 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) increases the plasma concentrations of ACTH and this effect is enhanced by the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino) tetralin hydrochloride (8-OH-DPAT; Li et al., 1992). Furthermore, 5-methoxy-dimethyltryptamine-induced head twitching (Darmani et al. 1989), an effect that is thought to be mediated by 5-HT2A receptor activation (Green et al. 1983), is enhanced by the 5-HT1A receptor agonist 8-OH-DPAT (Darmani et al. 1989). DOI increases 8-OH-DPAT induced forepaw treading (Arnt and Hyttel, 1989); on the other hand, DOI-induced head twitching is inhibited by 8-OH-DPAT (Darmani et al. 1989). Some drugs that have agonist activity at 5-HT1A receptors occasion some drug-appropriate responding in rats discriminating a 5-HT2A receptor agonist (1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane [DOM] or lysergic acid diethylamide [LSD]) and when studied in combination the same 5-HT1A receptor agonists enhance the discriminative stimulus effects of 5-HT2A receptor agonists (Khorana et al. 2009; Reissig et al. 2005). On the other hand, both 8-OH-DPAT and DOI decrease locomotion in rats and when the two drugs are administered together the interaction is infra-additive (Krebs-Thomson and Geyer 1998).
Interpretation of results from prior studies that examined interactions between 5-HT receptor agonists is, in some cases, limited by the following: 1) many 5-HT receptor agonists bind to and have activity at multiple receptor subtypes; 2) often antagonists are not used to confirm the receptor subtype through which a particular agonist produces a behavioral effect; 3) in some studies, particularly those examining elicited behavior, 5-HT1A and 5-HT2 receptor agonists can produce qualitatively different effects (under such conditions, apparent interactions between agonists that are thought to act at different receptor subtypes might reflect functional competition between incompatible behavioral effects and not pharmacodynamic interactions between agonist actions at different receptor subtypes); and 4) many studies have not analyzed behavioral data quantitatively and neither have they systematically varied the proportion of drugs studied in combination. For example, data from drug interaction studies often are presented as isobolograms which, although providing a visualization of results, are challenging to analyze statistically. The current study used a dose addition approach whereby different proportions of drugs were studied in combination and the empirically determined dose-response curves were compared to dose-response curves expected on the basis of additivity. As compared with isobolograms, the dose addition approach is more amenable to statistical analyses (Tallarida 2000). It is important to systematically vary proportions in drug interactions studies because different proportions of the same drugs can yield markedly different results.
This study used schedule-controlled responding in rats to examine possible interactions between agonists that are thought to act selectively at either 5-HT1A (3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-6-methylamino-pyridin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl-methanone fumaric acid salt [F13714] and 8-OH-DPAT) or 5-HT2A (DOM and DPT) receptors. Antagonists that are selective for 5-HT1A (WAY100635) or 5-HT2A (MDL100907) receptors were used to confirm the receptor(s) mediating the rate-decreasing effects of agonists. Subsequently, drugs acting at the same or different receptors were studied in combination to test whether their interaction was additive (as expected for drugs acting at the same receptor), infra-additive, or supra-additive.
Materials and Methods
Subjects
Eight adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed individually under a 12/12-h light/dark cycle (experiments were conducted during the light period) with free access to water in the home cage. Rats were maintained at a reduced, stable body weight (350 g) by providing rodent chow (Rodent sterilizable diet, Harlan Teklad, Madison, WI) in the home cage after daily sessions. Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
Schedule controlled responding
Experiments were conducted in commercially available chambers equipped with two levers, stimulus lights, and a food pellet dispenser (MED Associates Inc., St. Albans, VT, Model #ENV-008CT) located within sound-attenuating, ventilated enclosures (MED Associates Inc., Model #ENV-022M) that are described in detail elsewhere (Carter et al., 2003). Data were collected using MED-PC IV software and an interface (MED Associates, Inc.). Rats were trained to press the right lever in multiple-cycle sessions conducted 7 days per week. Each cycle consisted of a 10-min pretreatment period when stimulus lights were not illuminated and responding had no scheduled consequence, followed by a 5-min response period when the light above the right lever was illuminated and rats could respond under a fixed ratio 5 schedule to receive a maximum of 10 food pellets (45 mg; Research Diets, New Brunswick, NJ). When 10 food pellets were delivered before 5 min had elapsed, the light was extinguished and responses had no programmed consequence for the remainder of the response period. Throughout the study, responses on the left lever had no programmed consequence. Daily training sessions consisted of 5 cycles and testing began after rats satisfied the following criteria for five consecutive sessions: the average response rate across sessions varied by less than ± 20%; and the average response rate for all cycles within a session varied by less than ± 20%. Test sessions were separated by at least two consecutive training sessions that satisfied these criteria.
