Abstract
An increase in noncommunicable disease (NCD) in India has been attributed to an epidemiologic transition whereby, due to urbanization, there is an increase in traditional cardiovascular disease risk factors such as obesity. Accumulated biomarker data on the “Asian Indian phenotype” identify central obesity, which occurs at a lower body mass index (BMI), as a particularly potent risk factor in Asian Indians. A revised WHO case definition for obesity in India [BMI (in kg/m2) >25] has identified an obesity epidemic that exceeds 30% in some cities and rivals that in Western nations. This review summarizes 2 key lines of evidence: 1) the emergence of an obesity epidemic in urban and rural India and its contribution to the NCD burden and 2) the role of a “nutrition transition” in decreasing the whole plant food content of diets in India and increasing risk of obesity and NCDs. We then present new epidemiologic evidence from Asian Indians enrolled in the Adventist Health Study 2 that raises the possibility of how specific whole plant foods (eg, nuts) in a vegetarian dietary pattern could potentially prevent obesity and NCDs in a target population of >1 billion persons.
INTRODUCTION
The substantial increase in the prevalence of diabetes, coronary heart disease (CHD)5, and stroke in India points to the occurrence of an epidemiologic transition whereby, because of urbanization (1, 2), there is a higher prevalence of traditional cardiovascular disease risk factors such as obesity, physical inactivity, tobacco use, and high intake of saturated fat (3, 4). When considering the role of a “nutrition transition” in this trend, it is important to note that the Indian population has a long tradition of faith-based vegetarianism and remains ∼40% vegetarian (5), a rate that ranks India as the nation with the largest population of vegetarians (estimated at 300–400 million) in the world. Moreover, data from the FAO indicate that during the past 25 y, the annual per capita consumption of meats in India has only slightly increased (by ∼1 kg) to 5 kg (5, 6), primarily attributable to poultry consumption. Thus, of the known dietary risk factors for noncommunicable diseases (NCDs), meat intake does not appear to explain the major increase in NCD rates in India.
In this review, our purpose is to summarize accumulating evidence that a key component of the NCD risk attributable to a “nutrition transition” in India may be a decrease in the whole plant food content of the Indian vegetarian dietary pattern due to replacement with foods with known dietary risk factors (ie, processed foods, fried foods, unrefined carbohydrates). These data may explain why NCD rates are on the rise in a nation in which vegetarianism remains common despite urbanization. For the purpose of this review, we will define the practice of a “vegetarian” diet as being the avoidance of meat, poultry, and fish.
Specifically, the review summarizes the following lines of evidence from the recent literature on diet among Asian Indians: 1) the emergence of an obesity epidemic in the Indian subcontinent and its contribution to the NCD burden and 2) the role of a “nutrition transition” in decreasing the “whole plant food” content of dietary patterns of India, a trend that may be causal in increasing risk of obesity and NCDs. We then present new epidemiologic evidence from Asian Indians enrolled in the Adventist Health Study (AHS) 2 (7) that raises the possibility of how specific whole plant foods and preparation methods in a vegetarian dietary pattern could potentially prevent obesity and NCDs in a target population of >1 billion persons.
EPIDEMIC OF OBESITY IN ASIAN INDIANS
Asian Indian phenotype and the rationale for a new case definition for obesity
Studies of NCD biomarkers in Asian Indians have long raised the possibility of an “Asian Indian phenotype” that produces a high-risk metabolic profile consisting of high serum triglycerides, decreased HDL cholesterol and particle size (relative to whites), glucose intolerance, and metabolic syndrome. Other putative markers of NCD risk [lipoprotein(a), C-reactive protein, and homocysteine] are also elevated in Asian Indians (8–10). Other features of an Asian Indian phenotype include the following: 1) a younger age at first coronary event (mean age of 53 y) in Asian Indian migrants, which has been cited as evidence of this phenotype (9), and 2) a greater tendency toward depositing fat at sites promoting insulin resistance (ie, abdominal/intraabdominal, truncal subcutaneous, liver, skeletal muscle) (11, 12), an effect that promotes risk at a lower BMI.
In a study of cardiometabolic risk factors in a multiethnic sample, Razak et al (13) found that fasting glucose concentrations, LDL cholesterol, and blood pressure found in Europeans at a BMI (in kg/m2) of 30 could be found in Asian Indians at a much lower BMI. Taken together, these data on the global epidemiology of Asian Indians have been used to argue for lower cutoffs for overweight and obesity (13–15). Since 2000, the International Obesity Task Force of the WHO (IOTF-WHO) has proposed a modification of National Heart, Lung, and Blood Institute guidelines on overweight/obesity as follows (16): overweight, 23 to <25; class I obesity, 25 to <30; and class II obesity, ≥30. Redefining the obesity cutoff (BMI >25) creates an “obesity burden” in urban India that rivals Western nations and a “double burden” of obesity plus malnutrition in rural India.
