Abstract
Prostate cancer (PCa) is the most commonly diagnosed cancer in men in the United States and the second leading cause of cancer death. Death is not caused by the primary tumor but rather by the formation of distinct metastatic tumors. Therefore, prevention of metastasis is of utmost importance. The natural product genistein, found in high amounts in soy products, has been implicated in preventing PCa formation and metastasis in men who consume high amounts of soy. In vitro studies and in vivo rodent models that used human PCa cells, as well as prospective human clinical trials, provide a mechanistic explanation directly supporting genistein as an antimetastatic agent. Specifically, our group showed that genistein inhibits cell detachment, protease production, cell invasion, and human PCa metastasis at concentrations achieved in humans with dietary intake. Finally, phase I and phase II clinical trials conducted by us and others showed that concentrations of genistein associated with antimetastatic efficacy in preclinical models are achievable in humans, and treatment with genistein inhibits pathways that regulate metastatic transformation in human prostate tissue.
INTRODUCTION
Prostate cancer (PCa)5 is the most commonly diagnosed cancer among men in the United States, with a 1 in 6 chance of developing the disease throughout their lifetime (1). It is the second leading cause of cancer-related death in men and the fourth leading cause of cancer death overall. The natural product 4',5,7-trihydroxyisoflavone, or genistein, is a small molecule found in soy and soy products (2), and the consumption of soy products leads to increased blood concentrations of genistein (3). Several studies associated dietary soy consumption with a lower incidence of mortality from cancer, and from PCa in particular, compared with studies in individuals who consume low-soy, meat-based diets (4–8). Although such studies have inherent limitations, these have spurred investigations into the potential anticancer effects of soy products and genistein in particular. Because PCa death results from formation of metastasis, we evaluated the role of genistein in regulating metastatic pathways in PCa. In this article, we highlight the role of genistein in regulating several steps in the metastatic progression of human PCa. These include effects on cellular detachment, protease production, cellular invasion, and distinct metastasis formation. We also highlight relevant studies of genistein in humans in phase I and II clinical trials. Together, these findings indicate that genistein has the ability to inhibit human PCa metastasis, and that this activity can be achieved by amounts of genistein consumed in the diet.
EVALUATION OF GENISTEIN IN PRECLINICAL MODELS
Because death from PCa is attributable to the formation of metastasis, regulation of the metastatic cascade, ie, the series of steps cancer cells must complete to escape the primary organ of interest and implant and divide at a distant site in the body, is of utmost importance. Once a primary cancer has formed, it then may acquire the ability to metastasize. The earliest steps of the metastatic cascade involve changes in cellular adhesion and migration and then localized invasion into the surrounding tissue. This is followed by extravasation into the bloodstream, invasion into and implantation at distant site, and finally growth at that secondary site. In this section, we focus on how genistein regulates cellular adhesion, protease production leading to degradation of the extracellular matrix (ECM), cellular invasion, and formation of distinct metastases in animal models.