During the first minute of each training cycle rats received a vehicle or sham injection. Test sessions were identical to training sessions except that drug was administered. Dose-response curves were determined using a cumulative-dosing procedure. During the first cycle, rats received vehicle; on subsequent cycles, rats received increasing doses of drug with the cumulative dose increasing by 0.5 log unit per cycle. For antagonism studies, an antagonist was administered 5 min before the start of the session. Testing continued until rats received fewer than 5 pellets in a cycle. Agonists were first studied alone, then with antagonists, and finally in combination.
Data Analyses
Response rates were averaged among 8 rats (± SEM) and plotted as a function of dose. In order to determine the potency of drugs to decrease responding, response rates were converted to percentages for individual subjects. Control response rates for individual rats were determined by averaging response rate across all cycles in a training (i.e., no drug) session; the mean rates for 5 sessions were averaged to obtain the overall control rate. Linear regression was used to estimate the dose required to decrease response rate to 50% of the control rate (ED50) for individual rats. The 95% confidence limits (CL) were calculated from ED50 values averaged among rats.
Interactions between agonists and antagonists were assessed using Schild analysis. Dose ratios were determined for each rat by dividing the ED50 value for each agonist (8-OH-DPAT and DOM) studied in combination with an antagonist (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl) cyclohexanecarboxamide dihydrochloride [WAY100635] and MDL100907) by the ED50 value for the agonist studied alone (Li et al., 2009; Arunlakshana and Schild, 1959). Schild plots were constructed by plotting the log of the dose ratio -1 as a function of the negative log dose of antagonist (expressed in moles per kilogram body weight). Straight lines were simultaneously fitted to the Schild plots using GraphPad Prism version 5.00 for Windows (GraphPad Software Inc., San Diego, CA) using the following equation: log (dose ratio − 1) = −log (molar dose of antagonist) × slope + intercept; the apparent affinity (pA2) values and 95% CLs with unconstrained slopes were determined. Slopes of Schild plots were considered to conform to unity when the 95% CL included -1 and did not include 0. For the agonist-antagonist combination studies that only studied one dose of antagonist, apparent pKB values were obtained using the equation pKB= −log (molar of antagonist dose)/(dose ratio – 1).
Interactions between agonists were assessed using a fixed ratio dose addition analysis. For this analysis, two agonists were combined in a fixed proportion and administered using a cumulative dosing procedure. Dose-response curves for drugs administered alone and in combination were analyzed for parallelism with an F-ratio test (GraphPad Prism 5.0 for Windows; GraphPad Software, San Diego, CA; p<0.05). For drug mixtures, the dose-response curves were determined and the individual ED50 values of the two drugs in the mixture were calculated based upon the shared dose-response curves. The sum of the ED50 values of both drugs in the mixture was defined as Zmix. For statistical assessment of the drug interactions, the experimentally determined (observed) ED50 values (Zmix) were compared with the predicted additive ED50 values (Zadd) as described previously (Tallarida 2000; Stevenson et al. 2005) and calculated using Pharm Tools Pro version 1.1 for Windows (The McCary Group Inc., Wilmington, DE). Zadd values were determined for each rat from the following equation: Zadd = fA + (1−f)B, where A is the ED50 for one drug alone in the mixture, B is the ED50 for the other drug alone in the mixture, and f is the fractional multiplier of A in the computation of the additive total dose. The choice of f defines the proportion of drug A (ρA) in a mixture according to the following equation: ρA=fA/Zadd. The present study examined the interaction of four drug combinations: 8-OH-DPAT and F13714; DOM and DPT; DOM and 8-OH-DPAT; and DOM and F13714. For each drug mixture the first drug was defined as drug A and the second drug was defined as drug B; these studies examined effects produced by drug mixtures in which f=0.25, 0.5 and 0.75. Thus, f = 0.25 leads to ρA=A/(A + 3B), and the ratio of drug A concentration to drug B concentration is [(A/B)÷3] : 1; f = 0.5 leads to ρA=A/(A + B), and the ratio of drug A concentration to drug B concentration is (A/B) : 1; and f = 0.75 leads to ρA=3A/(3A + B), and the ratio of drug A concentration to drug B concentration is [(A/B) × 3]: 1. The Zadd and Zmix values were calculated and the ratio Zmix/Zadd was obtained for each individual subject. If the 95% CL of the mean of the ratio of the group did not include 1, then the ratio was considered to be significantly different from 1. A ratio significantly greater than 1 indicates that the drug mixture produces the effect in a manner that is less than additivity (infra-additivity); conversely, a ratio significantly smaller than 1 indicates that the drug mixture produces the effect in a manner that is significantly greater than additivity (supra-additivity).