Urban India
By using the IOTF-WHO obesity cutoff of >25 for Asian adults, prevalence surveys of urban India have identified an “obesity epidemic” that rivals that in Western nations. For example, in the Chennai Urban Rural Epidemiology study (n = 26,000) in the urban south (Chennai), 31.2% of women and 24.6% of men were found to meet the IOTF-WHO definition of obesity, an obesity burden similar to many states in the United States (17). In the New Delhi Birth Cohort Study, Huffman et al (10) reported findings from a birth cohort of 36-y-old adults, indicating a prevalence of IOTF-WHO obesity prevalence of 54% in men and 66% in women. By using a more sensitive waist circumference cutoff for abdominal obesity (≥80 cm in women, ≥90 cm in men) for Asian adults (18), 70% of the cohort was classified with central obesity (10).
In the Chennai Urban Population Study, 10 y of follow-up of the middle- and lower-income strata of adults during 1996–1998 (19) indicated that during the follow-up 1) the rates of obesity, hypertension, and dyslipidemia in low-income adults increased to the point of being similar to those in high-income adults and 2) 50.9% of men and 49.8% of women in the lower-income group experienced central obesity (19). Some of these temporal trends may be indicative of further urbanization occurring during the follow-up. In North India, Yadav et al (20) found a gradient of increasing BMI from rural to urban-slum to urban samples.
Rural India
The emergence of overweight/obesity and the accompanying NCD burden is quite evident in epidemiologic studies of rural India. In a sample of 105,000 adult subjects (ages 25–64 y) in rural Tamil Nadu, 32.8% of men and 38.2% of women had a BMI of ≥23 (21). Misra et al (22, 23) identified an immediate consequence of this epidemiologic transition as being higher rates of hypertension.
Most troubling is that the simultaneous occurrence of under- and overnutrition in rural households adds NCDs to what becomes a “double burden” of disease outcomes (24) and disability and the ensuing economic hardship. For maternal and child health, the effect can have the devastating consequences of both low- and high-BMI mothers giving birth to children with higher NCD rates through mechanisms of fetal hypoxia and fetal programming attributable to the stresses on the fetus from the extremes of the maternal BMI distribution (25–28).
WHAT IS THE EFFECT OF THE CURRENT PREVALENCE AND PRACTICE OF VEGETARIANISM IN INDIA ON OBESITY AND NCDs?
At the Third International Congress of Vegetarian Nutrition (29), Ganguli described a tradition of vegetarianism in India that dates back at least 3000 y (NK Ganguli, unpublished data, 2007). The tradition is deeply rooted in religion (ie, Hinduism, Vedic teachings) and continued in rural India out of economic necessity. Early vegetarian diets emphasized cereals, brown rice (“parboiled” with the husk), pulses, roots, and tubers and also included fermented milk (ie, curd) as a protein source. Age-old Vedic practices also promoted the use of fresh sprouting seeds such as mung bean and green gram, with minimal cooking of plant foods and the use of ground green bioingredients (“cooking without fire”). For rural cooking, Ganguli also described the tendency to use unsaturated cooking oils and, as a supplement for some foods, “ghee” (clarified butter). Such age-old, faith-based vegetarian traditions from rural India continue to result in a present-day Indian population that is 40% vegetarian (5), a rate that ranks India as the nation with the largest population of vegetarians (estimated to be 300–400 million) in the world.
How do we reconcile the seemingly contradictory epidemiologic trends of a nation that has the largest number of vegetarians in the world and yet ranks second globally in the number of patients with diabetes and has increasing rates of cardiovascular disease, stroke, and cancer? There is emerging evidence that the “nutrition transition” induced by urbanization has not only increased meat intake in India but also, among the sizable proportion of the population who remain vegetarian, has decreased the diet quality and whole plant food content of their diet. Four key features of a “nutrition transition” in vegetarians living in present-day India are described in the following sections.
Substitution of white rice for brown rice
Rice is a central component of the South Indian diet, and the type of rice used in a given household is often an indicator of socioeconomic status (highly polished, dehusked white rice compared with unmilled, parboiled brown rice that is cooked in the husk). Kumar et al (30) recently reported findings from qualitative studies in southern India indicating that brown rice is perceived as the food of poor and those in rural areas. In key informant interviews in Chennai, a subject states, “if we buy this kind of rice [referring to parboiled brown rice], it is a prestige issue. People will think poorly of us, it is a question of status.”
Thus, with economic improvement and urbanization, there is a strong sociocultural influence for families of India to purchase the most refined, “polished,” long-grain white rice accessible to their food budget. In the Chennai Urban Rural Epidemiology study, nutrition surveys identified that approximately half of the daily energy intake came from white rice (31).