GENISTEIN ALTERS CELLULAR DETACHMENT AND CELL FLATTENING
Changes in cellular adhesion are a critical step in the metastatic cascade. For a cell to move to another location, it must detach from the ECM, to which the primary tumor is attached. Our group showed that genistein inhibits human PCa cell detachment in a dose-dependent fashion both in vitro (9, 10) and in vivo (11). Genistein inhibits cell detachment in vitro at concentrations as low as 1 μmol/L in PC3-M, PC3, and DU-145 human PCa cell lines after 3 d (9), and as low as 10 nmol/L in PC3-M cells after 7 d (11). This effect corresponds with increased translocation of focal adhesion kinase (FAK) to the sites of focal adhesion complexes and its formation of a complex with β1-integrin. Formation of these focal adhesion complexes physically anchors cells to the ECM through β1-integrin. The ability of genistein to increase cell adhesion directly counteracts cell detachment and thereby inhibits an initial step in the metastatic cascade. In addition to these in vitro findings, our group also showed that genistein affects cellular adhesion in vivo (11). In this experiment, human PC3-M PCa cells were implanted into the prostate gland of athymic mice and allowed to form primary tumor and distinct organ metastases. Quantitative image analysis of nuclear morphometry was then used to measure nuclei size of human PCa cells residing within the prostate and lymph nodes of mice. When cells attach, they spread out and flatten, thereby also stretching out their nuclei. Although it is not possible to reliably measure the borders of cells residing in tissue, boundaries of nuclei can be measured and quantified by quantitative nuclear morphometric analysis. Thus, the use of quantitative image analysis to measure changes in nuclear shape provides a measure of changes in cell adhesion. In animals that ingested 250 mg genistein/kg feed pellets, resulting in plasma concentrations of 1.3 μmol/L, increased nuclei flattening, thus increased cellular adhesion, was observed. These concentrations are similar to mean plasma concentrations of total genistein of 0.28 μmol/L measured in soy-consuming Japanese men (3) and 1.42 μmol/L measured in men prospectively dosed with 150 mg genistein/d (12). Together, these findings indicate that genistein inhibits cell detachment in vivo at concentrations relevant to those attained with dietary consumption of genistein. Mechanistically relevant effects on molecular mediators of attachment accompany this inhibition. This directly supports the notion that genistein-mediated inhibition of cell detachment would prevent PCa cells from leaving the primary tumor and forming metastasis. If translatable to human patients, this would provide a mechanistic rationale for associations between dietary genistein consumption and decreased PCa mortality.
GENISTEIN DECREASES PRODUCTION OF PROTEASES
In addition to detaching from the ECM, cancer cells also must degrade the surrounding tissue to move outside the primary organ. This is accomplished primarily via increased production of matrix metalloproteinases (MMPs), a family of zinc endopeptidases whose normal physiologic function is tissue remodeling and embryonic development (13). Therapeutic inhibition of MMPs directly has not been successful because of a lack of specificity across the many subtypes of MMPs, which led to inhibition of normal physiologic processes and dose-limiting toxicity such as severe joint pain (14). Our group showed that genistein can directly decrease expression and activity of MMP-2 in a panel of human PCa cells at concentrations as low as 10 nmol/L (15). The panel included immortalized normal prostate 1532NPTX and 1542NPTX, immortalized primary PCa 1532CPTX and 1542CPTX, established cancer DU-145 and PC3, and established metastatic variant PC3-M cell lines, thus spanning the spectrum of carcinogenesis from early to metastatic disease. MMP-2 is critical for the degradation of collagen IV, a major component of the prostate gland basement membrane. Increased expression of MMP-2 in prostate tissue is associated with the future development of metastatic disease (13). Specific inhibition of MMP-2 mRNA expression by genistein was confirmed by other groups (16), as was reduction in overall MMP enzyme activity via zymography (17). Conflicting results were seen with the second member of the gelatinase family, MMP-9. We showed that genistein decreases MMP-9 expression in some PCa cell lines, specifically the PC3 and 1532NPTX lines, but not in others; and other groups reported that genistein can specifically decrease MMP-9 (15, 18). Therefore, genistein specifically decreases a subset of proteases that degrade a major component of the prostate ECM, are associated with the development of metastasis, and that have been difficult to therapeutically target without toxic side effects.
GENISTEIN INHIBITS PCa CELLULAR INVASION
The most robust measure of a cell's ability to drive metastasis in vitro is its ability to invade, as measured in an in vitro invasion assay. In this assay, a cell must both degrade a protein layer and move from one location to another. We first reported in 2005 that genistein can directly inhibit cellular invasion in a Boyden chamber assay in the panel of cell lines listed previously (15). Inhibition of invasion was observed at concentrations as low as 10 nmol/L. This concentration is highly relevant because, through regular dietary consumption of soy, this concentration of free genistein (ie, the form that has not been metabolically inactivated) can readily be achieved in individuals.