Drugs
The compounds used in this study were dissolved in saline, unless otherwise noted, and included the following: DOM hydrochloride (NIDA Research Technology Branch, Rockville, MD); DPT hydrochloride and MDL100907 were synthesized by Kenner Rice (Ullrich and Rice 2000); 8-OH-DPAT was purchased from Sigma-Aldrich (St. Louis, MO); F13714 and WAY100635 were gifts from Dr. Adrian Newman-Tancredi (Centre de Recherche Pierre Fabre, Castres, France). MDL100907 was dissolved in 20% dimethylsulfoxide (v/v). Drugs were administered intraperitoneally and doses are expressed as the form indicated above in mg per kg of body weight. Injection volumes were 0.2–1.0 ml.
Results
The average control response rate (± SEM) prior to the beginning of testing was 0.77 ± 0.02 responses/s. Response rates increased slightly, although not significantly, over the five cycles comprising daily sessions as follows: 0.73 ± 0.07 in cycle 1; 0.75 ± 0.05 in cycle 2; 0.79 ± 0.06 in cycle 3; 0.76 ± 0.07 in cycle 4; and 0.83 ± 0.06 in cycle 5.
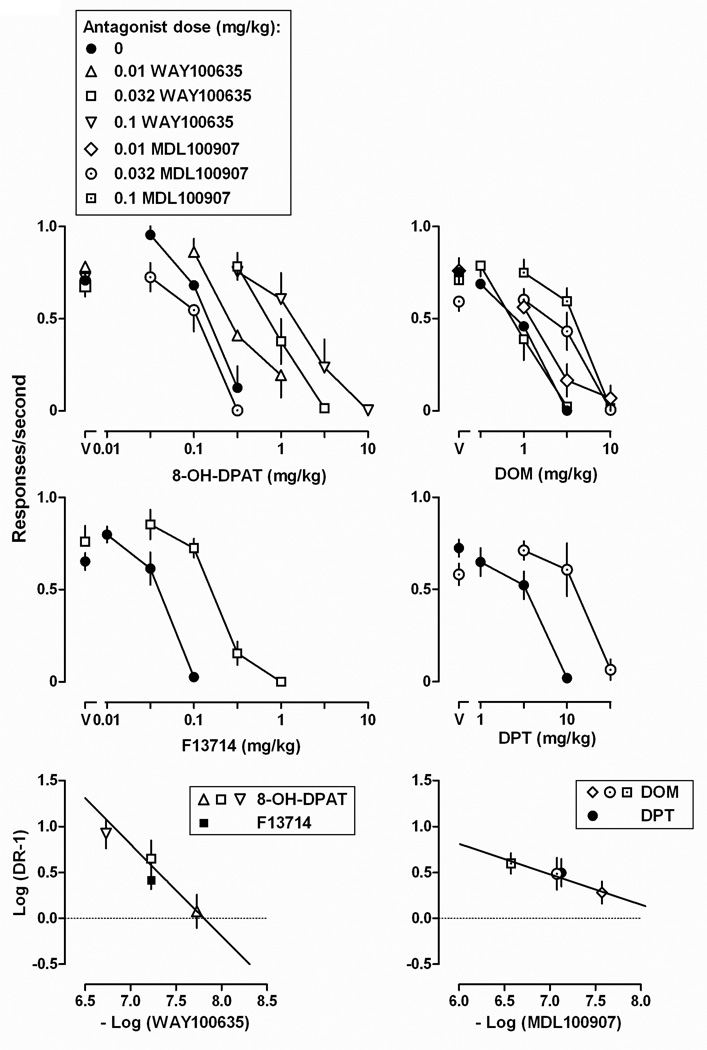
The 5-HT1A receptor agonists F13714 and 8-OH-DPAT as well as the 5-HT2A receptor agonists DOM and DPT decreased responding in a dose-related manner (Fig 1; see Table 1 for ED50 values). The rank order potency for decreasing responding was F13714 > 8-OH-DPAT > DOM > DPT. The relative potency of each drug (in comparison with DPT; Table 1) was used to calculate the proportions of each drug used in drug mixtures for subsequent studies.