This single element of the nutrition transition is postulated to have a major impact on NCDs. The brown rice once consumed by vast numbers of low-income households was hand-pounded and retained bran and germ constituents (32). These whole-food constituents of brown rice can improve insulin sensitivity and reduce the risk of type 2 diabetes (33–36). In contrast, the white rice consumed as half the diet in many urban areas of India is associated with insulin resistance, metabolic syndrome, and type 2 diabetes (31, 37). Some recent reports have attributed the steep increase in the number of patients with diabetes (38) to the simple transition from daily consumption of brown rice to daily consumption of white rice in large portion sizes (31, 39–41). Also, in contrast to ancient India, the variety of grains used in modern Asian Indian diets is greatly reduced, with whole grains such as barley, amaranth, and millet, which are high in protein and fiber, no longer contributing significantly to the diets (42). This transition from whole grains to the more-refined grains results in energy-dense but nutrient-poor diets that increase the risk of NCDs.
When considering the NCD impact, note that the heightened sensitivity to glucose intolerance under an “Asian Indian phenotype” (43) only further potentiates the risk created by a transition from brown to white rice in a large portion of the Indian population. Another potentiating factor of the Asian Indian phenotype is genetic predisposition to elevated homocysteine concentrations, which is a possible risk factor for cardiovascular disease found in some (44) but not all (45) studies.
Overconsumption of other refined carbohydrates
In addition to rice, the Asian Indian diet is high in other sources of carbohydrates, and the trend of the nutrition transition is toward overconsumption of refined carbohydrates. Secular trends and migration studies in Asian Indians indicated substitution of refined carbohydrates for lentils, fruit, vegetables, unrefined whole grains, nuts, and seeds (22, 23, 46, 47). A major source of refined carbohydrates in this “transition diet” is potatoes, which are included in fried foods and more recently in fast foods and snack foods (chips) (48–50). Interestingly, in a case-control study of CHD in urban India, Rastogi et al (51) found whole plant foods to be a protective factor only after exclusion of potatoes. Overall, when considering NCD impact, it is important to consider that total carbohydrate and glycemic load are also associated with increased risk of type 2 diabetes among Asian Indians (37).
Change in the amount and type of cooking oils
Another prominent feature of the nutrition transition attributable to urbanization is an increase in the use of ghee (clarified butter) in cooking (52, 53)—a practice that in low-resource rural areas was usually reserved for special occasions. Also, currently, palm oil is the most common cooking oil, followed by sunflower oil and the more traditional groundnut oil (21, 54). These trends of increased use of hydrogenated vegetable oil–based ghee (Vanaspati), palm oil, and safflower oil have decreased the content of n–3 PUFAs (47) and increased the content of n–6 polyunsaturated, trans, and saturated fats in the Indian diet. In a study in urban and rural North Indians, women and men who consumed trans fatty acids and ghee had a significantly higher prevalence of coronary artery disease than did those who substituted trans fats with vegetable oils (55). Mustard oil has been shown to have a protective effect when compared with sunflower oil for cooking after adjustment for age, sex, and smoking (51). In study of diet and hypertension in West Bengal, Das et al (56) found that diets high in fish and mustard oil were protective relative to vegetarian diets with no animal products. The authors attributed the effect to higher amounts of monounsaturated fats (cis-13-docosenoic acid) in mustard oil and n–3 fatty acids in fish oil (56).
Increased consumption of fast foods/processed foods
A key feature of the nutrition transition among Asian Indians also includes the increased consumption of low-cost fried foods and processed foods sold as packaged snacks or fast-food products similar to those found in Western nations (57–59). The higher content of trans fatty acids in these types of processed and snack foods is of note because of the association of this fatty acid with cardiovascular disease (55). Gulati et al (60) recently described in qualitative work how home-cooked meals are regarded as “old fashioned” and that in mother-child pairs there is a lack of understanding of the hazards of childhood obesity and its causal link to NCDs. Influences of marketing by transnational corporations, social media, globalization, and the economic shift resulting from rapid urbanization are notable in these trends (61).
INSIGHTS FROM STUDYING ASIAN INDIANS IN THE AHS-2
For the past 55 y, the NIH has funded a prospective investigation of the health effects of the vegetarian diet among US Seventh-day Adventists [1960 Adventist Mortality Study (62), 1976 AHS-1 (63), 2002 AHS-2 (7)], a group who provides a unique insight into the preventive effects of plant-based diets. On the basis of faith-based recommendations, ∼50% of Adventists are vegetarian (no weekly meat intake) and virtually all avoid smoking and alcohol (64). Faith-based counsels on diet also encourage 1) the consumption of specific plant foods (eg, nuts, legumes, grains, olives, whole-wheat bread) in place of animal products (64) and 2) the preparation of these foods in a simple manner, “free from grease” (65). The preventive effects of the vegetarian dietary pattern practiced by Adventists have been described in numerous reports that indicate an association with lower risk of CHD, diabetes, colon cancer, and all-cause mortality (64, 66). Protective NCD associations for nuts, legumes, green salads, tomatoes, and soy products have been reported and continue to be investigated in ongoing work (64, 66). The most recent cohort (AHS-2) represents a multiethnic sample from the United States and Canada (7). Two particular lines of evidence from the AHSs that provide insight into the global epidemiology of vegetarianism, obesity, and NCDs in Asian Indians are described.