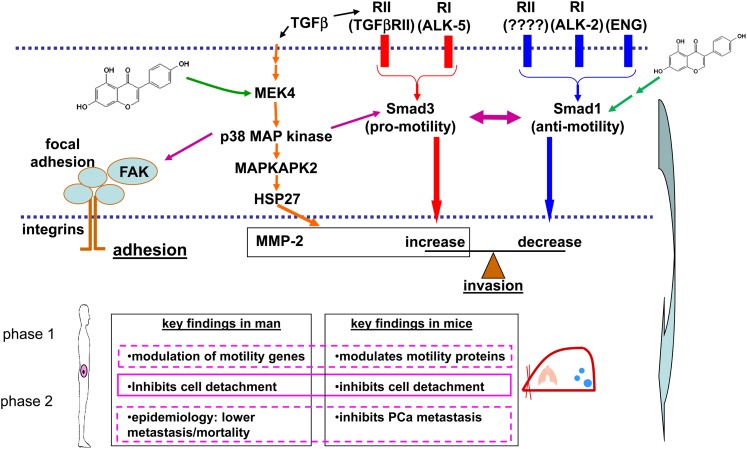
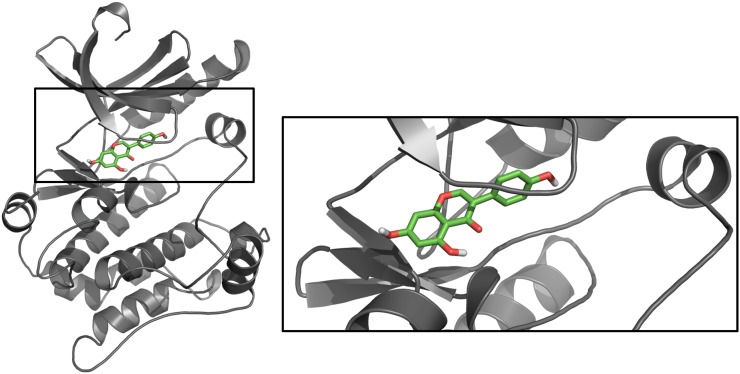
Through a series of studies, we used genistein as an investigative tool to uncover key pathways that regulate human PCa cell invasion and metastasis, as presented in Figure 1 (12, 15, 19, 20). Specifically, genistein directly binds to mitogen-activated protein kinase kinase 4 (MEK4; also abbreviated MKK4 or MAP2K4), inhibiting its kinase function. As a kinase, MEK4 phosphorylates downstream proteins—p38 mitogen-activated protein kinase (p38 MAPK) in this instance—and thereby activates or turns on those proteins. In this manner, genistein-mediated inhibition of MEK4 inhibits downstream signaling and activation of this pathway, thereby decreasing phosphorylation of mitogen-activated protein kinase–activated protein kinase 2 (MAPKAPK2) and of heat shock protein 27 (HSP27) and decreasing MMP-2 and cellular invasion at concentrations as low as 10 μmol/L (12). A model of genistein bound to the active site of MEK4 is shown in Figure 2. Many of the proteins in the pathway activated by MEK4 are upregulated in human PCa progression and are therapeutic targets in their own right.
FIGURE 1.
Genistein modulates a critical TGF-β signaling pathway in human prostate cancer. A schematic of this signaling network and relevant phenotypes associated with genistein treatment in both mice and men is shown. ALK, activin receptor–like kinase; ENG, endoglin; FAK, focal adhesion kinase; HSP27, heat shock protein 27; MAPKAPK2, mitogen-activated protein kinase–activated protein kinase 2; MEK4, mitogen-activated protein kinase kinase 4; MMP-2, matrix metalloproteinase 2; PCa, prostate cancer; p38 MAP, p38 mitogen-activated protein; RI, TGFb receptor type I ; RII, TGFb receptor type II; TGFβ, transforming growth factor β.
