Abstract
Plant-based macular xanthophylls (MXs; lutein and zeaxanthin) and the lutein metabolite meso-zeaxanthin are the major constituents of macular pigment, a compound concentrated in retinal areas that are responsible for fine-feature visual sensation. There is an unmet need to examine the genetics of factors influencing regulatory mechanisms and metabolic fates of these 3 MXs because they are linked to processes implicated in the pathogenesis of age-related macular degeneration (AMD). In this work we provide an overview of evidence supporting a molecular basis for AMD-MX associations as they may relate to DNA sequence variation in AMD- and lipoprotein-related genes. We recognize a number of emerging research opportunities, barriers, knowledge gaps, and tools offering promise for meaningful investigation and inference in the field. Overviews on AMD- and high-density lipoprotein (HDL)–related genes encoding receptors, transporters, and enzymes affecting or affected by MXs are followed with information on localization of products from these genes to retinal cell types manifesting AMD-related pathophysiology. Evidence on the relation of each gene or gene product with retinal MX response to nutrient intake is discussed. This information is followed by a review of results from mechanistic studies testing gene-disease relations. We then present findings on relations of AMD with DNA sequence variants in MX-associated genes. Our conclusion is that AMD-associated DNA variants that influence the actions and metabolic fates of HDL system constituents should be examined further for concomitant influence on MX absorption, retinal tissue responses to MX intake, and the capacity to modify MX-associated factors and processes implicated in AMD pathogenesis.
INTRODUCTION
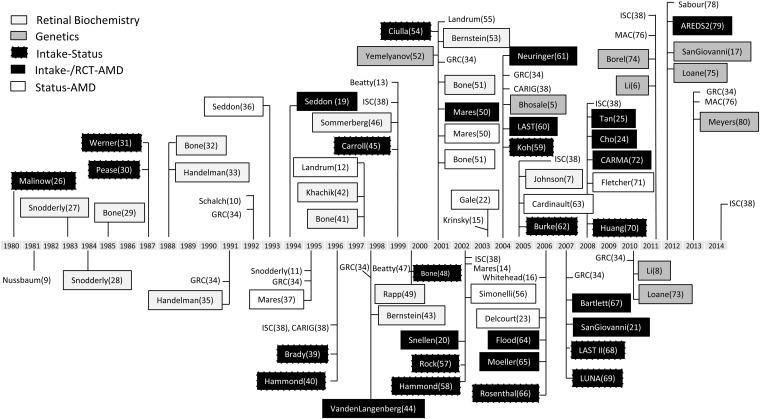
Age-related macular degeneration (AMD)5 is a common (1) and complex (2) disease of public health significance (3), manifesting sight-threatening pathology in the neural and vascular retina (4). The composition of the macula is notable for high concentrations of constituent plant-based xanthophyll carotenoids (lutein and zeaxanthin), their high-affinity binding proteins (5, 6), and the lutein metabolite meso-zeaxanthin (7). Among >600 naturally occurring carotenoids, 30–50 common dietary carotenoids, and 10–15 carotenoids commonly detected in serum, only lutein, zeaxanthin, and meso-zeaxanthin have been detected in appreciable quantities within the macula (reviewed in reference 8). Biochemical and biophysical properties of these macular xanthophylls (MXs), their metabolites, and cofactors have been implicated in protective capacities for >3 decades (9–17), and a number of large-scale human studies have yielded evidence for associations of AMD with the intake and status of lutein and zeaxanthin (18–25). A chronology of watershed events and publications addressing intake status–structure function axes in the AMD-MX field are shown in Figure 1. Events are classified in the figure by the nature of their design; those designated with the “Intake-Status” label examined the retinal response to MX intake; those with “Intake-/RCT-AMD” and “Status-AMD” designations are for respective investigations of dietary, nutrient supplement, or blood/macular pigment MX exposures on advanced AMD endpoints.
FIGURE 1.
Timeline of selected events and publications devoted to investigation of intake status–structure function relations in the field of AMD-macular xanthophyll research. Numbers in parentheses correspond to references in this article. Publications and events not enclosed in boxes are for literature reviews and conferences, respectively. Events and publications are named and classified in shaded boxes by event type or study design. Labels for review articles and scientific meetings are not surrounded by boxes. Studies in the “Intake-Status” category were observational in nature and applied dietary intake measures to estimate nutrient exposure and in or ex vivo retinal xanthophyll status measures as endpoints. Studies in the “Intake-/RCT-AMD” category were observational or experimental in nature and applied dietary intake questionnaires or nutrient supplement interventions as exposures and clinical classifications of AMD as endpoints. Studies in the “Status-AMD” category were observational in nature and applied blood or in vivo retinal xanthophyll status measures as exposures and clinical classifications of AMD as endpoints. AMD, age-related macular degeneration; AREDS2, Age-Related Eye Disease Study 2; CARIG, Carotenoids Research Interactive Group Conference; CARMA, Carotenoids in Age-Related Maculopathy study; GRC, Gordon Research Conference; ISC, International Symposium on Carotenoids; LAST, Lutein Antioxidant Supplementation Trial; LUNA, LUtein Nutrition effects measured by Autofluorescence Study; MAC, Conference on Macular Carotenoids and AMD; RCT, randomized clinical trial.
Primates are unable to synthesize lutein and zeaxanthin de novo and have developed the capacity for efficient retinal MX uptake (35, 81), transport (5, 6), and retention (47, 82–85). Genetic, dietary, and environmental factors influence aspects of these 3 processes, as shown by family-based studies (86, 87), biochemical analysis, and in vivo imaging of the retina (reviewed in reference 17). In addition to genetic influences on MX concentrations and distribution in retinal areas affected by AMD, reports on twins (88, 89) and first-degree relatives (90–92) have supported a genetic-basis for AMD (93). In the sections that follow we provide an overview on the molecular genetics of AMD in relation to actions of MXs on factors and processes implicated in AMD pathogenesis.
EXPANSION OF AN EMERGING CONCEPT
Large-scale genome-wide association studies (94, 95) have shown enrichment of AMD-related DNA sequence variants in genes encoding constituents of 1) complement regulatory systems (95) and 2) lipoprotein transport and metabolism systems (81, 95–98). Cholesterol (99, 100) and cholesterol metabolites (101) have been implicated in AMD pathogenesis due to their proinflammatory/immunoregulatory properties and presence in AMD-associated lesions. Sene et al (102) applied genetic and pharmacologic interventions influencing cholesterol efflux homeostasis to alter the severity of pathologic choroidal neovascularization (a hallmark of neovascular AMD) in mice; these authors acknowledged the complex nature of AMD-associated DNA variation in genes encoding proteins involved in cholesterol transport and metabolism, recognizing that allelic relations with advanced AMD do not always predict serum HDL or lipid status. When such observations are considered with the lack of evidence for protection of β-hydroxy-β-methylglutaryl coenzyme A reductase inhibitors (statins) against progression to advanced AMD (103, 104) and equivocal findings from large-scale observational studies on AMD endpoints examining dietary intake of cholesterol and saturated fat, it is clear that an expanded concept on the role of lipoprotein-related genes in AMD pathogenesis would offer valuable guidance for improving inquiry and inference in the field (17).
Findings on MX-lipoprotein relations may be a linchpin for inference on AMD-lipoprotein relations because MX uptake and transport is a facilitated process (105) involving many proteins that also act in cholesterol transport (77). The majority of circulating MXs are carried on HDL particles (106–108); as such, activity and distribution of lipoprotein constituents may influence the availability and accretion of MXs to the retina (77, 108, 109). The consequence of this condition can be considered in the context of central premises guiding AMD-MX research. These are as follows: 1) MX concentration is amplified 1000- to 10,000-fold from the circulation to the healthy retina (15, 110) via active transport mechanisms involving specific binding proteins (5, 6), 2) MXs are resident in retinal regions affected in AMD (46, 49), 3) MXs show a capacity to act on processes implicated in AMD pathogenesis (reviewed in references 11, 15, and 17), and 4) MX intake→MX status, MX status→AMD, MX intake→AMD relations have been observed in model systems and large-scale human studies (Figure 1 and Tables S1 and S2 under “Supplemental data” in the online issue). Variation in macular pigment optical density (MPOD; an in vivo measure of retinal MX status) has a hereditary component (86, 87, 111, 112). Meyers et al (80) noted the polygenic nature of MPOD variation and commented on the significance of extant works attributing genetic contributions to ∼70% of MPOD status (86) and ∼30% of variation in MPOD change, as associated with oral supplementation of MXs (112).
AMD-ASSOCIATED HDL-RELATED GENES AND PROTEINS AFFECTING MXs
AMD-associated polymorphisms in loci of genes encoding cholesteryl ester transfer protein (CETP); lipoprotein lipase (LPL); ATP-binding cassette, subfamily A member 1 (ABCA1); and hepatic lipase also yield variation in blood HDL-cholesterol concentrations (reviewed in reference 95). The observation that AMD-associated sequence variants exist in at least 3 other genes that encode proteins involved in HDL-resident systems [scavenger receptor class B type I (SCARB1), cluster determinant 36 (CD36), and apolipoprotein E (APOE)] supports an AMD-HDL nexus. In this work we discuss the confluence of evidence on a number of AMD-associated genes implicated in HDL transport and metabolism (94, 95, 113), retinal response to MX intake (73, 74, 80, 114), and alterations in the activity of MX-related molecular targets, regulatory mechanisms, and metabolic fate affecting retinal physiology (17). Our conclusion is that AMD-associated DNA variants that influence the actions and metabolic fates of HDL system constituents should be examined for concomitant influence on MX absorption, retinal tissue responses to MX intake, and the capacity to modify MX-related factors and processes implicated in AMD pathogenesis.
