Abstract
Background: Relations between the consumption of nuts and legumes and risk of ischemic heart disease (IHD), stroke, and diabetes have not been well established.
Objective: We systematically investigated and quantified associations of nut and legume consumption with incident IHD, stroke, and diabetes.
Design: We systematically searched multiple databases to identify randomized controlled trials or observational studies that examined the relations. Studies were excluded if they reported only intermediate physiologic measures, soft cardiovascular outcomes, or crude risk estimates. Data were extracted independently and in duplicate. We assessed pooled dose-response relations by using a generalized least-squares trend estimation, and prespecified sources of heterogeneity were assessed by using metaregression. The potential for publication bias was explored by using funnel plots, Begg's and Egger's tests, and Duval and Tweedie trim-and-fill methods.
Results: Of 3851 abstracts, 25 observational studies (23 prospective and 2 retrospective studies) and 2 trial reports met inclusion criteria and comprised 501,791 unique individuals and 11,869 IHD, 8244 stroke, and 14,449 diabetes events. The consumption of nuts was inversely associated with fatal IHD (6 studies; 6749 events; RR per 4 weekly 28.4-g servings: 0.76; 95% CI: 0.69, 0.84; I2 = 28%), nonfatal IHD (4 studies; 2101 events; RR: 0.78; 0.67, 0.92; I2 = 0%), and diabetes (6 studies; 13,308 events; RR: 0.87; 0.81,0.94; I2 = 22%) but not stroke (4 studies; 5544 events). Legume consumption was inversely associated with total IHD (5 studies; 6514 events; RR per 4 weekly 100-g servings: 0.86; 0.78, 0.94; I2 = 0%) but not significantly associated with stroke (6 studies; 6690 events) or diabetes (2 studies; 2746 events). A meta-regression did not identify the effect modification by age, duration of follow-up, study location, or study quality. Mixed evidence was seen for publication bias, but analyses by using the Duval and Tweedie trim-and-fill method did not appreciably alter results.
Conclusion: This systematic review supports inverse associations between eating nuts and incident IHD and diabetes and eating legumes and incident IHD.
See corresponding editorial on page 8.
INTRODUCTION
Although nuts and legumes (beans) have nutrient profiles that may reduce cardiometabolic risk, their associations with major clinical cardiometabolic endpoints have not been well established (1, 2). Nuts are rich in unsaturated fatty acids, plant protein, dietary fiber, antioxidant vitamins (eg, vitamin E and tocopherols), minerals (eg, magnesium and potassium), and phytochemicals (eg, flavonoids). Legumes are rich in protein, complex carbohydrates, fiber, and various micronutrients (eg, phytochemicals) (1). Controlled trials have shown beneficial effects of the consumption of nuts and legumes on cardiovascular disease (CVD)5 risk factors (1), and a recent trial in high risk adults showed that advice to consume a Mediterranean diet supplemented with nuts significantly reduced CVD events ∼30% (3). However, to our knowledge, only one previous meta-analysis has reviewed the relation between nut consumption and ischemic heart disease (IHD) and showed an inverse relation with fatal (but not nonfatal or total) IHD (4), and relations of nut or legume consumption with other cardiometabolic endpoints such as stroke or type 2 diabetes have not been systematically reviewed. Furthermore, the previous meta-analysis (4) included only 4 studies, did not use most–covariate-adjusted risk estimates from each study, and did not assess the potential dose-response relation. To address these key gaps in knowledge, we performed a systematic review and meta-analysis of associations between nut and legume intakes and incident IHD, stroke, and diabetes.
METHODS
We followed the Meta-analysis of Observational Studies in Epidemiology (5) guidelines for observational studies and Preferred Reporting Items for Systematic reviews and Meta-Analyses (6) guidelines for randomized control trials during all stages of design, implementation, and reporting of this meta-analysis.
Primary exposures and outcomes
Primary exposures of interest were the consumption of nuts, which was defined as intake of tree nuts (eg, walnut, almond, hazelnuts, pecan, cashew, and pistachio) and peanuts (including peanut butter), and legumes, which was defined as intake of beans, peas, lentil, and tofu, with the exclusion of studies focused on soy, soy milk, or soybean oil. Primary outcomes of interest were incident IHD, stroke, and type 2 diabetes. Subtypes of IHD and stroke were further evaluated including fatal and nonfatal IHD and ischemic and hemorrhagic strokes.
Search strategy
We searched multiple databases including PubMed (www.ncbi.nlm.nih.gov/pubmed), Ovid (www.ovid.com), the Cochrane Library (www.thecochranelibrary.com), the Web of Science (www.wokinfo.com), Cumulative Index to Nursing and Allied Health Literature (www.ebscohost.com/biomedical-libraries/the-cinahl-database), Faculty of 1000 (www.f1000.com), and Gray Literature sources (www.opengrey.eu). In addition, of full-text articles selected for review, we hand searched citation lists and performed additional electronic searches of the first 20 related articles on PubMed. We also directly contacted experts to identify recently published or potentially unpublished studies. Searches of databases were conducted through 25 December 2013. Search terms included nut, seeds, almond, pecan, cashew, pistachio, macadamia, legume, bean, soybeans, lentil, peas, peanut, Mediterranean diet, cardiovascular disease, heart disease, myocardial infarction, heart attack, sudden death, stroke, cerebrovascular accident, and diabetes; see supplemental text under “Supplemental data” in the online issue for full search terms. Titles and abstracts of all identified articles were screened by one investigator; full-texts of potentially relevant articles were assessed for eligibility independently and in duplicate by 2 investigators.
Study selection
Inclusion criteria
We included all randomized controlled trials (RCTs), prospective cohorts, or case-control studies that evaluated nut or legume consumption and risk of IHD, stroke, or diabetes in generally healthy adults. Studies were included if they provided corresponding multivariable-adjusted risk estimates such as RRs, HRs, or ORs with corresponding data to calculate the variance.
Exclusion criteria
We excluded studies with concomitant major interventions that could not be separated from nut or legume consumption. Studies were also excluded if they only reported soft CVD outcomes (eg, angina) or intermediate risk factors (eg, blood lipids, insulin resistance, or metabolic syndrome), only providing crude (unadjusted) risk estimates if observational, focused on comparing vegetarians with nonvegetarians, or had a follow-up duration <3 mo. When duplicate publications from the same study were identified, we included the report that included the largest number of cases for each endpoint of interest. We excluded commentaries, reviews, and case reports after reviewing their related articles and citation lists for relevant original investigations.
