Abstract
Background: Epidemiologic evidence for the relation between carbohydrate quality and risk of type 2 diabetes (T2D) has been mixed.
Objective: We prospectively examined the association of dietary glycemic index (GI) and glycemic load (GL) with T2D risk.
Design: We prospectively followed 74,248 women from the Nurses’ Health Study (1984–2008), 90,411 women from the Nurses’ Health Study II (1991–2009), and 40,498 men from the Health Professionals Follow-Up Study (1986–2008) who were free of diabetes, cardiovascular disease, and cancer at baseline. Diet was assessed by using a validated questionnaire and updated every 4 y. We also conducted an updated meta-analysis, including results from our 3 cohorts and other studies.
Results: During 3,800,618 person-years of follow-up, we documented 15,027 cases of incident T2D. In pooled multivariable analyses, those in the highest quintile of energy-adjusted GI had a 33% higher risk (95% CI: 26%, 41%) of T2D than those in the lowest quintile. Participants in the highest quintile of energy-adjusted GL had a 10% higher risk (95% CI: 2%, 18%) of T2D. Participants who consumed a combination diet that was high in GI or GL and low in cereal fiber had an ∼50% higher risk of T2D. In the updated meta-analysis, the summary RRs (95% CIs) comparing the highest with the lowest categories of GI and GL were 1.19 (1.14, 1.24) and 1.13 (1.08, 1.17), respectively.
Conclusion: The updated analyses from our 3 cohorts and meta-analyses provide further evidence that higher dietary GI and GL are associated with increased risk of T2D.
See corresponding editorial on page 1.
INTRODUCTION
Type 2 diabetes (T2D)4 has remained a significant public health problem for several decades. The number of adults with diabetes worldwide increased from 153 million in the year 1980 to 347 million in 2008 (1). The WHO projects diabetes to be the seventh leading cause of death in 2030 (2). Furthermore, the total costs of diagnosed diabetes have increased by 41% from $174 billion in 2007 to $245 billion in 2012 in the United States (3). Well-designed randomized controlled trials such as the Diabetes Prevention Program (4, 5) have shown that following a healthy dietary pattern along with lifestyle modifications are as effective as or even better than pharmacologic interventions in preventing T2D. Carbohydrates are the dietary components that have the greatest effect on blood glucose concentrations. Because carbohydrates differ in their ability to increase blood glucose, the concept of glycemic index (GI) was introduced in the early 1980s. The GI is a ranking of carbohydrates according to their effect on postprandial glycemia (6). Although the GI can effectively rank foods on the basis of their blood glucose response, it does not account for the amount of carbohydrate in a typical serving. The glycemic load (GL) was therefore developed as the product of the GI and the amount of carbohydrate in a serving.
Despite the existence of these 2 indexes for many years, the role of GI and GL in preventing T2D remains controversial. Although 3 meta-analyses (7–9) of prospective cohort studies found a higher risk of T2D with higher GI and GL, a more recent meta-analysis of 8 European countries found no association with T2D (10). Furthermore, the most recent American Diabetes Association's nutrition recommendations for management of adults with T2D indicated that, to date, supportive evidence for a role of GI and GL in glycemic control has only been from poorly controlled or uncontrolled studies or observational studies with a high potential for bias (11).
Given the inconsistencies in the literature, the aims of the current study were as follows: 1) to update our previous analyses of GI and GL and T2D risk using much longer follow-up in the 3 Harvard cohorts [Nurses’ Health Study (NHS), NHS II, and the Health Professionals Follow-Up Study (HPFS)] and 2) to conduct an updated meta-analysis of the previous literature including results from our 3 cohorts.
METHODS
Study population
The NHS began in the early 1970s as a long-term prospective investigation of the health effects of various contraceptive methods of 121,701 married female nurses, aged 30–55 y, from 11 US states. The NHS II is a prospective cohort study in 116, 671 younger female nurses, aged 25–42 y, that began in 1989. The HPFS began in 1986 as a prospective cohort study to evaluate the association of nutritional factors and the incidence of several chronic diseases in a group of 51, 529 male health professionals. In all 3 cohorts, participants are followed biennially through validated questionnaires that obtain updated information on their medical history, lifestyle factors, and occurrence of chronic diseases. For the current study, we used baseline as 1984 in NHS, 1986 in HPFS, and 1991 in NHS II when detailed information on diet was first collected. We excluded participants with a baseline history of diabetes (including type 1 diabetes and T2D), cardiovascular disease, or cancer because these diagnoses may result in changes in diet (12). We also excluded women who left ≥10 items blank on the food-frequency questionnaire (FFQ) or who had implausible energy intakes (<800 or >4200 kcal/d for men and <500 or >3500 kcal/d for women). Men who left ≥70 items blank on the FFQ were also excluded. The final analytic sample included 74,248 women from the NHS, 90,411 women from the NHS II, and 40,498 men from the HPFS with complete information. The study was approved by the Human Research Committee of Brigham and Women's Hospital and the Harvard School of Public Health in Boston.
Ascertainment of exposure
With the use of a 126-item semiquantitative FFQ, diet was first assessed in 1984 in NHS and in 1986 in HPFS. By using a 133-item semiquantitative FFQ, diet was first assessed in NHS II in 1991. In all 3 cohorts, subsequent FFQs were administered every 4 y to update dietary information. In all FFQs, participants were asked how often, on average (never to ≥6 times/d), during the previous year they had consumed a commonly used unit or portion size of foods and beverages. The FFQs were reviewed every 4 y with the use of extensive pilot studies and analyses of open-ended sections to determine whether changes were needed. Nutrient intakes were computed by multiplying the frequency of consumption of each food or beverage by the nutrient content of the specified portion and summing the contributions from all items. Nutrient intakes were obtained by using the USDA food-composition database supplemented with information from manufacturers and information from our own analyses of foods. The GI values for single food items on the FFQ were derived from available databases and publications (6, 13, 14). Certain foods not represented in the database were sent to the University of Sydney for GI analysis. These foods included breakfast cereals, cakes, cookies, muffin mixes, pancake mixes, and candy bars. For the remaining foods not represented in the database, we imputed their GI values from similar foods in the database. For each participant, we calculated the average dietary GI by summing the products of the carbohydrate content per serving for each food item times the average number of servings of that food per day, times its GI, and divided by the total daily carbohydrate content (15). Because the amount of carbohydrate in an overall diet can vary, we derived a global dietary GL score by multiplying the amount of carbohydrates in the diet by the average GI. GL is an indicator of insulin demand or glucose response induced by total carbohydrate intake (15). GI, GL, and intakes of all nutrients were energy-adjusted by using the residual method (16).
The validity and reliability of the FFQs have been described elsewhere (17–22). In a validation study conducted in a subsample of 173 NHS participants, FFQ assessments of total carbohydrate and dietary fiber were highly correlated with diet record assessments (total carbohydrate, r = 0.64; fiber, r = 0.56) (17, 22). Similar correlation coefficients were seen in a validation study in a subsample of 40- to 74-y-old HPFS participants (total carbohydrate, r = 0.73; fiber, r = 0.68) (19).
Ascertainment of diabetes
The primary endpoint for this study was incident T2D. Participants who reported a diagnosis of T2D on the biennial main questionnaire were mailed a supplementary questionnaire with regard to symptoms, diagnostic tests, and hypoglycemic therapy. In accordance with the National Diabetes Data Group criteria (23), a case of T2D was confirmed if at least one of the following was reported on the supplementary questionnaire: 1) one or more classic symptoms (excessive thirst, polyuria, weight loss, hunger) and fasting plasma glucose concentrations of ≥11.1 mmol/L, 2) ≥2 elevated plasma glucose concentrations on different occasions (fasting concentrations of ≥7.8 mmol/L, random plasma glucose concentrations of ≥11.1 mmol/L, and/or concentrations of ≥11.1 mmol/L after ≥2 h shown by an oral-glucose-tolerance test) in the absence of symptoms, or 3) treatment with hypoglycemic medication (insulin or oral hypoglycemic agent). For cases identified after 1998, we applied the American Diabetes Association criteria (24) in which the threshold for diagnosis of diabetes changed from a fasting plasma glucose concentration of 7.8 to 7.0 mmol/L. The current analysis includes only the cases confirmed by the supplementary questionnaire.
The validity of the supplementary questionnaire was established in 2 previous studies through medical record reviews. In both studies, diagnosis of T2D was confirmed in >97% of the cases (25, 26).
Assessment of covariates
In the biennial follow-up questionnaires, we updated information on a participant's age, weight, smoking status, physical activity, menopausal status and use of postmenopausal hormone therapy (for women), oral contraceptive use (for women), and personal history of chronic diseases. Height was ascertained on the 1976 enrollment questionnaire in the NHS and the 1986 enrollment questionnaire in the HPFS. We calculated BMI as weight in kilograms divided by height in meters squared. Self-reports of body weight have been shown to be highly correlated with measured weights (r = 0.96) in the NHS and HPFS (27). The presence or absence of a family history of diabetes (in first-degree relatives) was assessed in 1982 and 1988 in the NHS; in 1989, 1997, 2001, and 2005 in the NHS II; and in 1987 in the HPFS. Information on intakes of alcohol and other beverages was updated every 4 y by using the FFQ.
Statistical analysis
We calculated each individual's person-time from the return of the baseline questionnaire (1984 in NHS, 1991 in NHS II, and 1986 in HPFS) to the date of diagnosis of T2D, death, date of loss to follow-up, or the cutoff date (30 June 2008 in NHS, 30 June 2009 in NHS II, and 1 January 2008 in HPFS), whichever occurred first. We used Cox proportional hazards regression models to examine the association between quintiles of GI and GL and risk of T2D. The regression models included age in years as the time scale, stratified by calendar time in 2-y intervals, and allowed for possible interaction between calendar time and age in the baseline hazards to be accounted for nonparametrically (model 1). Our first multivariable model (model 2) adjusted for race (white or nonwhite) and several lifestyle factors, including family history of diabetes (yes or no), menopausal status, and postmenopausal hormone use (women only; premenopausal, postmenopausal with no history of hormone replacement, or postmenopausal with current hormone replacement), oral contraceptive use (women only; never user, past user, or current user), physical activity (<3.0, 3.0–8.9, 9.0–17.9, 18.0–26.9, or ≥27.0 metabolic equivalents/wk), BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–32.9, 33–34.9, 35–39.9, or ≥40), smoking status (never; past; current: 1–14, 14–25, or ≥25 cigarettes/d), and total energy intake (quintiles). In model 3, we further adjusted for intakes of total coffee, energy-adjusted intakes of cereal fiber, trans fatty acids, PUFAs, SFAs, and MUFAs (quintiles). For models with GI as the exposure, we additionally adjusted for total protein intake (quintiles, model 4).