The subsections that follow begin with overviews on the state-of-science for AMD-associated and HDL-related genes encoding receptors, transporters, and enzymes affecting or affected by MXs, their metabolites, and cofactors. The overviews are followed with information on localization of the respective HDL-related proteins to retinal cell types manifesting AMD-related pathophysiology. Evidence on the relation of each gene or gene product with retinal MX response to intake is then presented. This information is followed by a review of findings from mechanistic studies designed to investigate retinal disease-gene relations. We then present findings on the relation of AMD and retinal MX status with DNA sequence variations in these HDL-related genes (Table 1). In the final section, we comment on the promise of this emerging evidence base for informing applied clinical research projects.
TABLE 1.
Genes and selected sequence variants associated with retinal status of MXs and AMD1
| Sequence variant (ref) |
||||
| Symbol | HDL function | Retinal MX status | AMD association | Model system (ref) |
| SCARB1 | LPB | rs10744182 (80) | rs5888 (97) | ARPE (118), mouse (122) |
| CD36 | LPI | rs1761667 (74) | rs3173789/rs3211883 (130) | ARPE (118), mouse (126–128) |
| ABCA1 | LPSBC | rs1929841 (80) | rs1883025 (81, 95) | hRPE (134), WHAM chick (106) |
| APOE | LPM | rs429358/rs7412 (73) | rs429358/rs7412 (113, 137) | Mouse (140) |
| LPL | LPM | — | rs12678919 (95) | Human (153) |
| CETP | LPT | — | rs1864163 (94) | Human (146) |
| LIPC | LPU | rs6078 (80) | rs920915 (94) | — |
Associations were determined with logistic regression, examining the likelihood of having advanced AMD, relative to the distribution of specific nucleotide bases for each of the sequence variants listed. The list of sequence variants is not comprehensive (see References for complete list). Full names and additional details on genes can be found at http://www.ncbi.nlm.nih.gov/gene. An additional AMD-associated single-nucleotide polymorphism exists in APOE rs4420638 (94). AMD, age-related macular degeneration; ARPE, differentiated human retinal pigment epithelial–derived cell line; hRPE, human retinal pigment epithelial cells; LPB, lipoprotein binding; LPI, lipoprotein internalization; LPM, lipoprotein metabolism; LPSBC, lipoprotein secretion by (efflux from) cells; LPT, transfer of lipoproteins; LPU, lipoprotein uptake at the cell surface; MX, macular xanthophyll; ref, reference number.
HDL-RELATED GENES AND MX UPTAKE IN RETINA
SCARB1
The scavenger receptor class B type I (SR-BI), a cell surface glycoprotein of CD36 superfamily with high affinity to HDL and localized to the apical surface of the enterocyte, has been implicated in nonspecific binding and absorption of MXs at the intestinal brush border (115). SR-BI is encoded by SCARB1 (12q24.31), a gene expressed in primary human retinal pigment epithelium (RPE) cells (116). SR-BI mRNA has been detected in human neural retina using reverse transcriptase–polymerase chain reaction (117). Immunohistochemical localization of the protein in monkey retina showed the strongest signal in retinal ganglion cells, outer segments of photoreceptor rods and cones, and the choriocapillaris (the vascular interface to the RPE and neural retina) (Figure 2). Equivocal evidence exists for strong expression of the protein in primate RPE. A specific SR-BI antibody-blocking technique and small interfering RNA on a differentiated human RPE–derived cell line (ARPE-19) showed that zeaxanthin uptake can be driven by an SR-BI–dependent process (118). The authors of the ARPE-19 study (118) discuss a gene-MX-disease link, pointing to a mutation in ninaD, an insect gene with high sequence identity to SCARB1 that is associated with reduced carotenoid uptake, reduced zeaxanthin deposition, and blindness in Drosophila (119–121). The retinal ultrastructure of SR-BI−/− and wild-type (WT) mice manifested differences after a feeding regimen enriched in components carried on HDL (122). Relative to the WT mice, SR-BI−/− animals showed increased lipid inclusions and disorganization of photoreceptor outer segments and areas within the outer nuclear layer. Also, Bruch's membrane, a permeable 5-layer structure of basement membranes, collagen, and elastin existing between the choroid and RPE, was thickened in the SR-BI−/− mice and showed sparse sub-RPE deposits. The relevance to AMD is that altered flow of essential compounds across Bruch's membrane has been implicated in the progression to advanced forms of the disease. In addition to these changes, the choroid of the SR-BI−/− mice manifested abnormal distribution of collagen fibers and a vacuolization associated with local inflammation in the subretinal space (linked to the infiltration of macrophages); SR-BI−/− mice did not exhibit abnormal choroidal neovascularization. However, induction of vascular endothelial growth factor (VEGF; a molecule involved in retinal angiogenesis) expression in the outer nuclear layer of the SR-BI−/− mice was observed.
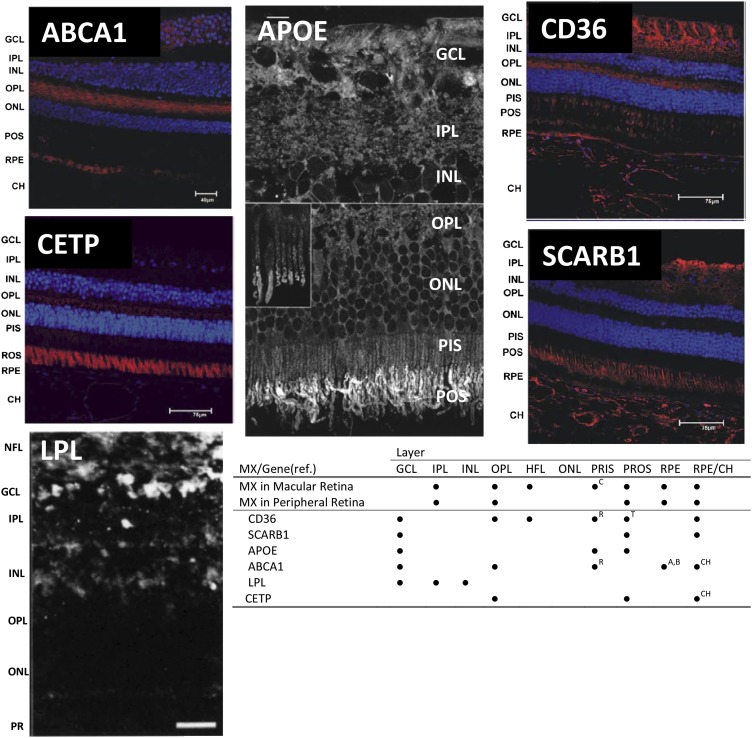
FIGURE 2.
Retinal localization of proteins involved in uptake, transport, and cleavage of MXs. Immunohistochemical localization studies in primate retina were reported by Tserentsoodol et al (117) for ABCA1, CD36, CETP, and SCARB1; by Anderson et al (152) for APOE; and by Casaroli-Marano et al (147) for LPL. For micrographs of ABCA1, CD36, CETP, and SCARB1, areas in red indicate regions of the respective MX-related protein localization. For APOE and LPL, lucent areas indicate protein localization. Reproduced with permission from references 117, 147, and 152. CH, retinal choroid layer; GCL, ganglion cell layer; HFL, Henle Fiber layer; INL, inner nuclear layer; IPL, inner plexiform layer (interneurons); MX, macular xanthophyll; NFL, nerve fiber layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PR, photoreceptors; PRIS or PIS, photoreceptor inner segments; PROS, photoreceptor outer segments; PROS or POS, photoreceptor outer segments; ref., reference; RPE, retinal pigmented epithelium.
A locus in SCARB1 (rs10744182) has been associated with alterations in MPOD within the Carotenoids in Age-related Eye Disease Study, a project involving 1585 women participating in the Women's Health Initiative Observational Study (80). An exonic sequence variant in SCARB1 (rs5888) linked to lower SR-BI expression (123) has been associated with advanced AMD in a large-scale genotyping project in French- and US-based cohorts (97). We have reported relations of AMD with a common intronic variant (rs989892; P = 0.010) coinherited (D′ = 0.98, r2 = 0.82) with rs5888 and another single-nucleotide polymorphism (SNP; rs838878; P = 0.007) in complete linkage disequilibrium with an SNP (rs838884) proximal to the 3′ untranslated region of SCARB1 (124).