FIGURE 2.
Genistein binds MEK4’s ATP pocket. A homology model of MEK4 [gray; based on a structure of MEK1 as the template (Protein Data Bank entry code: 1s9j)] was constructed by using the Multiple Mapping Method with Multiple Templates (M4T) server interface. To this model, genistein (green) was docked by using Molegro Virtual Docker, and the highest scoring pose was selected. The image was generated by using PyMol (Schroendinger). MEK, mitogen-activated protein kinase kinase.
In conjunction with this cellular pathway, the endoglin signaling pathway can cross-talk and thereby regulate cellular invasion in response to genistein activity (21–24). Endoglin is a tumor suppressor that serves as an accessory transforming growth factor β (TGF-β) superfamily receptor. In PCa, this molecule is lost during progression (22). The degree to which cells invade is regulated by the ratio of the downstream targets of endoglin, Smad1 and Smad3. Smad1 is an antimotility protein and is activated by endoglin. Conversely, Smad3 is a promotility protein and can be activated by TGF-β–mediated activation of the TGF-β superfamily receptors, or through p38 MAPK via signaling pathway cross-talk. Because p38 MAPK is a direct target of MEK4, which is directly inhibited by genistein, genistein therefore has the ability to decrease activation of Smad3 in a p38-dependent manner (21). Independently of this interaction, Smad1 can also be regulated by genistein by a mechanism that is not fully understood. On treatment with genistein for 24 h at 25 or 50 μmol/L, the activation of Smad1 increases in a manner dependent on activin receptor–like kinase 2 (ALK-2), another TGF-β–adrenergic receptor family member that works in conjunction with endoglin (24). Recently, we showed that endoglin-mediated regulation of Smad1 and cell invasion is dependent on 2 additional type II TGF-β superfamily receptors, activin A receptor type IIA (ActRIIA) and bone morphogenetic protein receptor type II (BMPRII) (25). Therefore, genistein acts to decrease the function of promotility pathways via MEK4 inhibition, including inhibition of MMP-2 production and Smad3 inactivation, whereas it also increases the function of antimotility pathways, including Smad1 activation.
Other groups also showed changes in cellular invasion in PCa in response to genistein treatment. By using androgen-responsive early-stage PCa cell lines IA8-ARCaP and LNCaP, treatment with genistein at concentrations ≥0.2 μmol/L significantly decreased cellular invasion (26, 27). Similarly, others showed that genistein will decrease the invasion of advanced PCa cell lines PC3, DU-145, and TRAMP-C2 (18, 26, 28, 29). A variety of mechanisms have been proposed, including changes related to prevention of the epithelial to mesenchymal transition, as well as regulation of microRNAs. Therefore, genistein is likely regulating a variety of pathways, all contributing to decreased cellular invasion; and as more research is conducted, these may be more intrinsically linked than is initially apparent.
GENISTEIN DECREASES METASTATIC FORMATION IN VIVO
We also showed that genistein regulates the formation of metastasis in a murine model of PCa (11). In this model, the PC3-M human PCa cell line is orthotopically implanted into the prostate; after tumor progression, the presence of lung metastasis is quantified by microscopic examination of serial tissue sections. In addition to drug discovery efforts, this model has also been used to evaluate the ability of molecular changes, such as changes in endoglin expression, in the primary tumor to alter the formation of metastasis (30). Genistein was tested in this model by incorporating genistein at increasing doses of 0, 100, or 250 mg genistein/kg feed pellets into soy-free feed. The mean plasma concentrations of total genistein in the animals posttreatment were 0, 290, and 1307 nmol total genistein/L, respectively. These values approximate the concentrations of total genistein that are measured in Japanese men who consume a high-soy diet (276 nmol/L) or in US men prospectively dosed with 150 mg genistein daily (1420 nmol/L) (3, 12). Because of the small volume of blood available, it was not possible to measure the free form of genistein in these studies. However, from other dedicated pharmacology studies of genistein in mice, the concentrations of free genistein would be one-tenth of the total, giving estimated concentrations of free genistein of 0, 29, and 131 nmol/L for the 0-, 100-, and 250-mg-dose cohorts, respectively.