For each 2-y time period, we updated information on all nondietary covariates to account for changes in risk factors over time. For dietary measures, we used the cumulative average of food intakes from baseline to the censoring events to best represent long-term diet and minimize within-person variation (12). However, we stopped updating dietary variables on a report of cancer or cardiovascular disease because changes in diet after development of these conditions may confound the relation between diet and diabetes (12, 28).We performed several sensitivity analyses to test the robustness of our results. First, we tested associations by using baseline diet alone. Second, to test if our results were biased by selectively stopping updating diet after an intermediate outcome, we continuously updated diet until the end of follow-up. Third, we excluded incident cases of cardiovascular disease or cancer during follow-up from the analysis. Finally, instead of using repeated measures of diet, we used the most recent measure of diet or the intake reported at the beginning of each FFQ cycle.
The proportional hazards assumption was tested by including an interaction term between the main exposures and months to events (P > 0.05 for all tests). We tested for potential effect modification by BMI, family history of diabetes, and physical activity by including cross-product terms with the exposure variables in our fully adjusted model. Tests for linear trend were conducted by assigning the median value to each quintile or category and treating this as a continuous variable in the regression model. To evaluate potential joint effects of cereal fiber and BMI with GI and GL, we cross-classified participants into categories of BMI (<25, 25–29.9, or ≥30) and tertiles of cereal fiber, GI, and GL.
Because of differences in sex, follow-up time, and questionnaires in the 3 cohorts, all analyses were performed separately in each cohort to achieve better control of confounding. For the primary analyses, to obtain overall estimates for both sexes and to increase statistical power, the RRs from the multivariable-adjusted models from the 3 cohorts were combined with the use of an inverse variance–weighted meta-analysis by the fixed-effects model (29). The cohort analyses were not prespecified at the time of cohort initiation. All statistical tests were 2-sided and performed by using SAS version 9.2 for UNIX (SAS Institute).
Updated meta-analysis on GI, GL, and incident T2D
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol to conduct an updated systematic review and meta-analysis of prospective studies assessing the association between GI, GL, and risk of developing T2D in adults. We performed a systematic search of MEDLINE (http://www.ncbi.nlm.nih.gov/pubmed) and EMBASE (www.embase.com) (until August 2013) and a manual search of bibliographies of key retrieved articles and relevant reviews. To be included in the meta-analysis, studies had to fulfill the following criteria: 1) be prospective in design, 2) have T2D as an endpoint (self-reported or clinically assessed), 3) provide assessment method for GI and GL, 4) present RRs and the corresponding variance estimates, and 5) describe the adjustment for potential confounders. Detailed information on search criteria is presented as Supplemental Material under “Supplemental data” in the online issue. One author (SNB) defined the study criteria, assessed study eligibility, extracted the data, and assessed study quality by using the Newcastle-Ottawa quality scale. A second author (DKT) independently double-checked the extracted data. Discrepancies were resolved by mutual consultation. The following data items were extracted: author, year of publication, journal, cohort name, country, comorbidities excluded at baseline, percentage of population as male, mean age, mean BMI, length of follow-up, mean GI and GL, dietary instrument, number of dietary assessments, number of T2D cases, method of ascertainment of outcome, and covariates adjusted for in Cox models. The RRs were used as the common measure of association across studies. We considered HRs and ORs to be equivalent to RRs. Because random-effects meta-analysis tends to give disproportionally more weight to small, statistically less robust studies (30), we used a fixed-effects model to compute the summary RR of T2D for the highest category of GI or GL compared with the lowest category. However, we also provided summary RRs with the use of random-effects models. For one study (31) that analyzed GI as a continuous variable, we estimated the RR for the highest compared with lowest categories of exposures by determining the difference in GI between the highest and lowest categories and scaling the risk estimates accordingly. For another study that provided estimates per 10-unit increases in GI (32) and stated that these approximated the difference between the 87.5th and the 12.5th percentiles, we used the published estimates for comparing the highest with the lowest quartiles. In sensitivity analyses, we examined if the summary RR for the highest compared with lowest categories of GI changed appreciably with the inclusion of these 2 studies. Forest plots were produced to visually assess the RRs and their corresponding 95% CIs across studies. Heterogeneity in the association of the main exposures with T2D risk was assessed by using the I2 statistic. I2 values of ∼25%, 50%, and 75% were considered to indicate low, moderate, and high heterogeneity, respectively. When heterogeneity was considered to be present, we conducted univariate meta-regressions by using study-level data to explore potential sources of heterogeneity. Study quality (Newcastle-Ottawa quality scale), use of a validated dietary instrument, follow-up time, number of dietary assessments (baseline compared with repeated measures), and type of assessment of T2D (self-reported or confirmed by medical review) were examined as potential modifiers when heterogeneity was present. We also examined the influence of each study on the overall estimate by systematically removing one study at a time and examining the influence on the overall risk estimate and the 95% CI. We assessed the possibility of publication bias by using the Begg's test and Egger's test and a visual inspection of funnel plots (33, 34). The meta-analysis was performed with the use of the STATA statistical program, version 9.2 (StataCorp).
RESULTS
Cohort analyses
During 3,800,618 person-years of follow-up, we documented 15,027 cases of T2D. The distribution of age-adjusted baseline characteristics according to quintiles of energy-adjusted GI is shown in Table 1. In all 3 cohorts, compared with those who consumed diets with the lowest GI, those in the highest category of GI were younger, less physically active, consumed a higher GL diet, and had lower intakes of alcohol, magnesium, total protein, and total coffee. At the same time, they also had higher intakes of cereal fiber and trans fatty acids. The distribution of baseline characteristics according to quintiles of energy-adjusted GL is shown in Table 2. Like their GI counterparts, those in the highest quintile of GL had lower intakes of alcohol, protein, and total coffee compared with those with the lowest GL diets. They also consumed a high-GI diet and had higher intakes of cereal fiber.
TABLE 1.
Age-adjusted baseline characteristics by quintile of glycemic index1
| Quintile of glycemic index |
|||||
| Variable | 1 | 2 | 3 | 4 | 5 |
| Nurses’ Health Study (1984) | |||||
| No. of participants | 14,830 | 14,826 | 14,854 | 14,883 | 14,855 |
| Median glycemic index | 48.8 | 51.8 | 53.6 | 55.2 | 57.6 |
| Age (y) | 51.1 ± 6.92 | 50.7 ± 7.1 | 50.2 ± 7.2 | 49.8 ± 7.2 | 49.6 ± 7.4 |
| White (%) | 98 | 98 | 98 | 98 | 96 |
| BMI (kg/m2) | 24.8 ± 4.3 | 24.9 ± 4.5 | 24.9 ± 4.6 | 24.8 ± 4.7 | 24.8 ± 4.8 |