Nutrition transition away from faith-based vegetarianism
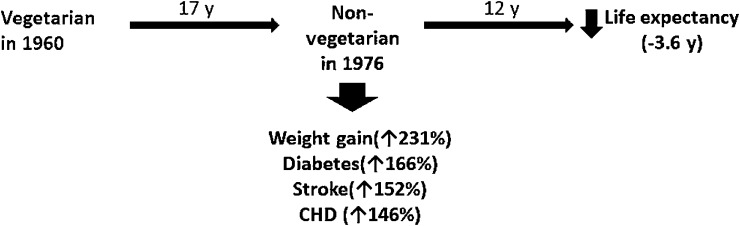
Of particular relevance to assessing the effect of secular trends away from what is typically faith-based vegetarianism in India are the AHS findings shown in Figure 1, which summarizes outcomes among those Adventists who, over a 17-y interval, experienced a “nutrition transition” away from faith-based vegetarianism. During 17–29 y of follow-up of Adventist Mortality Study and AHS-1 cohort members, Singh et al (67, 68) and Vang et al (69) studied how this nutrition transition away from vegetarianism affected weight gain, NCD outcomes, and longevity. Change from a vegetarian (no meat intake) to a nonvegetarian (weekly meat intake) dietary pattern over a 17-y interval was associated with significant increases in the likelihood of weight gain (OR: 3.31; 95% CI: 2.26, 4.86) (67), diabetes (OR: 2.66; 95% CI: 1.79, 3.95) (67), stroke (OR: 2.52; 95% CI: 1.30, 4.90; PN Singh, unpublished data, 2014), and CHD (OR: 2.46; 95% CI: 1.62, 3.73; previously unpublished). During the 12 y after the transition, the exposure was associated with a 3.6 y (95% CI: 1.4, 5.8 y) decrease in life expectancy (68).
FIGURE 1.
Nutrition transition from a vegetarian to nonvegetarian diet over 17 y among cohort members of the Adventist Health Study 1: effects on weight gain, noncommunicable diseases, and longevity (67, 68, 70). CHD, coronary heart disease.
Asian Indian cohort members of the AHS-2
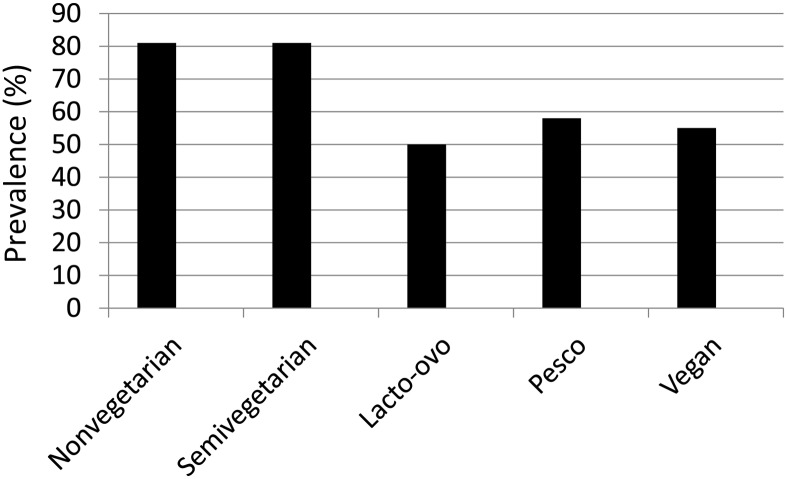
Among US-based Asian Indian cohort members of the AHS-2 (n = 239), we examined the association between vegetarian dietary patterns and overweight/obesity under the IOTF-WHO definition (Figure 2). Similar to reports on the parent cohort, we found that lactoovovegetarian and vegan dietary patterns were associated with a lower prevalence of overweight/obesity (BMI >23) by using the IOTF-WHO definition. The higher consumption of nuts and seeds in vegans and lactoovovegetarians shown among Asian Indian AHS-2 cohort members in Table 1 is noteworthy. Further study of the dietary pattern of Asian Indian vegetarian Adventists is needed to characterize whole plant food intake. Current smoking and alcohol use were very rare in this sample (<5%).
FIGURE 2.
Prevalence of overweight/obesity [BMI (in kg/m2] >23] with the use of the International Obesity Task Force–WHO definition (16) among Asian Indians from the Adventist Health Study 2 according to dietary pattern (nonvegetarian, n = 119; semivegetarian, n = 14; lactoovovegetarian, n = 44; pescovegetarian, n = 35; vegan, n = 9). Lacto-ovo, lactoovovegetarian; Pesco, pescovegetarian.
TABLE 1.
