Abstract
Background: Hypertension and dyslipidemia have traditionally been associated with dietary sodium and fat intakes, respectively; however, they have recently been associated with the consumption of added sugars in adults and older adolescents, but there is no clear indication of how early in the life span this association manifests.
Objective: This study explored the cross-sectional association between added sugar (sugars not naturally occurring in foods) consumption in children, blood pressure (BP), and fasting blood lipids [triglycerides and total, low-density lipoprotein, and high-density lipoprotein (HDL) cholesterol].
Design: BP, blood lipids, and dietary intakes were obtained in a multiethnic pediatric sample aged 7–12 y of 122 European American (EA), 106 African American (AA), 84 Hispanic American (HA), and 8 mixed-race children participating in the Admixture Mapping of Ethnic and Racial Insulin Complex Outcomes (AMERICO) study—a cross-sectional study conducted in the Birmingham, AL, metro area investigating the effects of racial-ethnic differences on metabolic and health outcomes. Multiple regression analyses were performed to evaluate the relations of added sugars and sodium intakes with BP and of added sugars and dietary fat intakes with blood lipids. Models were controlled for sex, race-ethnicity, socioeconomic status, Tanner pubertal status, percentage body fat, physical activity, and total energy intake.
Results: Added sugars were positively associated with diastolic BP (P = 0.0462, β = 0.0206) and serum triglycerides (P = 0.0206, β = 0.1090). Sodium was not significantly associated with either measure of BP nor was dietary fat with blood lipids. HA children had higher triglycerides but lower added sugar consumption than did either the AA or EA children. The AA participants had higher BP and HDL but lower triglycerides than did either the EA or HA children.
Conclusions: These data suggest that increased consumption of added sugars may be associated with adverse cardiovascular health factors in children, specifically elevated diastolic BP and triglycerides. Identification of dietary factors influencing cardiovascular health during childhood could serve as a tool to reduce cardiovascular disease risk. This trial was registered at clinicaltrials.gov as NCT00726778.
See corresponding article on page 4.
INTRODUCTION
Cardiovascular disease (CVD)4 is the number one cause of death in adults in the United States (1) and costs an estimated $445 billion/y (2). Although the incidence of CVD primarily occurs in adulthood, CVD precursors such as atherosclerotic lesions have been shown to begin as early as childhood (3). Among the major risk factors for CVD are hypertension and dyslipidemia (4), both of which have become prevalent in children in the United States (5, 6). Hypertension during childhood is correlated with hypertension in adulthood (7), and elevated blood pressure (BP) in children and young adults further exacerbates the development of atherosclerotic plaques (8). Likewise, dyslipidemia during childhood is associated with dyslipidemia in adulthood and an increased risk of CVD (9–11).
Because of the early nature of the development of CVD, it is imperative to identify effective lifestyle interventions for the prevention and treatment of hypertension and dyslipidemia. Dietary interventions for BP reduction have traditionally included the decreased intake of sodium, and interventions for dyslipidemia have included the decreased intake of dietary fats, with emphasis on cholesterol, saturated fat, and trans fat (12, 13). More recently, however, added sugars in the diet (ie, sugars not naturally occurring in foods) have been associated with these conditions. The main sources of added sugars in the diets of US children and adolescents are sugar-sweetened beverages (SSBs), grain desserts, dairy desserts, cold cereal, and candy (14). Studies have shown positive associations between SSB and BP in both adults and older adolescents (15–17); furthermore, the reduction of SSB consumption has been associated with lower diastolic and systolic BP in adults (18). Components of dyslipidemia have also been associated with SSBs and added sugars in the diet in adolescents (19, 20) and with SSB alone in children (21).
Although the relations between some added sugars, hypertension, and dyslipidemia have been explored in various age groups, it is not clear whether, when examining added sugars from all sources, these relations are present in children. Therefore, we investigated the relation between added sugars, BP (diastolic and systolic), and blood lipids (total cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol) in children between the ages of 7 and 12 while controlling for potentially confounding lifestyle and societal factors. We hypothesized that the consumption of added sugars in children would be positively associated with BP, total cholesterol, and triglycerides.