Treatment with 100 and 250 mg genistein/kg feed pellets decreased lung metastasis to 44% and 4% of control levels, respectively, although it had no effect on tumor size. These findings show that genistein specifically inhibits human PCa metastasis formation in a dose-responsive manner at concentrations relevant to dietary consumption. This finding was corroborated by other groups by using 2 different transgenic rodent models of spontaneous PCa (28, 31–33). In the TRAMP-FVB murine model, treatment with 250 mg genistein/kg feed pellets significantly decreased pelvic lymph node metastasis (18). By using a Simian virus 40 transgenic rat model of treatment with 250 mg genistein/kg feed pellets, cell proliferation decreased and apoptosis increased, resulting in an overall increase in survival of rats treated with genistein (31).
CLINICAL TRIALS OF GENISTEIN IN PCa
Our group has performed both phase I and phase II trials of genistein in men with PCa (12, 34). In the phase I trial, the pharmacokinetics and toxicity of genistein were evaluated; in the phase II trial, the efficacy of genistein in regulating MMP-2 transcript levels was measured. In the phase I study of genistein, we administered doses of 2, 4 or 8 mg genistein/kg body weight orally to men with PCa, which was well tolerated in all groups observed (34). These treatment conditions correspond to ∼4–16 times the average daily consumption of high soy consumers. The peak concentrations of total genistein were 4.3–16.3 μmol/L, with ˃98% of the genistein in the conjugated form. Importantly, the clearance of genistein was not affected by body mass, indicating that dosing does not need to be altered for body weight. These concentrations correspond with treatment concentrations in the in vitro studies previously mentioned, as well as with the concentrations observed in animals in the studies evaluating metastatic changes attributable to genistein. This indicates that concentrations of genistein required to induce reversion of the metastatic phenotype are attainable in humans. Similar concentrations were observed in other phase I studies, which also showed minimal side effects (35, 36). In one study, 300–600 mg genistein/d was administered to men with PCa for 84 d, and therapy was generally well tolerated with minimal side effects (35). Another study administered a single-dose soy isoflavone treatment composed of ∼90% genistein, 10% daidzein, and 1% glycitein, resulting in genistein doses of 1–16 mg genistein/kg body weight (34). The goal of this study was to determine the metabolism and clearance of genistein in healthy men aged 40–69 y old. Even at high concentrations of genistein, there were minimal side effects, and the genistein was rapidly metabolized and cleared by the body.
In a randomized controlled phase II study conducted by our group, the efficacy of genistein was evaluated (12). Patients undergoing radical prostatectomy for localized PCa were randomly assigned to receive 150 mg genistein or no treatment. This dose corresponded to 2 mg genistein/kg body weight, the lowest dose evaluated in the phase I study. Genistein was given for a mean of 4 wk before surgery (minimum of 2 wk) and resulted in total plasma concentrations of 1.42 μmol genistein/L in the genistein treatment group, compared with undetectable concentrations in the control group. The efficacy of genistein was measured by removing normal prostate epithelial cells from fresh-frozen prostate tissue samples by laser-capture microdissection and then measuring changes in the mRNA levels of MMP-2 by quantitative real-time reverse transcription polymerase chain reaction. Targeted tissue for capture was confirmed histologically by a genitourinary pathologist. Because histologically normal cells present within a prostate gland containing PCa are at high risk for progression to PCa, we therefore wanted to determine the therapeutic effect of genistein on these high-risk cells. In these cells, genistein significantly decreased the expression of MMP-2 by 76%. As described above, MMP-2 has been linked to cell invasion and poor patient prognosis and has been difficult to therapeutically target. Therefore, we show that it is possible to modulate prometastatic pathways in humans with high specificity, a long-sought goal of cancer therapeutics. We are further evaluating markers of metastasis from clinical samples from this trial, including those related to cellular adhesion and detachment.