Unadjusted weight of major food categories according to dietary pattern among Asian Indian cohort members of the Adventist Health Study 21
| Dietary pattern |
||||||
| Vegan (n = 9) | Lactoovovegetarian (n = 44) | Pescovegetarian (n = 35) | Semivegetarian (n = 14) | Nonvegetarian (n = 119) | P2 | |
| g | ||||||
| Fruit | 409.9 ± 303.1 | 425.5 ± 269.8 | 558.7 ± 473.3 | 276.0 ± 258.4 | 339.8 ± 241.4 | 0.043 |
| Vegetables | 331.7 ± 253.7 | 390.2 ± 211.1 | 429.7 ± 259.5 | 319.8 ± 206.9 | 358.6 ± 247.5 | 0.655 |
| Avocados | 8.3 ± 11.7 | 3.8 ± 6.4 | 4.1 ± 6.0 | 3.7 ± 9.4 | 3.6 ± 8.0 | 0.825 |
| Potatoes | 144.3 ± 72.2 | 97.7 ± 98.0 | 57.9 ± 68.7 | 44.4 ± 40.3 | 78.7 ± 84.9 | 0.004 |
| Legumes | 120.4 ± 64.9 | 120.2 ± 104.2 | 127.6 ± 100.7 | 92.7 ± 46.3 | 94.5 ± 96.7 | 0.216 |
| Soy foods and meat analogs | 215.6 ± 170.8 | 168.8 ± 176.3 | 181.4 ± 163.3 | 144.2 ± 129.4 | 144.1 ± 188.3 | 0.532 |
| Added fats | 20.4 ± 11.5 | 31.9 ± 20.6 | 31.1 ± 27.8 | 29.2 ± 13.7 | 40.5 ± 32.4 | <0.001 |
| Nuts and seeds | 35.5 ± 35.8 | 33.6 ± 30.4 | 26.1 ± 18.2 | 14.1 ± 10.3 | 21.6 ± 21.4 | 0.015 |
| Meats | 0.0 ± 0.0 | 2.1 ± 9.9 | 10.8 ± 7.3 | 6.2 ± 4.1 | 46.5 ± 46.2 | <0.001 |
| Dairy products | 2.6 ± 3.1 | 151.8 ± 222.9 | 299.8 ± 809.8 | 154.5 ± 199.1 | 252.2 ± 250.1 | <0.001 |
| Eggs | 1.9 ± 2.4 | 8.1 ± 7.0 | 9.5 ± 6.7 | 8.6 ± 5.3 | 10.9 ± 9.3 | <0.001 |
| Grains | 150.5 ± 82.3 | 221.6 ± 110.4 | 200.6 ± 116.4 | 204.2 ± 84.3 | 221.8 ± 126.5 | 0.157 |
| Sweets | 13.2 ± 31.8 | 13.6 ± 19.9 | 19.6 ± 32.1 | 30.6 ± 73.9 | 23.8 ± 36.6 | 0.236 |
| Beverages (not water) | 45.7 ± 40.0 | 155.1 ± 210.0 | 164.1 ± 200.1 | 234.4 ± 333.8 | 244.2 ± 264.5 | <0.001 |
| Water | 1465.1 ± 660.1 | 1485.4 ± 473.3 | 1634.1 ± 641.9 | 1544.2 ± 518.2 | 1381.6 ± 602.5 | 0.192 |
All values are means ± SDs.
ANOVA comparing all other dietary pattern categories with nonvegetarians.
In Figure 2, the extremely high rate (>80%) of overweight/obesity in non- and semivegetarian Asian Indian AHS-2 cohort members is notable relative to the ∼50% rate in vegans and lactoovovegetarians. Prospective studies of the plant foods underlying these trends in Asian Indian Adventists could yield insights into a preventive diet for Asian Indians.
We note that the AHS-2 baseline measures were designed and validated primarily for black and white subjects who account for >90% of the cohort. Further studies in Asian Indian Adventists to identify preventive vegetarian diet practices will require design of dietary exposures to measure the intersection of culture, faith, and diet in this unique subgroup of a long-lived cohort with low NCD rates. Also, further prospective studies of larger samples are needed to control for a range of possible confounder variables.
CONCLUSIONS
Current evidence indicates that obesity and overweight can be a particularly potent risk factor for Asian Indians and, under a revised case definition for obesity of a BMI >25, an epidemic of obesity is underway in urban India and is emerging in rural India. Underlying this epidemic is a complex nutrition transition in vegetarians in which whole plant foods (fruit, vegetables, nuts, seeds, unrefined whole grains) are being replaced by refined carbohydrates, fast foods/snack foods/processed foods, and fried foods. A transition to cooking oils with more atherogenic effects is also evident. We have presented evidence that such a nutrition transition is increasing the rate of NCDs despite the continued high prevalence of vegetarianism. We posit that one strategy to inform the design of dietary interventions to control obesity and NCDs in India is to include a “global epidemiology“ approach of studying Asian Indians who live beyond the nutrition transition in regions and contexts where preventive dietary habits are more common.
Acknowledgments
The authors’ responsibilities were as follows—PNS: conceived the project and analyzed AHS-1 data; PNS and KNA: wrote the manuscript; MJO: analyzed AHS-2 data on Asian Indians and edited the manuscript; JS, JSJ, AP, and WJ: edited the manuscript; and SR: contributed to the writing on secular trends in Asian Indian diets and editing of the manuscript. The authors had no conflicts of interest.