| Physical activity (MET-h/wk) | 17.4 ± 25.6 | 15.6 ± 20.8 | 14.1 ± 20.2 | 12.6 ± 17.8 | 11.4 ± 18.2 |
| Family history of diabetes (%) | 28 | 28 | 28 | 28 | 29 |
| History of hypertension (%) | 8 | 7 | 7 | 8 | 8 |
| History of hypercholesterolemia (%) | 3 | 3 | 3 | 3 | 4 |
| Current smoker (%) | 25 | 23 | 24 | 24 | 26 |
| Current postmenopausal hormone use (%) | 25 | 25 | 25 | 24 | 23 |
| Dietary factors | |||||
| Total energy intake (kcal/d) | 1706 ± 547 | 1791 ± 535 | 1803 ± 534 | 1765 ± 520 | 1651 ± 493 |
| Alcohol (g/d) | 10.0 ± 14.8 | 7.9 ± 11.4 | 6.8 ± 10.4 | 6.0 ± 9.6 | 4.6 ± 8.6 |
| Glycemic load | 83.4 ± 17.8 | 94.1 ± 15.5 | 99.4 ± 15.4 | 105 ± 16 | 114 ± 19 |
| Cereal fiber (g/d) | 3.5 ± 2.3 | 4.1 ± 2.3 | 4.3 ± 2.2 | 4.4 ± 2.3 | 4.5 ± 2.5 |
| Magnesium (mg/d) | 335 ± 87 | 305 ± 67 | 287 ± 62 | 271 ± 62 | 245 ± 64 |
| PUFAs (g/d) | 11.9 ± 3.8 | 11.9 ± 3.1 | 11.9 ± 2.9 | 11.8 ± 2.8 | 11.4 ± 2.9 |
| SFAs (g/d) | 22.6 ± 5.3 | 22.4 ± 4.5 | 22.3 ± 4.3 | 22.0 ± 4.2 | 21.3 ± 4.4 |
| trans Fatty acids (g/d) | 3.0 ± 1.0 | 3.3 ± 1.0 | 3.5 ± 1.0 | 3.5 ± 1.1 | 3.6 ± 1.1 |
| Protein (g/d) | 78.2 ± 14.9 | 73.5 ± 11.8 | 70.9 ± 11.0 | 68.5 ± 10.9 | 65.1 ± 11.6 |
| Total coffee (cups/d)3 | 2.9 ± 2.0 | 2.7 ± 1.9 | 2.5 ± 1.8 | 2.3 ± 1.7 | 1.9 ± 1.7 |
| Nurses’ Health Study II (1991) | |||||
| No. of participants | 18,084 | 18,081 | 18,104 | 18,101 | 18,041 |
| Median glycemic index | 49.7 | 52.3 | 54.0 | 55.6 | 57.9 |
| Age (y) | 36.7 ± 4.6 | 36.3 ± 4.6 | 36.1 ± 4.6 | 35.9 ± 4.7 | 35.5 ± 4.7 |
| White (%) | 98 | 98 | 97 | 97 | 93 |
| BMI (kg/m2) | 24.7 ± 5.0 | 24.5 ± 5.1 | 24.5 ± 5.2 | 24.4 ± 5.3 | 24.4 ± 5.5 |
| Physical activity (MET-h/wk) | 26.0 ± 32.1 | 22.6 ± 28.6 | 20.5 ± 25.8 | 18.9 ± 25.8 | 16.6 ± 23.0 |
| Family history of diabetes (%) | 34 | 34 | 34 | 34 | 35 |
| History of hypertension (%) | 6 | 6 | 6 | 6 | 6 |
| History of hypercholesterolemia (%) | 14 | 14 | 14 | 15 | 15 |
| Current smoker (%) | 14 | 12 | 11 | 11 | 12 |
| Menopausal status and postmenopausal hormone use (%) | |||||
| Premenopausal | 96.4 | 96.6 | 96.6 | 96.5 | 96.3 |
| Postmenopausal + never user | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Postmenopausal + past user | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 |
| Postmenopausal + current user | 2.6 | 2.6 | 2.5 | 2.6 | 2.6 |
| Missing information | 0.4 | 0.4 | 0.4 | 0.4 | 0.5 |
| Oral contraceptive use (%) | |||||
| Never user | 15.6 | 15.5 | 15.2 | 15.1 | 16.0 |
| Past user | 74.4 | 74.7 | 74.1 | 73.8 | 71.9 |
| Current user | 10.0 | 9.8 | 10.7 | 11.1 | 12.1 |
| Dietary factors | |||||
| Total energy intake (kcal/d) | 1753 ± 547 | 1827 ± 546 | 1831 ± 546 | 1814 ± 548 | 1722 ± 540 |
| Alcohol (g/d) | 4.8 ± 8.6 | 3.7 ± 6.3 | 3.0 ± 5.3 | 2.5 ± 4.7 | 1.7 ± 3.9 |
| Glycemic load | 105 ± 18 | 115 ± 16 | 121 ± 17 | 127 ± 18 | 139 ± 22 |
| Cereal fiber (g/d) | 5.2 ± 3.7 | 5.7 ± 3.0 | 5.8 ± 2.8 | 5.8 ± 2.8 | 5.6 ± 2.9 |
| Magnesium (mg/d) | 356 ± 76 | 334 ± 69 | 317 ± 66 | 299 ± 64 | 272 ± 70 |
| PUFAs (g/d) | 11.3 ± 3.1 | 11.4 ± 2.7 | 11.4 ± 2.6 | 11.3 ± 2.6 | 10.8 ± 2.6 |
| SFAs (g/d) | 23.2 ± 5.3 | 22.9 ± 4.8 | 22.6 ± 4.6 | 22.3 ± 4.6 | 21.1 ± 4.6 |
| trans Fatty acids (g/d) | 2.9 ± 1.1 | 3.2 ± 1.1 | 3.3 ± 1.2 | 3.4 ± 1.2 | 3.5 ± 1.3 |
| Protein (g/d) | 93.3 ± 16.1 | 89.0 ± 14.1 | 86.5 ± 13.7 | 83.8 ± 13.9 | 79.5 ± 15.2 |
| Total coffee (cups/d) | 2.1 ± 1.9 | 1.8 ± 1.7 | 1.5 ± 1.6 | 1.3 ± 1.5 | 1.0 ± 1.4 |
| Health Professionals Follow-Up Study (1986) | |||||
| No. of participants (n) | 8101 | 8095 | 8093 | 8118 | 8091 |
| Median glycemic index | 48.8 | 51.7 | 53.4 | 55.1 | 57.4 |
| Age (y) | 54.2 ± 9.3 | 53.3 ± 9.3 | 52.8 ± 9.5 | 52.2 ± 9.5 | 51.9 ± 9.5 |
| White (%) | 96 | 96 | 96 | 95 | 93 |
| BMI (kg/m2) | 25.1 ± 5.0 | 25.0 ± 4.9 | 25.0 ± 4.6 | 24.8 ± 4.9 | 24.5 ± 5.0 |
| Physical activity (MET-h/wk) | 23.6 ± 31.9 | 23.4 ± 31.3 | 21.7 ± 29.7 | 20.6 ± 29.0 | 18.3 ± 25.3 |
| Family history of diabetes (%) | 21 | 20 | 20 | 20 | 20 |
| History of hypertension (%) | 20 | 19 | 19 | 18 | 19 |
| History of hypercholesterolemia (%) | 10 | 10 | 10 | 10 | 10 |
| Current smoker (%) | 11 | 9 | 8 | 9 | 8 |
| Dietary factors | |||||
| Total energy intake (kcal/d) | 1960 ± 643 | 2023 ± 623 | 2028 ± 617 | 2024 ± 615 | 1942 ± 600 |
| Alcohol (g/d) | 16.7 ± 19.9 | 12.7 ± 15.7 | 11.0 ± 13.8 | 9.4 ± 12.8 | 7.5 ± 11.6 |
| Glycemic load | 104 ± 23 | 118 ± 21 | 124 ± 20 | 132 ± 21 | 144 ± 25 |
| Cereal fiber (g/d) | 4.7 ± 4.0 | 5.7 ± 4.5 | 6.0 ± 3.4 | 6.3 ± 3.4 | 6.7 ± 3.9 |
| Magnesium (mg/d) | 389 ± 89 | 368 ± 79 | 351 ± 74 | 336 ± 72 | 315 ± 78 |
| PUFAs (g/d) | 13.7 ± 4.3 | 13.4 ± 3.4 | 13.3 ± 3.2 | 13.0 ± 3.1 | 12.6 ± 3.1 |
| SFAs (g/d) | 25.4 ± 7.0 | 24.8 ± 6.1 | 24.8 ± 5.9 | 24.5 ± 5.7 | 23.5 ± 5.8 |
| trans Fatty acids (g/d) | 2.5 ± 1.1 | 2.7 ± 1.1 | 2.9 ± 1.1 | 3.0 ± 1.1 | 3.1 ± 1.2 |
| Protein (g/d) | 96.6 ± 18.5 | 93.9 ± 15.9 | 92.1 ± 15.1 | 90.1 ± 14.9 | 86.9 ± 15.5 |
| Total coffee (cups/d) | 2.4 ± 2.0 | 2.1 ± 1.8 | 1.9 ± 1.8 | 1.8 ± 1.7 | 1.5 ± 1.6 |
Values are age-standardized with the exception of age. MET-h, metabolic equivalent task hours.
Mean ± SD (all such values).
1 cup = 240 mL.
TABLE 2.
Age-adjusted baseline characteristics by quintile of glycemic load1
| Quintile of glycemic load |
|||||
| Variable | 1 | 2 | 3 | 4 | 5 |
| Nurses’ Health Study (1984) | |||||
| No. of participants | 14,689 | 14,849 | 14,849 | 14,920 | 14,941 |
| Median glycemic load | 75.0 | 90.0 | 99.0 | 108.0 | 123.0 |
| Age (y) | 50.2 ± 6.92 | 50.0 ± 7.0 | 50.0 ± 7.2 | 50.3 ± 7.3 | 50.8 ± 7.4 |
| White (%) | 99 | 98 | 98 | 98 | 96 |
| BMI (kg/m2) | 25.0 ± 4.6 | 25.1 ± 4.7 | 24.9 ± 4.5 | 24.8 ± 4.6 | 24.3 ± 4.4 |
| Physical activity (MET-h/wk) | 14.2 ± 21.7 | 14.1 ± 19.6 | 14.0 ± 19.9 | 14.2 ± 19.8 | 14.5 ± 23.2 |
| Family history of diabetes (%) | 28 | 29 | 29 | 28 | 28 |
| History of hypertension (%) | 8 | 7 | 7 | 7 | 8 |
| History of hypercholesterolemia (%) | 3 | 3 | 3 | 3 | 4 |
| Current smoker (%) | 33 | 25 | 22 | 21 | 20 |
| Current postmenopausal hormone use (%) | 26 | 26 | 24 | 24 | 24 |
| Dietary factors | |||||
| Total energy intake (kcal/d) | 1699 ± 536 | 1782 ± 527 | 1795 ± 527 | 1767 ± 523 | 1677 ± 524 |
| Alcohol (g/d) | 15.2 ± 17.7 | 7.9 ± 10.5 | 5.5 ± 7.9 | 4.1 ± 6.4 | 2.6 ± 4.8 |
| Glycemic index | 50.3 ± 4.2 | 52.4 ± 3.0 | 53.4 ± 2.8 | 54.4 ± 2.7 | 56.1 ± 2.9 |
| Cereal fiber (g/d) | 2.8 ± 1.5 | 3.7 ± 1.7 | 4.2 ± 1.9 | 4.7 ± 2.2 | 5.3 ± 3.1 |
| Magnesium (mg/d) | 293 ± 85 | 291 ± 69 | 288 ± 70 | 287 ± 71 | 281 ± 82 |
| PUFAs (g/d) | 12.9 ± 3.9 | 12.3 ± 3.0 | 11.9 ± 2.7 | 11.4 ± 2.6 | 10.4 ± 2.6 |
| SFAs (g/d) | 25.1 ± 5.3 | 23.6 ± 4.0 | 22.4 ± 3.5 | 21.0 ± 3.3 | 18.4 ± 3.6 |
| trans Fatty acids (g/d) | 3.5 ± 1.1 | 3.5 ± 1.1 | 3.5 ± 1.1 | 3.4 ± 1.0 | 3.0 ± 1.1 |
| Protein (g/d) | 79.0 ± 15.0 | 75.1 ± 11.4 | 71.8 ± 10.5 | 68.4 ± 9.9 | 61.8 ± 10.3 |
| Total coffee (cups/d)3 | 2.9 ± 1.9 | 2.7 ± 1.9 | 2.5 ± 1.8 | 2.3 ± 1.8 | 1.8 ± 1.7 |
| Nurses’ Health Study II (1991) | |||||
| No. of participants | 18,082 | 18,084 | 18,091 | 18,065 | 18,089 |
| Median glycemic load | 95.8 | 110.3 | 120.3 | 131.0 | 148.7 |
| Age (y) | 36.5 ± 4.6 | 36.3 ± 4.6 | 36.0 ± 4.6 | 35.8 ± 4.7 | 35.8 ± 4.7 |
| White (%) | 98 | 97 | 98 | 97 | 93 |
| BMI (kg/m2) | 25.6 ± 5.8 | 25.0 ± 5.4 | 24.5 ± 5.0 | 24.0 ± 4.8 | 23.5 ± 4.8 |
| Physical activity (MET-h/wk) | 19.5 ± 25.9 | 19.9 ± 24.9 | 20.6 ± 26.0 | 21.5 ± 28.3 | 23.0 ± 30.9 |
| Family history of diabetes (%) | 35 | 36 | 34 | 33 | 33 |
| History of hypertension (%) | 8 | 6 | 5 | 5 | 6 |
| History of hypercholesterolemia (%) | 15 | 14 | 14 | 14 | 15 |
| Current smoker (%) | 18 | 12 | 11 | 9 | 10 |
| Menopausal status and postmenopausal hormone use (%) | |||||
| Premenopausal | 96.4 | 96.7 | 96.6 | 96.5 | 96.2 |
| Postmenopausal + never user | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Postmenopausal + past user | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 |
| Postmenopausal + current user | 2.7 | 2.4 | 2.5 | 2.6 | 2.8 |