Phase II trials were also performed by other groups in men with PCa. Separate studies measuring the effect of genistein in different soy preparations showed that, with genistein treatment, serum concentrations of prostate-specific antigen (PSA), which is commonly used as a marker for PCa progression, decreased (37, 38). In the first nonrandomized trial, men were administered soy milk containing 47 mg isoflavanoid per 8 ounces 3 times/d for 12 mo after localized PCa therapy (37). The rate of increase in PSA was reduced as compared with before genistein treatment in these patients. The second study was a block-randomized double-blind study wherein patients were either administered 30 mg genistein daily or a placebo 3–6 wk before prostatectomy. This amount resulted in a plasma concentration of genistein that was 100-fold higher in the treatment group than in the control group. Although both groups showed a decrease in PSA concentrations, the genistein-treatment group had a significantly greater reduction. Therefore, in several independent studies, genistein treatment significantly decreases different markers of PCa progression in humans.
CONCLUSIONS
Epidemiologic studies raised the possibility that genistein may have antimetastatic activity against PCa in humans, prompting prospective investigations of the use of genistein as an antimetastatic agent. From these studies, there is substantial evidence that genistein can modulate the metastatic capabilities of human PCa. Our laboratory's findings in cell lines, animal models, and human clinical trials are summarized in Table 1. At concentrations of genistein similar to those consumed in a soy-based diet, this small molecule can decrease prometastatic phenotypes in vitro, including those related to cellular adhesion, production of MMP-2, and cellular invasion. In an orthotopic model of human PCa, genistein treatment reduced metastatic burden, without altering primary tumor size, and decreased cell detachment. Finally, prospective human studies showed that genistein is well tolerated in patients with PCa and that therapeutic modulation of relevant molecular targets of metastasis, specifically MMP-2, is possible. These results highlight the potential of genistein as an antimetastatic agent for patients with PCa, and thereby its potential to decrease PCa mortality.
TABLE 1.
Antimetastatic action of genistein across preclinical and clinical model systems1
| Cell detachment | Cell invasion/metastasis | MMP expression | Efficacy at low nmol/L concentrations | Inhibits prometastatic pathways | Toxicity | Ref | |
| In vitro cell lines | Inhibits | Inhibits | Decreases MMP-2 consistently, MMP-9 in some cell lines | Yes | Yes | No | (9, 10, 12, 19–25) |
| Preclinical animal models | Inhibits | Inhibits | Future work | Yes | Yes | No | (11) |
| Phase I human trials | N/A | N/A | N/A | N/A | N/A | No | (12, 34) |
| Phase II human trials | In progress | Future work | Decreases MMP-2 | Yes | Yes | No | (12) |
MMP, matrix metalloproteinase; N/A, not available; Ref, reference.
Acknowledgments
The authors’ responsibilities were as follows—JMP and RCB: wrote and edited the manuscript and prepared the figures for the manuscript; and SNK: prepared figures, provided research, and edited the manuscript. The authors stated that they had no conflicts of interest to disclose.