SUBJECTS AND METHODS
Population and data collection
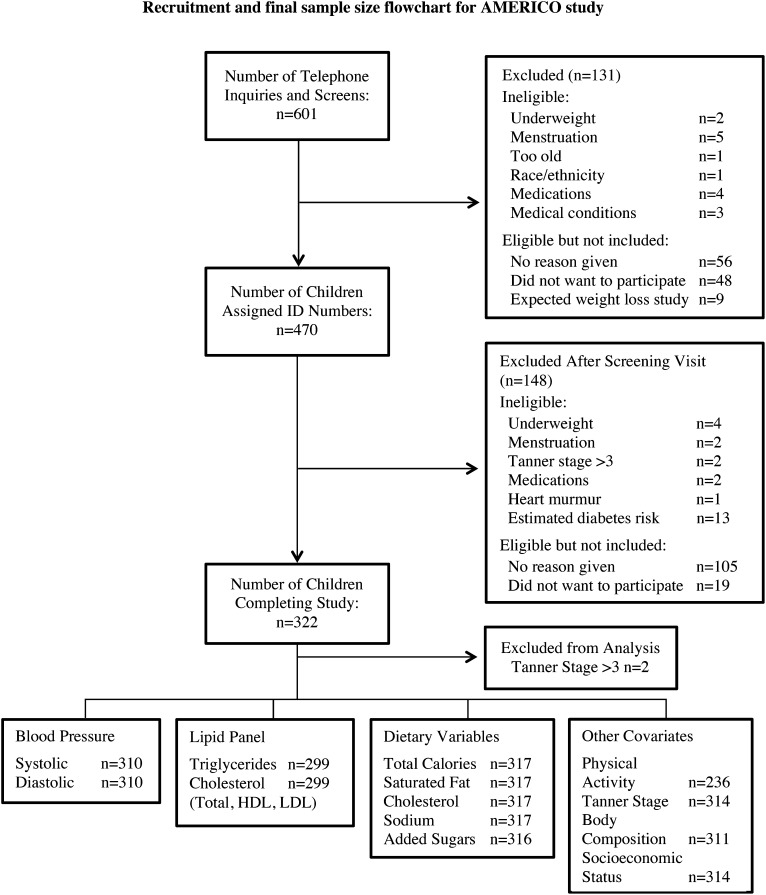
Data were collected from October 2004 to December 2008 as part of the AMERICO study—a cross-sectional study investigating the effects of racial-ethnic differences on metabolic and health outcomes (Figure 1). The final sample included 320 children aged 7–12 y who were self-identified as European American (EA; n = 122), African American (AA; n = 106), Hispanic American (HA; n = 84), and mixed-race ethnicity (n = 8). The children who were peripubertal [pubertal stage ≤3 as assessed by a pediatrician according to the criteria of Marshall and Tanner (22, 23)] and not taking any medications contraindicated for study participation (eg, medication known to affect body composition, metabolism, or cardiac function) were eligible for participation in the study. Participants were recruited from the Birmingham, AL, area via newspaper advertisements, community fliers, and presentations at schools, churches, and health fairs. A total of 601 children were telephone screened; 131 were either self-selected out or were excluded because they did not meet the study criteria. From this stage, 470 participants came in for the study visit, but only 320 fulfilled the study criteria and completed the study. The study was approved by the University of Alabama at Birmingham (UAB) Institutional Review Board for Human Use (children and parents provided informed assent and consent, respectively, before participation).
FIGURE 1.
Recruitment and final sample size flowchart for the AMERICO study. Reasons for participants not being included between recruitment/study stages are given to the right of the arrows denoting the transitions between stages. Final sample sizes for all variables of interest in the study are provided at the bottom of the figure. AMERICO, Admixture Mapping of Ethnic and Racial Insulin Complex Outcomes; ID, identification.
Data collection occurred across 2 study visits: the first was outpatient and the second took place at the UAB General Clinical Research Center (GCRC). Dual-energy X-ray absorptiometry (DXA) scans and the assessment of pubertal status were conducted at the first visit, and the children were given an accelerometer to wear to measure physical activity (collected at the second visit). The second visit occurred ∼7 d later and included an overnight stay at the UAB GCRC; all participants received the same meal and snacks, and only receiving water after 2000 until after the morning blood and BP tests. The 24-h dietary recalls were conducted during both visits. The detailed variable information below parallels the order found in Table 1 (descriptive statistics).
TABLE 1.