| Missing information | 0.4 | 0.4 | 0.4 | 0.5 | 0.5 |
| Oral contraceptive use (%) | |||||
| Never user | 13.2 | 14.7 | 15.8 | 16.2 | 17.4 |
| Past user | 76.4 | 74.7 | 73.7 | 72.9 | 71.1 |
| Current user | 10.4 | 10.6 | 10.5 | 10.9 | 11.5 |
| Dietary factors | |||||
| Total energy intake (kcal/d) | 1760 ± 560 | 1820 ± 541 | 1819 ± 539 | 1799 ± 535 | 1748 ± 556 |
| Alcohol (g/d) | 5.8 ± 9.9 | 3.4 ± 5.6 | 2.7 ± 4.5 | 2.2 ± 4.0 | 1.5 ± 2.9 |
| Glycemic index | 51.2 ± 3.4 | 52.9 ± 2.7 | 53.8 ± 2.6 | 54.8 ± 2.6 | 56.5 ± 2.8 |
| Cereal fiber (g/d) | 4.2 ± 1.8 | 5.2 ± 2.1 | 5.7 ± 2.5 | 6.2 ± 3.0 | 6.8 ± 4.4 |
| Magnesium (mg/d) | 311 ± 67 | 316 ± 67 | 318 ± 69 | 319 ± 75 | 314 ± 92 |
| PUFAs (g/d) | 12.7 ± 3.2 | 11.9 ± 2.5 | 11.4 ± 2.3 | 10.8 ± 2.2 | 9.7 ± 2.2 |
| SFAs (g/d) | 26.7 ± 4.8 | 24.2 ± 3.7 | 22.6 ± 3.4 | 20.9 ± 3.3 | 17.8 ± 3.7 |
| trans Fatty acids (g/d) | 3.6 ± 1.3 | 3.5 ± 1.2 | 3.3 ± 1.2 | 3.1 ± 1.1 | 2.8 ± 1.1 |
| Protein (g/d) | 97.1 ± 15.9 | 91.3 ± 12.8 | 87.1 ± 12.0 | 83.0 ± 11.7 | 73.6 ± 12.8 |
| Total coffee (cups/d) | 2.0 ± 1.9 | 1.7 ± 1.7 | 1.6 ± 1.7 | 1.4 ± 1.6 | 1.1 ± 1.5 |
| Health Professionals Follow-Up Study (1986) | |||||
| No. of participants | 8132 | 7927 | 7941 | 8388 | 8110 |
| Median glycemic load | 93 | 111 | 124 | 137 | 156s |
| Age (y) | 53.7 ± 9.1 | 52.8 ± 9.3 | 52.7 ± 9.4 | 52.5 ± 9.6 | 52.7 ± 9.8 |
| White (%) | 96 | 96 | 96 | 95 | 92 |
| BMI (kg/m2) | 25.5 ± 5.0 | 25.2 ± 5.0 | 24.9 ± 5.0 | 24.6 ± 4.7 | 24.2 ± 4.6 |
| Physical activity (MET-h/wk) | 18.6 ± 26.2 | 20.2 ± 26.4 | 21.8 ± 32.1 | 22.2 ± 30.6 | 24.5 ± 31.3 |
| Family history of diabetes (%) | 20 | 21 | 20 | 20 | 20 |
| History of hypertension (%) | 22 | 19 | 18 | 18 | 18 |
| History of hypercholesterolemia (%) | 9 | 9 | 10 | 11 | 12 |
| Current smoker (%) | 15 | 11 | 8 | 6 | 5 |
| Dietary factors | |||||
| Total energy intake (kcal/d) | 1963 ± 638 | 2020 ± 621 | 2032 ± 611 | 2014 ± 613 | 1951 ± 619 |
| Alcohol (g/d) | 22.9 ± 22.0 | 13.5 ± 14.8 | 9.4 ± 11.3 | 6.9 ± 9.1 | 4.3 ± 6.9 |
| Glycemic index | 50.2 ± 3.9 | 52.3 ± 2.9 | 53.3 ± 2.8 | 54.2 ± 2.8 | 55.7 ± 3.0 |
| Cereal fiber (g/d) | 3.7 ± 2.3 | 5.0 ± 2.7 | 5.8 ± 3.0 | 6.6 ± 3.6 | 8.1 ± 5.6 |
| Magnesium (mg/d) | 344 ± 80 | 348 ± 75 | 348 ± 76 | 352 ± 79 | 367 ± 99 |
| PUFAs (g/d) | 14.4 ± 4.3 | 13.8 ± 3.3 | 13.4 ± 3.0 | 12.9 ± 2.9 | 11.5 ± 2.9 |
| SFAs (g/d) | 28.6 ± 6.7 | 26.7 ± 5.2 | 25.3 ± 4.7 | 23.3 ± 4.5 | 19.3 ± 4.9 |
| trans Fatty acids (g/d) | 2.9 ± 1.1 | 3.0 ± 1.1 | 3.0 ± 1.1 | 2.9 ± 1.1 | 2.4 ± 1.1 |
| Protein (g/d) | 99.2 ± 19.3 | 95.9 ± 15.1 | 92.7 ± 13.9 | 89.4 ± 13.4 | 82.7 ± 14.2 |
| Total coffee (cups/d) | 2.5 ± 1.9 | 2.2 ± 1.8 | 1.9 ± 1.7 | 1.7 ± 1.7 | 1.3 ± 1.6 |
Values are age-standardized with the exception of age. MET-h, metabolic equivalent task hours.
Mean ± SD (all such values).
1 cup = 240 mL.
Higher GI was associated with a progressively higher risk of T2D (Table 3). In the pooled analysis of estimates from the 3 studies that used fixed-effects models, in age- and lifestyle-adjusted models (model 2) the RRs (95% CIs) across quintiles 1–5 were 1.00, 1.10 (1.04, 1.16), 1.09 (1.04, 1.15), 1.18 (1.12, 1.24), and 1.28 (1.21, 1.35) (P-trend < 0.0001). Further adjustment for intakes of total coffee, cereal fiber, and different kinds of fatty acids did not materially change this observation. However, additional adjustment for total dietary protein intake strengthened this association. Participants in the highest quintile of GI had a 33% higher risk (95% CI: 26%, 41%) compared with those in the lowest quintile. The RRs (95% CIs) across quintiles of energy-adjusted GL are shown in Table 4. Those in the highest quintile of GL had a higher risk of T2D, but this association was only significant in the NHS. However, in pooled analysis, compared with those with the lowest GL diet, those in the highest quintile of energy-adjusted GL had a 10% higher risk (95% CI: 2%, 18%) for T2D after adjustment for age, lifestyle, and dietary factors (model 3). Further adjustment for total protein intake strengthened the associations, and risk estimates were similar to those of GI. The RRs (95% CIs) across quintiles 1–5 were 1.00, 1.09 (1.03, 1.15), 1.09 (1.02, 1.17), 1.21 (1.12, 1.32), and 1.36 (1.23, 1.50) (P-trend < 0.0001).
TABLE 3.
RRs (95% CIs) for type 2 diabetes according to quintile of energy-adjusted glycemic index with diet no longer updated after incident cardiovascular disease or cancer1
| Quintile of glycemic index |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend | |
| Nurses’ Health Study | ||||||
| Median | 49.1 | 51.5 | 52.9 | 54.4 | 56.5 | |
| No. of cases/person-years | 1279/313,389 | 1412/313,443 | 1392/313,612 | 1565/313,277 | 1752/312,732 | |
| Model 1 | 1.00 | 1.12 (1.03, 1.20) | 1.11 (1.03, 1.20) | 1.26 (1.17, 1.35) | 1.42 (1.32, 1.52) | <0.0001 |
| Model 2 | 1.00 | 1.11 (1.03, 1.20) | 1.11 (1.03, 1.19) | 1.23 (1.14, 1.33) | 1.37 (1.27, 1.48) | <0.0001 |
| Model 3 | 1.00 | 1.12 (1.04, 1.21) | 1.12 (1.04, 1.21) | 1.24 (1.14, 1.34) | 1.34 (1.24, 1.45) | <0.0001 |
| Model 4 | 1.00 | 1.14 (1.06, 1.23) | 1.16 (1.07, 1.25) | 1.30 (1.20, 1.41) | 1.44 (1.33, 1.57) | <0.0001 |
| Nurses’ Health Study II | ||||||
| Median | 49.9 | 52.2 | 53.6 | 55.1 | 57.2 | |
| No. of cases/person-years | 857/301,114 | 866/301,223 | 858/300,754 | 903/300,883 | 1031/299,752 | |
| Model 1 | 1.00 | 1.05 (0.96, 1.16) | 1.07 (0.98, 1.18) | 1.15 (1.05, 1.27) | 1.35 (1.23, 1.48) | <0.0001 |
| Model 2 | 1.00 | 1.04 (0.95, 1.15) | 1.06 (0.96, 1.16) | 1.07 (0.98, 1.18) | 1.20 (1.09, 1.31) | 0.0002 |
| Model 3 | 1.00 | 1.06 (0.96, 1.17) | 1.07 (0.97, 1.18) | 1.07 (0.97, 1.18) | 1.16 (1.05, 1.28) | 0.007 |
| Model 4 | 1.00 | 1.08 (0.98, 1.19) | 1.10 (0.99, 1.21) | 1.11 (1.00, 1.22) | 1.20 (1.08, 1.34) | 0.001 |
| Health Professionals Follow-Up Study | ||||||
| Median | 49.4 | 51.8 | 53.2 | 54.6 | 56.7 | |
| No. of cases/person-years | 591/146,003 | 650/146,284 | 617/146,203 | 638/146,231 | 616/145,719 | |
| Model 1 | 1.00 | 1.12 (1.00, 1.25) | 1.08 (0.96, 1.21) | 1.14 (1.02, 1.27) | 1.11 (1.00, 1.25) | 0.06 |
| Model 2 | 1.00 | 1.16 (1.04, 1.30) | 1.11 (0.99, 1.25) | 1.22 (1.09, 1.36) | 1.19 (1.06, 1.34) | 0.002 |
| Model 3 | 1.00 | 1.19 (1.06, 1.34) | 1.15 (1.02, 1.29) | 1.26 (1.12, 1.42) | 1.24 (1.09, 1.40) | 0.0005 |
| Model 4 | 1.00 | 1.22 (1.09, 1.37) | 1.19 (1.05, 1.33) | 1.31 (1.16, 1.48) | 1.30 (1.15, 1.47) | <0.0001 |
| Pooled analysis2 | ||||||
| Model 1 | 1.00 | 1.10 (1.04, 1.15) | 1.09 (1.03, 1.15) | 1.20 (1.14, 1.26) | 1.33 (1.26, 1.40) | <0.0001 |
| Model 2 | 1.00 | 1.10 (1.04, 1.16) | 1.09 (1.04, 1.15) | 1.18 (1.12, 1.24) | 1.28 (1.21, 1.35) | <0.0001 |
| Model 3 | 1.00 | 1.12 (1.06, 1.18) | 1.11 (1.05, 1.17) | 1.19 (1.13, 1.25) | 1.26 (1.20, 1.34) | <0.0001 |
| Model 4 | 1.00 | 1.14 (1.08, 1.20) | 1.14 (1.08, 1.21) | 1.24 (1.17, 1.31) | 1.33 (1.26, 1.41) | <0.0001 |
Median values of glycemic index for each category were used to test for a linear trend across categories. Model 1 adjusted for age (y); model 2 adjusted as for model 1 + race (white or nonwhite), smoking (never; past; current: 1–14, 15–24, or >24 cigarettes/d), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/d), postmenopausal hormone use (women only; premenopausal, postmenopausal current user, or postmenopausal never/past user), oral contraceptive use (women only; never, past, or current), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task hours/wk), family history of diabetes (yes or no), BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–34.9, 35–39.9, or ≥40), and total energy intake (quintiles); model 3 adjusted as for model 2 + intakes of total coffee (quintiles), energy-adjusted cereal fiber (quintiles), energy-adjusted PUFAs (quintiles), energy-adjusted trans fatty acids (quintiles), and SFAs (quintiles); model 4 adjusted as for model 3 + total protein intake (quintiles). All statistical tests were conducted by using Cox proportional hazards regression models.