Footnotes
Abbreviations used: AHS, Adventist Health Study; CHD, coronary heart disease; IOTF, International Obesity Task Force; NCD, noncommunicable disease.
REFERENCES
- 1.Wagner KH, Brath H. A global view on the development of non communicable diseases. Prev Med 2012;54(suppl):S38–41. [DOI] [PubMed] [Google Scholar]
- 2.Shetty PS. Nutrition transition in India. Public Health Nutr 2002;5(1A):175–82. [DOI] [PubMed] [Google Scholar]
- 3.Singh RB, Pella D, Mechirova V, Kartikey K, Demeester F, Tomar RS, Beegom R, Mehta AS, Gupta SB, De Amit K, et al. Prevalence of obesity, physical inactivity and undernutrition, a triple burden of diseases during transition in a developing economy. The Five City Study Group. Acta Cardiol 2007;62:119–27. [DOI] [PubMed] [Google Scholar]
- 4.Singh RB, Singh S, Chattopadhya P, Singh K, Singhz V, Kulshrestha SK, Tomar RS, Kumar R, Singh G, Mechirova V, et al. Tobacco consumption in relation to causes of death in an urban population of north India. Int J Chron Obstruct Pulmon Dis 2007;2:177–85. [PMC free article] [PubMed] [Google Scholar]
- 5.FAOSTAT. Food and Agriculture Organization of the United Nations, statistical database. Available from: http://faostat.fao.org/ (cited 19 June 2013).
- 6.FAO. World agriculture: towards 2015/2030. An FAO perspective. Available from: http://www.fao.org/docrep/005/y4252e/y4252e05c.htm (cited 19 June 2013).
- 7.Butler TL, Fraser GE, Beeson WL, Knutsen SF, Herring RP, Chan J, Sabaté J, Montgomery S, Haddad E, Preston-Martin S, et al. Cohort profile: the Adventist Health Study-2 (AHS-2). Int J Epidemiol 2008;37:260–5. [DOI] [PubMed] [Google Scholar]
- 8.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res 2007;125:217–30. [PubMed] [Google Scholar]
- 9.Mohan V, Venkatraman JV, Pradeepa R. Epidemiology of cardiovascular disease in type 2 diabetes: the Indian scenario. J Diabetes Sci Technol 2010;4:158–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huffman MD, Prabhakaran D, Osmond C, Fall CH, Tandon N, Lakshmy R, Ramji S, Khalil A, Gera T, Prabhakaran P, et al. Incidence of cardiovascular risk factors in an Indian urban cohort results from the New Delhi birth cohort. J Am Coll Cardiol 2011;57:1765–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misra A, Shrivastava U. Obesity and dyslipidemia in South Asians. Nutrients. 2013;5:2708–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D, Joshi SR, Sadikot S, Gupta R, Gulati S, et al. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India 2009;57:163–70. [PubMed] [Google Scholar]
- 13.Razak F, Anand SS, Shannon H, Vuksan V, Davis B, Jacobs R, Teo KK, McQueen M, Yusuf S. Defining obesity cut points in a multiethnic population. Circulation 2007;115:2111–8. [DOI] [PubMed] [Google Scholar]
- 14.Wen CP, David Cheng TY, Tsai SP, Chan HT, Hsu HL, Hsu CC, Eriksen MP. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr 2009;12:497–506. [DOI] [PubMed] [Google Scholar]
- 15.Low S, Chin MC, Ma S, Heng D, Deurenberg-Yap M. Rationale for redefining obesity in Asians. Ann Acad Med Singapore 2009;38:66–9. [PubMed] [Google Scholar]
- 16.Anuurad E, Shiwaku K, Nogi A, Kitajima K, Enkhmaa B, Shimono K, Yamane Y. The new BMI criteria for Asians by the regional office for the Western Pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health 2003;45:335–43. [DOI] [PubMed] [Google Scholar]
- 17.Deepa M, Farooq S, Deepa R, Manjula D, Mohan V. Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47). Eur J Clin Nutr 2009;63:259–67. [DOI] [PubMed] [Google Scholar]
- 18.International Diabetes Foundation. The IDF consensus worldwide definition of the metabolic syndrome. Brussels, Belgium: International Diabetes Foundation. Available from: http://www.idf.org/webdata/docs/MetSyndrome_FINAL.pdf (cited 28 April 2013).