Descriptive statistics of the AMERICO study population: overall, by sex, and by race-ethnicity1
| Total(n = 320) | Male(n = 170) | Female(n = 150) | EA(n = 122) | AA(n = 106) | HA(n = 84) | MR(n = 8) | |
| Age | 9.5 ± 0.1 | 9.7 ± 0.1a | 9.3 ± 0.1b | 9.7 ± 0.2 | 9.6 ± 0.1 | 9.4 ± 0.2 | 9.1 ± 0.7 |
| Socioeconomic status2 | 38.7 ± 0.8 | 38.7 ± 1.1 | 38.8 ± 1.3 | 49.4 ± 0.9a | 37.0 ± 1.1b | 25.4 ± 1.3c | 39.4 ± 7.3a,b |
| Tanner stage3 | 1.5 ± 0.0 | 1.4 ± 0.1b | 1.6 ± 0.1a | 1.4 ± 0.1b | 1.7 ± 0.1a | 1.4 ± 0.1b | 1.3 ± 0.2a,b |
| Percentage body fat (%) | 23.4 ± 0.5 | 21.0 ± 0.8b | 26.1 ± 0.7a | 22.5 ± 0.8b | 20.4 ± 1.0b | 28.5 ± 0.9a | 21.6 ± 2.7a,b |
| Physical activity4 | 2012.3 ± 22.5 | 2015.0 ± 32.1 | 2009.3 ± 31.4 | 2016.5 ± 32.7 | 2036.0 ± 45.0 | 1965.1 ± 43.3 | 2201.6 ± 113.9 |
| Total energy intake (kcal/d) | 1894.0 ± 26.4 | 1950.5 ± 37.4a | 1831.1 ± 36.7b | 1877.3 ± 38.5 | 1889.6 ± 50.7 | 1906.0 ± 52.4 | 2080.3 ± 156.4 |
| Added sugars (g/d) | 76.9 ± 2.4 | 79.4 ± 3.2 | 74.2 ± 3.7 | 82.9 ± 3.6a | 84.0 ± 4.8a | 59.5 ± 4.0b | 77.4 ± 15.2a.b |
| Dietary sodium (mg/d) | 3207.3 ± 59.0 | 3299.7 ± 80.8a | 3104.3 ± 85.7b | 3050.1 ± 80.3 | 3279.8 ± 116.7 | 3339.6 ± 118.8 | 3258.9 ± 292.4 |
| Dietary fat (g/d) | 74.4 ± 1.4 | 75.6 ± 2.0 | 73.2 ± 1.9 | 71.3 ± 1.9 | 78.2 ± 2.7 | 73.1 ± 2.6 | 86.5 ± 10.1 |
| Diastolic blood pressure (mm Hg) | 60.1 ± 0.4 | 60.9 ± 0.5a | 59.2 ± 0.6b | 59.0 ± 0.6b | 62.4 ± 0.7a | 58.6 ± 0.6b | 62.3 ± 2.4a,b |
| Systolic blood pressure (mm Hg) | 103.3 ± 0.6 | 104.0 ± 0.8 | 102.6 ± 0.9 | 102.4 ± 1.0a,b | 105.8 ± 1.1a | 101.2 ± 1.0b | 108.2 ± 3a,b |
| Total cholesterol (mg/dL) | 154.0 ± 1.6 | 155.0 ± 2.2 | 152.8 ± 2.4 | 153.2 ± 2.3 | 153.8 ± 2.8 | 154.6 ± 3.6 | 164 ± 10.6 |
| Triglycerides (mg/dL) | 66.7 ± 2.1 | 63.1 ± 2.5b | 70.8 ± 3.4a | 66.1 ± 2.9b | 54.3 ± 2.7b | 83.4 ± 5.3a | 68.2 ± 19.3a,b |
| LDL (mg/dL) | 90.2 ± 1.5 | 90.4 ± 2.1 | 89.9 ± 2.3 | 91.6 ± 2.2 | 87.6 ± 2.8 | 91.9 ± 3.4 | 83.9 ± 6.1 |
| HDL (mg/dL) | 50.3 ± 0.7 | 52.0 ± 1.0a | 48.3 ± 1.0b | 48.4 ± 1.0b | 55.5 ± 1.4a | 46.1 ± 1.4b | 55.2 ± 6.7a,b |
All values are means ± SEs. Means with different superscript letters are significantly different, P < 0.05 (Tukey's post hoc test). AA, African American; AMERICO, Admixture Mapping of Ethnic and Racial Insulin Complex Outcomes; EA, European American; HA, Hispanic American; MR, mixed race.
Assessed via the Hollingshead 4-factor index; potential scores ranged from 8 to 66.
Pubertal stages of 1 to 3 as assessed by a pediatrician according to the criteria of Marshall and Tanner.
Physical activity includes total minutes per week of light, moderate, and vigorous activities.
Socioeconomic status
Socioeconomic status (SES) was calculated via the Hollingshead 4-factor index of social status (24), which includes educational level, occupational prestige, marital status, and sex. Educational level was on a 7-point scale (1 = less than seventh grade completed; 7 = graduate degree) and was weighted by a factor of 3; occupational prestige was on a 9-point scale (1 = farm laborers/menial service workers; 9 = higher executives, proprietors of large businesses, and major professionals) and was weighted by a factor of 5. Overall scores ranged from 8 to 66 and were determined for the participant's working parents(s); higher scores indicate higher SES.
Pubertal status
Pubertal status was determined via physician assessment by using the Marshall and Tanner pubertal status evaluation criteria (22, 23). The 5 stages of pubertal status are based on pubic hair development in both sexes, breast development in females, and genital development in males. The higher value of the 2 developmental criteria is used to assign the Tanner stage. In this study, only children of Tanner stages 1–3 were used.