CD36
CD36 is a major glycoprotein that acts as a primary antiangiogenic receptor of thrombospondin-1. CD36 binds long-chain fatty acids, collagen, anionic phospholipids, and oxidized LDL in macrophages. CD36 is involved in internalization of HDL and transport of oxidized LDL particles and may act in the transport of and/or as a regulator of fatty acid transport. In the retina, CD36 acts in phagocytosis of photoreceptor outer segments. CD36 is encoded by the CD36 gene (7q11.2); the protein is localized in the primate retina within RPE, tips of rod outer segments, rod inner segments, the choriocapillaris, the outer plexiform layer, and in the ganglion cell layer (117) (Figure 2). In a study on human retina, the expression of CD36 varied by >8-fold in the neural retina and by >20-fold in RPE between donors (125). During et al (118) did not detect MX transport actions of CD36 in their work on differentiated human RPE cells: a CD36-specific antibody did not prevent accumulation of zeaxanthin in ARPE-19 cells after the addition of MX to the medium.
CD36−/− mice manifest a progressive age-related choroidal involution typically involving a 100–300% increase in the avascular area within the choriocapillaris (126). Progressive choroidal degeneration accompanies reduced cyclooxygenase-2 (PTGS2) and VEGF (126) expression. CD36 activating antibody stimulates PTGS2 expression in RPE cell cultures, whereas CD36 deficiency was associated with inhibition of COX-2 and subsequent lack of VEGF response to outer segment or antibody stimulation in vitro (126). Picard et al (127) used CD36−/− and CD36+/+ mice to show an age-related CD36-dependent process of deposition and clearance of subretinal deposits that bears similarity to those typically seen in AMD; in this study, CD36−/− animals developed basal laminar deposits. In a mouse model showing similar pathology (ApoE−/−) to the CD36−/− model, the administration of a CD36 agonist inhibited the formation of pathologic subretinal deposits. CD36 may be linked to AMD-like retinal pathology in spontaneous hypertensive rats as well. These animals develop retinal and choroidal degeneration independent of hypertension (128). CD36 mutations exist in some spontaneous hypertensive rat strains (129).
Borel et al (74) examined 5 sequence variants in CD36 for the association of MPOD response to a 6-mo regimen of daily supplementation with a formula containing 10 mg lutein esters in a 30-person French cohort. A CD36 locus (rs1761667) was associated with variation in macular status of MXs; persons homozygous for the major allele (G) had significantly higher MPOD than those carrying the minor allele (A). This SNP was tested by Meyers et al (80) and did not yield MPOD variations in their US-based cohort of women. Kondo et al (130) examined the allelic frequency of 19 SNPs resident in CD36 for association with neovascular AMD in a Japanese cohort of 109 people with neovascular AMD and 182 unrelated controls. There was a 50% lower likelihood of having neovascular AMD among carriers of the minor allele for 2 intronic sequence variants in the gene (rs3173798 and rs3211883).
The central messages on SCARB1 and CD36 (HDL-related genes both expressed in retina and implicated in retinal uptake of intake-based MXs within model systems) are as follows: 1) animal models applying gene deletions of either SCARB1 or CD36 manifest AMD-like pathology, 2) DNA variation (in this case, SNPs) in SCARB1 and CD36 is associated with variation in MPOD in a number of human studies, and 3) SCARB1-AMD relations have been reported in multiple cohorts.
HDL-RELATED GENES AND MX TRANSPORT AND METABOLISM IN THE RETINA
ABCA1
The ATP-binding cassette, subfamily A member 1 (ABCA1) protein, encoded by the ABCA1 gene (9q31.1), is a member of the superfamily of ATP-binding cassette transporters. The protein acts as a major lipoprotein transporter and works with apolipoprotein A-I (APOA1) in the process of cholesterol and phospholipid metabolism as an efflux pump from tissue to nascent HDL. ABCA1 controls the intracellular transport and secretion of APOE and APOA1 (117). A mutation mapped to ABCA1 exists in persons with Tangier disease [Online Mendelian Inheritance in Man (OMIM) 205400], an autosomal recessive disorder characterized by extremely reduced concentrations of plasma HDL, leading to tissue accumulation of cholesterol esters. Immunolocalization of ABCA1 in primate retina indicates that the highest concentrations of the protein are found in the macular ganglion cell layer, the MX-rich outer plexiform layer, and RPE (117). Within the polarized RPE cell, both basal (side of choroidal apposition) and apical (side of photoreceptor apposition) aspects showed specific ABCA1 staining (Figure 2).
Connor et al (106) applied an avian model expressing a sex-linked recessive mutation in ABCA1 [the Wisconsin hypo α mutant (WHAM) chick] to show the critical role of HDL-mediated MX transport to the retina. The WHAM chick exhibits similar concentrations of VLDL and LDL to WT Leghorn chicks but shows a 90% reduction in HDL cholesterol. Hepatic accumulation of MXs in the WHAM chick was not appreciably different from that in the WT birds; however, differences were seen in plasma, heart, adipose, and retina. Repletion of all tissues except for retina was attained with a 1-mo lutein-rich feeding regimen. With the lutein-rich diet, the absolute concentration of retinal MXs remained 15-fold lower than those in the WT chicks. The WHAM mutation acts by inhibiting APOA1-mediated efflux of hydrophobic molecules from peripheral tissue to HDL. APOA1 is rapidly degraded when low in lipid content.
Lakkaraju et al (131) used immortalized human and bovine primary RPE cell cultures to show that activation of ABCA1 by the peroxisome proliferator–activated receptor γ (PPAR-γ) agonist pioglitazone and liver X receptor (LXR) agonist TO901317 is effective in hydrolyzing A2E, a quaternary amine and retinoid by-product of the visual cycle responsible for pathologic accumulation of free and esterified cholesterol in RPE cells. Blue-light exposure in RPE cells induces A2E to generate singlet oxygen that has the capacity to damage DNA and lead to apoptotic cell death (132); lutein, which is present in inner retinal layers, acts as a filter for blue light. The implications of these findings for AMD-MX research may not be readily apparent. Pioglitazone acts on PPAR-γ, which forms a bioactive heterodimer with the retinoid X receptor (RXR). RXR heterodimerizes with the retinoid A receptors (RARs). RXR heterodimerizes with PPAR-γ and LXR; this process activates ABCA1, leading to A2E hydrolysis. Lutein is a ligand to the RARs and may thus influence the activation of the RAR-RXR-PPAR-γ-LXR complex (133). This putative relation ties the ABCA1-MX relation to a process implicated in AMD pathogenesis and raises the possibility that lutein (or a lutein metabolite) may activate ABCA1 to influence A2E catabolism. Work by Matsumoto et al (133) indicates that β-cryptoxanthin (a mono-hydroxy xanthophyll with similar bonding structure and functional groups to MXs) was effective in activating ABCA1. Duncan et al (134) reported that exposure of human RPE cell cultures to glyburide, a nonspecific ABCA1 inhibitor (also inhibiting SCARB1), prevents HDL-stimulated basal transport of photoreceptor-derived lipids implicated in AMD pathogenesis. These findings are intriguing, considering the MX-binding capacity of SCARB1, the role of ABCA1 in MX transport, and the putative activation of ABCA1 by MXs via RARA and RARG.
Meyers et al (80) reported variations in MPOD related to allelic variants in the ABCA1 SNP rs1929841; persons carrying the CC genotype showed a 20% lower value for MPOD than persons with AA or AC genotypes. Sequence variants in ABCA1 have been associated with AMD in numerous large-scale genotyping projects (81, 95, 98, 124, 135, 136).
Apolipoprotein E
Apolipoprotein E (apo E) is an apolipoprotein acting in lipid metabolism as a ligand to lipoprotein receptors and in response to injury within the central nervous system (137, 138). apo E is necessary for the normal catabolism of HDL, triglyceride-rich components of chylomicrons, and VLDL (73). SNPs in the APOE gene (19q13.2) are associated with increased plasma concentrations of cholesterol and triglycerides. DNA sequence variants in APOE have been a focus of work on AMD, because apo E is a component of drusen (retinal lipid deposits first evident in early AMD). In the retina, APOE is expressed primarily in astrocytes and Müller cells; it has been localized to Bruch's membrane, the RPE, and the photoreceptor outer segment layer (reviewed in reference 117) (Figure 2). MXs are transported in serum on apo E in HDL and LDL (73, 139).
Six-month-old ApoE−/− C57BL/6 mice fed standard laboratory feed pellets (9605/8; Harlan Teklad TRM) showed a 50% lower concentration of lutein in the neural retina, relative to WT animals of the same inbred strain fed the same diet. Zeaxanthin concentrations were unchanged (140). Retinal ultrastructure in the ApoE−/− mice was characterized by AMD-associated lesions, including severe basal laminar deposits/vacuolization and thickening of Bruch's membrane. Choroid-RPE homogenates of these animals showed 42% higher VEGF concentrations than those in WT mice, as analyzed by Western blots. Abnormal lipid accumulation in the RPE and Bruch's membrane in a transgenic mouse expressing the human APOEϵ2 risk variant has been reported. Compared with WT mice, the APOEϵ2 transgenic animals exhibited an overexpression of VEGF and basic fibroblast growth factor (bFGF). Dysregulation of VEGF and bFGF are central events in diseases characterized by pathologic retinal angiogenesis.