Results were combined with the use of a fixed-effect model.
TABLE 4.
RRs (95% CIs) for type 2 diabetes according to quintile of energy-adjusted glycemic load with diet no longer updated after incident cardiovascular disease or cancer1
| Quintile of glycemic load |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend | |
| Nurses’ Health Study | ||||||
| Median | 81.4 | 94.5 | 102.8 | 111.0 | 123.5 | |
| No. of cases/person-years | 1486/312,181 | 1588/312,959 | 1472/315,849 | 1459/310,499 | 1395/314,964 | |
| Model 1 | 1.00 | 1.07 (1.00, 1.15) | 0.99 (0.92, 1.06) | 0.99 (0.92, 1.06) | 0.93 (0.87, 1.00) | 0.01 |
| Model 2 | 1.00 | 0.99 (0.92, 1.06) | 0.94 (0.88, 1.02) | 1.00 (0.92, 1.07) | 1.05 (0.97, 1.13) | 0.27 |
| Model 3 | 1.00 | 1.02 (0.95, 1.11) | 1.00 (0.92, 1.09) | 1.08 (0.99, 1.19) | 1.18 (1.06, 1.31) | 0.003 |
| Nurses’ Health Study II | ||||||
| Median | 98.8 | 112.5 | 121.7 | 131.2 | 146.5 | |
| No. of cases/person-years | 1164/300,470 | 1040/300,655 | 810/301,083 | 776/301,073 | 725/300,445 | |
| Model 1 | 1.00 | 0.93 (0.85, 1.01) | 0.73 (0.67, 0.80) | 0.71 (0.65, 0.77) | 0.66 (0.61, 0.73) | <0.0001 |
| Model 2 | 1.00 | 1.00 (0.92, 1.09) | 0.87 (0.79, 0.95) | 0.94 (0.86, 1.03) | 1.02 (0.93, 1.12) | 0.66 |
| Model 3 | 1.00 | 1.05 (0.96, 1.15) | 0.93 (0.83, 1.03) | 1.01 (0.89, 1.14) | 1.05 (0.92, 1.21) | 0.67 |
| Health Professionals Follow-Up Study | ||||||
| Median | 99.0 | 116.5 | 128.0 | 140.0 | 157.2 | |
| No. of cases/person-years | 760/145,549 | 686/144,995 | 631/146,062 | 573/147,446 | 462/146,387 | |
| Model 1 | 1.00 | 0.93 (0.84, 1.03) | 0.85 (0.76, 0.94) | 0.77 (0.69, 0.86) | 0.63 (0.56, 0.70) | <0.0001 |
| Model 2 | 1.00 | 0.96 (0.86, 1.06) | 0.92 (0.83, 1.03) | 0.90 (0.80, 1.01) | 0.83 (0.73, 0.94) | 0.003 |
| Model 3 | 1.00 | 0.99 (0.88, 1.11) | 0.99 (0.87, 1.13) | 0.98 (0.85, 1.14) | 0.96 (0.81, 1.15) | 0.71 |
| Pooled analysis2 | ||||||
| Model 1 | 1.00 | 0.99 (0.94, 1.04) | 0.87 (0.83, 0.92) | 0.85 (0.80, 0.89) | 0.78 (0.74, 0.82) | <0.0001 |
| Model 2 | 1.00 | 0.98 (0.94, 1.03) | 0.91 (0.87, 0.96) | 0.96 (0.91, 1.01) | 0.99 (0.94, 1.05) | 0.50 |
| Model 3 | 1.00 | 1.02 (0.97, 1.08) | 0.98 (0.92, 1.04) | 1.04 (0.97, 1.11) | 1.10 (1.02, 1.18) | 0.02 |
Median values of glycemic load for each category were used to test for a linear trend across categories. Model 1 adjusted for age (y); model 2 adjusted as for model 1 + race (white or nonwhite), smoking (never; past; current: 1–14, 15–24, or >24 cigarettes/d), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/d), postmenopausal hormone use (women only; premenopausal, postmenopausal current user, or postmenopausal never/past user), oral contraceptive use (women only; never, past, or current), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task hours/wk), family history of diabetes (yes or no), BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–34.9, 35–39.9, or ≥40), and total energy intake (quintiles); model 3 adjusted as for model 2 + intakes of total coffee (quintiles), energy-adjusted cereal fiber (quintiles), energy-adjusted PUFAs (quintiles), energy-adjusted trans fatty acids (quintiles), and SFAs (quintiles). All statistical tests were conducted by using Cox proportional hazards regression models.
Results were combined with the use of a fixed-effects model.
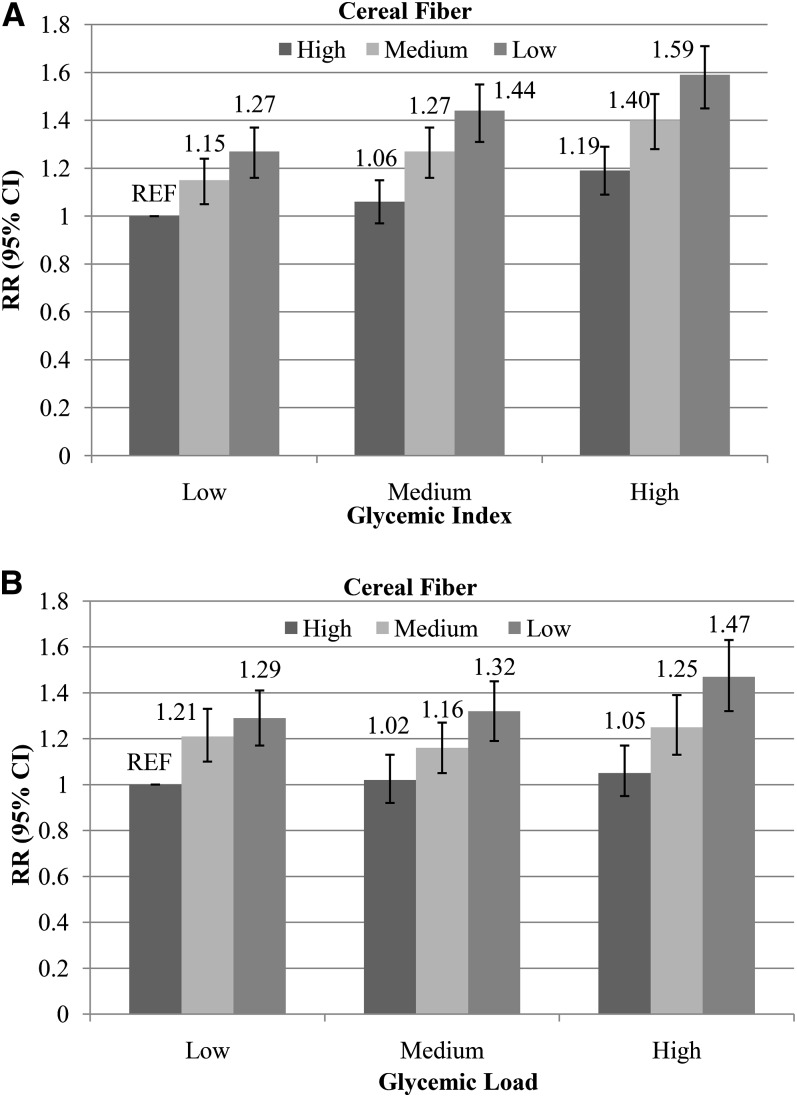
We examined the joint effects of GI and GL with cereal fiber by cross-classifying participants by both variables (Figure 1, A and B). The pooled RR for the combination of a high GI and a low cereal fiber intake compared with the opposite extreme was 1.59 (95% CI: 1.47, 1.73; P-interaction > 0.20 in all 3 cohorts). Similarly, those with a high-GL and a low-cereal-fiber diet had a 1.47-fold risk (95% CI: 1.32, 1.63) compared with those with a low-GL and a high-cereal-fiber diet (P-interaction > 0.10 in all 3 cohorts). We also evaluated the joint effects of GI and GL with BMI on the risk of T2D. Compared with those who were normal weight and had low-GI or -GL diets, obese participants with high-GI (RR: 12.28; 95% CI: 11.09, 13.60; P-interaction > 0.05) or high-GL (RR: 10.80; 95% CI: 9.63, 12.11; P-interaction > 0.05) diets had a >10-fold higher risk of T2D.