Percentage body fat
Body composition was assessed via DXA by using a GE Lunar Prodigy densitometer (GE Lunar Radiation Corp) with pediatric software (version 1.5e). DXA scans were conducted while the participants were wearing light clothing and lying flat on their back with arms at their sides. Total calculated fat mass from the scan was divided by total body weight to determine percentage body fat.
Physical activity
Participants were given a uniaxial ActiGraph accelerometer (GT1M, standard model 198–0100-02; ActiGraph LLC) to wear to capture physical activity levels and patterns (7 d of data were used). Actigraph monitors have previously shown high interinstrument reliability and the ability to distinguish between varying levels of physical activity in children (25). Epoch length was configured at 1 min, and the data are expressed as counts/min. Data were characterized as average time (min/wk) spent on moderate, hard, and very hard activities.
Dietary recalls
Two 24-h dietary recalls were administered and analyzed by a registered dietitian using the triple-pass method (26). A parent or guardian was present for, and assisted with, each recall; visual aids were used to help in portion size estimation, and all 24-h dietary recalls were conducted on weekdays. Data were entered by a registered dietitian into the Nutrition Data System for Research (software version 2006; Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). Output from the same participant's dietary recalls were averaged, and the variables of interest included the following: total calories (kcal/d), total fat (g/d), saturated fat (g/d), dietary cholesterol (mg/d), dietary sodium (mg/d), and added sugars (g/d). Added sugars were defined as those not naturally occurring in foods, but were added as a result of processing or preparation and did not include 100% fruit juices. The Nutrition Data System for Research software derived added sugars in the diet by using data from sources such as the USDA (eg, provisional tables and agricultural handbooks), scientific literature, manufacturers, calculations from recipes, and calculations from ingredient lists or similar food products (27).
Blood pressure
During the overnight visit at the UAB GCRC, trained nurses took 2 BP measurements at 1800 and 2 more the next day at 0700. An automated pediatric BP cuff (Dinamap Pro 200; GE Medical Systems) was used for this purpose, and appropriate child-sized cuffs were used based on participant arm size. Participants were seated at rest, with feet flat on the floor, for ≥10 min before measurements were taken, and the first and second measurements were separated by a 5-min seated rest. Evening and morning measurements were not significantly different. The systolic and diastolic BP measurements were averaged from a total of 4 measurements of each.
Blood lipids and lipoproteins
Fasting blood samples were collected at 0700 after the participants’ overnight stay at the UAB GCRC. Concentrations of all serum-derived analytes were measured at the UAB in the Metabolism Core Laboratory that services the GCRC and Nutrition Obesity Research Center. Lipids (total cholesterol, HDL cholesterol, and triglycerides) were measured by using a Stanbio SIRRUS analyzer. LDL cholesterol was calculated by using the method of Friedewald (28).
Statistical analysis
Multiple variable linear regression analyses were performed, with control for covariates, to evaluate the main associations of interest. The independent variables in our analyses were total dietary added sugar intake (g/d), total dietary sodium intake (mg/d), total dietary fat intake (g/d), total dietary saturated fat intake (g/d), and total dietary cholesterol intake (mg/d). Dependent variables included average diastolic BP (mm Hg), average systolic BP (mm Hg), total cholesterol (mg/dL), triglycerides (mg/dL), LDL cholesterol (mg/dL), and HDL cholesterol (mg/dL). Covariates included sex, race-ethnicity, SES, Tanner pubertal status, percentage body fat, physical activity (min/wk), and total energy intake (kcal/d). All variables were continuous in nature, other than sex and race-ethnicity. Race-ethnicity was entered as a dummy-coded variable because it is nominal in nature; EA participants were used as the reference group because they are the largest group and have the least variance for the greatest number of variables of interest.
The associations between added sugars and BP (diastolic and systolic run independently) and between added sugars and blood lipids (total cholesterol, triglycerides, HDL cholesterol, and LDL cholesterol run independently) were tested by using multiple-variable linear regression analyses. Identical models were also run between dietary sodium and BP and also between total dietary fat and blood lipids to evaluate their potentially confounding effects on associations between added sugars and CVD risk factors. Only those participants with values for all variables in the models were included in the analyses.
Descriptive statistics were analyzed by race-ethnicity and then by sex by using ANOVA with Tukey's post hoc test. Participants with missing data were not included in the analyses. To comply with assumptions of regression, all models were evaluated for normality of residuals before any potential statistical transformations of the dependent variable. In all regression models, residuals exceeding 3 SDs were removed; consequently, no statistical transformations of dependent variables were necessary. All statistical analyses were performed in SAS version 9.3 (SAS Institute Inc).