Fig. 1.
Effects of F13714, 8-OH-DPAT, DOM and DPT on schedule-controlled responding in rats. Abscissa, drug in milligrams per kilogram body weight. Ordinate, response rate in responses per second. All entries represent the mean (± SEM) from 8 rats; entries above “V” represent response rate after vehicle administration.
Table 1.
ED50 values (mg/kg, 95% CL) and relative potency (compared with DPT) for DPT, DOM, 8-OH-DPAT, and F13714 in decreasing food-maintained responding in rats.
| Agonist | ED50 Value (95% CL) | Relative Potency |
|---|---|---|
| DPT | 3.07 (2.01, 4.13) | 1 |
| DOM | 0.86 (0.43, 1.29) | 3.6 |
| 8-OH-DPAT | 0.14 (0.10, 0.19) | 22 |
| F13714 | 0.04 (0.03, 0.05) | 77 |
When administered alone, WAY100635 had no effect on responding (points above “V”, left panels, Fig 2); however, WAY100635 attenuated the rate-decreasing effect of 8-OH-DPAT with doses of 0.01, 0.032, and 0.1 mg/kg shifting the 8-OH-DPAT dose-response curve 2.4-, 6.9-, and 13.3-fold to the right, respectively (top left panel, Fig 2). Schild analysis yielded a slope (95% CL) not different from -1 (-0.93 [-1.25, -0.71]) and a pA2 value (95% CL) of 7.95 (7.62, 8.28; bottom left panel, Fig 2). Similarly, a dose of 0.032 mg/kg WAY100907 attenuated the rate-decreasing effects of F13714, shifting the F13714 dose-response curve 4.6-fold to the right (middle left panel, Fig 2); the apparent pKB for WAY100907 in attenuating F13714 was 7.78. A dose of 0.032 mg/kg of the selective 5-HT2A receptor antagonist MDL100907 did not attenuate the rate-decreasing effect of 8-OH-DPAT (upper left panel, Fig 2).
Fig. 2.
Upper four panels: effects of 8-OH-DPAT, F13714, DOM and DPT alone or in combination with the 5-HT1A receptor antagonist WAY100635 or the 5-HT2A receptor antagonist MDL100907. See Fig 1 for other details. Bottom two panels: Schild plots constructed from the same data shown in the upper and middle panels. Abscissae, negative log of the dose of antagonist in moles per kilogram of body weight. Ordinates, log of the dose ratio – 1.
When administered alone, MDL100907 did not significantly affect the rate of responding (points above “V”, right panels, Fig 2); however, MDL100907 attenuated the rate-decreasing effects of DOM with doses of 0.01, 0.032, and 0.1 mg/kg shifting the DOM dose-response curve 2.9-, 3.9-, and 4.5-fold to the right, respectively (upper right panel, Fig 2). Schild analysis yielded a slope significantly different from -1 (-0.30 [-0.69, 0.09]; bottom right panel, Fig 2); thus, a pA2 value was not determined. The pKB value for MDL10907 (0.032 mg/kg) in antagonizing DOM was of 7.68. A dose of 0.032 mg/kg MDL100907 also shifted the dose-response curve of DPT 4.1-fold to the right, yielding a pKB value of 7.72 (middle right panel, Fig 2). A dose of 0.032 mg/kg of the selective 5-HT1A receptor antagonist WAY100635 did not attenuate the rate-decreasing effects of DOM (upper right panel, Fig 2).