Data extraction
Data were extracted by using a standardized electronic format independently and in duplicate by 2 investigators. Information included the first author name, contact information, publication year, study name, location, design, population (age, sex, race, and sample size), duration of follow-up, exposure definition, exposure assessment, exposure categories, dose in each category, outcome definition, outcome ascertainment, statistical analysis method, and covariates. Also, for each exposure category, we collected data on the median exposure, number of participants, person-years, number of events, and risk estimate and its corresponding uncertainty. For nuts, analyses were standardized to 4 weekly servings [28.4 g (1 oz)/serving (7)] on the basis of intakes associated with lowest risk in observational studies (8) and dietary targets set by advocacy organization (9). For consistency, analyses for legumes were also standardized to 4 weekly servings [100 g/serving (7)]. Differences in data extraction between investigators were unusual and were resolved by consensus.
Quality assessment
We assessed study quality on the basis of 5 criteria including the appropriateness and reporting of inclusion and exclusion criteria, assessment of exposure, assessment of outcome, control of confounding, and evidence of bias as previously reported (10). Each study received a score of zero or one (one being better) for each criterion, and an overall quality score was calculated as the sum of individual scores. For descriptive purposes, quality scores from 0 to 3 were generally considered lower quality, and scores from 4 to 5 were considered higher quality.
Statistical analysis
To maximize the use of information across all categories of exposure in each study, we conducted dose-response meta-analyses by using the 2-step generalized least-squares trend model (11, 12). Study-specific dose-response risk estimates were computed from the ln of risk estimates across categories of nut and legume intakes, with the consumption level in each category accounted for; and these study-specific risk estimates were pooled to derive an overall risk estimate. HRs and ORs were considered approximations of RRs. For each category of exposure, required inputs were the multivariable-adjusted risk estimate, its corresponding SE, person-years of follow-up (for RCTs and prospective cohorts) or number of subjects (for case-control studies), median exposure, and number of cases. When only sex-stratified results were reported by a study, these estimates were first pooled within each study by using the 2-step generalized least-squares trend method to derive the overall study-specific risk estimate. Analyses were performed by using random-effects inverse-variance weights. We compared these findings to fixed-effects inverse-variance weights in sensitivity analyses.
The between-study heterogeneity was assessed by using Cochran's Q and the I2 statistic. I2 values of 25%, 50%, and 75% were considered to represent low, moderate, and high heterogeneity, respectively (13). Potential explanatory sources of heterogeneity were explored by using meta-regression including age (continuous; and categorical <50 compared with ≥50 y), follow-up duration (continuous), study location (United States compared with non–United States), and study-quality score (continuous). Publication bias was assessed statistically by using Egger's and Begg's tests (14) and visual inspection of funnel plots. The influence of a potential publication bias on findings was explored by using the Duval and Tweedie trim-and-fill procedure (15). Analyses were conducted with STATA 13.0 software (StataCorp).
RESULTS
Of 3851 articles identified, 27 studies met inclusion and exclusion criteria (Figure 1), including 2 RCT reports, 23 prospective cohort reports, and 2 retrospective case-control studies. These represented one unique trial, 13 unique prospective cohorts, and 2 unique retrospective case-control studies that comprised 501,791 unique participants. The weighted mean (±SD) age of populations was 53.4 ± 4.0 y, with a weighted mean follow-up of 13.1 ± 6.3 y (Tables 1 and 2). Sixteen studies were from North America, 8 studies were from Europe, and 3 studies were from Asia. Median intakes of nuts across categories within each study ranged from 0 to 213 g/wk and of legumes from 0 to 938 g/wk. Nearly all studies adjusted for major potential confounders including age, sex, tobacco use, physical activity, alcohol intake, and intakes of saturated fat, trans fat, fiber, vegetables, and fruit. Several studies also adjusted for factors that could be either confounders or intermediates in the causal pathway (eg, blood pressure and hypercholesterolemia) (16–21); such adjustment could inappropriately attenuate observed risk estimates. Nearly all studies received a high-quality score (≥4).
FIGURE 1.
Screening and selection process of studies evaluating the consumption of nuts or legumes and incidence of ischemic heart disease, stroke, or diabetes. CVD, cardiovascular disease.
TABLE 1.
Identified studies evaluating the consumption of nuts or legumes and incidence of IHD, stroke, or diabetes1
| First author (year of publication) (ref) | Study name | Location | Design | Exposure assessment | Median consumption in lowest/highest categories | Endpoint | Disease ascertainment |
| Nuts (g/wk) | |||||||
| IHD | |||||||
| Albert (2002) (17) | PHS | US | Prospective cohort | FFQ | 4/71 | Fatal, nonfatal | Central adjudication2 |
| Bao (2013) (22) | NHS | US | Prospective cohort | FFQ | 0/159 | Fatal | Central adjudication |
| HPFS | US | Prospective cohort | FFQ | 0/159 | Fatal | Central adjudication | |
| Bernstein (2010) (23) | NHS | US | Prospective cohort | FFQ | 0/79 | Total | Central adjudication |
| Estruch (2013) (3) | PREDIMED | Spain | Randomized trial | FFQ | 47/194 | Total3 | Central adjudication |
| Fraser (1992) (24) | AHS | US | Prospective cohort | FFQ | 14/156 | Fatal, nonfatal | Central adjudication |