Footnotes
Abbreviations used: ECM, extracellular matrix; MEK4, mitogen-activated protein kinase kinase 4; MMP, matrix metalloproteinase; PCa, prostate cancer; PSA, prostate-specific antigen; p38 MAPK, p38 mitogen-activated protein kinase; TGF-β, transforming growth factor β.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer 2006;55:1–12. [DOI] [PubMed] [Google Scholar]
- 3.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 1993;342:1209–10. [DOI] [PubMed] [Google Scholar]
- 4.Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest Suppl 1990;201:3–23. [PubMed] [Google Scholar]
- 5.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res 1989;49:1857–60. [PubMed] [Google Scholar]
- 6.Shimizu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 1991;63:963–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM. Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer 2009;61:598–606. [DOI] [PubMed] [Google Scholar]
- 8.Yan L, Spitznagel EL. Soy consumption and prostate cancer risk in men: a revisit of a meta-analysis. Am J Clin Nutr 2009;89:1155–63. [DOI] [PubMed] [Google Scholar]
- 9.Bergan R, Kyle E, Nguyen P, Trepel J, Ingui C, Neckers L. Genistein-stimulated adherence of prostate cancer cells is associated with the binding of focal adhesion kinase to beta-1-integrin. Clin Exp Metastasis 1996;14:389–98. [DOI] [PubMed] [Google Scholar]
- 10.Kyle E, Neckers L, Takimoto C, Curt G, Bergan R. Genistein-induced apoptosis of prostate cancer cells is preceded by a specific decrease in focal adhesion kinase activity. Mol Pharmacol 1997;51:193–200. [DOI] [PubMed] [Google Scholar]
- 11.Lakshman M, Xu L, Ananthanarayanan V, Cooper J, Takimoto CH, Helenowski I, Pelling JC, Bergan RC. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res 2008;68:2024–32. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Ding Y, Catalona WJ, Yang XJ, Anderson WF, Jovanovic B, Wellman K, Killmer J, Huang X, Scheidt KA, et al. MEK4 function, genistein treatment, and invasion of human prostate cancer cells. J Natl Cancer Inst 2009;101:1141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vihinen P, Ala-aho R, Kahari VM. Matrix metalloproteinases as therapeutic targets in cancer. Curr Cancer Drug Targets 2005;5:203–20. [DOI] [PubMed] [Google Scholar]
- 14.Wojtowicz-Praga SM, Dickson RB, Hawkins MJ. Matrix metalloproteinase inhibitors. Invest New Drugs 1997;15:61–75. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Chen S, Xu L, Liu Y, Deb DK, Platanias LC, Bergan RC. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res 2005;65:3470–8. [DOI] [PubMed] [Google Scholar]
- 16.Kumi-Diaka JK, Hassanhi M, Merchant K, Horman V. Influence of genistein isoflavone on matrix metalloproteinase-2 expression in prostate cancer cells. J Med Food 2006;9:491–7. [DOI] [PubMed] [Google Scholar]
- 17.Skogseth H, Follestad T, Larsson E, Halgunset J. Transcription levels of invasion-related genes in prostate cancer cells are modified by inhibitors of tyrosine kinase. Acta Pathol Microbiol Immunol Scand A 2006;114(5):364–71. [DOI] [PubMed] [Google Scholar]
- 18.El Touny LH, Banerjee PP. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res 2009;69:3695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu L, Bergan RC. Genistein inhibits matrix metalloproteinase type 2 activation and prostate cancer cell invasion by blocking the transforming growth factor beta-mediated activation of mitogen-activated protein kinase-activated protein kinase 2-27-kDa heat shock protein pathway. Mol Pharmacol 2006;70:869–77. [DOI] [PubMed] [Google Scholar]
- 20.Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene 2006;25:2987–98. [DOI] [PubMed] [Google Scholar]
- 21.Hayes SA, Huang X, Kambhampati S, Platanias LC, Bergan RC. p38 MAP kinase modulates Smad-dependent changes in human prostate cell adhesion. Oncogene 2003;22:4841–50. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Jovanovic B, Pins M, Lee C, Bergan RC. Over expression of endoglin in human prostate cancer suppresses cell detachment, migration and invasion. Oncogene 2002;21:8272–81. [DOI] [PubMed] [Google Scholar]