DNA variants in APOE are associated with MPOD concentrations. Loane et al (73) reported that persons carrying at least one APOEϵ4 allele have higher MPOD values across the macula than did noncarriers. Meyers et al (80) did not observe MPOD variation with haplotypes of the SNPs (rs7142, rs429358) examined by Loane et al. The APOE-AMD relations reported by Souied et al (113) and Klaver et al (137) in 1998 were among the first gene-AMD associations published. Findings have been replicated numerous times (138, 141–145). An age-, sex-, and smoking-adjusted pooled analysis of 15 studies (n = 21,160) confirmed the protective association of the APOEϵ4 alleles and risk of the APOEϵ2 alleles on late AMD (96). Our findings on rs405509 are similar to those of the pooled analysis. A large multicenter study incorporating data from >77,000 people identified advanced AMD associations with the presence of the A allele in rs4420638 (94).
CETP
CETP is a secreted soluble protein that acts with ABCA1 in the transfer of cholesteryl esters between lipoproteins during reverse cholesterol transport; it is encoded by the CETP gene (16q21) and expressed in retina mainly within the photoreceptor outer segments and the outer plexiform layer (117). A study examining exchange of carotenoids between human VLDL and HDL (146) showed that CETP inhibitors significantly increased the proportion of lutein in HDL. To our knowledge. there are no studies using in vivo model systems to examine the role of CETP on health and disease of the retina or the actions of MXs.
Meyers et al (80) examined the influence of 3 intronic CETP SNPs (rs173539, rs3764261, and rs708272) on retinal MX status. In no case were these variants associated with MPOD. AMD-associated DNA sequence variants in CETP (rs173539, rs3764261, rs1864163) have been reported in numerous studies (94, 95, 135).
LPL
LPL is a water-soluble enzyme that hydrolyzes triglycerides in lipoproteins and enables cellular uptake of chylomicron remnants, cholesterol-dense lipoproteins, and free fatty acids. The enzyme is encoded by the LPL gene (8p22) and requires the apolipoprotein C2 (encoded by the APOC2 gene) as a cofactor in these processes. In the primate, the LPL protein is localized within the inner retinal layers (nerve fiber, ganglion cell, inner plexiform, and inner nuclear layers) and the choroid (147) (Figure 2). Within the choroid, it is most likely to be attached to the luminal epithelium. Carriers of an exonic sequence variant X447 (rs328) in LPL show a 20% reduction in serum MXs, relative to carriers of the S447S alleles (148). The authors of this work suggest that this stop polymorphism alters shedding of MXs from their surface positions on chylomicrons during lipolysis. To our knowledge, there are no reported relations of LPL sequence variants with MPOD, although one (rs328) was tested in this capacity by Meyers et al (80). A sequence variant in LPL known to influence HDL cholesterol (rs12678919) has been reported in association with advanced (95), but not early (136), AMD. We have reported on another variant (rs10099160) also associated with advanced AMD (124).
The state of evidence on HDL-related genes both expressed in retina and implicated in retinal transport of intake-based MXs within model systems indicates the following: 1) animal models of ABCA1 and APOE gene deletions yield substantial reductions in retinal lutein, and in the case of APOE, manifest aspects of AMD-like lesions; 2) human MPOD has varied with DNA variants in ABCA1 and APOE in some studies; and 3) ABCA1-, APOE-, and CETP-AMD relations have been replicated in large cohorts. We also reported on a HDL-related gene (LPL) expressed in retina and implicated in MX release from its carrier protein. While LPL-AMD relationships have been reported for advanced forms of the disease, there is no current support for influence on human macular status of MXs.
CONCLUSIONS AND FUTURE DIRECTIONS
MX concentrations in the retina are, in some cases, dependent on and modifiable by MX intake. In this report, we recognize converging fields of evidence that implicate the influence of genes encoding constituents of HDL metabolism and transport systems with both with retinal MX status (106, 108) and AMD risk (95). At least 4 of the 6 AMD-associated HDL-related genes we discussed are known to carry DNA polymorphisms linked to variation in MPOD, a measure of retinal MX concentration in the retinal area sustaining AMD pathology. Animal models characterized by gene knockouts in SCARB1, ABCA1, and APOE yielded both alterations in retinal MX amounts and AMD-like pathology. Extensive testing of sequence variants in the 6 reviewed genes for effects on MPOD has not been applied in diverse cohorts and it is necessary to replicate findings while accounting for predictors and correlates (see Table 2) of MPOD status. Future studies should be designed with the provisions from a number of expert reviews that offer insightful commentary on strengths and limitations of in vivo imaging modalities for AMD-MX research (110, 149).
TABLE 2.
Factors associated with advanced AMD and macular pigment optical density1
| Factor | AMD relation | Direction of MX-MPOD relation |
| Older age | Harm | ↓ (86, 154–157); ↔ (54, 62, 80, 158, 159) |
| Female | Harm | ↓ (40, 157); ↔ (54, 62, 156, 160, 161) |
| White race | Harm | ↓ (161) |
| Obesity/high BMI | Harm | ↓ (58, 60, 62, 80, 155, 162) |
| History of smoking | Harm | ↓ (157, 163); ↔(54, 161) |
| Circulating MX concentrations | See Table S1 under “Supplemental data” in the online supplement | ↑ (40, 48, 54, 62, 80, 155, 164–166); ↓ (167) |
| MX dietary intake | See Table S2 under “Supplemental data” in the online supplement | ↑ AMD-free (54, 62, 80, 82, 162, 168, 169); ↔ (82) |
| ↑ Elderly (170) | ||
| MX supplement use | Suggestive benefit | ↑ AMD-free (83, 171–175) |
| ↑ Elderly (59, 60, 69, 156, 162, 168, 176, 177); ↔ (69) |
AMD, age-related macular degeneration; MPOD, macular pigment optical density; MX, diet-based macular xanthophylls (lutein + zeaxanthin); ↑, increase; ↔, no change; ↓, decrease.
Neovascular AMD has been associated consistently with lower reported intakes of lutein + zeaxanthin (Supplemental Table S2 under “Supplemental data” in the online issue). We believe that this is the endpoint on which gene-MPOD studies are most likely to yield informative results. A recent work combining data from >77,000 people has shown neovascular AMD-ABCA1 and -CETP relations (94). The AMD-associated SNPs in these genes (and SNPs coinherited with these) should be examined for influence on MPOD response to MX intake. Many proteins encoded by AMD-associated HDL-related genes act together; as such, it is essential to evaluate gene-gene relations for both MPOD and AMD endpoints. Epistasis (gene-gene interactions) for AMD- and MPOD-related SNPs should be examined within and between genes. The first AMD-APOE relations reported (113, 137) were based on allele combinations from 2 independent SNPs; this may be the case with other gene-based effect modifiers of AMD risk.
Diet influences the expression of CD36 (150); to our knowledge, the other genes discussed above have not been tested for this capacity, and such information would aid in inference on retinal response to MX intake. The interindividual variation in CD36 expression within the RPE is formidable, with an up to a 20-fold difference between donor eyes analyzed by Zheng et al (125).
Cholesterol efflux is important for preventing cytotoxic oxysterol production in the retina (101). Actions of ABCA1 appear to influence this process, and there is a link here with pathologic choroidal neovascularization (102). Oxidative degradation products of MXs exist in the retina (42), and we suspect that ABCA1 may also work in efflux of these compounds. The issue may be germane for AMD prevention because some oxidative degradation products of lutein have been shown to damage DNA of an immortalized RPE cell line in a dose- and time-dependent manner (151). An important opportunity now exists to examine the influence of ABCA1 on the clearance of oxidized xanthophylls from retinal cells.
In summary, a more-thorough investigation of AMD- and HDL-associated loci for their putative actions in the intake status–structure function axis in AMD-MX relations is reasonable given the following: 1) the joint actions of lipoprotein-related genes in cholesterol and MX transport and metabolism and 2) the biological plausibility of cholesterol and MXs and their metabolites for influencing processes implicated in AMD pathogenesis.
Acknowledgments
The authors’ responsibilities were as follows—JPS and MN: conceived and designed the study; EK, MN, and JPS: wrote the manuscript and contributed meaningful commentary on all concepts addressed; and JPS: had primary responsibility for final content. All of the authors read and approved the final manuscript. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: ABCA1, ATP-binding cassette, subfamily A member 1; AMD, age-related macular degeneration; apo E, apolipoprotein E; CD36, cluster determinant 36; CETP, cholesteryl ester transfer protein; COX-2, cyclooxygenase-2; LPL, lipoprotein lipase; LXR, liver X receptor; MPOD, macular pigment optical density; MX, macular xanthophyll; PPAR-γ, peroxisome proliferator-activated receptor γ RAR, retinoid A receptor; RPE, retinal pigment epithelium; RXR, retinoid X receptor; SNP, single-nucleotide polymorphism; SR-BI, scavenger receptor class B type I; VEGF, vascular endothelial growth factor; WT, wild-type.