FIGURE 1.
RRs (95% CIs) for type 2 diabetes according to joint categories of GI (tertiles; A), GL (tertiles; B), and cereal fiber (tertiles) in all 3 cohorts combined. Multivariable RRs were adjusted for age (y), BMI (in kg/m2; <21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–34.9, 35–39.9, or ≥40), race (white or nonwhite), smoking (never; past; current: 1–14, 15–24, or >24 cigarettes/d), alcohol intake (0, 0.1–4.9, 5–9.9, 10–14.9, or ≥15 g/d), postmenopausal hormone use (women only; premenopausal, postmenopausal current user, or postmenopausal never/past user), oral contraceptive use (women only; never, past, or current), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 metabolic equivalent task hours/wk), family history of diabetes (yes or no), total energy intake (quintiles), and intakes of total coffee (quintiles), energy-adjusted PUFAs (quintiles), energy-adjusted trans fatty acids (quintiles), energy-adjusted SFAs (quintiles), energy-adjusted MUFAs (quintiles), and energy-adjusted protein (quintiles; only for panel A). Results were combined with the use of a fixed-effects model. All statistical tests were conducted by using Cox proportional hazards regression models. Low GI, high cereal fiber: n = 19,524; medium GI, high cereal fiber: n = 24,491; high GI, high cereal fiber: n = 24,367; low GI, medium cereal fiber: n = 21,392; medium GI, medium cereal fiber: n = 24,029; high GI, medium cereal fiber: n = 22,793; low GI, low cereal fiber: n = 27,474; medium GI, low cereal fiber: n = 19,862; high GI, low cereal fiber: n = 21,224. P-interaction >0.20 in all 3 cohorts. Low GL, high cereal fiber: n = 9826; medium GL, high cereal fiber: n = 23,669; high GL, high cereal fiber: n = 34,888; low GL, medium cereal fiber: n = 22,326; medium GL, medium cereal fiber: n = 26,486; high GL, medium cereal fiber: n = 19,403; low GL, low cereal fiber: n = 36,270; medium GL, low cereal fiber: n = 18,150; high GL, low cereal fiber: n = 14,140. P-interaction >0.10 in all 3 cohorts. GI, glycemic index; GL, glycemic load; REF, reference.
Our results remained robust in several sensitivity analyses (see Supplemental Tables S1 and S2 under “Supplemental data” in the online issue). When we used baseline diet alone to examine associations between GI, GL, and T2D, risk estimates were largely attenuated. In fully adjusted pooled models, those in the highest quintile of GI and GL had RRs of 1.26 (95% CI: 1.19, 1.33) and 1.02 (95% CI: 0.94, 1.09) for T2D compared with those in quintile 1. Results remained largely unchanged when we continuously updated diet until the end of follow-up (RR for quintile 5 compared with quintile 1 for GI: 1.33; 95% CI: 1.25, 1.41; RR for quintile 5 compared with quintile 1 for GL: 1.09; 95% CI: 1.01, 1.18) when we used the most recent diet as our primary exposure (RR for quintile 5 compared with quintile 1 for GI: 1.31; 95% CI: 1.24, 1.39; RR for quintile 5 compared with quintile 1 for GL: 1.12; 95% CI: 1.04, 1.21) or if we censored cases of cardiovascular disease or cancer that occurred during follow-up (RR for quintile 5 compared with quintile 1 for GI: 1.34; 95% CI: 1.26, 1.43; RR for quintile 5 compared with quintile 1 for GL: 1.07; 95% CI: 0.99, 1.16). Associations remained unchanged among nonsmokers and after excluding those with very low body weights (see Supplemental Table S3 under “Supplemental data” in the online issue). Results remained unchanged when we excluded participants with gestational diabetes at baseline (data not shown).
Meta-analysis
We further conducted an updated meta-analysis that included updated results from our 3 cohorts together with those of previous published studies. Our search on MEDLINE and EMBASE identified a total of 2424 citations. After the first level of screening based on titles and abstracts with the aforementioned criteria, 70 articles remained for further evaluation. After examining these articles in more detail, 56 articles were excluded for reasons shown in Supplemental Figure S1 under “Supplemental data” in the online issue. A total of 10 articles were identified for inclusion in the meta-analysis of GI and T2D (10, 35–43) and a total of 14 articles for the analysis on GL and T2D (10, 31, 32, 35–45).
Detailed characteristics of the studies (not including our cohorts) included in our meta-analysis are shown in Table 5. The association between GI and GL and T2D risk was the primary outcome in all but one study (45). Six studies were conducted in the United States, 4 in Europe, 1 each in the United Kingdom, Japan, and China. The number of incident T2D cases in the studies ranged from 99 to 11,559. Seven studies enrolled both sexes; 2 included only men, whereas 3 studies included only women. All studies assessed diet by using an FFQ. The median length of follow-up ranged from 4 to 14 y. Diabetes ascertainment was based on medical confirmation in all but 4 studies. Study quality scores based on the Newcastle-Ottawa scale were all >6.
TABLE 5.
Characteristics of studies included in the meta-analysis of the association of glycemic index and glycemic load with T2D1
| Study (reference) | Country | Sex | Baseline age2 | Comorbidities excluded at baseline | No. of T2D cases | Follow-up | Diet assessment | T2D assessment | Confounders | NOS |
| y | y | |||||||||
| Hodge et al (32)3 | Australia | M, F | 54.5 | Diabetes, angina, heart attack | 365 | 4 | 121-item FFQ | Self-report + confirmation from doctor | Age, sex, country of birth, physical activity, family history of diabetes, alcohol intake, educational level, weight change in the past 5 y, energy intake, BMI, and WHR | 7 |
| Hopping et al (44) | USA | M, F | 45–75 | Diabetes | 8587 | 14 | FFQ | Self-report + confirmation by health plan | BMI, physical activity, education, calories | 7 |
| Krishnan et al (35) | USA | F | 21–69 | Diabetes, gestational diabetes, cancer, cardiovascular disease | 1938 | 8 | 68-item FFQ | Self-report of physician-diagnosed diabetes | Age, BMI, energy intake, family history of diabetes, physical activity, cigarette use, cereal fiber intake, protein intake, fat intake | 6 |
| Meyer et al (36) | USA | F | 55–69 | Diabetes | 1141 | 6 | 127-item FFQ | Self-report | Age, total energy intake, BMI, WHR, education, pack-years of smoking, alcohol intake, physical activity, total dietary fiber | 6 |
| Mosdøl et al (37) | UK | M, F | 39–63 | Diabetes | 329 | 9–13 | 127-item FFQ | Self-report of doctor's diagnosis, diabetic medication use, and 2-h oral-glucose-tolerance test | Sex, age group, ratio of energy intake to energy expenditure, employment grade, physical activity, smoking, baseline BMI and WHR, intakes of alcohol, fiber, and carbohydrates | 8 |
| Patel et al (45)4 | USA | M, F | 50–74 | Diabetes, cancer | NR | 9 | 68-item FFQ | Self-report | Age, sex, race, history of gallstones, smoking, family history of pancreatic cancer, total energy intake, location of weight gain, sedentary behavior, BMI | 6 |
| Rossi et al (38) | Greece | M, F | 50.4 | Diabetes, cancer, cardiovascular disease, stroke | 2330 | 11.345 | 150-item FFQ | Medical records, discharge diagnosis, or death certificate | Age, sex, educational level, BMI, physical activity, WHR, total noncarbohydrate energy intake | 8 |
| Sahyoun et al (39) | USA | M, F | 70–79 | Diabetes | 99 | 4 | 108-item FFQ | Annual report of physician diagnosis, reported use of exogenous insulin or oral hypoglycemic medication use, or fasting serum glucose ≥126 mg/dL | Age, sex, race, clinical site, education, physical activity, baseline fasting glucose, BMI, alcohol consumption, and smoking status | 8 |
| Sakurai et al (40) | Japan | M | 46 | 133 | 6 | 147-item FFQ | Fasting plasma glucose ≥126 mg/dL, 2-h glucose ≥200 mg/dL in a 75-g oral-glucose-tolerance test, or treatment with insulin or an oral hypoglycemic agent | Age, BMI, family history of diabetes, smoking, alcohol intake, habitual exercise, presence of hypertension and hyperlipidemia at baseline, total energy, total fiber | 8 | |
| Similä et al (41) | Finland | M | 50–69 | Diabetes | 1098 | 12 | 276-item FFQ | Registry of reimbursement for medication use | Age, intervention group, BMI, smoking, physical activity, intakes of total energy and alcohol, energy-adjusted intakes of fat, fiber, and coffee | 8 |
| Sluijs et al (10) | Europe | M, F | 35–70 | Diabetes | 11,559 | 125 | FFQ | Self-report, linkage to primary care registers, secondary care registers, medication use, and hospital admissions and mortality data | Center, age, sex, educational level, physical activity, BMI, menopausal status, smoking status, alcohol consumption, energy intake, dietary protein, P:S, fiber (all energy-adjusted) | 9 |
| Stevens et al (31)6 | USA | M, F | 45–64 | Diabetes | 14487 | 95 | 66-item FFQ | Fasting glucose ≥126 mg/dL, or nonfasting glucose ≥200 mg/dL, physician report of diabetes, or reported taking diabetes medication within 2 wk preceding their examination | Age, BMI, sex, field center, educational level, smoking status, physical activity, cereal fiber (glycemic index and glycemic load were energy-adjusted) | 8 |
| van Woudenbergh et al (42) | Netherlands | M, F | 67.38 | Diabetes | 456 | 12.45 | 170-item FFQ | Confirmation from general practitioner and 1) plasma glucose ≥7.0 mmol/L, 2) random plasma glucose ≥11.1 mmol/L, 3) antidiabetes medication use, or 4) treatment by diet | Age, sex, smoking, family history of diabetes, BMI, C-reactive protein, and intakes of energy, protein, saturated fat, alcohol, and fiber | 8 |
| Villegas et al (43) | China | F | 40–70 | Diabetes, cardiovascular disease, cancer | 1605 | 5 | 77-item FFQ | Self-report of T2D and at least one of the American Diabetes Association criteria | Age, energy, BMI, WHR, smoking status, alcohol, physical activity, income, educational level, occupation, diagnosis of hypertension | 8 |
FFQ, food-frequency questionnaire; NOS, Newcastle-Ottawa scale; NR, not reported; P:S, PUFA to SFA ratio; T2D, type 2 diabetes; WHR, waist-to-hip ratio.