| Hu (1998) (16) | NHS | US | Prospective cohort | FFQ | 0/170 | Fatal, nonfatal, total4 | Central adjudication |
| Blomhoff (2006) (25) | IWHS | US | Prospective cohort | FFQ | 0/111 | Fatal | State Health Registry |
| Stroke | |||||||
| Bao (2013) (22) | NHS | US | Prospective cohort | FFQ | 0/159 | Total fatal5 | Central adjudication |
| HPFS | US | Prospective cohort | FFQ | 0/159 | Total fatal5 | Central adjudication | |
| Bernstein (2012) (26) | NHS | US | Prospective cohort | FFQ | 0/67 | Total, ischemic, hemorrhagic | Central adjudication |
| HPFS | US | Prospective cohort | FFQ | 0/119 | Total, ischemic, hemorrhagic | Central adjudication | |
| Djoussé (2010) (27) | PHS | US | Prospective cohort | FFQ | 0/213 | Total, ischemic, hemorrhagic | Central adjudication |
| Estruch (2013) (3) | PREDIMED | Spain | Randomized trial | FFQ | 47/194 | Total | Central adjudication |
| He (2003) (28) | HPFS | US | Prospective cohort | FFQ | 14/213 | Ischemic, hemorrhagic6 | Central adjudication |
| Diabetes | |||||||
| Jiang (2002) (29) | NHS | US | Prospective cohort | FFQ | 0/170 | Diabetes5 | Self-report |
| Kochar (2010) (30) | PHS | US | Prospective cohort | FFQ | 0/213 | Diabetes | Self-report |
| Montonen (2005) (31) | FMCHES | Finland | Prospective cohort | Dietary history interview | 7/607 | Diabetes | The Social Insurance Institution register |
| Pan (2013) (32) | NHS | US | Prospective cohort | FFQ | 0/170 | Diabetes | Self-report |
| NHS II | US | Prospective cohort | FFQ | 0/170 | Diabetes | Self-report | |
| Salas-Salvadó (2014) (33) | PREDIMED | Spain | Randomized trial | FFQ | 62/201 | Diabetes | Central adjudication |
| Villegas (2008) (34) | SWHS | China | Prospective cohort | FFQ | 1/228 | Diabetes | Self-report |
| Legumes (g/d) | |||||||
| IHD | |||||||
| Bazzano (2001) (21) | NHEFS | US | Prospective cohort | FFQ | 7/63 | Total | Central adjudication |
| Bernstein (2010) (23) | NHS | US | Prospective cohort | FFQ | 0/12 | Total | Central adjudication |
| Buckland (2009) (35) | EPIC-Heart | Spain | Prospective cohort | Dietary history interview | 16/66 | Total | Central adjudication |
| Dilis(2012) (36) | EPIC-Greece | Greece | Prospective cohort | FFQ | 10/17 | Total, fatal | Central adjudication |
| Kabagambe (2005) (37) | Cost Rica | Case-control | FFQ | 0/129 | Nonfatal | Central adjudication | |
| Kelemen (2005) (38) | IWHS | US | Prospective cohort | FFQ | 14/96 | Fatal | National Death Index |
| Kokubo (2007) (19) | JPHC | Japan | Prospective cohort | FFQ | 0/30 | Total | Central adjudication |
| Martínez-González (2002) (18) | Spain | Case-control | FFQ | 11/35 | Nonfatal | Central adjudication | |
| Nagura (2009) (39) | JACC | Japan | Prospective cohort | FFQ | 4/26 | Fatal | Death certificates |
| Stroke | |||||||
| Bernstein (2012) (26) | NHS | US | Prospective cohort | FFQ | 6/37 | Total, ischemic, hemorrhagic | Central adjudication |
| HPFS | US | Prospective cohort | FFQ | 6/49 | Total, ischemic, hemorrhagic | Central adjudication | |
| Kokubo (2007) (19) | JPHC | Japan | Prospective cohort | FFQ | 0/30 | Total | Central adjudication |
| Misirli (2012) (40) | EPIC-Greece | Greece | Prospective cohort | FFQ | 9/15 | Total | Central adjudication |
| Mizrahi (2009) (20) | FMCHES | Finland | Prospective cohort | Dietary history interview | 0/10 | Total, ischemic, hemorrhagic | Death certificates |
| Nagura (2009) (39) | JACC | Japan | Prospective cohort | FFQ | 4/26 | Total, ischemic, hemorrhagic | Death certificates |
| Diabetes | |||||||
| Meyer (2000) (41) | IWHS | US | Prospective cohort | FFQ | 12/80 | Diabetes | Self-report |
| Villegas (2008) (34) | SWHS | China | Prospective cohort | FFQ | 12/65 | Diabetes | Self-report |
We used the reported median intake or midpoint of each exposure category to define the median intake in that category. For studies with open-ended lower or higher categories that did not report median intake, we set the lower boundary to zero for lower categories and used the difference from the median to the upper range in the closest adjacent category to impute the median intake in the highest category. When reported intakes were energy adjusted (eg, per 1000 kcal), we estimated absolute intakes on the basis of the mean or median total energy intake. All intakes were standardized across studies to 4 weekly servings (28.4 g) for nuts and 4 weekly servings (100 g) for legumes. AHS, The Adventist Health Study; EPIC, European Prospective Investigation into Cancer and Nutrition; FFQ, Food-frequency questionnaire; FMCHES, Finnish Mobile Clinic Health Examination Survey; HPFS, Health Professionals Follow-Up Study; IHD, ischemic heart disease; IWHS, Iowa Women's Health Study; JACC, Japan Collaborative Cohort Study; JPHC, The Japan Public Health Center–Based Study; NHEFS, NHANES Epidemiologic Follow-up Study; NHS, Nurses’ Health Study; PHS, Physicians’ Health Study; PREDIMED, Prevencion con Dieta Mediterranea; ref, reference; SWHS, Shanghai Women's Health Study.
Disease endpoints were ascertained by an endpoints committee of physicians whereby a physician, who was unaware of the subjects’ risk factor status, reviewed medical records and death certificates (for fatal events). If the cause of death was not adequately documented in the medical records, the next of kin was interviewed about the circumstances under which death occurred.
The study did not separately report risk estimates for fatal and nonfatal IHD, and the risk estimate of total IHD was used for both fatal and nonfatal IHD.
Fatal IHD was not included in the analysis because a report with a larger number of cases from the same cohort was included in the analysis.
Not included in the analysis because a report with a larger number of cases on the same association from the same cohort was included in the analysis.
Ischemic and hemorrhagic stroke were not included in the analysis because a report with a larger number of cases from the same cohort was included in the analysis.
Total intakes of nuts and peas.
Intake of peanuts.
TABLE 2.