- 23.Craft CS, Romero D, Vary CP, Bergan RC. Endoglin inhibits prostate cancer motility via activation of the ALK2-Smad1 pathway. Oncogene 2007;26:7240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craft CS, Xu L, Romero D, Vary CP, Bergan RC. Genistein induces phenotypic reversion of endoglin deficiency in human prostate cancer cells. Mol Pharmacol 2008;73:235–42. [DOI] [PubMed] [Google Scholar]
- 25.Breen MJ, Moran DM, Liu W, Huang X, Vary CP, Bergan RC. Endoglin-mediated suppression of prostate cancer invasion is regulated by activin and bone morphogenetic protein type II receptors. PLoS ONE 2013;8(8):e72407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng W, Zhang Y, Ma D, Shi Y, Liu C, Wang P. (+/−)Equol inhibits invasion in prostate cancer DU145 cells possibly via down-regulation of matrix metalloproteinase-9, matrix metalloproteinase-2 and urokinase-type plasminogen activator by antioxidant activity. J Clin Biochem Nutr 2012;51:61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang LL, Li L, Wu DP, Fan JH, Li X, Wu KJ, Wang XY, He DL. A novel anti-cancer effect of genistein: reversal of epithelial mesenchymal transition in prostate cancer cells. Acta Pharmacol Sin 2008;29:1060–8. [DOI] [PubMed] [Google Scholar]
- 28.El Touny LH, Banerjee PP. Genistein induces the metastasis suppressor kangai-1 which mediates its anti-invasive effects in TRAMP cancer cells. Biochem Biophys Res Commun 2007;361:169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiyomaru T, Yamamura S, Zaman MS, Majid S, Deng G, Shahryari V, Saini S, Hirata H, Ueno K, Chang I, et al. Genistein suppresses prostate cancer growth through inhibition of oncogenic microRNA-151. PLoS ONE 2012;7:e43812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakshman M, Huang X, Ananthanarayanan V, Jovanovic B, Liu Y, Craft CS, Romero D, Vary CP, Bergan RC. Endoglin suppresses human prostate cancer metastasis. Clin Exp Metastasis 2011;28:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harper CE, Cook LM, Patel BB, Wang J, Eltoum IA, Arabshahi A, Shirai T, Lamartiniere CA. Genistein and resveratrol, alone and in combination, suppress prostate cancer in SV-40 tag rats. Prostate 2009;69:1668–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP). Cancer Res 2001;61:6777–82. [PubMed] [Google Scholar]
- 33.El Touny LH, Banerjee PP. Akt GSK-3 pathway as a target in genistein-induced inhibition of TRAMP prostate cancer progression toward a poorly differentiated phenotype. Carcinogenesis 2007;28:1710–7. [DOI] [PubMed] [Google Scholar]
- 34.Takimoto CH, Glover K, Huang X, Hayes SA, Gallot L, Quinn M, Jovanovic BD, Shapiro A, Hernandez L, Goetz A, et al. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol Biomark Prev 2003;12(11 pt 1):1213–21. [PubMed] [Google Scholar]
- 35.Fischer L, Mahoney C, Jeffcoat AR, Koch MA, Thomas BE, Valentine JL, Stinchcombe T, Boan J, Crowell JA, Zeisel SH. Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer 2004;48:160–70. [DOI] [PubMed] [Google Scholar]
- 36.Busby MG, Jeffcoat AR, Bloedon LT, Koch MA, Black T, Dix KJ, Heizer WD, Thomas BF, Hill JM, Crowell JA, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr 2002;75:126–36. [DOI] [PubMed] [Google Scholar]
- 37.Pendleton JM, Tan WW, Anai S, Chang M, Hou W, Shiverick KT, Rosser CJ. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 2008;8:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazarevic B, Boezelijn G, Diep LM, Kvernrod K, Ogren O, Ramberg H, Moen A, Wessel N, Berg RE, Egge-Jacobsen W, et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo-controlled, double-blind phase 2 clinical trial. Nutr Cancer 2011;63:889–98. [DOI] [PubMed] [Google Scholar]