REFERENCES
- 1.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–72. [DOI] [PubMed] [Google Scholar]
- 2.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 2003;48:257–93. [DOI] [PubMed] [Google Scholar]
- 3.Congdon N, O'Colmain B, Klaver CCW, Klein R, Muoz B, Friedman D, Kempen J, Taylor H, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004;122:477–85. [DOI] [PubMed] [Google Scholar]
- 4.Hageman GS, Gehrs K, Johnson LV, Anderson D. Age-related macular degeneration (AMD). Webvision. 1995. Available from: http://www.ncbi.nlm.nih.gov/books/NBK27323/(cited 5 May 2014). [PubMed]
- 5.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem 2004;279:49447–54. [DOI] [PubMed] [Google Scholar]
- 6.Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry 2011;50:2541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas. III: effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci 2005;46:692–702. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Vachali P, Bernstein PS. Human ocular carotenoid-binding proteins. Photochem Photobiol Sci 2010;9:1418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nussbaum JJ, Pruett RC, Delori FC. Historic perspectives. Macular yellow pigment: the first 200 years. Retina 1981;1:296–310. [PubMed] [Google Scholar]
- 10.Schalch W. Carotenoids in the retina—a review of their possible role in preventing or limiting damage caused by light and oxygen. EXS 1992;62:280–98. [DOI] [PubMed] [Google Scholar]
- 11.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr 1995;62(suppl):1448S–61S. [DOI] [PubMed] [Google Scholar]
- 12.Landrum JT, Bone RA, Kilburn MD. The macular pigment: a possible role in protection from age-related macular degeneration. Adv Pharmacol 1997;38:537–56. [DOI] [PubMed] [Google Scholar]
- 13.Beatty S, Boulton M, Henson D, Koh HH, Murray IJ. Macular pigment and age related macular degeneration. Br J Ophthalmol 1999;83:867–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mares-Perlman JA, Millen AE, Ficek TL, Hankinson SE. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease: overview. J Nutr 2002;132(suppl):518S–24S. [DOI] [PubMed] [Google Scholar]
- 15.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 2003;23:171–201. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead AJ, Mares JA, Danis RP. Macular pigment: a review of current knowledge. Arch Ophthalmol 2006;124:1038–45. [DOI] [PubMed] [Google Scholar]
- 17.SanGiovanni JP, Neuringer M. The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: promise of molecular genetics for guiding mechanistic and translational research in the field. Am J Clin Nutr 2012;96(suppl):1223S–33S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Eye Disease Case-Control Study Group. Risk factors for neovascular age-related macular degeneration. Arch Ophthalmol 1992;110:1701–8. [DOI] [PubMed] [Google Scholar]
- 19.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994;272:1413–20. [PubMed] [Google Scholar]
- 20.Snellen EL, Verbeek AL, Van Den Hoogen GW, Cruysberg JR, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol Scand 2002;80:368–71. [DOI] [PubMed] [Google Scholar]
- 21.SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, III, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS report no. 22. Arch Ophthalmol 2007;125:1225–32. [DOI] [PubMed] [Google Scholar]
- 22.Gale CR, Hall NF, Phillips DI, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci 2003;44:2461–5. [DOI] [PubMed] [Google Scholar]
- 23.Delcourt C, Carriere I, Delage M, Barberger-Gateau P, Schalch W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA study. Invest Ophthalmol Vis Sci 2006;47:2329–35. [DOI] [PubMed] [Google Scholar]
- 24.Cho E, Hankinson SE, Rosner B, Willett WC, Colditz GA. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am J Clin Nutr 2008;87:1837–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology 2008;115:334–41. [DOI] [PubMed] [Google Scholar]
- 26.Malinow MR, Feeney-Burns L, Peterson LH, Klein ML, Neuringer M. Diet-related macular anomalies in monkeys. Invest Ophthalmol Vis Sci 1980;19:857–63. [PubMed] [Google Scholar]
- 27.Snodderly DM, Delori FC, Auran JD. Macular pigment density in primate retinas. Invest Ophthalmol Vis Sci 1983;24(suppl):256. [PubMed] [Google Scholar]
- 28.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci 1984;25:674–85. [PubMed] [Google Scholar]
- 29.Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res 1985;25:1531–5. [DOI] [PubMed] [Google Scholar]
- 30.Pease PL, Adams AJ, Nuccio E. Optical density of human macular pigment. Vision Res 1987;27:705–10. [DOI] [PubMed] [Google Scholar]
- 31.Werner JS, Donnelly SK, Kliegl R. Aging and human macular pigment density. Appended with translations from the work of Max Schultze and Ewald Hering. Vision Res 1987;27:257–68. [DOI] [PubMed] [Google Scholar]
- 32.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci 1988;29:843–9. [PubMed] [Google Scholar]
- 33.Handelman GJ, Dratz EA, Reay CC, van Kuijk JG. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci 1988;29:850–5. [PubMed] [Google Scholar]
- 34.Gordon Research Conference on Carotenoids. Carotenoids. Available from: http://www.grc.org/conferences.aspx?id=0000309 (cited 5 May 2014).
- 35.Handelman GJ, Snodderly DM, Krinsky NI, Russett MD, Adler AJ. Biological control of primate macular pigment: biochemical and densitometric studies. Invest Ophthalmol Vis Sci 1991;32:257–67. [PubMed] [Google Scholar]
- 36.Eye Disease Case-Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol 1993;111:104–9. [DOI] [PubMed] [Google Scholar]
- 37.Mares-Perlman JA, Brady WE, Klein R, Klein BE, Bowen P, Stacewicz-Sapuntzakis M, Palta M. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol 1995;113:1518–23. [DOI] [PubMed] [Google Scholar]
- 38. The International Carotenoid Society. Meetings and conferences. Available from: http://www.carotenoidsociety.org/meetings-and-conferences (cited 5 May 2014).
- 39.Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr 1996;126:129–37. [DOI] [PubMed] [Google Scholar]
- 40.Hammond BR, Jr, Curran-Celentano J, Judd S, Fuld K, Krinsky NI, Wooten BR, Snodderly DM. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Res 1996;36:2001–12. [DOI] [PubMed] [Google Scholar]
- 41.Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res 1997;64:211–8. [DOI] [PubMed] [Google Scholar]
- 42.Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci 1997;38:1802–11. [PubMed] [Google Scholar]
- 43.Bernstein PS, Yoshida MD, Katz NB, McClane RW, Gellermann W. Raman detection of macular carotenoid pigments in intact human retina. Invest Ophthalmol Vis Sci 1998;39:2003–11. [PubMed] [Google Scholar]
- 44.VandenLangenberg GM, Mares-Perlman JA, Klein R, Klein BE, Brady WE, Palta M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the Beaver Dam Eye Study. Am J Epidemiol 1998;148:204–14. [DOI] [PubMed] [Google Scholar]
- 45.Carroll YL, Corridan BM, Morrissey PA. Carotenoids in young and elderly healthy humans: dietary intakes, biochemical status and diet-plasma relationships. Eur J Clin Nutr 1999;53:644–53. [DOI] [PubMed] [Google Scholar]
- 46.Sommerburg OG, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk FJ. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res 1999;19:491–5. [DOI] [PubMed] [Google Scholar]
- 47.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000;45:115–34. [DOI] [PubMed] [Google Scholar]
- 48.Bone RA, Landrum JT, Dixon Z, Chen Y, Llerena CM. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp Eye Res 2000;71:239–45. [DOI] [PubMed] [Google Scholar]
- 49.Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci 2000;41:1200–9. [PubMed] [Google Scholar]
- 50.Mares-Perlman JA, Fisher AI, Klein R, Palta M, Block G, Millen AE, Wright JD. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2001;153:424–32. [DOI] [PubMed] [Google Scholar]
- 51.Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci 2001;42:235–40. [PubMed] [Google Scholar]
- 52.Yemelyanov AY, Katz NB, Bernstein PS. Ligand-binding characterization of xanthophyll carotenoids to solubilized membrane proteins derived from human retina. Exp Eye Res 2001;72:381–92. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res 2001;72:215–23. [DOI] [PubMed] [Google Scholar]
- 54.Ciulla TA, Curran-Celantano J, Cooper DA, Hammond BR, Jr, Danis RP, Pratt LM, Riccardi KA, Filloon TG. Macular pigment optical density in a midwestern sample. Ophthalmology 2001;108:730–7. [DOI] [PubMed] [Google Scholar]
- 55.Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys 2001;385:28–40. [DOI] [PubMed] [Google Scholar]
- 56.Simonelli F, Zarrilli F, Mazzeo S, Verde V, Romano N, Savoia M, Testa F, Vitale DF, Rinaldi M, Sacchetti L. Serum oxidative and antioxidant parameters in a group of Italian patients with age-related maculopathy. Clin Chim Acta 2002;320:111–5. [DOI] [PubMed] [Google Scholar]