Values are means or ranges at baseline.
The study by Hodge et al (32) was included in the sensitivity meta-analysis of glycemic index and T2D. Risk estimates for the 10-unit difference in glycemic index were approximated to represent the difference between the highest and lowest quartiles. Risk estimates for the association of highest compared with lowest glycemic load quartiles and T2D were obtained from online supplemental material of Livesey et al (9), who obtained these estimates by direct e-mail correspondence from the author.
Confounders reported in the table are for associations between glycemic load and pancreatic cancer risk. Results for T2D are presented in the text.
Median follow-up time.
The study by Stevens et al was included in the sensitivity meta-analysis on the association of glycemic index and T2D. Risk estimates for the highest and lowest quartile were computed from continuous measures by first calculating the unit difference in median glycemic index in extreme quartiles. Risk estimates were then scaled accordingly.
Calculated on the basis of incidence of diabetes in African American and white adults.
Calculated from Table 1 in van Woudenbergh et al (42) as the weighted average of age in tertiles of glycemic index.
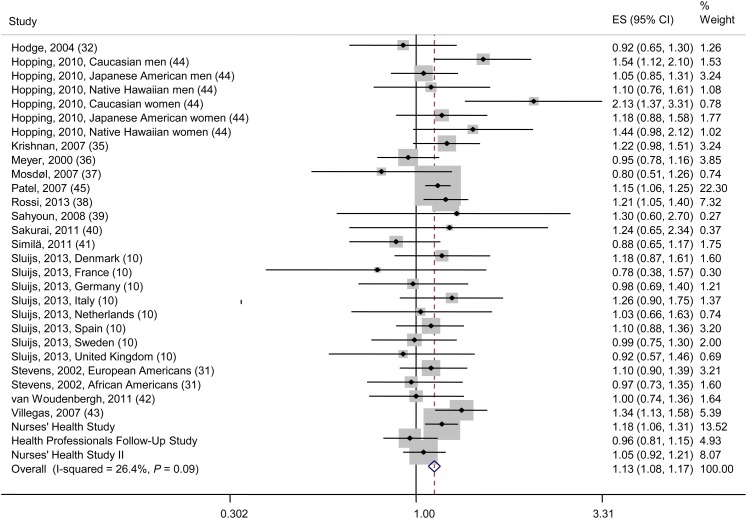
Those in the highest categories of GI and GL had a significantly higher risk of T2D (Figures 2 and 3). The RRs (95% CIs) from the fixed-effects model comparing the highest categories of GI or GL with their corresponding lowest categories were 1.19 (1.14, 1.24) and 1.13 (1.08, 1.17), respectively. However, we found evidence of moderate heterogeneity among studies included in the meta-analysis of GI and T2D (I2 = 68.5%, P < 0.001; Figure 3). When we used a random-effects model to address the issue of heterogeneity, the summary risk estimate for the association of GI with T2D was attenuated but remained significant (RR: 1.12; 95% CI: 1.03, 1.21; Supplemental Figure S2 under “Supplemental data” in the online issue). On the other hand, the summary risk estimate comparing the highest with the lowest category of GL and T2D from our random-effects model was similar to that of our fixed-effects model (RR: 1.12; 95% CI: 1.06, 1.17; Supplemental Figure S3 under “Supplemental data” in the online issue).
FIGURE 2.
Association of glycemic index with type 2 diabetes risk (highest compared with lowest category) and I2 for the proportion of heterogeneity between studies. The dashed vertical line represents the pooled estimate. The pooled estimate is based on fixed-effects meta-analysis. ES, effect size.
FIGURE 3.
Association of glycemic load with type 2 diabetes risk (highest compared with lowest category) and I2 for the proportion of heterogeneity between studies. The dashed vertical line represents the pooled estimate. The pooled estimate is based on fixed-effects meta-analysis. ES, effect size.
We documented significant publication bias for the association between GI and risk of T2D (see Supplemental Figure S4A under “Supplemental data” in the online issue). However, no publication bias was detected for the association between GL and T2D (see Supplemental Figure S4B under “Supplemental data” in the online issue). We found that the number of dietary assessments (baseline compared with repeated), length of follow-up, and type of T2D assessments (self-report compared with medical confirmation) were significant sources of heterogeneity (see Supplemental Table S4 under “Supplemental data” in the online issue). However, given the limited power and the small number of studies, when analyses were stratified by these variables, heterogeneity remained in the various subgroups (data not shown). Therefore, results are presented with all groups combined. When we systematically removed one study at a time, excluding any single study did not change the risk estimate or the 95% CIs to include the null value of one, suggesting that no single study markedly affected the overall conclusion. In sensitivity analyses, when we included scaled estimates for the 2 studies that reported results that used GI as a continuous exposure, the summary RR for the highest compared with the lowest category of GI and T2D remained largely unchanged (RR: 1.18; 95% CI: 1.14, 1.23; see Supplemental Figure S5 under “Supplemental data” in the online issue).
DISCUSSION
In these 3 large prospective cohorts of US men and women, we documented a positive association between GI, GL, and the risk of T2D. However, GI appears to be more strongly associated with T2D than does GL. Participants who consumed diets with high GI or high GL and low cereal fiber had a nearly 40% higher risk of T2D compared with those whose diets were high in cereal fiber and low in GI or GL.
Our findings are consistent with results of some (35, 38, 43) but not all (10, 36, 37, 39–42) prospective cohort studies that examined the association between GI and T2D. RRs comparing extreme quintiles of intake of GI ranged from 1.14 (95% CI: 1.01, 1.30) in the European Prospective Investigation into Cancer and Nutrition–Greek cohort (38) to 1.96 (95% CI: 1.04, 3.67) in a cohort of male Japanese factory workers (40). In contrast, no associations between GI and risk of T2D were observed in the Iowa Women's Health Study (36), the Whitehall II Study (37), the Health, Aging, and Body Composition Study (39), the Alpha-Tocopherol, Beta-Cancer Prevention Study (41), the European Prospective Investigation into Cancer and Nutrition–InterAct consortium (10), and the Rotterdam Study (42). Interestingly, all of the studies that found no association between GI and T2D assessed diet only at baseline. This may be because any measurement error (random or systematic) in GI would attenuate the risk estimates toward the null. However, when we used only baseline diet to examine the association with T2D in our cohorts, we still documented a 26% higher risk in pooled analysis. The inconsistencies in findings between cohorts could also be a result of differences in databases used to assign GI values to individual foods. Although most cohorts used the 2002 international table of GI values by Foster-Powell et al (46), 2 studies (10, 42) used the more updated international tables of GI and GL published in 2008 by Atkinson et al (47). Another important limitation was the subjectivity involved in assigning GI values to locally grown foods with different processing and ingredient compositions than those present in the available databases. There is also considerable debate around the issue of aggregating GI values of individual foods to calculate the GI of a mixed meal. Although some studies showed no association between calculated GI and measured GI of mixed meals (48, 49), others showed that the glycemic response to mixed meals can be predicted from summing the weighted GI values of foods in the meal (50). Although fat and protein in mixed meals affect the absolute glycemic response, the relative differences between carbohydrate-containing foods remain unaffected.
In all 3 cohorts, we observed that participants who consumed a high-GL diet had a more health-conscious lifestyle because they were less likely to smoke, had a lower BMI, and consumed more cereal fiber. Interestingly, these participants also consumed a low-fat, high-carbohydrate dietary pattern as evidenced by lower intakes of saturated fat, polyunsaturated fat, and total protein. This may explain the lower risk of T2D observed in age-adjusted GL models. However, when we accounted for the negative confounding introduced by the factors above, the direction of the association between GL and T2D changed, and a high GL was associated with a higher risk of T2D. When we further adjusted for dietary protein, risk estimates became similar to those of GI because the β-coefficient for GL represents the effect of substituting high-GI carbohydrate foods with low-GI carbohydrate foods while keeping protein, saturated fat, polyunsaturated fat, and trans fat constant (51). The overall summary risk estimate from our meta-analysis for the association of GL with T2D is similar but somewhat more attenuated compared with 2 previous meta-analyses on GL and T2D (8, 9). This may be because the meta-analysis by Livesey et al (9), double-counted data from the same cohorts that were published separately and included gestational diabetes as an outcome. In addition, our meta-analysis included updated results with longer follow-up from our cohorts.
Our results show that the effects of cereal fiber and GI and GL are additive. Although no studies have examined the joint effects of cereal fiber and GI and GL in relation to risk of T2D, a recent systematic review of prospective cohort studies that examined the association of cereal fiber with T2D risk concluded that consuming foods rich in cereal fiber is associated with a modestly reduced risk of T2D (52). Our findings support current dietary recommendations to consume a diet rich in whole grains and indicate that a diet low in GI and rich in fiber and minimally processed whole grains may lower the risk of T2D.
Several physiologic mechanisms have been proposed to explain the positive association of GI and GL with T2D (53, 54). High-GI and -GL diets are known to stimulate the increased production of insulin, resulting in a state of hyperinsulinemia, which, in turn, can induce insulin resistance. The consumption of high-GI and -GL diets for several years can increase the demand on β-cells and lead to β-cell exhaustion and failure (55). Furthermore, because high-GI and -GL diets increase concentrations of blood glucose and free fatty acids (6, 54), chronic exposure to these elevated concentrations can also induce β-cell failure. The biological plausibility of an association between GI and T2D is evident from results of metabolic and intervention studies. In a meta-analysis of 12 randomized controlled trials that comprised 612 subjects, low-GI diets reduced glycated hemoglobin by 0.4% (95% CI: −0.7%, −0.2%) over that produced by the control diets (56). Furthermore, the Study to Prevent NonInsulin Dependent Diabetes Mellitus (STOP-NIDDM) trial, which showed that acarbose, an oral α-glucosidase inhibitor, which effectively converts the diet to a low-GI/GL diet, reduced T2D risk by 25% over a mean follow-up of 3 y provides a proof-of-concept for low-GI diets (57).