RESULTS
Baseline characteristics of study participants are given in Table 1 for the overall sample and by sex and race-ethnicity. No statistically significant differences were observed between the sexes in terms of SES, physical activity, added sugars, dietary fat, or systolic BP, and no significant differences were observed among race-ethnicity in terms of physical activity, total energy intake, total cholesterol, or LDL cholesterol. Relative to males, females had a higher Tanner stage, percentage body fat, and triglycerides but had lower total energy intake, dietary sodium, average diastolic BP, and HDL cholesterol. HA participants had higher percentage body fat and triglycerides but lower SES and added sugar consumption than did the AA or EA children. AA participants had a higher Tanner stage, average diastolic and systolic BPs, and HDL cholesterol but lower triglycerides than did either the EA or HA children.
A significant positive relation was observed between added sugars and diastolic BP (P = 0.0462), as described in Table 2, with a significant contribution of sex in the model (P = 0.0259). Sodium was not significantly associated with diastolic BP; however, both sex (P = 0.0301) and total energy intake (P = 0.0436) contributed significantly to the model. No significant relations were observed between systolic BP and either added sugars or sodium. When the relation between added sugars and systolic blood pressure was modeled, the following covariates contributed significantly to the model: sex (P = 0.0233), Tanner stage (P = 0.0127), and percentage body fat (P = 0.0107). When the relation between sodium and systolic blood pressure was modeled, the following covariates contributed significantly to the model: sex (P = 0.0246), Tanner stage (P = 0.0092), and percentage body fat (P = 0.0101). In all models of blood pressure, female sex was associated with lower values. When they contributed significantly to models of blood pressure, Tanner stage, percentage body fat, and total energy intake were associated with higher values.
TABLE 2.
Regression analyses of diastolic and systolic blood pressures compared with added sugars and dietary sodium, run separately1
| Diastolic blood pressure |
Systolic blood pressure |
|||
| β Coefficient | P value | β Coefficient | P value | |
| Added sugars (g/d) | 0.0206 | 0.0462 | 0.0126 | 0.4827 |
| Dietary sodium (mg/d) | −0.0009 | 0.0645 | −0.0001 | 0.8745 |
Models were linear regressions with statistically significant associations at P < 0.05. All analyses were controlled for the following covariates: sex, race-ethnicity (dummy coded), socioeconomic status, Tanner pubertal status, percentage body fat, total physical activity, and total energy intake. In the above models, the sample size for which all variables were available was 220.
Added sugars were positively associated with triglycerides (P = 0.0206), but no other blood lipids, as can be seen in Table 3. Tanner stage contributed significantly in the following models: total cholesterol (P = 0.0254), triglycerides (P = 0.0020), and HDL cholesterol (P = 0.0038). Percentage body fat contributed significantly in the following models: total cholesterol (P = 0.0009), triglycerides (P = 0.0104), LDL cholesterol (P < 0.0001), and HDL cholesterol (P < 0.0001).
TABLE 3.
Regression analyses of blood lipids and lipoproteins compared with added sugars and dietary fat, run separately1
| Total cholesterol | Triglycerides | LDL cholesterol | HDL cholesterol | |||||
| β Coefficient | P value | β Coefficient | P value | β Coefficient | P value | β Coefficient | P value | |
| Added sugars (g/d) | 0.0276 | 0.5995 | 0.1090 | 0.0206 | 0.0318 | 0.5259 | −0.0127 | 0.5516 |
| Dietary fat (g/d) | 0.1087 | 0.4480 | −0.1351 | 0.2740 | 0.0769 | 0.5735 | 0.0496 | 0.3947 |
Models were linear regressions with statistically significant associations at P < 0.05. All analyses were controlled for the following covariates: sex, race-ethnicity (dummy coded), socioeconomic status, Tanner pubertal status, percentage body fat, total physical activity, and total energy intake. In the above models, the sample size for which all variables were available was 210.
Total dietary fat was not significantly associated with any of the blood lipids investigated, as can be seen in Table 3. Tanner stage contributed significantly in the following models: total cholesterol (P = 0.0397), triglycerides (P = 0.0083), and HDL cholesterol (P = 0.0040). Percentage body fat contributed significantly in the following models: total cholesterol (P = 0.0009), triglycerides (P = 0.0129), LDL cholesterol (P < 0.0001), and HDL cholesterol (P < 0.0001). When it contributed significantly to models of blood lipids, Tanner stage was associated with higher values of triglycerides but lower values of total cholesterol and HDL cholesterol. When it contributed significantly to models of blood lipids, percentage body fat was associated with higher values of total cholesterol, triglycerides, and LDL cholesterol but with lower values of HDL cholesterol. Results from the aforementioned regression analyses are alternatively presented with 95% CIs elsewhere (see Supplemental Tables 1 and 2 under “Supplemental data” in the online issue).