Characteristics of the participants and quality scores of the identified studies1
| First author (year of publication) (ref) | Population | Age range | Sample size | Follow-up | Person-years | No. of events | Degree of covariate adjustment | Quality score |
| y | y | |||||||
| Nuts | ||||||||
| IHD | ||||||||
| Albert (2002) (17) | US male physicians | 40–84 | 21,454 | 17 | 366,751 | 1603 | +++ | 5 |
| Bao (2013) (22) | US female registered nurses | 34–59 | 76,464 | 30 | 2,135,482 | 2208 | +++ | 5 |
| US male health care professionals | 40–75 | 42,498 | 24 | 903,371 | 2698 | +++ | 5 | |
| Bernstein (2010) (23) | US female registered nurses | 30–55 | 84,136 | 26 | 2,050,071 | 3162 | +++ | 5 |
| Estruch (2013) (3) | Adults at high risk of CVD in Spain | 55–80 | 7447 | 4.8 | 31,980 | 106 | ++ | 5 |
| Fraser (1992) (24) | US non-Hispanic white Adventists in California | 25+ | 26,473 | 6 | 158,838 | 463 | ++ | 5 |
| Hu (1998) (16) | US female registered nurses | 34–59 | 86,016 | 14 | 1,132,229 | 1255 | +++ | 5 |
| Blomhoff (2006) (25) | US postmenopausal women in Iowa | 55–69 | 31,778 | 15 | 472,354 | 948 | +++ | 5 |
| Stroke | ||||||||
| Bao (2013) (22) | US female registered nurses | 34–59 | 76,464 | 30 | 2,135,482 | 878 | +++ | 5 |
| US male health care professionals | 40–75 | 42,498 | 24 | 903,371 | 687 | +++ | 5 | |
| Bernstein (2012) (26) | US female registered nurses | 30–55 | 84,010 | 26 | 2,041,679 | 2633 | +++ | 5 |
| US male health care professionals | 40–75 | 43,150 | 22 | 833,660 | 1397 | +++ | 5 | |
| Djoussé (2010) (27) | US male physicians | 40–86 | 21,078 | 21.1 | 455,246 | 1424 | +++ | 5 |
| Estruch (2013) (3) | Adults at high risk of CVD in Spain | 55–80 | 7447 | 4.8 | 31,980 | 139 | ++ | 5 |
| He (2003) (28) | US male health care professionals | 40–75 | 43,732 | 14 | 612,248 | 578 | +++ | 5 |
| Diabetes | ||||||||
| Jiang (2002) (29) | US female registered nurses | 34–59 | 83,818 | 16 | 1,283,547 | 3206 | +++ | 5 |
| Kochar (2010) (30) | US male physicians | 40–87 | 20,224 | 19.2 | 388,301 | 1828 | +++ | 5 |
| Montonen (2005) (31) | Finnish men and women | 40–69 | 4304 | 23 | 98,992 | 383 | ++ | 4 |
| Pan (2013) (32) | US female registered nurses | 52–77 | 58,063 | 10 | 1,164,248 | 5121 | +++ | 5 |
| US female registered nurses | 35–52 | 79,893 | 10 | 1,599,667 | 4098 | +++ | 5 | |
| Salas-Salvadó (2014) (33) | Adults at high risk of CVD in Spain | 55–80 | 3541 | 4 | 14,173 | 273 | ++ | 5 |
| Villegas (2008) (34) | Chinese women residing in Shanghai | 40–70 | 64,191 | 4.6 | 297,744 | 1605 | +++ | 5 |
| Legumes | ||||||||
| IHD | ||||||||
| Bazzano (2001) (21) | US NHANES I participants | 25–74 | 9632 | 19 | 159,599 | 1802 | +++ | 5 |
| Bernstein (2010) (23) | US female registered nurses | 34–59 | 84,136 | 26 | 2,050,071 | 3162 | +++ | 5 |
| Buckland (2009) (35) | Healthy volunteers living in Spain | 29–69 | 40,757 | 10.4 | 423, 873 | 606 | ++ | 4 |
| Dilis(2012) (36) | Adults with no CVD or cancer in Greece | 20–86 | 23,929 | 10 | 229,894 | 636 | +++ | 5 |
| Kabagambe (2005) (37) | Hispanic Americans in the central valley of Costa Rica | — | 4238 | — | — | 2119 | +++ | 3 |
| Kelemen (2005) (38) | US postmenopausal women in Iowa | 55–69 | 29,017 | 17 | 475,755 | 739 | +++ | 4 |
| Kokubo (2007) (19) | Japanese men and women | 40–59 | 40,450 | 12.5 | 503,998 | 308 | +++ | 5 |
| Martínez-González (2002) (18) | Spanish male or female admitted to hospitals of Pamplona | — | 342 | — | — | 171 | +++ | 3 |
| Nagura (2009) (39) | Japanese men and women who participated in municipal health screening examinations | 40–79 | 59,485 | 12.7 | 756,054 | 452 | +++ | 5 |
| Stroke | ||||||||
| Bernstein (2012) (26) | US female registered nurses | 34–59 | 84,010 | 26 | 2,041,679 | 633 | +++ | 5 |
| US male health care professionals | 40–75 | 43,150 | 22 | 833,660 | 1397 | +++ | 5 | |
| Kokubo (2007) (19) | Japanese men and women | 40–59 | 40,450 | 12.5 | 503,998 | 578 | +++ | 5 |
| Misirli (2012) (40) | Adults with no CVD or cancer in Greece | 20–86 | 23,601 | 10.6 | 227,448 | 395 | +++ | 5 |
| Mizrahi (2009) (20) | Finnish men and women | 40–74 | 3932 | 24 | 94,368 | 625 | ++ | 4 |
| Nagura (2009) (39) | Japanese men and women who participated in municipal health screening examinations | 40–79 | 59,485 | 12.7 | 756,054 | 1053 | +++ | 5 |
| Diabetes | ||||||||
| Meyer (2000) (41) | US postmenopausal women in Iowa | 55–69 | 35,988 | 6 | 202,673 | 1141 | ++ | 4 |
| Villegas (2008) (34) | Chinese women residing in Shanghai | 40–70 | 64,191 | 4.6 | 297,744 | 1605 | +++ | 5 |
Degrees of adjustment for confounders were as follows: sociodemographics (+), sociodemographics plus either other risk factors or dietary variables (++), and sociodemographics plus other risk factors and dietary variables (+++). For quality scores, a quality assessment was performed by a review of the study design, including inclusion and exclusion criteria, assessment of exposure, assessment of outcome, control of confounding, and evidence of bias. Each criterion of the 5 quality criteria was evaluated and scored on an integer scale (zero or one, with one being better) and summed; quality scores from 0 to 3 were considered lower quality, and quality scores from 4 to 5 were considered higher quality. CVD, cardiovascular disease; IHD, ischemic heart disease; ref, reference.