The strengths of the current analysis include the availability of repeated measures of diet in all 3 cohorts, the long duration and high-proportion of follow-up, and the large sample size. However, a few important limitations need to be considered. First, the use of an FFQ to measure GI and GL introduces some degree of measurement error. However, given the prospective nature of the study, measurement error in GI and GL is unrelated to diabetes case status and is therefore more likely to attenuate the risk estimates toward the null. Second, the FFQ was validated in a sample of nurses at an earlier time and in male health professionals and the validation data may not directly apply to those in the NHS II. Furthermore, FFQs in our study were not specifically designed to measure GI and GL of foods. However, previous validation studies in a subsample of older nurses and male health professionals showed a reasonable degree of correlation between our FFQ and multiple dietary records for both carbohydrate and fiber. In addition, dietary GI and GL in the NHS were found to be associated with plasma HDL and fasting triglyceride concentrations, indicating the biological validity of the FFQ in assessing dietary GI and GL (58). Third, our study population mainly consisted of health professionals of European ancestry with a high educational status. Although the lack of racial diversity limits our ability to generalize our results to other populations, the consistency of our findings in other ethnic groups along with existence of strong biological mechanisms may make our results readily generalizable. The high educational status can be perceived as an advantage because high-quality and reliable data can be collected from our study participants. Furthermore, although we carefully adjusted for several known dietary and lifestyle factors that could confound the association between dietary GI and GL and T2D, residual confounding remains a possibility. Finally, given the observational nature of our study, we cannot establish true causality.
In conclusion, results from our study confirm that consuming a high-GI/GL diet is associated with a higher risk of T2D. Participants who consume diets that are low in cereal fiber but with a high GI/GL have an elevated risk of T2D. Given that the lifetime risk of developing T2D is 32.8% for men and 38.5% for women (59), even small excess RRs such as those observed in our study will translate to large differences in absolute risk and have important public health implications. Given the consistency of our results and the findings of randomized controlled trials of low-GI diets on measures of glycemia, a large randomized controlled trial should be considered to evaluate the role of low-GI and -GL diets in preventing T2D.
Supplementary Material
Acknowledgments
We are indebted to the participants in the NHS, the NHS II, and the HPFS for their continuing outstanding support and colleagues working in these studies for their valuable help.
The authors’ responsibilities were as follows—SNB: designed and conducted the analysis, interpreted the data, and wrote the manuscript; DKT, VSM, AP, AH, JEM, WCW, and FBH: assisted in interpreting the data and edited the manuscript; JEM, WCW, and FBH: obtained funding; managed and conducted the NHS, the NHS II, and the HPFS; and critically reviewed the manuscript for important intellectual content; and SNB and FBH: had primary responsibility for final content. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: FFQ, food-frequency questionnaire; GI, glycemic index; GL, glycemic load; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study; T2D, type 2 diabetes.
REFERENCES
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011;378:31–40. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global status report on noncommunicable diseases 2010. Geneva, Switzerland: World Health Organization, 2011. [Google Scholar]
- 3.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–50. [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins DJ, Wolever TM, Taylor RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AL, Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–6. [DOI] [PubMed] [Google Scholar]
- 7.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, Brand-Miller JC. Glycemic index, glycemic load, and chronic disease risk—a meta-analysis of observational studies. Am J Clin Nutr 2008;87:627–37. [DOI] [PubMed] [Google Scholar]
- 8.Dong JY, Zhang L, Zhang YH, Qin LQ. Dietary glycaemic index and glycaemic load in relation to the risk of type 2 diabetes: a meta-analysis of prospective cohort studies. Br J Nutr 2011;106:1649–54. [DOI] [PubMed] [Google Scholar]
- 9.Livesey G, Taylor R, Livesey H, Liu S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am J Clin Nutr 2013;97:584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sluijs I, Beulens JWJ, Van Der Schouw YT, Van Der ADL, Buckland G, Kuijsten A, Schulze MB, Amiano P, Ardanaz E, Balkau B, et al. Dietary glycemic index, glycemic load, and digestible carbohydrate intake are not associated with risk of type 2 diabetes in eight European countries. J Nutr 2013;143:93–9. [DOI] [PubMed] [Google Scholar]
- 11.Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, Neumiller JJ, Nwankwo R, Verdi CL, Urbanski P, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2013;36:3821–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 13.Miller JB, Pang E, Broomhead L. The glycaemic index of foods containing sugars: comparison of foods with naturally-occurring v. added sugars. Br J Nutr 1995;73:613–23. [DOI] [PubMed] [Google Scholar]
- 14.The University of Sydney. Glycemic index database. Available from: www.glycemicindex.com (cited 2013).
- 15.Salmerón J, Ascherio A, Rimm EB, Colditz GA, Spiegelman D, Jenkins DJ, Stampfer MJ, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997;20:545–50. [DOI] [PubMed] [Google Scholar]
- 16.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 18.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6. [DOI] [PubMed] [Google Scholar]
- 19.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26; discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 20.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Browne ML, Stampfer MJ, Rosner B, Hennekens CH, Speizer FE. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol 1988;127:188–99. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC. Nutritional epidemiology. Monographs in epidemiology and biostatistics. New York, NY: Oxford University Press, 1998. [Google Scholar]
- 23.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–57. [DOI] [PubMed] [Google Scholar]
- 24.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–97. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161(12):1542–8. [DOI] [PubMed] [Google Scholar]
- 26.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, Rosner B, Hennekens CH, Speizer FE. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–8. [DOI] [PubMed] [Google Scholar]
- 27.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 28.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–9. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 30.Peto R, Awasthi S, Read S, Clark S, Bundy D. Vitamin A supplementation in Indian children—authors’ reply. Lancet 2013;382:594–6. [DOI] [PubMed] [Google Scholar]
- 31.Stevens J, Ahn K, Juhaeri J, Houston D, Steffan L, Couper D. Dietary fiber intake and glycemic index and incidence of diabetes in African-American and white adults: the ARIC study. Diabetes Care 2002;25:1715–21. [DOI] [PubMed] [Google Scholar]
- 32.Hodge AM, English DR, O'Dea K, Giles GG. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004;27:2701–6. [DOI] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnan S, Rosenberg L, Singer M, Hu FB, Djoussé L, Cupples LA, Palmer JR. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch Intern Med 2007;167:2304–9. [DOI] [PubMed] [Google Scholar]
- 36.Meyer KA, Kushi LH, Jacobs DR, Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30. [DOI] [PubMed] [Google Scholar]
- 37.Mosdøl A, Witte DR, Frost G, Marmot MG, Brunner EJ. Dietary glycemic index and glycemic load are associated with high-density-lipoprotein cholesterol at baseline but not with increased risk of diabetes in the Whitehall II study. Am J Clin Nutr 2007;86:988–94. [DOI] [PubMed] [Google Scholar]
- 38.Rossi M, Turati F, Lagiou P, Trichopoulos D, Augustin LS, La Vecchia C, Trichopoulou A. Mediterranean diet and glycaemic load in relation to incidence of type 2 diabetes: results from the Greek cohort of the population-based European Prospective Investigation into Cancer and Nutrition (EPIC). Diabetologia 2013;56:2405–13. [DOI] [PubMed] [Google Scholar]
- 39.Sahyoun NR, Anderson AL, Tylavsky FA, Jung SL, Sellmeyer DE, Harris TB. Dietary glycemic index and glycemic load and the risk of type 2 diabetes in older adults. Am J Clin Nutr 2008;87:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakurai M, Nakamura K, Miura K, Takamura T, Yoshita K, Morikawa Y, Ishizaki M, Kido T, Naruse Y, Suwazono Y, et al. Dietary glycemic index and risk of type 2 diabetes mellitus in middle-aged Japanese men. Metabolism 2012;61:47–55. [DOI] [PubMed] [Google Scholar]
- 41.Similä ME, Valsta LM, Kontto JP, Albanes D, Virtamo J. Low-, medium- and high-glycaemic index carbohydrates and risk of type 2 diabetes in men. Br J Nutr 2011;105:1258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Woudenbergh GJ, Kuijsten A, Sijbrands EJ, Hofman A, Witteman JC, Feskens EJ. Glycemic index and glycemic load and their association with C-reactive protein and incident type 2 diabetes. J Nutr Metab 2011;. 2011:623076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villegas R, Liu S, Gao YT, Yang G, Li H, Zheng W, Xiao OS. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch Intern Med 2007;167:2310–6. [DOI] [PubMed] [Google Scholar]
- 44.Hopping BN, Erber E, Grandinetti A, Verheus M, Kolonel LN, Maskarinec G. Dietary fiber, magnesium, and glycemic load alter risk of type 2 diabetes in a multiethnic cohort in Hawaii. J Nutr 2010;140:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel AV, McCullough ML, Pavluck AL, Jacobs EJ, Thun MJ, Calle EE. Glycemic load, glycemic index, and carbohydrate intake in relation to pancreatic cancer risk in a large US cohort. Cancer Causes Control 2007;18:287–94. [DOI] [PubMed] [Google Scholar]
- 46.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5–56. [DOI] [PubMed] [Google Scholar]
- 47.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laine DC, Thomas W, Levitt MD, Bantle JP. Comparison of predictive capabilities of diabetic exchange lists and glycemic index of foods. Diabetes Care 1987;10:387–94. [DOI] [PubMed] [Google Scholar]
- 49.Henry CJ, Lightowler HJ, Newens KJ, Pata N. The influence of adding fats of varying saturation on the glycaemic response of white bread. Int J Food Sci Nutr 2008;59:61–9. [DOI] [PubMed] [Google Scholar]
- 50.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr 1986;43:167–72. [DOI] [PubMed] [Google Scholar]
- 51.Liu S, Chou EL. Dietary glycemic load and type 2 diabetes: modeling the glucose-raising potential of carbohydrates for prevention. Am J Clin Nutr 2010;92:675–7. [DOI] [PubMed] [Google Scholar]
- 52.Cho SS, Qi L, Fahey GC, Jr, Klurfeld DM. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am J Clin Nutr 2013;98:594–619. [DOI] [PubMed] [Google Scholar]
- 53.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002;287:2414–23. [DOI] [PubMed] [Google Scholar]
- 54.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr 2002;76(suppl):274S–80S. [DOI] [PubMed] [Google Scholar]
- 55.Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet 2004;364:778–85. [DOI] [PubMed] [Google Scholar]
- 56.Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr 2010;104:797–802. [DOI] [PubMed] [Google Scholar]
- 57.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 2002;359:2072–7. [DOI] [PubMed] [Google Scholar]
- 58.Liu S, Manson JE, Stampfer MJ, Holmes MD, Hu FB, Hankinson SE, Willett WC. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. Am J Clin Nutr 2001;73:560–6. [DOI] [PubMed] [Google Scholar]
- 59.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.