- 19.Deepa M, Anjana RM, Manjula D, Narayan KM, Mohan V. Convergence of prevalence rates of diabetes and cardiometabolic risk factors in middle and low income groups in urban India: 10-year follow-up of the Chennai Urban Population Study. J Diabetes Sci Technol 2011;5:918–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav K, Krishnan A. Changing patterns of diet, physical activity and obesity among urban, rural and slum populations in north India. Obesity Rev 2008;9(5):400–8. [DOI] [PubMed] [Google Scholar]
- 21.Kaur P, Rao SR, Radhakrishnan E, Ramachandran R, Venkatachalam R, Gupte MD. High prevalence of tobacco use, alcohol use and overweight in a rural population in Tamil Nadu, India. J Postgrad Med 2011;57:9–15. [DOI] [PubMed] [Google Scholar]
- 22.Misra A, Khurana L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metab Syndr Relat Disord 2009;7:497–514. [DOI] [PubMed] [Google Scholar]
- 23.Misra A, Khurana L, Isharwal S, Bhardwaj S. South Asian diets and insulin resistance. Br J Nutr 2009;101:465–73. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian SV, Kawachi I, Smith GD. Income inequality and the double burden of under- and overnutrition in India. J Epidemiol Community Health 2007;61:802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corsi DJ, Finlay JE, Subramanian SV. Global burden of double malnutrition: has anyone seen it? PLoS ONE 2011;6:e25120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993;341:938–41. [DOI] [PubMed] [Google Scholar]
- 27.Barker DJ, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. BMJ 1993;307:1524–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barker DJ, Osmond C, Simmonds SJ, Wield GA. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ 1993;306:422–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston PK, Sabaté J. Preface. Am J Clin Nutr 1999;70(3 suppl):429S. [PubMed]
- 30.Kumar S, Mohanraj R, Sudha V, Wedick NM, Malik V, Hu FB, Spiegelman D, Mohan V. Perceptions about varieties of brown rice: a qualitative study from southern India. J Am Diet Assoc 2011;111:1517–22. [DOI] [PubMed] [Google Scholar]
- 31.Radhika G, Van Dam RM, Sudha V, Ganesan A, Mohan V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57). Metabolism 2009;58:675–81. [DOI] [PubMed] [Google Scholar]
- 32.Achaya K. New Delhi, India: India Oxford University Press, 2009. [Google Scholar]
- 33.Fung TT, Hu FB, Pereira MA, Liu S, Stampfer MJ, Colditz GA, Willett WC. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr 2002;76:535–40. [DOI] [PubMed] [Google Scholar]
- 34.Pereira MA, Jacobs DR, Jr, Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr 2002;75:848–55. [DOI] [PubMed] [Google Scholar]
- 35.Montonen J, Knekt P, Jarvinen R, Aromaa A, Reunanen A. Whole-grain and fiber intake and the incidence of type 2 diabetes. Am J Clin Nutr 2003;77:622–9. [DOI] [PubMed] [Google Scholar]
- 36.Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, Hu FB. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 2010;170:961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohan V, Radhika G, Sathya RM, Tamil SR, Ganesan A, Sudha V. Dietary carbohydrates, glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59). Br J Nutr 2009;102:1498–506. [DOI] [PubMed] [Google Scholar]
- 38.Singh AK, Mani K, Krishnan A, Aggarwal P, Gupta SK. Prevalence, awareness, treatment and control of diabetes among elderly persons in an urban slum of Delhi. Indian J Comm Med 2012;37(4):236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isharwal S, Misra A, Wasir JS, Nigam P. Diet & insulin resistance: a review & Asian Indian perspective. Indian J Med Res 2009;129:485–99. [PubMed] [Google Scholar]
- 40.Pande A, Krishnamoorthy G, Moulick ND. Hypoglycaemic and hypolipidaemic effects of low GI and medium GL Indian diets in type 2 diabetics for a period of 4 weeks: a prospective study. Int J Food Sci Nutr 2012;63:649–58. [DOI] [PubMed] [Google Scholar]
- 41.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011;34:1249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dixit AA, Azar KM, Gardner CD, Palaniappan LP. Incorporation of whole, ancient grains into a modern Asian Indian diet to reduce the burden of chronic disease. Nutr Rev 2011;69:479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi L, Cornelis MC, Zhang C, van Dam RM, Hu FB. Genetic predisposition, Western dietary pattern, and the risk of type 2 diabetes in men. Am J Clin Nutr 2009;89:1453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weisberg I, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 1998;64:169–72. [DOI] [PubMed] [Google Scholar]
- 45.Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, Bonaa KH, Spence JD, Nygard O, Jamison R. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med 2010;170:1622–31. [DOI] [PubMed] [Google Scholar]
- 46.Holmboe-Ottesen G, Wandel M. Changes in dietary habits after migration and consequences for health: a focus on South Asians in Europe. Food Nutr Res 2012;Nov 6 (Epub ahead of print; DOI:10.3402/fnr.v56i0.18891). [DOI] [PMC free article] [PubMed]
- 47.Misra A, Singhal N, Khurana L. Obesity, the metabolic syndrome, and type 2 diabetes in developing countries: role of dietary fats and oils. J Am Coll Nutr 2010;29(suppl):289S–301S. [DOI] [PubMed] [Google Scholar]
- 48.Gujral UP, Pradeepa R, Weber MB, Narayan KM, Mohan V. Type 2 diabetes in South Asians: similarities and differences with white Caucasian and other populations. Ann N Y Acad Sci 2013;1281:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wandel M, Raberg M, Kumar B, Holmboe-Ottesen G. Changes in food habits after migration among South Asians settled in Oslo: the effect of demographic, socio-economic and integration factors. Appetite 2008;50:376–85. [DOI] [PubMed] [Google Scholar]
- 50.Garduño-Diaz SD, Khokhar S. Prevalence, risk factors and complications associated with type 2 diabetes in migrant South Asians. Diabetes Metab Res Rev 2012;28:6–24. [DOI] [PubMed] [Google Scholar]
- 51.Rastogi T, Reddy KS, Vaz M, Spiegelman D, Prabhakaran D, Willett WC, Stampfer M, Ascherio A. Diet and risk of ischemic heart disease in India. Am J Clin Nutr 2004;79:582–92. [DOI] [PubMed] [Google Scholar]
- 52.Lad V. The complete book of Ayurvedic home remedies. New York, NY: Three Rivers Press, 1998.