DISCUSSION
This study evaluated the cross-sectional associations between added sugars in the diet, as reported via guardian-supervised children's 24-h dietary recalls, and CVD risk factors—specifically blood pressure and blood lipids. Whereas previous studies have shown positive cross-sectional associations between added sugars in the diet and elevated BP in adolescents and adults (15–17), and also between added sugars and triglycerides in adolescents (29), our study evaluated this relation in children (Marshall and Tanner pubertal status ≤3).
In our study population, a positive statistically significant association was observed between added sugars and diastolic BP, but not systolic BP. This dichotomy is particularly noteworthy because of the young age of our population, given that isolated diastolic hypertension is much more common in younger than in older adults (30). The relation between added sugars and diastolic BP suggested a modest increase of 0.0206 mm Hg per gram of added sugars. The average daily consumption of added sugars for our participants was 308 kcal (76.9 g), for boys in the United States is 362 kcal (90.5 g), and for girls in the United States is 282 kcal (70.5 g) (31)—well above the range of 12 to 32 g recommended by the American Heart Association for the age range of our study population (32). (Range derivations for added sugars are available under “Supplemental data” in the online issue.) This level of added sugar consumption by US children may parlay into an increase of 1.8643 mm Hg in boys and of 1.4523 mm Hg in girls, which may be clinically relevant given that a sustained increase of only 5–6 mm Hg in diastolic BP in older adults over a few years may increase the risk of stroke by 67% (33).
A dietary factor traditionally thought to increase BP, dietary sodium (34), did not have a statistically significant association with either measure of BP in our sample. We hypothesized that the lack of association in our population between dietary sodium and BP may have been attributable to the age of our population and the possibility that their vascular tone and renal function have not yet been impaired. In terms of blood lipids, although added sugars were associated with triglycerides in our study, dietary fat did not show this association. When further examined, neither dietary cholesterol nor saturated fats were significantly associated with triglycerides (data not shown).
Data from the Framingham study show that patients with elevated BP also have other metabolic conditions, such as high cholesterol and triglycerides, lower HDL cholesterol, obesity, and insulin resistance (30). Other studies have suggested that hypertriglyceridemia and hypertension tend to coexist in adult individuals (35). These observations suggest that metabolic diseases could respond to a common etiology rooted in genetic and environmental factors that alter the normal functionality of the body. Our data support that the consumption of added sugars might represent a modifiable risk factor that deserves further exploration in the identification of preventive strategies for such complex diseases, particularly among the pediatric population.
Understanding how risk factors affect disease progression is critical in the development of effective interventions and treatments. Added sugars in the diet may affect BP by acting on the kidneys to increase blood concentrations of uric acid, which could, in turn, reduce the production and/or availability of nitric oxide, a powerful vasodilator, and thereby raise BP (36). In fact, an increased OR of elevated BP has been seen in those adolescents with higher serum uric acid concentrations (37). Alternatively, the mechanism by which added sugars may raise BP could involve the renin-angiotensin pathway, as has been observed in rat studies (38, 39), or the interplay between this pathway and nitric oxide production (39). Elevation of triglycerides from added sugars, on the other hand, may have a less convoluted etiology. A likely mechanism by which added sugars may increase triglyceride concentrations is through stimulation of hepatic de novo lipogenesis (DNL) (40, 41). Although glucose alone does not appear to significantly increase DNL (42), fructose has been shown to significantly increase DNL within hours (43, 44). (Both glucose and fructose are found in commonly used added sugars such as cane sugar and high-fructose corn syrup.)
The strengths of our study include the use of a diverse cohort in terms of race-ethnicity and the ability to more accurately isolate the association between dietary sugars, BP, and blood lipids by controlling for lifestyle (physical activity and dietary intakes) and SES, race-ethnicity, sex, Tanner stage, and percentage body fat. In addition to the inclusion of these covariates, robust measures of body composition and physical activity were used. For instance, percentage body fat was calculated from body-composition variables and weight obtained via DXA technology instead of estimated by using BMI, because BMI has been shown to be inconsistent in classifying adiposity status across racial-ethnic groups in children, and <50% of children in the 85th–95th BMI percentile (ie, overweight) show high adiposity levels when assessed via DXA (45). Physical activity was directly measured via the use of accelerometers, as opposed to self-reported activity. Tanner status was used as a proxy for maturation status, instead of age alone, to better control for hormonal and developmental differences among the children.
Limitations of the study include the sample size (n = 320), the cross-sectional nature of the study, and the inability to obtain certain data. Given that our sample was from children living in the Birmingham, AL, metro area, the results may not be readily generalized to other geographic contexts. As we were not able to follow the children over time, it is not possible to determine how the observed relations between added sugars, BP, and blood lipids may change throughout the life span. The use of self-reported dietary assessments may raise concerns about the potential underreporting of added sugars—as observed in adults (46)—that could have affected the associations reported herein. Although 24-h dietary recalls may introduce error because of their reliance on memory and truthfulness in reporting, a previous review has shown repeated 24-h dietary recalls to be the most accurate self-reported measure in children as compared with doubly labeled water (47). Despite control for SES (as determined via the Hollingshead index), limited parental income data were available for us to evaluate the possible effects of purchasing power constraints on diet quality and their associations with foods high in added sugars. We did not have measures of serum uric acid, nitric oxide, or the renin-angiotensin system in our population to evaluate the potential physiologic mechanisms by which added sugars may raise BP.