Nut consumption and cardiometabolic endpoints
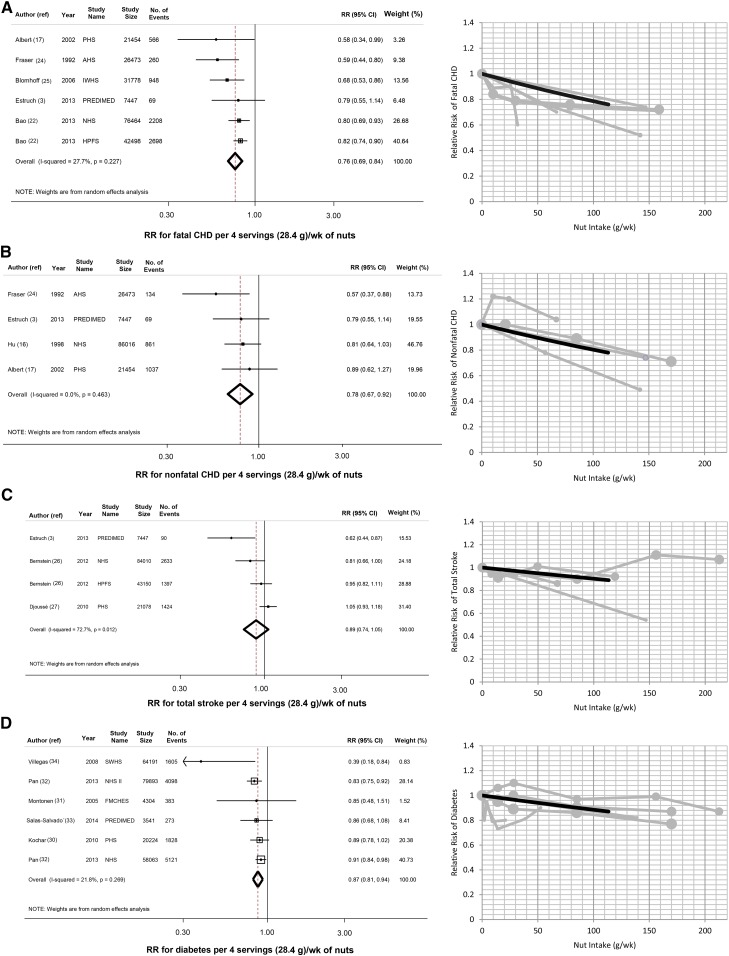
Five prospective cohorts and one RCT evaluated the relation of nut intake with fatal IHD (206,114 participants; 6749 events), and 3 prospective cohorts and one RCT assessed nut intake and nonfatal IHD (141,390 participants; 2101 events) (Figure 2, A and B). Pooling all studies, 4 weekly 28.4-g servings of nuts were associated with 24% lower risk of fatal IHD (RR: 0.76; 95% CI: 0.69, 0.84) and 22% lower risk of nonfatal IHD (RR: 0.78; 95% CI: 0.67, 0.92).
FIGURE 2.
Nut consumption and risk of cardiometabolic endpoints. Nut intake and risk of fatal IHD (A), nonfatal IHD (B), stroke (C), and diabetes (D). The figure shows pooled estimates (dashed line) and 95% CIs (open diamond) of risk per 4 weekly servings (28.4 g) of nuts (left) and plots of pooled (solid black lines) and study-specific (solid gray lines) RRs (right). AHS, The Adventist Health Study; CHD, coronary heart disease; FMCHES, Finnish Mobile Clinic Health Examination Survey; HPFS, Health Professionals Follow-Up Study; IHD, ischemic heart disase; IWHS, Iowa Women's Health Study; NHS, Nurses’ Health Study; PHS, Physicians’ Health Study; PREDIMED, Prevencion con Dieta Mediterranea; ref, reference; SWHS, Shanghai Women's Health Study.
Three prospective cohorts and one RCT study reported on nut consumption and stroke (155,685 participants; 5544 events). When we pooled these studies, nut intake was not significantly associated with total stroke (RR: 0.89; 95% CI: 0.74, 1.05) (Figure 2C) or stroke subtypes (see Supplemental Figure 1, A and B, under “Supplemental data” in the online issue). In 5 prospective cohorts and one RCT that assessed nut intake and incident diabetes (230,216 participants; 13,308 events), an inverse association with risk was seen (per 4 weekly servings, RR: 0.87; 95% CI: 0.81, 0.94) (Figure 2D).
All findings were similar with a fixed-effects inverse-variance weighting. For example, 4 weekly servings of nuts was associated with 22% lower risk of fatal IHD (95% CI: 16%, 27%), 22% lower risk of nonfatal IHD (95% CI: 8%, 33%), and 12% lower risk of diabetes (95% CI: 7%, 17%) and was not associated with total stroke (RR: 0.95; 95% CI: 0.87, 1.03).
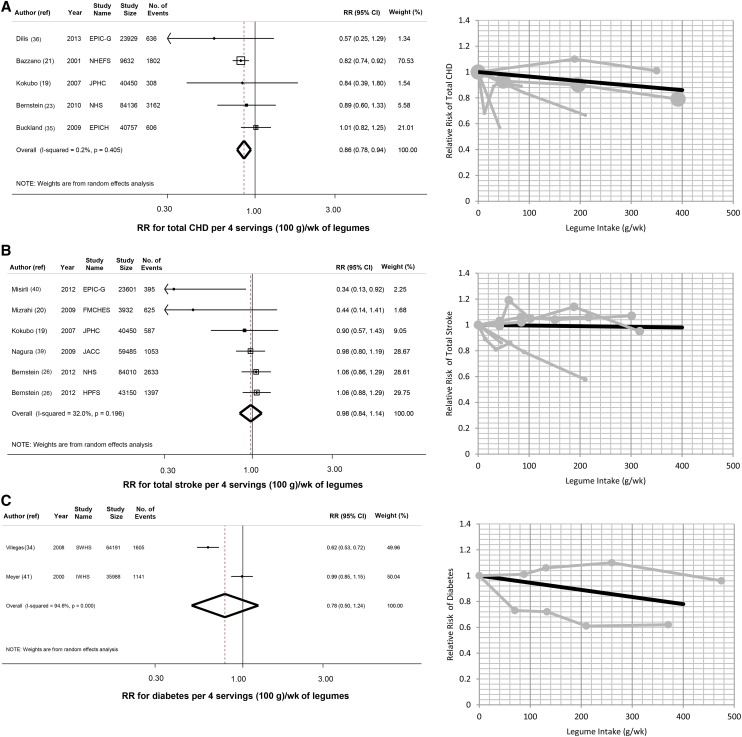
Legume consumption and cardiometabolic endpoints
When 5 prospective cohorts (198,904 participants; 6514 events) were pooled, 4 weekly 100-g servings of legumes was associated with 14% lower risk of total IHD (RR: 0.86; 95% CI: 0.78, 0.94) (Figure 3A). Six prospective cohorts evaluated legume consumption and stroke (254,628 participants; 6690 events). Pooling all studies, legume intake was not significantly associated with total stroke (RR: 0.98; 95% CI: 0.84, 1.14) (Figure 3B) or stroke subtypes (see Supplemental Figure 2, A and B, under “Supplemental data” in the online issue). Two prospective cohorts reported on legume consumption and incident diabetes (100,179 participants; 2746 events) (Figure 3C). When these studies were pooled, legume consumption was not significantly associated with incident diabetes (RR: 0.78; 95% CI: 0.50, 1.24).