- 57.Rock CL, Thornquist MD, Neuhouser ML, Kristal AR, Neumark-Sztainer D, Cooper DA, Patterson RE, Cheskin LJ. Diet and lifestyle correlates of lutein in the blood and diet. J Nutr 2002;132:525S–30S. [DOI] [PubMed] [Google Scholar]
- 58.Hammond BR, Jr, Ciulla TA, Snodderly DM. Macular pigment density is reduced in obese subjects. Invest Ophthalmol Vis Sci 2002;43:47–50. [PubMed] [Google Scholar]
- 59.Koh HH, Murray IJ, Nolan D, Carden D, Feather J, Beatty S. Plasma and macular responses to lutein supplement in subjects with and without age-related maculopathy: a pilot study. Exp Eye Res 2004;79:21–7. [DOI] [PubMed] [Google Scholar]
- 60.Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75:216–30. [DOI] [PubMed] [Google Scholar]
- 61.Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas. I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Invest Ophthalmol Vis Sci 2004;45:3234–43. [DOI] [PubMed] [Google Scholar]
- 62.Burke JD, Curran-Celentano J, Wenzel AJ. Diet and serum carotenoid concentrations affect macular pigment optical density in adults 45 years and older. J Nutr 2005;135:1208–14. [DOI] [PubMed] [Google Scholar]
- 63.Cardinault N, Abalain JH, Sairafi B, Coudray C, Grolier P, Rambeau M, Carre JL, Mazur A, Rock E. Lycopene but not lutein nor zeaxanthin decreases in serum and lipoproteins in age-related macular degeneration patients. Clin Chim Acta 2005;357:34–42. [DOI] [PubMed] [Google Scholar]
- 64.Flood V, Rochtchina E, Wang JJ, Mitchell P, Smith W. Lutein and zeaxanthin dietary intake and age related macular degeneration. Br J Ophthalmol 2006;90:927–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moeller SM, Parekh N, Tinker L, Ritenbaugh C, Blodi B, Wallace RB, Mares JA. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-related Eye Disease Study (CAREDS): ancillary study of the Women's Health Initiative. Arch Ophthalmol 2006;124:1151–62. [DOI] [PubMed] [Google Scholar]
- 66.Rosenthal JM, Kim J, de Monasterio F, Thompson DJ, Bone RA, Landrum JT, de Moura FF, Khachik F, Chen H, Schleicher RL, et al. Dose-ranging study of lutein supplementation in persons aged 60 years or older. Invest Ophthalmol Vis Sci 2006;47:5227–33. [DOI] [PubMed] [Google Scholar]
- 67.Bartlett HE, Eperjesi F. Effect of lutein and antioxidant dietary supplementation on contrast sensitivity in age-related macular disease: a randomized controlled trial. Eur J Clin Nutr 2007;61:1121–7. [DOI] [PubMed] [Google Scholar]
- 68.Richer S, Devenport J, Lang JC. LAST II: differential temporal responses of macular pigment optical density in patients with atrophic age-related macular degeneration to dietary supplementation with xanthophylls. Optometry 2007;78:213–9. [DOI] [PubMed] [Google Scholar]
- 69.Trieschmann M, Beatty S, Nolan JM, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res 2007;84:718–28. [DOI] [PubMed] [Google Scholar]
- 70.Huang LL, Coleman HR, Kim J, de Monasterio F, Wong WT, Schleicher RL, Ferris FL, 3rd, Chew EY. Oral supplementation of lutein/zeaxanthin and omega-3 long chain polyunsaturated fatty acids in persons aged 60 years or older, with or without AMD. Invest Ophthalmol Vis Sci 2008;49:3864–9. [DOI] [PubMed] [Google Scholar]
- 71.Fletcher AE, Bentham GC, Agnew M, Young IS, Augood C, Chakravarthy U, de Jong PT, Rahu M, Seland J, Soubrane G, et al. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch Ophthalmol 2008;126:1396–403. [DOI] [PubMed] [Google Scholar]
- 72.Neelam K, Hogg RE, Stevenson MR, Johnston E, Anderson R, Beatty S, Chakravarthy U. Carotenoids and Co-antioxidants in Age-related Maculopathy: design and methods. Ophthalmic Epidemiol 2008;15:389–401. [DOI] [PubMed] [Google Scholar]
- 73.Loane E, McKay GJ, Nolan JM, Beatty S. Apolipoprotein E genotype is associated with macular pigment optical density. Invest Ophthalmol Vis Sci 2010;51:2636–43. [DOI] [PubMed] [Google Scholar]
- 74.Borel P, de Edelenyi FS, Vincent-Baudry S, Malezet-Desmoulin C, Margotat A, Lyan B, Gorrand JM, Meunier N, Drouault-Holowacz S, Bieuvelet S. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann Med 2011;43:47–59. [DOI] [PubMed] [Google Scholar]
- 75.Loane E, Nolan JM, McKay GJ, Beatty S. The association between macular pigment optical density and CFH, ARMS2, C2/BF, and C3 genotype. Exp Eye Res 2011;93:592–8. [DOI] [PubMed] [Google Scholar]
- 76.Macular Carotenoids Conference. Homepage. Available from: http://www.macularcarotenoids.org/ (cited 5 May 2014).
- 77.Borel P. Genetic variations involved in interindividual variability in carotenoid status. Mol Nutr Food Res 2012;56:228–40. [DOI] [PubMed] [Google Scholar]
- 78.Sabour-Pickett S, Nolan JM, Loughman J, Beatty S. A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Mol Nutr Food Res 2012;56:270–86. [DOI] [PubMed] [Google Scholar]
- 79.Chew EY, Clemons T, SanGiovanni JP, Danis R, Domalpally A, McBee W, Sperduto R, Ferris FL. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012;119:2282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, Liu Z, Igo RP, Jr, Truitt B, Klein ML, et al. Genetic determinants of macular pigments in women of the Carotenoids in Age-related Eye Disease Study. Invest Ophthalmol Vis Sci 2013;54:2333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc Natl Acad Sci USA 2010;107:7395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hammond BR, Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, Snodderly DM. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci 1997;38:1795–801. [PubMed] [Google Scholar]
- 83.Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr 2003;133:992–8. [DOI] [PubMed] [Google Scholar]
- 84.Hammond BR, Jr, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A Opt Image Sci Vis 1997;14:1187–96. [DOI] [PubMed] [Google Scholar]
- 85.Zeimer M, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. Kurz- und mittelfristige Änderungen der makulären Pigmentdichte infolge der Supplementation mit Lutein und Zeaxanthin sowie Koantioxidanzien. [The macular pigment: short- and intermediate-term changes of macular pigment optical density following supplementation with lutein and zeaxanthin and co-antioxidants. The LUNA Study.] Ophthalmologe 2009;106:29–36 (in German). [DOI] [PubMed] [Google Scholar]
- 86.Liew SH, Gilbert CE, Spector TD, Mellerio J, Marshall J, van Kuijk FJ, Beatty S, Fitzke F, Hammond CJ. Heritability of macular pigment: a twin study. Invest Ophthalmol Vis Sci 2005;46:4430–6. [DOI] [PubMed] [Google Scholar]
- 87.Wenzel AJ, Sheehan JP, Burke JD, Lefsrud MG, Curran-Celentano J. Dietary intake and serum concentrations of lutein and zeaxanthin, but not macular pigment optical density, are related in spouses. Nutr Res 2007;27:462–9. [Google Scholar]
- 88.Klein ML, Mauldin WM, Stoumbos VD. Heredity and age-related macular degeneration: Observations in monozygotic twins. Arch Ophthalmol 1994;112:932–7. [DOI] [PubMed] [Google Scholar]
- 89.Seddon JM, Cote J, Page WF, Aggen SH, Neale MC. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol 2005;123:321–7. [DOI] [PubMed] [Google Scholar]
- 90.Heiba IM, Elston RC, Klein BE, Klein R. Sibling correlations and segregation analysis of age-related maculopathy: the Beaver Dam Eye Study. Genet Epidemiol 1994;11:51–67. [DOI] [PubMed] [Google Scholar]
- 91.Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age-related maculopathy. Am J Ophthalmol 1997;123:199–206. [DOI] [PubMed] [Google Scholar]
- 92.Klaver CC, Wolfs RC, Assink JJ, van Duijn CM, Hofman A, de Jong PT. Genetic risk of age-related maculopathy: population-based familial aggregation study. Arch Ophthalmol 1998;116:1646–51. [DOI] [PubMed] [Google Scholar]
- 93.Swaroop A, Chew EY, Rickman CB, Abecasis GR. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet 2009;10:19–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S, et al. Seven new loci associated with age-related macular degeneration. Nat Genet 2013;45:433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak-Vance MA, Campochiaro PA, Klein ML, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci USA 2010;107:7401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McKay GJ, Patterson CC, Chakravarthy U, Dasari S, Klaver CC, Vingerling JR, Ho L, de Jong PT, Fletcher AE, Young IS, et al. Evidence of association of APOE with age-related macular degeneration—a pooled analysis of 15 studies. Hum Mutat 2011;32:1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zerbib J, Seddon JM, Richard F, Reynolds R, Leveziel N, Benlian P, Borel P, Feingold J, Munnich A, Soubrane G, et al. rs5888 Variant of SCARB1 gene is a possible susceptibility factor for age-related macular degeneration. PLoS ONE 2009;4:e7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu Y, Reynolds R, Fagerness J, Rosner B, Daly MJ, Seddon JM. Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:4663–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Curcio CA, Johnson M, Huang JD, Rudolf M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res 2009;28:393–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Curcio CA, Johnson M, Huang JD, Rudolf M. Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration. J Lipid Res 2010;51:451–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodríguez IR, Larrayoz IM. Cholesterol oxidation in the retina: implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J Lipid Res 2010;51:2847–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sene A, Khan AA, Cox D, Nakamura RE, Santeford A, Kim BM, Sidhu R, Onken MD, Harbour JW, Hagbi-Levi S, et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metab 2013;17:549–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maguire MG, Ying GS, McCannel CA, Liu C, Dai Y. Statin use and the incidence of advanced age-related macular degeneration in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology 2009;116:2381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peponis V, Chalkiadakis SE, Bonovas S, Sitaras NM. The controversy over the association between statins use and progression of age-related macular degeneration: a mini review. Clin Ophthalmol 2010;4:865–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr 2005;135:2305–12. [DOI] [PubMed] [Google Scholar]