- 53.Sharma H, Zhang X, Dwivedi C. The effect of ghee (clarified butter) on serum lipid levels and microsomal lipid peroxidation. Ayu 2010;31(2):134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lakshmipriya N, Rajagopal G, Gayathri K, Praseena P, Vijayalakshmi P, Geetha G, Sudha V, Krishnaswamy K, Anjana RM, Henry J, et al. Type of vegetable oils used in cooking and risk of metabolic syndrome among Asian Indians. Int J Food Sci Nutr 2013;64:131–9. [DOI] [PubMed] [Google Scholar]
- 55.Singh RB, Niaz MA, Ghosh S, Beegom R, Rastogi V, Sharma JP, Dube GK. Association of trans fatty acids (vegetable ghee) and clarified butter (Indian ghee) intake with higher risk of coronary artery disease in rural and urban populations with low fat consumption. Int J Cardiol 1996;56:289–98; discussion 99–300. [DOI] [PubMed] [Google Scholar]
- 56.Das SK, Sanyal K, Basu A. Study of urban community survey in India: growing trend of high prevalence of hypertension in a developing country. Int J Med Sci 2005;2:70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta N, Shah P, Goel K, Misra A, Rastogi K, Vikram NK, Kumari V, Pandey RM, Kondal D, Wasir JS. Imbalanced dietary profile, anthropometry, and lipids in urban Asian Indian adolescents and young adults. J Am Coll Nutr 2010;29:81–91. [DOI] [PubMed] [Google Scholar]
- 58.Enas EA, Singh V, Munjal YP, Bhandari S, Yadave RD, Manchanda SC. Reducing the burden of coronary artery disease in India: challenges and opportunities. Indian Heart J 2008;60:161–75. [PubMed] [Google Scholar]
- 59.Misra A, Singhal N, Sivakumar B, Bhagat N, Jaiswal A, Khurana L. Nutrition transition in India: secular trends in dietary intake and their relationship to diet-related non-communicable diseases. J Diabetes 2011;3(4):278–92. [DOI] [PubMed] [Google Scholar]
- 60.Gulati S, Misra A, Colles SL, Kondal D, Gupta N, Goel K, Bansal S, Mishra M, Madkaikar V. Dietary intakes and familial correlates of overweight/obesity: a four-cities study in India. Ann Nutr Metab 2013;62:279–90. [DOI] [PubMed] [Google Scholar]
- 61.Kapil U, Sachdev HP. Urgent need to orient public health response to rapid nutrition transition. Indian J Comm Med 2012;37(4):207–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lemon FR, Walden RT. Death from respiratory system disease among Seventh-Day Adventist men. JAMA 1966;198:117–26. [PubMed] [Google Scholar]
- 63.Beeson WL, Mills PK, Phillips RL, Andress M, Fraser GE. Chronic disease among Seventh-day Adventists, a low-risk group: rationale, methodology, and description of the population. Cancer 1989;64:570–81. [DOI] [PubMed] [Google Scholar]
- 64.Fraser GE. Diet, life expectancy, and chronic disease: studies of Seventh-Day Adventists and other vegetarians. New York, NY: Oxford University Press, 2003. [Google Scholar]
- 65.White EG. Washington, DC: Review and Herald Publishing Association, 1938. [Google Scholar]
- 66.Fraser GE. Vegetarian diets: what do we know of their effects on common chronic diseases? Am J Clin Nutr 2009;89(suppl):1607S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh PN, Nguyen D, Sabaté J. Trends in meat intake over 17 years in relation to diabetes, weight gain, and weight loss. Am J Epidemiol 2013;177(suppl):S141. [Google Scholar]
- 68.Singh PN, Sabaté J, Fraser GE. Does low meat consumption increase life expectancy in humans? Am J Clin Nutr 2003;78(suppl):526S–32S. [DOI] [PubMed] [Google Scholar]
- 69.Vang A, Singh PN, Lee JW, Haddad EH, Brinegar CH. Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: findings from Adventist Health Studies. Ann Nutr Metab 2008;52:96–104. [DOI] [PubMed] [Google Scholar]
- 70.Singh PN. Does low meat consumption contribute to greater longevity? In:Sabaté J, ed. Vegetarian nutrition. Modern nutrition. Boca Raton, FL: CRC Press, 2001:135–70. [Google Scholar]