FIGURE 3.
Legume intake and risk of cardiometabolic endpoints. Legume intake and risk of total IHD (A), stroke (B), and diabetes (C). In each panel, the left figure shows the pooled estimates (dashed line) and 95% CIs (open diamond) of the risk per 4 weekly servings (100 g) of legumes, and the right figure shows the plot of the pooled (solid black line) and study-specific (solid gray lines) RRs. EPIC-G, European Prospective Investigation into Cancer and Nutrition-Greece; EPICH, European Prospective Investigation into Cancer and Nutrition-Heart; FMCHES, Finnish Mobile Clinic Health Examination Survey; HPFS, Health Professionals Follow-Up Study; IHD, ischemic heart disease; IWHS, Iowa Women's Health Study; JACC, Japan Collaborative Cohort Study; JPHC, The Japan Public Health Center–Based Study; NHEFS, NHANES Epidemiologic Follow-up Study; NHS, Nurses’ Health Study; ref, reference; SWHS, Shanghai Women's Health Study.
Findings with fixed-effects inverse-variance weighting were similar for IHD (RR: 0.86; 95% CI: 0.78, 0.94) and stroke (RR: 1.00; 95% CI: 0.90, 1.12). In contrast, with fixed-effects inverse-variance weighting, legume consumption was inversely associated with diabetes (RR: 0.79; 95% CI: 0.71, 0.87).
Evaluation of heterogeneity
For several diet-disease relations, at least a moderate statistical heterogeneity was evident (Figures 2 and 3). However, no statistically significant source of heterogeneity was identified in a meta-regression analysis of age, follow-up duration, study location, and study-quality score (P > 0.05 for each).
Publication bias
The visual inspection of funnel plots (see Supplemental Figure 3 under “Supplemental data” in the online issue) and Begg's and Egger's tests provided mixed evidence for a publication bias toward small, protective studies for the relation of nut consumption with fatal IHD (Begg's P = 0.260, Eggers's P = 0.059) and total stroke (Begg's P = 0.089, Eggers's P = 0.006) and legume consumption with total stroke (Begg's P = 0.06, Eggers's P = 0.009). When we explored the influence of a potential publication bias using the trim-and-fill method, findings were generally similar with inverse associations of nut consumption with fatal IHD and no significant association of nut consumption or legume consumption with total stroke (see Supplemental Figure 4 under “Supplemental data” in the online issue). Evidence of a publication bias was not seen for studies that examined nut consumption and nonfatal IHD or diabetes or legume consumption and total IHD or diabetes. However, such testing could have had limited power in the setting of relatively few studies.
DISCUSSION
For the first time to our knowledge, we systematically reviewed the relations between consumption of nuts and legumes and incidence of IHD, stroke, and diabetes. Our investigation identified 27 studies that included 501,791 unique individuals from 3 continents, including 11,869 IHD, 8244 stroke, and 14,449 diabetes events. We showed that 4 weekly 28-g servings of nuts were associated with 24% lower risk of fatal IHD, 22% lower risk of nonfatal IHD, and 13% lower risk of diabetes. Also, the consumption of 4 weekly 100-g servings of legumes was associated with 14% lower risk of IHD. Conversely, neither nut or legume intake was significantly associated with incident stroke, and legume consumption was not significantly associated with incident diabetes, although each assessment was based on relatively few studies.
Most findings were robust to the choice of random- or fixed-effects weights and fill-and-trim methods to explore the potential influence of a publication bias. The exception was legume consumption and diabetes risk, for which results varied on the basis of weight; therefore, significant fixed-effects findings, which were derived from only 2 studies, should be interpreted with caution. Otherwise, each pooled analysis included hundreds of thousands of participants and thousands of clinical events. Thus, our study represents the most complete available evidence to-date on how intakes of these foods relate to the incidence of major cardiometabolic events.
Cardiovascular and metabolic benefits of nut consumption have been supported by several lines of evidence. Nuts contain many healthful components including unsaturated fatty acids, vegetable proteins, fiber, folate, minerals, antioxidants, and phytochemicals, which, in isolation or as part of enriched foods, improve cardiometabolic risk factors (42–45). For example, in a pooled analysis of 25 controlled trials, daily nut consumption reduced the total cholesterol concentrations by 10.9 mg/dL, the LDL-cholesterol concentration by 10.2 mg/dL, the LDL-to-HDL ratio by 0.22, and the total-to-HDL ratio by 0.24 (46). The consumption of nuts has also reduced triglycerides in subjects with high baseline concentrations (>150 mg/dL) (46). Furthermore, a recent meta-analysis of 8 controlled feeding trials showed that nut consumption lowered systolic blood pressure (−2.25 mm Hg; 95% CI: −4.22, −0.28 mm Hg) and mean blood pressure (−0.75 mm Hg; 95% CI: −1.44, −0.06 mm Hg) (45). The consumption of nuts may also improve the antioxidant capacity and reduce systemic inflammation (47). In addition, low carbohydrate and high unsaturated fat contents of nuts produce lower postprandial glucose and insulin responses when consumed alone or in combination with carbohydrate-rich foods (48, 49). Nuts are also a rich source of magnesium, which may have antiarrhythmic effects (50). Notably, although nuts are relatively calorie-dense (20–30 kJ/g) because of their higher total fat content (46–76%), nut consumption has been associated with less adiposity in observational studies (51) and did not contribute to weight gain in trials (47, 51). In sum, improvements in CVD risk factors in trials, benefits of a nut-focused dietary intervention rich in the Prevencion con Dieta Mediterranea (PREDIMED) trial (3), and our current results together provide compelling evidence that nut consumption is cardioprotective.
Like nuts, legumes contain multiple bioactive constituents that could improve cardiometabolic health, including fiber, folate, and phytochemicals (52). In a meta-analysis of 11 clinical trials, the consumption of nonsoy legumes lowered total cholesterol, LDL cholesterol, and triglycerides by 7%, 6%, and 17%, respectively, without significant changes in body weight (52). Legumes also produce lower glycemic responses, which may protect against diabetes (53). Although the identified studies did not attempt to model specific food replacements, it is plausible that metabolic benefits of legume consumption could at least partly be related to the replacement of iron-containing meats or higher glycemic grains, starches, and sugars (10, 54). Our results showed an inverse association between eating legumes and risk of IHD while also highlighting the paucity of studies on legumes and stroke or diabetes.