Our results support the hypothesis that increased consumption of added sugars may contribute to the development of poor cardiovascular health before maturity. Further research is needed in humans, especially randomized control trials and longitudinal studies, to verify whether the relations between added sugars, BP, blood lipids, and cardiovascular health are definitively causative or only correlative. However, in light of the current obesity epidemic—because added sugars in food products may further increase caloric load—it would be advisable to limit the consumption of added sugars, especially in children.
Supplementary Material
Acknowledgments
We thank all of the participants who took part in the AMERICO study.
The authors’ responsibilities were as follows—KPK and JRF: designed the research analyses; KPK, MIC, MMBB, and JRF: analyzed the data; KPK: wrote the manuscript; and MIC, JRF, and MMBB: provided editing support. All authors read and approved the final manuscript. None of the authors reported any conflicts of interest.
Footnotes
Abbreviations used: AA, African American; BP, blood pressure; CVD, cardiovascular disease; DNL, de novo lipogenesis; DXA, dual-energy X-ray absorptiometry; EA, European American; HA, Hispanic American; GCRC, General Clinical Research Center; SES, socioeconomic status; SSB, sugar-sweetened beverage; UAB, University of Alabama at Birmingham.
REFERENCES
- 1.Miniño A, Murphy S, Xu J, Kochanek K. Deaths: final data for 2008. Natl Vital Stat Rep 2011;59:1–126. [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon J, Khavjou O, Butler J, Dracup K, Ezekowitz M, Finkelstein E, Hong Y, Johnston S, Khera A, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933–44. [DOI] [PubMed] [Google Scholar]
- 3.Stary HC. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis 1989;9(suppl):I19–32. [PubMed] [Google Scholar]
- 4.Kannel WB. Hypertension as a risk factor for cardiac events-epidemiologic results of long-term studies. J Cardiovasc Pharmacol 1993;21(suppl 2):S27–37. [DOI] [PubMed] [Google Scholar]
- 5.Kit BK, Carroll M, Lacher D, Sorlie P, DeJesus J, Ogden C. Trends in serum lipids among US youths aged 6 to 19 years, 1988-2010. JAMA 2012;308:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorof JM, Lai D, Turner J, Poffenbarger T, Portman R. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics 2004;113:475–82. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation 2008;117:3171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berenson GS, Srinivasan S, Bao W, Newman W, Tracy R, Wattigney W. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med 1998;338:1650–6. [DOI] [PubMed] [Google Scholar]
- 9.Juonala M, Järvisalo M, Mäki-Torkko N, Kähönen M, Viikari J, Raitakari O. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation 2005;112:1486–93. [DOI] [PubMed] [Google Scholar]
- 10.Berenson GS. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The Bogalusa Heart Study. Am J Cardiol 2002;90(10C). [DOI] [PubMed] [Google Scholar]
- 11.Kwiterovich P, Gidding S. Universal screening of cholesterol in children. Clin Cardiol 2012;35:662–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appel LJ, Brands M, Daniels S, Karanja N, Elmer P, Sacks F. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47:296–308. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher B, Berra K, Ades P, Braun L, Burke L, Durstine J, Fair J, Fletcher G, Goff D, Hayman L, et al. Managing abnormal blood lipids: a collaborative approach. Circulation 2005;112:3184–209. [DOI] [PubMed] [Google Scholar]
- 14.Reedy J, Krebs-Smith S. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. J Am Diet Assoc 2010;110:1477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Høstmark A. The Oslo health study: soft drink intake is associated with the metabolic syndrome. Appl Physiol Nutr Metab 2010;35:635–42. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen S, Choi H, Lustig R, Hsu C-y. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr 2009;154:807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown IJ, Stamler J, Van Horn L, Robertson C, Chan Q, Dyer A, Huang C-C, Rodriguez B, Zhao L, Daviglus M, et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: international study of macro/micronutrients and blood pressure. Hypertension 2011;57:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Caballero B, Mitchell D, Loria C, Lin P-H, Champagne C, Elmer P, Ard J, Batch B, Anderson C, et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation 2010;121:2398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsh JA, Sharma A, Cunningham S, Vos M. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation 2011;123:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambrosini GL, Oddy W, Huang R, Mori T, Beilin L, Jebb S. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am J Clin Nutr 2013;98:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosova EC, Auinger P, Bremer A. The relationships between sugar-sweetened beverage intake and cardiometabolic markers in young children. J Acad Nutr Diet 2013;113:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall WA, Tanner J. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner J. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cirino PT, Chin C, Sevcik R, Wolf M, Lovett M, Morris R. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment 2002;9:145–55. [DOI] [PubMed] [Google Scholar]
- 25.Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci 2008;26:1557–65. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RK, Driscoll P, Goran M. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc 1996;96:1140–4. [DOI] [PubMed] [Google Scholar]
- 27.Nutrition Data System for Research. User manual. Appendix 22. Sources of food and nutrient data. Version current 1 March 2014. Available from: http://wwwnccumnedu/ndsrsupport/usermanualexcerpts/13appendix22_sourcesoffoodandnutrientdatapdf (cited 1 March 2014).