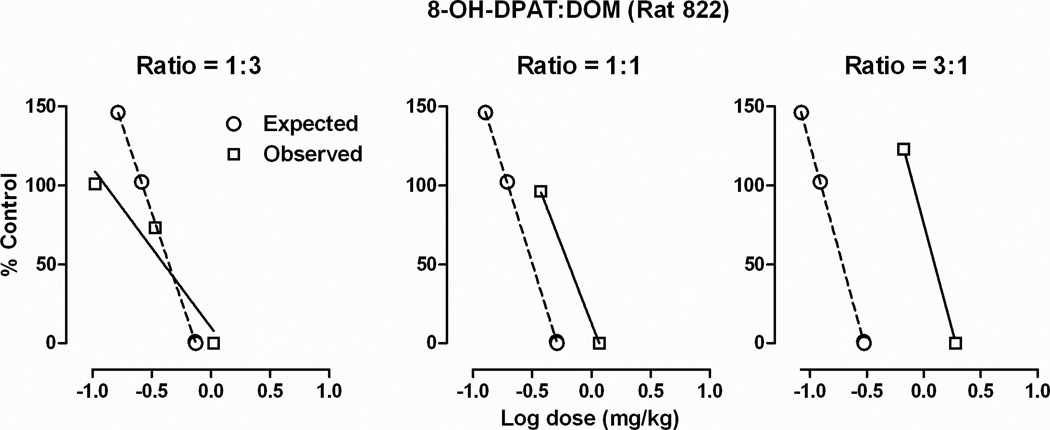
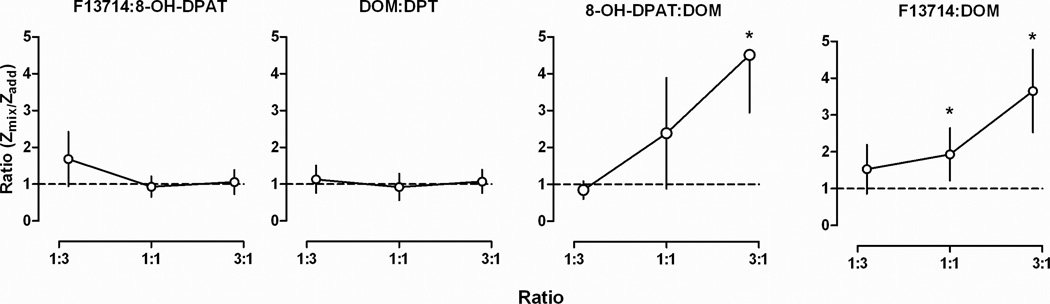
Slopes of dose-response curves for drug combinations were not significantly different from slopes of dose-response curves for drugs administered alone (data not shown). Representative composite additive curves showing response rate as a function of the mixture dose for an individual rat (822) are shown in Fig 3 for proportions of 1:3, 1:1, and 3:1 of 8-OH-DPAT and DOM. Comparison between expected (circles) and observed (squares) dose-response curves indicate a difference (i.e. not additive) for a proportion of 3:1 but not for 1:3. Similar results were obtained in other rats (data not shown) and the mean ED50 values for all drug mixtures are shown in Table 2. Presented in the table are the observed ED50 values (Zmix), expected ED50 values (Zadd), and a ratio of the observed/expected values (Zmix/Zadd). Mixtures of F13714 and 8-OH-DPAT and of DOM and DPT yielded ED50 values that were not different from expected values (i.e. additivity), regardless of the proportion of each drug in the mixture (Table 2, Fig 4). In contrast, one combination of 8-OH-DPAT and DOM as well as two combinations of F13714 and DOM yielded ED50 values that were significantly different from the expected (additive) values. As shown in Fig 4 for a proportion of 3:1 of 8-OH-DPAT and a proportion of 1:1 and 3:1 of F13714 and DOM, the 95% CL for the ratio of the observed (Zmix) and the expected (Zadd) ED50 values did not include 1, indicating an infra-additive interaction. Other proportions of these mixtures were not different from the expected values.
Fig. 3.
Composite additive curves from a representative rat for three mixtures (1:3, 1:1, and 3:1) of 8-OH-DPAT and DOM. Abscissae, log dose of the drug mixture in milligrams per kilogram body weight. Ordinate, response rate expressed as a percentage of the control (vehicle) rate.
Table 2.