A previous meta-analysis of 4 prospective cohorts reported an inverse association between nut consumption and fatal IHD (4). However, the study did not assess nonfatal IHD, stroke, diabetes, or legume consumption. Also, only data in highest compared with lowest categories of intakes were used rather than the use of all categories; and extracted risk estimates in 3 of 4 studies (16, 17, 24) were not the most multivariable adjusted (ie, including dietary covariates). Our findings have built on and substantially expanded these previous results by evaluating a range of cardiometabolic endpoints, assessing legume consumption, using fully multivariable-adjusted estimates; and incorporating all categories of data to quantify the overall pooled dose response.
Compared with the PREDIMED trial (3), our pooled risk estimates for nuts were of similar magnitude for IHD and diabetes and were not significant for stroke. Because total CVD was the primary outcome of the PREDIMED trial, the specific findings in that trial for secondary endpoints such as IHD, stroke, and diabetes should be interpreted cautiously, and our pooled results may provide the most robust overall estimate of how nut consumption influences these endpoints. Conversely, PREDIMED participants achieved other small dietary changes that could alter stroke risk, which perhaps accounted for a larger benefit than seen in our meta-analysis. In addition, some of the observational studies in our analysis adjusted for potential mediating pathways (eg, blood pressure) that might have attenuated the true magnitude of the effect.
An insufficient consumption of nuts has been estimated to be a major contributor to global cardiometabolic mortality (8). On the basis of risk estimates from a preliminary version of this meta-analysis (55), nearly 2.5 million global deaths in 2010 were estimated to be attributable to low nut intake (8). Compared with our previous preliminary results (55), our current findings showed modestly smaller inverse associations of nut consumption with IHD and diabetes. Future analyses should incorporate these new findings to provide the best estimates of the global burden of diseases attributable to insufficient nut consumption.
Our analysis had several strengths. We systematically searched multiple databases to identify relevant studies. Our pooled findings included and were consistent with results of a large randomized trial (3, 33). We used available information across all exposure categories to estimate the pooled dose response. We evaluated and accounted for potential heterogeneity by several characteristics including study quality. Although relatively few studies were identified for each diet-disease association, large numbers of participants and events were included, which increased the statistical power to detect clinically meaningful associations. We identified studies from North America, Europe, and Asia, which increased the generalizability.
Potential limitations should be considered. We did not identify any controlled trials that evaluated legumes and incident IHD, stroke, or diabetes. Although all observational studies in our analysis adjusted for multiple major risk factors, the possibility of residual confounding by imprecisely or unmeasured factors could not be excluded. Yet, our findings were consistent with cardiometabolic benefits of nuts and legumes in short-term trials and, for nuts, with a clinical endpoint trial. Dietary habits were self-reported, definitions of nuts and legumes were not identical across studies, and most studies did not account for nuts or legumes consumed as an ingredient within other foods. These limitations would have caused an exposure misclassification, which could have attenuated the true effects toward the null.
In conclusion, we showed inverse associations of nut consumption with fatal IHD, nonfatal IHD, and diabetes and of legume consumption with incident IHD. Our findings also highlight key knowledge gaps, such as for stroke subtypes and legumes and diabetes. In light of their constituents and benefits on cardiometabolic risk factors in trials, our results support a role for nuts and possibly legumes as part of a cardiometabolically healthy diet.
Supplementary Material
Acknowledgments
We thank Liana Del Gobbo for her comments.
The authors’ responsibilities were as follows—AA, RM, and DM: conceived the study design and aims; AA, RM, and SK: performed the systematic review and data extraction; AA: performed the analysis; AA and DM: interpreted the results and drafted the manuscript; and RM and SK: contributed to manuscript revisions. AA and DM are guarantors. All authors had full access to all data and took responsibility for the integrity of data and accuracy of the data analysis. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: CVD, cardiovascular disease; IHD, ischemic heart disease; PREDIMED, Prevencion con Dieta Mediterranea; RCT, randomized controlled trial.
REFERENCES
- 1.Mozaffarian D, Appel LJ, Van Horn L. Components of a cardioprotective diet: new insights. Circulation 2011;123:2870–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Department of Agriculture and US Department of Health and Human Services. Dietary guidelines for Americans. 7th ed. Washington, DC: US Government Printing Office, 2010. [Google Scholar]
- 3.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 4.Kelly JH, Jr, Sabaté J. Nuts and coronary heart disease: an epidemiological perspective. Br J Nutr 2006;96(suppl 2):S61–7. [DOI] [PubMed] [Google Scholar]
- 5.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. QUOROM Group. Br J Surg 2000;87:1448–54. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Agriculture and US Department of Health and Human Services. Dietary guidelines for Americans 2010. 7th ed. Washington, DC: US Government Printing Office, 2010..