- 106.Connor WE, Duell PB, Kean R, Wang Y. The prime role of HDL to transport lutein into the retina: evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Invest Ophthalmol Vis Sci 2007;48:4226–31. [DOI] [PubMed] [Google Scholar]
- 107.Parker RS. Absorption, metabolism, and transport of carotenoids. FASEB J 1996;10:542–51. [PubMed] [Google Scholar]
- 108.Wang W, Connor SL, Johnson EJ, Klein ML, Hughes S, Connor WE. Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration. Am J Clin Nutr 2007;85:762–9. [DOI] [PubMed] [Google Scholar]
- 109.Loane E, Nolan JM, O'Donovan O, Bhosale P, Bernstein PS, Beatty S. Transport and retinal capture of lutein and zeaxanthin with reference to age-related macular degeneration. Surv Ophthalmol 2008;53:68–81. [DOI] [PubMed] [Google Scholar]
- 110.Bernstein PS, Delori FC, Richer S, van Kuijk FJ, Wenzel AJ. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision Res 2010;50:716–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hogg RE, Ong EL, Chamberlain M, Dirani M, Baird PN, Guymer RH, Fitzke F. Heritability of the spatial distribution and peak density of macular pigment: a classical twin study. Eye (Lond) 2012;26:1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hammond CJ, Liew SH, Van Kuijk FJ, Beatty S, Nolan JM, Spector TD, Gilbert CE. The heritability of macular response to supplemental lutein and zeaxanthin: a classic twin study. Invest Ophthalmol Vis Sci 2012;53:4963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Souied EH, Benlian P, Amouyel P, Feingold J, Lagarde JP, Munnich A, Kaplan J, Coscas G, Soubrane G. The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol 1998;125:353–9. [DOI] [PubMed] [Google Scholar]
- 114.Aleman TS, Cideciyan AV, Windsor EA, Schwartz SB, Swider M, Chico JD, Sumaroka A, Pantelyat AY, Duncan KG, Gardner LM, et al. Macular pigment and lutein supplementation in ABCA4-associated retinal degenerations. Invest Ophthalmol Vis Sci 2007;48:1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li B, Ahmed F, Bernstein PS. Studies on the singlet oxygen scavenging mechanism of human macular pigment. Arch Biochem Biophys 2010;504:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Duncan KG, Bailey KR, Kane JP, Schwartz DM. Human retinal pigment epithelial cells express scavenger receptors BI and BII. Biochem Biophys Res Commun 2002;292:1017–22. [DOI] [PubMed] [Google Scholar]
- 117.Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis 2006;12:1319–33. [PubMed] [Google Scholar]
- 118.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res 2008;49:1715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, von Lintig J. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry 2006;45:13429–37. [DOI] [PubMed] [Google Scholar]
- 120.Kiefer C, Sumser E, Wernet MF, Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci USA 2002;99:10581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Giovannucci DR, Stephenson RS. Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC. Vision Res 1999;39:219–29. [DOI] [PubMed] [Google Scholar]
- 122.Provost AC, Vede L, Bigot K, Keller N, Tailleux A, Jais JP, Savoldelli M, Ameqrane I, Lacassagne E, Legeais JM, et al. Morphologic and electroretinographic phenotype of SR-BI knockout mice after a long-term atherogenic diet. Invest Ophthalmol Vis Sci 2009;50:3931–42. [DOI] [PubMed] [Google Scholar]
- 123.Constantineau J, Greason E, West M, Filbin M, Kieft JS, Carletti MZ, Christenson LK, Rodriguez A. A synonymous variant in scavenger receptor, class B, type I gene is associated with lower SR-BI protein expression and function. Atherosclerosis 2010;210:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.SanGiovanni JP, Neuringer M. Macular xanthophylls and age-related macular degeneration. In: Landrum JT, Nolan J, eds. Carotenoids and retinal disease. Boca Raton, FL: CRC Press, 2013.
- 125.Zheng W, Reem RE, Omarova S, Huang S, DiPatre PL, Charvet CD, Curcio CA, Pikuleva IA. Spatial distribution of the pathways of cholesterol homeostasis in human retina. PLoS ONE 2012;7:e37926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Houssier M, Raoul W, Lavalette S, Keller N, Guillonneau X, Baragatti B, Jonet L, Jeanny JC, Behar-Cohen F, Coceani F, et al. CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents. PLoS Med 2008;5:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Picard E, Houssier M, Bujold K, Sapieha P, Lubell W, Dorfman A, Racine J, Hardy P, Febbraio M, Lachapelle P, et al. CD36 plays an important role in the clearance of oxLDL and associated age-dependent sub-retinal deposits. Aging (Albany NY) 2010;2:981–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Funk R, Rohen JW. Comparative morphological studies on blood vessels in eyes of normotensive and spontaneously hypertensive rats. Exp Eye Res 1985;40:191–203. [DOI] [PubMed] [Google Scholar]
- 129.Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, et al. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet 1999;21:76–83. [DOI] [PubMed] [Google Scholar]
- 130.Kondo N, Honda S, Kuno S, Negi A. Positive association of common variants in CD36 with neovascular age-related macular degeneration. Aging (Albany NY) 2009;1:266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lakkaraju A, Finnemann SC, Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc Natl Acad Sci USA 2007;104:11026–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res 2005;80:595–606. [DOI] [PubMed] [Google Scholar]
- 133.Matsumoto A, Mizukami H, Mizuno S, Umegaki K, Nishikawa J, Shudo K, Kagechika H, Inoue M. beta-Cryptoxanthin, a novel natural RAR ligand, induces ATP-binding cassette transporters in macrophages. Biochem Pharmacol 2007;74:256–64. [DOI] [PubMed] [Google Scholar]
- 134.Duncan KG, Hosseini K, Bailey KR, Yang H, Lowe RJ, Matthes MT, Kane JP, LaVail MM, Schwartz DM, Duncan JL. Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. Br J Ophthalmol 2009;93:1116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yu Y, Bhangale TR, Fagerness J, Ripke S, Thorleifsson G, Tan PL, Souied EH, Richardson AJ, Merriam JE, Buitendijk GH, et al. Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum Mol Genet 2011;20:3699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fauser S, Smailhodzic D, Caramoy A, van de Ven JP, Kirchhof B, Hoyng CB, Klevering BJ, Liakopoulos S, den Hollander AI. Evaluation of serum lipid concentrations and genetic variants at high-density lipoprotein metabolism loci and TIMP3 in age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:5525–8. [DOI] [PubMed] [Google Scholar]
- 137.Klaver CC, Kliffen M, van Duijn CM, Hofman A, Cruts M, Grobbee DE, van Broeckhoven C, de Jong PT. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet 1998;63:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zareparsi S, Reddick AC, Branham KE, Moore KB, Jessup L, Thoms S, Smith-Wheelock M, Yashar BM, Swaroop A. Association of apolipoprotein E alleles with susceptibility to age-related macular degeneration in a large cohort from a single center. Invest Ophthalmol Vis Sci 2004;45:1306–10. [DOI] [PubMed] [Google Scholar]
- 139.Loane E, Nolan JM, Beatty S. The respective relationships between lipoprotein profile, macular pigment optical density, and serum concentrations of lutein and zeaxanthin. Invest Ophthalmol Vis Sci 2010;51:5897–905. [DOI] [PubMed] [Google Scholar]
- 140.Fernández-Robredo P, Recalde S, Arnaiz G, Salinas-Alaman A, Sadaba LM, Moreno-Orduna M, Garcia-Layana A. Effect of zeaxanthin and antioxidant supplementation on vascular endothelial growth factor (VEGF) expression in apolipoprotein-E deficient mice. Curr Eye Res 2009;34:543–52. [DOI] [PubMed] [Google Scholar]