Observed (experimentally determined) ED50 values (Zmix) and expected additive ED50 values (Zadd) and the ratio of observe/expected ED50 values for drug mixtures in decreasing food-maintained responding in rats.
| Mixture | Zmix (95% CL) | Zadd (95% CL) | Ratio Zmix/Zadd |
|---|---|---|---|
| Relative dose (proportion) |
|||
| F13714:8-OH-DPAT | |||
| 0.10:1 (1:3) | 0.192 (0.175, 0.208) | 0.154 (0.124, 0.184) | 1.25 |
| 0.29:1 (1:1) | 0.085 (0.077, 0.093) | 0.101 (0.079, 0.123) | 0.84 |
| 0.88:1 (3:1) | 0.073(0.055, 0.090) | 0.072 (0.059, 0.086) | 1.01 |
| DOM:DPT | |||
| 0.09:1 (1:3) | 3.02 (1.83, 4.20) | 2.58 (1.80, 3.35) | 1.17 |
| 0.28:1 (1:1) | 2.29 (0.62, 3.97) | 2.05 (1.42, 2.69) | 1.11 |
| 0.84:1 (3:1) | 1.83 (0.88, 2.78) | 1.53 (1.02, 2.05) | 1.93 |
| 8-OH-DPAT:DOM | |||
| 0.06:1 (1:3) | 0.89 (0.50, 1.28) | 0.80 (0.48, 1.12) | 1.11 |
| 0.17:1 (1:1) | 1.15 (0.63, 1.67) | 0.59 (0.37, 0.80) | 1.95 |
| 0.51:1 (3:1) | 1.45 (0.87, 2.02) | 0.37 (0.26, 0.48) | 3.92a |
| F13714:DOM | |||
| 0.016:1 (1:3) | 0.89 (0.38, 1.40) | 0.77 (0.45, 1.09) | 1.16 |
| 0.048:1 (1:1) | 1.15 (0.51, 1.79) | 0.53 (0.31, 0.75) | 2.17a |
| 0.145:1 (3:1) | 1.02 (0.65, 1.40) | 0.29 (0.17, 0.40) | 3.52a |
p<.05
Fig. 4.
Ratios of observed (experimentally determined) ED50 values (Zmix) and expected (additive) ED50 values (Zadd) for different proportions of drug mixtures. Ordinates, ratio of Zmix and Zadd. Abscissae, proportion ratios of each pair of the drug
Discussion
Indirect-acting 5-HT agonists (e.g., SSRIs) are used widely to treat various psychiatric disorders although the specific mechanism(s) accounting for the therapeutic effects of these drugs is not fully understood. For example, it is not clear which 5-HT receptor subtype(s) mediates the therapeutic effects of indirect-acting agonists and, relevant to the current study, it is not clear to what extent activity at one 5-HT receptor subtype impacts activity at other 5-HT receptor subtypes. This study examined interactions between agonists acting selectively at either 5-HT1A or 5-HT2A receptors, both of which are believed to play a role in the therapeutic actions of SSRIs, and the results demonstrate that agonist actions at one 5-HT receptor subtype can significantly attenuate agonist actions at a different 5-HT receptor subtype. However, the nature of the interaction between drugs can vary markedly depending on the relative activity (e.g. drug proportions in the mixture) at different receptor subtypes. Thus, the combined neuropharmacological actions and therapeutic effects of indirect-acting agonists are not likely to be adequately characterized by examining in isolation activity at particular 5-HT receptor subtypes.
Prior studies that examined interactions between drugs acting selectively at different 5-HT receptor subtypes have often used experimental conditions where the two drugs of interest do not share the same effect; however, under such conditions interactions might be due to functional competition between incompatible behavioral effects and not to pharmacodynamic interactions. Similarly, the analysis and interpretation of interactions between agonists can be challenging when one of the drugs of interest is without effect when administered alone. Thus, there can be advantages to studying agonists that share a common effect, even if that effect occurs by actions at different receptors, because full dose-response curves can be determined for each agonist alone and for specific combinations (proportions) of two agonists. It is critical that the measured effects are mediated by the receptors of interest, particularly for agonists that are thought to have activity at more than one receptor. Many 5-HT receptor agonists have activity at more than one receptor subtype; for example, 8-OH-DPAT induced hypothermia in rats is mediated by both 5-HT1A and 5-HT7 receptors (Hedlund et al., 2004). DOM has similar affinity for 5-HT2A and 5-HT2C receptors and reportedly exerts agonist activity at both receptor subtypes (Eckler et al., 2004; Fiorella et al., 1995; Li et al., 2009).