- 8.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2224–60 (Published erratum appears in Lancet 201;381:628.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 10.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010;121:2271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. [DOI] [PubMed] [Google Scholar]
- 12.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J 2006;6:40–557. [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 14.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 15.Duval S, Tweedie R. A non parametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc 2000;95:89–98. [Google Scholar]
- 16.Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BA, Speizer FE, Hennekens CH, Willett WC. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ 1998;317:1341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert CM, Gaziano JM, Willett WC, Manson JE. Nut consumption and decreased risk of sudden cardiac death in the Physicians’ Health Study. Arch Intern Med 2002;162:1382–7. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Gonzalez MA, Fernandez-Jarne E, Martinez-Losa E, Prado-Santamaria M, Brugarolas-Brufau C, Serrano-Martinez M. Role of fibre and fruit in the Mediterranean diet to protect against myocardial infarction: a case-control study in Spain. Eur J Clin Nutr 2002;56:715–22. [DOI] [PubMed] [Google Scholar]
- 19.Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S, Grp JS. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation 2007;116:2553–62. [DOI] [PubMed] [Google Scholar]
- 20.Mizrahi A, Knekt P, Montonen J, Laaksonen MA, Heliovaara M, Jarvinen R. Plant foods and the risk of cerebrovascular diseases: a potential protection of fruit consumption. Br J Nutr 2009;102:1075–83. [DOI] [PubMed] [Google Scholar]
- 21.Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch Intern Med 2001;161:2573–8. [DOI] [PubMed] [Google Scholar]
- 22.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med 2013;369:2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser GE, Sabaté J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart-disease - the Adventist Health Study. Arch Intern Med 1992;152:1416–24. [PubMed] [Google Scholar]
- 25.Blomhoff R, Carlsen MH, Andersen LF, Jacobs DR., Jr Health benefits of nuts: potential role of antioxidants. Br J Nutr 2006;96(suppl 2):S52–60. [DOI] [PubMed] [Google Scholar]
- 26.Bernstein AM, Pan A, Rexrode KM, Stampfer M, Hu FB, Mozaffarian D, Willett WC. Dietary protein sources and the risk of stroke in men and women. Stroke 2012;43:637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Djoussé L, Gaziano JM, Kase CS, Kurth T. Nut consumption and risk of stroke in US male physicians. Clin Nutr 2010;29:605–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ezzati M, Hoorn SV, Rodgers A, Lopez AD, Mathers CD, Murray CJ. Estimates of global and regional potential health gains from reducing multiple major risk factors. Lancet 2003;362:271–80. [DOI] [PubMed] [Google Scholar]
- 29.Jiang R, Manson JE, Stampfer MJ, Liu SM, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA 2002;288:2554–60. [DOI] [PubMed] [Google Scholar]
- 30.Kochar J, Gaziano JM, Djoussé L. Nut consumption and risk of type II diabetes in the Physicians’ Health Study. Eur J Clin Nutr 2010;64:75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montonen J, Jarvinen R, Heliovaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr 2005;59:441–8. [DOI] [PubMed] [Google Scholar]
- 32.Pan A, Sun Q, Manson JE, Willett WC, Hu FB. Walnut consumption is associated with lower risk of type 2 diabetes in women. J Nutr 2013;143:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salas-Salvadó J, Bullo M, Estruch R, Ros E, Covas MI, Ibarrola-Jurado N, Corella D, Aros F, Gómez-Gracia E, Ruiz-Gutiérrez V, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med 2014;160: 1–10. [DOI] [PubMed] [Google Scholar]
- 34.Villegas R, Gao Y-T, Yang G, Li H-L, Elasy TA, Zheng W, Shu XO. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr 2008;87:162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckland G, Gonzalez CA, Agudo A, Vilardell M, Berenguer A, Amiano P, Ardanaz E, Arriola L, Barricarte A, Basterretxea M, et al. Adherence to the Mediterranean diet and risk of coronary heart disease in the Spanish EPIC Cohort Study. Am J Epidemiol 2009;170:1518–29. [DOI] [PubMed] [Google Scholar]
- 36.Dilis V, Katsoulis M, Lagiou P, Trichopoulos D, Naska A, Trichopoulou A. Mediterranean diet and CHD: the Greek European Prospective Investigation into Cancer and Nutrition cohort. Br J Nutr 2012;108:699–709. [DOI] [PubMed] [Google Scholar]
- 37.Lemaitre RN, Tanaka T, Tang W, Manichaikul A, Foy M, Kabagambe EK, Nettleton JA, King IB, Weng LC, Bhattacharya S, et al. Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome-wide association studies from the CHARGE Consortium. PLoS Genet 2011;7:e1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelemen LE, Kushi LH, Jacobs DR, Jr, Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol 2005;161:239–49. [DOI] [PubMed] [Google Scholar]
- 39.Nagura J, Iso H, Watanabe Y, Maruyama K, Date C, Toyoshima H, Yamamoto A, Kikuchi S, Koizumi A, Kondo T, et al. Fruit, vegetable and bean intake and mortality from cardiovascular disease among Japanese men and women: the JACC Study. Br J Nutr 2009;102:285–92. [DOI] [PubMed] [Google Scholar]
- 40.Misirli G, Benetou V, Lagiou P, Bamia C, Trichopoulos D, Trichopoulou A. Relation of the traditional Mediterranean diet to cerebrovascular disease in a Mediterranean population. Am J Epidemiol 2012;176:1185–92. [DOI] [PubMed] [Google Scholar]
- 41.Meyer KA, Kushi LH, Jacobs DR, Jr, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30. [DOI] [PubMed] [Google Scholar]
- 42.Blomhoff R. Dietary antioxidants and cardiovascular disease. Curr Opin Lipidol 2005;16:47–54. [DOI] [PubMed] [Google Scholar]
- 43.Brown AA, Hu FB. Dietary modulation of endothelial function: implications for cardiovascular disease. Am J Clin Nutr 2001;73:673–86. [DOI] [PubMed] [Google Scholar]
- 44.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr 2009;89:1649S–56S. [DOI] [PubMed] [Google Scholar]
- 45.Jayalath VH, de Souza RJ, Sievenpiper JL, Ha V, Chiavaroli L, Mirrahimi A, Di Buono M, Bernstein AM, Leiter LA, Kris-Etherton PM, et al. Effect of dietary pulses on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Am J Hypertens 2014;27:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med 2010;170:821–7. [DOI] [PubMed] [Google Scholar]
- 47.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr 2009;90:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Josse AR, Kendall CW, Augustin LS, Ellis PR, Jenkins DJ. Almonds and postprandial glycemia–a dose-response study. Metabolism 2007;56:400–4. [DOI] [PubMed] [Google Scholar]
- 49.Jenkins DJ, Kendall CW, Josse AR, Salvatore S, Brighenti F, Augustin LS, Ellis PR, Vidgen E, Rao AV. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr 2006;136:2987–92. [DOI] [PubMed] [Google Scholar]
- 50.Chakraborti S, Chakraborti T, Mandal M, Mandal A, Das S, Ghosh S. Protective role of magnesium in cardiovascular diseases: a review. Mol Cell Biochem 2002;238:163–79. [DOI] [PubMed] [Google Scholar]
- 51.Mattes RD, Kris-Etherton PM, Foster GD. Impact of peanuts and tree nuts on body weight and healthy weight loss in adults. J Nutr 2008;138:1741S–5S. [DOI] [PubMed] [Google Scholar]
- 52.Anderson JW, Major AW. Pulses and lipaemia, short- and long-term effect: potential in the prevention of cardiovascular disease. Br J Nutr 2002;88(suppl 3):S263–71. [DOI] [PubMed] [Google Scholar]
- 53.Anderson JW, Smith BM, Washnock CS. Cardiovascular and renal benefits of dry bean and soybean intake. Am J Clin Nutr 1999;70(suppl):464S–74S. [DOI] [PubMed] [Google Scholar]
- 54.Mozaffarian D, Kamineni A, Carnethon M, Djoussé L, Mukamal KJ, Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med 2009;169:798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Abstract MP21: consumption of nuts and beans and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2013;127(supplement):AMP21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.