- 141.Baird PN, Guida E, Chu DT, Vu HT, Guymer RH. The epsilon2 and epsilon4 alleles of the apolipoprotein gene are associated with age-related macular degeneration. Invest Ophthalmol Vis Sci 2004;45:1311–5. [DOI] [PubMed] [Google Scholar]
- 142.Francis PJ, Hamon SC, Ott J, Weleber RG, Klein ML. Polymorphisms in C2, CFB and C3 are associated with progression to advanced age related macular degeneration associated with visual loss. J Med Genet 2009;46:300–7. [DOI] [PubMed] [Google Scholar]
- 143.Tikellis G, Sun C, Gorin MB, Klein R, Klein BE, Larsen EK, Siscovick DS, Hubbard LD, Wong TY. Apolipoprotein E gene and age-related maculopathy in older individuals: the Cardiovascular Health Study. Arch Ophthalmol 2007;125:68–73. [DOI] [PubMed] [Google Scholar]
- 144.Wong TY, Shankar A, Klein R, Bray MS, Couper DJ, Klein BE, Sharrett AR, Folsom AR. Apolipoprotein E gene and early age-related maculopathy: the Atherosclerosis Risk in Communities Study. Ophthalmology 2006;113:255–9. [DOI] [PubMed] [Google Scholar]
- 145.Zareparsi S, Buraczynska M, Branham KE, Shah S, Eng D, Li M, Pawar H, Yashar BM, Moroi SE, Lichter PR, et al. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet 2005;14:1449–55. [DOI] [PubMed] [Google Scholar]
- 146.Tyssandier V, Choubert G, Grolier P, Borel P. Carotenoids, mostly the xanthophylls, exchange between plasma lipoproteins. Int J Vitam Nutr Res 2002;72:300–8. [DOI] [PubMed] [Google Scholar]
- 147.Casaroli-Marano RP, Peinado-Onsurbe J, Reina M, Staels B, Auwerx J, Vilaro S. Lipoprotein lipase in highly vascularized structures of the eye. J Lipid Res 1996;37:1037–44. [PubMed] [Google Scholar]
- 148.Herbeth B, Gueguen S, Leroy P, Siest G, Visvikis-Siest S. The lipoprotein lipase serine 447 stop polymorphism is associated with altered serum carotenoid concentrations in the Stanislas Family Study. J Am Coll Nutr 2007;26:655–62. [DOI] [PubMed] [Google Scholar]
- 149.Beatty S, van Kuijk FJ, Chakravarthy U. Macular pigment and age-related macular degeneration: longitudinal data and better techniques of measurement are needed. Invest Ophthalmol Vis Sci 2008;49:843–5. [DOI] [PubMed] [Google Scholar]
- 150.Llorente-Cortés V, Estruch R, Mena MP, Ros E, Gonzalez MA, Fito M, Lamuela-Raventos RM, Badimon L. Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 2010;208:442–50. [DOI] [PubMed] [Google Scholar]
- 151.Kalariya NM, Ramana KV, Srivastava SK, van Kuijk FJ. Genotoxic effects of carotenoid breakdown products in human retinal pigment epithelial cells. Curr Eye Res 2009;34:737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Anderson DH, Ozaki S, Nealon M, Neitz J, Mullins RF, Hageman GS, Johnson LV. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am J Ophthalmol 2001;131:767–81. [DOI] [PubMed] [Google Scholar]
- 153.Dichek HL, Fojo SS, Beg OU, Skarlatos SI, Brunzell JD, Cutler GB, Jr, Brewer HB., Jr Identification of two separate allelic mutations in the lipoprotein lipase gene of a patient with the familial hyperchylomicronemia syndrome. J Biol Chem 1991;266:473–7. [PubMed] [Google Scholar]
- 154.Beatty S, Murray IJ, Henson DB, Carden D, Koh H, Boulton ME. Macular pigment and risk for age-related macular degeneration in subjects from a northern European population. Invest Ophthalmol Vis Sci 2001;42:439–46. [PubMed] [Google Scholar]
- 155.Nolan J, O'Donovan O, Kavanagh H, Stack J, Harrison M, Muldoon A, Mellerio J, Beatty S. Macular pigment and percentage of body fat. Invest Ophthalmol Vis Sci 2004;45:3940–50. [DOI] [PubMed] [Google Scholar]
- 156.Bernstein PS, Zhao DY, Wintch SW, Ermakov IV, McClane RW, Gellermann W. Resonance Raman measurement of macular carotenoids in normal subjects and in age-related macular degeneration patients. Ophthalmology 2002;109:1780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hammond BR, Jr, Caruso-Avery M. Macular pigment optical density in a Southwestern sample. Invest Ophthalmol Vis Sci 2000;41:1492–7. [PubMed] [Google Scholar]
- 158.Chen SF, Chang Y, Wu JC. The spatial distribution of macular pigment in humans. Curr Eye Res 2001;23:422–34. [DOI] [PubMed] [Google Scholar]
- 159.Chang Y, Lee FL, Chen SJ, Chen SF. Optical measurement of human retinal macular pigment and its spatial distribution with age. Med Phys 2002;29:2621–8. [DOI] [PubMed] [Google Scholar]
- 160.Berendschot TT, Willemse-Assink JJ, Bastiaanse M, de Jong PT, van Norren D. Macular pigment and melanin in age-related maculopathy in a general population. Invest Ophthalmol Vis Sci 2002;43:1928–32. [PubMed] [Google Scholar]
- 161.Iannaccone A, Mura M, Gallaher KT, Johnson EJ, Todd WA, Kenyon E, Harris TL, Harris T, Satterfield S, Johnson KC, et al. Macular pigment optical density in the elderly: findings in a large biracial Midsouth population sample. Invest Ophthalmol Vis Sci 2007;48:1458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Johnson EJ, Hammond BR, Yeum KJ, Qin J, Wang XD, Castaneda C, Snodderly DM, Russell RM. Relation among serum and tissue concentrations of lutein and zeaxanthin and macular pigment density. Am J Clin Nutr 2000;71:1555–62. [DOI] [PubMed] [Google Scholar]
- 163.Hammond BR, Jr, Wooten BR, Snodderly DM. Cigarette smoking and retinal carotenoids: implications for age-related macular degeneration. Vision Res 1996;36:3003–9. [DOI] [PubMed] [Google Scholar]
- 164.Curran-Celentano J, Hammond BR, Jr, Ciulla TA, Cooper DA, Pratt LM, Danis RB. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am J Clin Nutr 2001;74:796–802. [DOI] [PubMed] [Google Scholar]
- 165.Nolan JM, Stack J, O'Connell E, Beatty S. The relationships between macular pigment optical density and its constituent carotenoids in diet and serum. Invest Ophthalmol Vis Sci 2007;48:571–82. [DOI] [PubMed] [Google Scholar]
- 166.Broekmans WM, Berendschot TT, Klopping-Ketelaars IA, de Vries AJ, Goldbohm RA, Tijburg LB, Kardinaal AF, van Poppel G. Macular pigment density in relation to serum and adipose tissue concentrations of lutein and serum concentrations of zeaxanthin. Am J Clin Nutr 2002;76:595–603. [DOI] [PubMed] [Google Scholar]
- 167.Nolan JM, Stack J, Mellerio J, Godhinio M, O'Donovan O, Neelam K, Beatty S. Monthly consistency of macular pigment optical density and serum concentrations of lutein and zeaxanthin. Curr Eye Res 2006;31:199–213. [DOI] [PubMed] [Google Scholar]
- 168.Francoise JL, Askew EW, Lang LC, Bernstein PS. Serum and macular responses to antioxidant supplementation versus a carotenoid-rich dietary intervention in the elderly. Curr Topics Nutraceutical Res 2006;4:69–78. [Google Scholar]
- 169.Kopsell DA, Lefsrud MG, Kopsell DE, Wenzel AJ, Gerweck C, Curran-Celentano J. Spinach cultigen variation for tissue carotenoid concentrations influences human serum carotenoid levels and macular pigment optical density following a 12-week dietary intervention. J Agric Food Chem 2006;54:7998–8005. [DOI] [PubMed] [Google Scholar]
- 170.Vishwanathan R, Goodrow-Kotyla EF, Wooten BR, Wilson TA, Nicolosi RJ. Consumption of 2 and 4 egg yolks/d for 5 wk increases macular pigment concentrations in older adults with low macular pigment taking cholesterol-lowering statins. Am J Clin Nutr 2009;90:1272–9. [DOI] [PubMed] [Google Scholar]
- 171.Berendschot TT, Goldbohm RA, Klopping WA, van de Kraats J, van Norel J, van Norren D. Influence of lutein supplementation on macular pigment, assessed with two objective techniques. Invest Ophthalmol Vis Sci 2000;41:3322–6. [PubMed] [Google Scholar]
- 172.Bone RA, Landrum JT, Cao Y, Howard AN, Alvarez-Calderon F. Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr Metab (Lond) 2007;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Landrum JT, Bone RA, Joa H, Kilburn MD, Moore LL, Sprague KE. A one year study of the macular pigment: the effect of 140 days of a lutein supplement. Exp Eye Res 1997;65:57–62. [DOI] [PubMed] [Google Scholar]
- 174.Rodriguez-Carmona M, Kvansakul J, Harlow JA, Kopcke W, Schalch W, Barbur JL. The effects of supplementation with lutein and/or zeaxanthin on human macular pigment density and colour vision. Ophthalmic Physiol Opt 2006;26:137–47. [DOI] [PubMed] [Google Scholar]
- 175.Schalch W, Cohn W, Barker FM, Kopcke W, Mellerio J, Bird AC, Robson AG, Fitzke FF, van Kuijk FJ. Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin—the LUXEA (LUtein Xanthophyll Eye Accumulation) study. Arch Biochem Biophys 2007;458:128–35. [DOI] [PubMed] [Google Scholar]
- 176.Cardinault N, Gorrand JM, Tyssandier V, Grolier P, Rock E, Borel P. Short-term supplementation with lutein affects biomarkers of lutein status similarly in young and elderly subjects. Exp Gerontol 2003;38:573–82. [DOI] [PubMed] [Google Scholar]
- 177.Johnson EJ, Chung HY, Caldarella SM, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum, lipoproteins, and macular pigmentation. Am J Clin Nutr 2008;87:1521–9. [DOI] [PubMed] [Google Scholar]