The current study used schedule-controlled responding to study interactions between drugs acting selectively at different 5-HT receptor subtypes because all of the drugs studied decrease responding in this procedure and they do so, at least in part, by actions at the receptor subtype of interest. Thus, the rate-decreasing effects of DOM and DPT (agonists at 5-HT2A receptors), and not those of the selective 5-HT1A receptor agonist F13714 (Koek et al., 2001) or 8-OH-DPAT (agonist at 5-HT1A receptors), were attenuated by MDL100907 (selective 5-HT2A receptor antagonist) and not by WAY100635 (selective 5-HT1A receptor antagonist). Conversely, the rate-decreasing effects of F13714 and 8-OH-DPAT, and not those of DOM or DPT, were attenuated by WAY100635 and not by MDL100907. The apparent affinity estimate for WAY100635 in combination with 8-OH-DPAT in the current study was similar to apparent affinities reported by others for the same drugs (e.g. 7.8 [7.4, 8.2]; Koek et al. 2000) and confirm the role of 5-HT1A receptors. Moreover, the relative potency of the agonists in decreasing schedule-controlled responding was the same as their relative potency in other (e.g., drug discrimination) studies where it has been shown that they act selectively through a 5-HT receptor subtype (e.g., Li et al., 2010). Thus, in the current study interactions between drugs were likely due to actions at 5-HT1A and 5-HT2A receptors because those are the receptor subtypes that mediate the rate-decreasing effects of the agonists studied.
Based on receptor theory, it was expected that the interaction between two agonists acting at the same 5-HT receptor subtype (either 5-HT1A or 5-HT2A) would be additive. That is, when two drugs produce the same effect by acting at the same receptor, pretreatment with one drug should increase the effects of the second drug in an additive manner. For example, in rats discriminating DOM, DPT enhances the discriminative stimulus effects of DOM in an additive manner (Li et al., 2010). In the current study, for drugs acting at the same receptor (as demonstrated by antagonism studies), the interaction for rate-decreasing effects was additive (i.e., between 8-OH-DPAT and F13714, on the one hand, and between DOM and DPT, on the other).
There are reports of non-additive (i.e., infra- or supra-additive) interactions between drugs acting at different 5-HT receptor subtypes (e.g., 5-HT1A and 5-HT2A). The current study used a dose addition analysis to investigate interactions between agonists acting at different 5-HT receptor subtypes. The interactions between either 8-OH-DPAT or F13714 and DOM were additive or infra-additive, depending on the proportion of each drug in the mixture. When a smaller proportion of 8-OH-DPAT and F13714 was used in the drug mixture, their interaction with DOM was additive; however, when the proportion of 8-OH-DPAT and F13714 in the mixture was increased, their interaction with DOM was infra-additive. These results extend previous reports (Krebs-Thompson and Geyer 1998; Tallarida 2000) showing that the interaction between two drugs can vary significantly depending on the proportion of each drug in the mixture. It is unclear why interactions between agonists vary according to the relative activity at different receptors, although this would appear to be a fundamentally important area of research for understanding the in vivo actions of drugs that simultaneously activate more than one receptor (e.g. indirect acting agonists).
In summary, this study used schedule-controlled responding in rats and quantitative pharmacological analyses to examine interactions between drugs acting selectively at 5-HT1A or 5-HT2A receptors. Antagonism studies confirmed that the rate-decreasing effects of 8-OH-DPAT and DOM are mediated predominantly, if not exclusively, by 5-HT1A and 5-HT2A receptors, respectively, and dose addition analyses showed that, depending on the relative proportion of drugs in the mixture, the interaction between 5-HT1A and 5-HT2A receptor agonists is additive or infra-additive. These data suggest that the neuropharmacology of indirect-acting 5-HT receptor agonists (e.g., SSRIs) includes possibly complex interactions between activities at different receptor subtypes and, therefore, that the therapeutic effects of such drugs cannot be fully understood by examining in isolation agonism at specific receptor subtypes.
Acknowledgements
The authors thank Christopher Cruz, Sonia Cano, Margarita Gardea and Jennifer Kite for excellent technical assistance.