- 28.Friedewald WTLR, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of an ultracentrifuge. Clin Chem 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Welsh JA, Sharma A, Abramson J, Vaccarino V, Gillespie C, Vos M. Caloric sweetener consumption and dyslipidemia among US adults. JAMA 2010;303:1490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kannel WB. Historic perspectives on the relative contributions of diastolic and systolic blood pressure elevation to cardiovascular risk profile. Am Heart J 1999;138:205–10. [DOI] [PubMed] [Google Scholar]
- 31.Ervin RB, Kit BK, Carroll MD, Ogden CL. Consumption of added sugar among U.S. children and adolescents, 2005-2008. Adv Nutr 2012;3:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson RK, Appel L, Brands M, Howard B, Lefevre M, Lustig R, Sacks F, Steffen L, Wylie-Rosett J, American Heart Association Nutrition Committee of the Council on Nutrition PA, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2009;120:1011–20. [DOI] [PubMed] [Google Scholar]
- 33.Johnson A. NSAIDs and increased blood pressure. What is the clinical significance? Drug safety: an international journal of medical toxicology and drug experience 1997;17:277–89. [DOI] [PubMed] [Google Scholar]
- 34.Frisoli TM, Schmieder R, Grodzicki T, Messerli F. Salt and hypertension: is salt dietary reduction worth the effort? Am J Med 2012;125:433–9. [DOI] [PubMed] [Google Scholar]
- 35.Karasek D, Vaverkova H, Halenka M, Jackuliakova D, Frysak Z, Orsag J, Novotny D. Prehypertension in dyslipidemic individuals; relationship to metabolic parameters and intima-media thickness. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2013;157:41–9. [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa T, Hu H, Zharikov S, Tuttle K, Short R, Glushakova O, Ouyang X, Feig D, Block E, Herrera-Acosta J, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 2006;290:F625–31. [DOI] [PubMed] [Google Scholar]
- 37.Loeffler LF, Navas-Acien A, Brady T, Miller E, Fadrowski J. Uric acid level and elevated blood pressure in US adolescents: National Health and Nutrition Examination Survey, 1999-2006. Hypertension 2012;59:811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freitas RR, Lopes K, Carillo B, Bergamaschi C, Carmona A, Casarini D, Furukawa L, Heimann J, Campos R, Dolnikoff M. Sympathetic and renin-angiotensin systems contribute to increased blood pressure in sucrose-fed rats. Am J Hypertens 2007;20:692–8. [DOI] [PubMed] [Google Scholar]
- 39.Chou C-L, Pang C-Y, Lee T, Fang T-C. Direct renin inhibitor prevents and ameliorates insulin resistance, aortic endothelial dysfunction and vascular remodeling in fructose-fed hypertensive rats. Hypertens Res 2013;36:123–8. [DOI] [PubMed] [Google Scholar]
- 40.Parks EJ, Hellerstein M. Carbohydrate-induced hypertriacylglycerolemia: historical perspective and review of biological mechanisms. Am J Clin Nutr 2000;71:412–33. [DOI] [PubMed] [Google Scholar]
- 41.Hellerstein MK. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur J Clin Nutr 1999;53(suppl 1):S53–65. [DOI] [PubMed] [Google Scholar]
- 42.Stanhope KL, Havel P. Fructose consumption: recent results and their potential implications. Ann N Y Acad Sci 2010;1190:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hudgins LC, Parker T, Levine D, Hellerstein M. A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J Clin Endocrinol Metab 2011;96:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr 1996;16:523–57. [DOI] [PubMed] [Google Scholar]
- 45.Flegal KM, Ogden C, Yanovski J, Freedman D, Shepherd J, Graubard B, Borrud L. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr 2010;91:1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millen AE, Tooze J, Subar A, Kahle L, Schatzkin A, Krebs-Smith S. Differences between food group reports of low-energy reporters and non-low-energy reporters on a food frequency questionnaire. J Am Diet Assoc 2009;109:1194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burrows TL, Martin R, Collins C. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J Am Diet Assoc 2010;110:1501–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.