CPF is supported by a Senior Scientist Award from the National Institute on Drug Abuse, National Institutes of Health (K05 DA17918). This research was supported, in part, by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to declare
References
- Arnt J, Hyttel J. Facilitation of 8-OH-DPAT-induced forepaw treading of rats by the 5-HT2agonist DOI. Eur J Pharmacol. 1989;161:45–51. doi: 10.1016/0014-2999(89)90178-7. [DOI] [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, France CP. The role of GABABreceptors in the discriminative stimulus effects of gamma-hydroxybutyrate in rats: time course and antagonism studies. J Pharmacol Exp Ther. 2003;305:668–674. doi: 10.1124/jpet.102.047860. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1Aand 5-HT2Areceptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1Aand 5-HT2receptors? Pharmacol Biochem Behav. 1989;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Eckler JR, Reissig CJ, Rabin RA, Winter JC. A 5-HT(2C) receptor-mediated interaction between 2,5-dimethoxy-4-methylamphetamine and citalopram in the rat. Pharmacol Biochem Behav. 2004;79:25–30. doi: 10.1016/j.pbb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2Aand 5-HT2Creceptors in the stimulus effects of hallucinogenic drugs: I. Antagonist correlation analysis. Psychopharmacology. 1995;121:347–356. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- Green AR, O’Shaughnessy K, Hammond M, Schachter M, Grahame-Smith DG. Inhibition of 5-hydroxytryptamine-mediated behaviour by the putative 5-HT2antagonist pirenperone. Neuropharmacology. 1983;22:573–578. doi: 10.1016/0028-3908(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1Aand 5-HT7receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Khorana N, Young R, Glennon RA. Effect of 8-hydroxy-2-(N,N-di-n-propylamino) tetralin and MDMA on the discriminative stimulus effects of the classical hallucinogen DOM in rats. Pharmacol Biochem Behav. 2009;91:385–392. doi: 10.1016/j.pbb.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Assie M, Zernig G, France CP. In vivo estimates of efficacy at 5-HT1Areceptors: effects of EEDQ on the ability of agonists to produce lower lip retraction in rats. Psychopharmacology. 2000;149:377–387. doi: 10.1007/s002130000374. [DOI] [PubMed] [Google Scholar]
- Koek W, Vacher B, Cosi C, Assié MB, Patoiseau JF, Pauwels PJ, Colpaert FC. 5-HT1Areceptor activation and antidepressant-like effects: F 13714 has high efficacy and marked antidepressant potential. Eur J Pharmacol. 2001;420:103–112. doi: 10.1016/s0014-2999(01)01011-1. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA. Evidence for a functional interaction between 5-HT1Aand 5-HT2Areceptors in rats. Psychopharmacology. 1998;140:69–74. doi: 10.1007/s002130050740. [DOI] [PubMed] [Google Scholar]
- Li J-X, Koek W, Rice KC, France CP. Differential effects of 5-HT1A receptor agonists on the discriminative stimulus effects of the 5-HT2A receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rats and rhesus monkeys. J Pharmacol Exp Ther. 2010;333:244–252. doi: 10.1124/jpet.109.163451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-X, Rice KC, France CP. Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rhesus monkeys: antagonism and apparent pA2analyses. J Pharmacol Exp Ther. 2009;328:976–981. doi: 10.1124/jpet.108.145458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Rittenhouse PA, Levy AD, Alvarez Sanz MC, Van de Kar LD. Neuroendocrine responses to the serotonin 2 agonist DOI are differentially modified by three 5-HT1Aagonists. Neuropharmacology. 1992;31:983–989. doi: 10.1016/0028-3908(92)90098-a. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. The selective 5-HT2Areceptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology. 2005;30:2205–2215. doi: 10.1038/sj.npp.1300762. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Eckler JR, Rabin RA, Winter JC. The 5-HT1Areceptor and the stimulus effects of LSD in the rat. Psychopharmacology. 2005;182:197–204. doi: 10.1007/s00213-005-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson GW, Folk JE, Rice KC, Negus SS. Interactions between delta and mu opioid agonists in assays of schedule-controlled responding, thermal nociception, drug self-administration, and drug versus food choice in rhesus monkeys: studies with SNC80 and heroin. J Pharmacol Exp Ther. 2005;314:221–231. doi: 10.1124/jpet.104.082685. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ, Stone DJ, Jr, Raffa RB. Efficient designs for studying synergistic drug combinations. Life Sci. 1997;61:PL417–PL425. doi: 10.1016/s0024-3205(97)01030-8. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug synergism and dose-effect data analysis. Boca Raton, FL: Chapman and Hall/CRC; 2000. [Google Scholar]
- Ullrich T, Rice KC. A practical synthesis of the serotonin 5-HT2Areceptor antagonist MDL 100907, its enantiomer and their 3-phenolic derivatives as precursors for11C labeled PET ligands. Bioorg Med Chem. 2000;8:2427–2432. doi: 10.1016/s0968-0896(00)00175-9. [DOI] [PubMed] [Google Scholar]