Abstract
Background: The relation between carbohydrate intake and risk of ischemic heart disease (IHD) has not been fully explored in Asian populations known to have high-carbohydrate diets.
Objective: We assessed whether intakes of total carbohydrates, different types of carbohydrates, and their food sources were associated with IHD mortality in a Chinese population.
Design: We prospectively examined the association of carbohydrate intake and IHD mortality in 53,469 participants in the Singapore Chinese Health Study with an average follow-up of 15 y. Diet was assessed by using a semiquantitative food-frequency questionnaire. HRs and 95% CIs were calculated by using a Cox proportional hazards analysis.
Results: We documented 1660 IHD deaths during 804,433 person-years of follow-up. Total carbohydrate intake was not associated with IHD mortality risk [men: HR per 5% of energy, 0.97 (95% CI: 0.92, 1.03); women: 1.06 (95% CI: 0.99, 1.14)]. When types of carbohydrates were analyzed individually, starch intake was associated with higher risk [men: 1.03 (95% CI: 0.99, 1.08); women: 1.08, (95% CI: 1.02, 1.14)] and fiber intake with lower risk of IHD mortality [men: 0.94 (95% CI: 0.82, 1.08); women: 0.71 (95% CI: 0.60, 0.84)], with stronger associations in women than men (both P-interaction < 0.01). In substitution analyses, the replacement of one daily serving of rice with one daily serving of noodles was associated with higher risk (difference in HR: 26.11%; 95% CI: 10.98%, 43.30%). In contrast, replacing one daily serving of rice with one of vegetables (−23.81%; 95% CI: −33.12%, −13.20%), fruit (−11.94%; 95% CI: −17.49%, −6.00%), or whole-wheat bread (−19.46%; 95% CI: −34.28%, −1.29%) was associated with lower risk of IHD death.
Conclusions: In this Asian population with high carbohydrate intake, the total amount of carbohydrates consumed was not substantially associated with IHD mortality. In contrast, the shifting of food sources of carbohydrates toward a higher consumption of fruit, vegetables, and whole grains was associated with lower risk of IHD death.
See corresponding article on page 4.
INTRODUCTION
The rising prevalence of type 2 diabetes and cardiovascular diseases (CVDs)5 in Asia and the projected public health and economic consequences have made identifying and addressing modifiable risk factors a priority (1). Carbohydrate intakes are typically higher in Asian (2, 3) than Western (3) populations and understanding their impact on ischemic heart disease (IHD) risk may have considerable implications for prevention efforts. Current evidence on the impact of total carbohydrate intake on IHD has primarily come from studies in Western populations and has been equivocal. Metabolic study results have suggested that the isoenergetic replacement of carbohydrates with unsaturated fats or proteins can reduce insulin resistance (4) and blood pressure (5) and improve plasma lipoprotein profiles (5, 6). However, associations between long-term carbohydrate intake on IHD (7–9) and CVD (8, 10–15) in prospective cohort studies have been inconsistent.
Some of these discrepancies in findings with regards to total carbohydrate intake and cardiovascular health may have been related to the type of carbohydrates consumed or food sources of carbohydrates. On ingestion, intake of carbohydrates from foods with a high glycemic index (GI) cause rapid surges in blood glucose and has been associated with higher CVD risk (9, 16, 17), whereas carbohydrates from low-GI food sources (GI ≤57) was not associated with IHD risk (9). A high dietary glycemic load (reflecting both the amount of carbohydrates and dietary GI) has also been associated with higher CVD risk in several studies (16, 17). Similarly, refined food sources of carbohydrates with higher GI such as sugar-sweetened beverages and refined-grain cereals have been related to higher risk of type 2 diabetes and IHD in some studies (18, 19), whereas the consumption of whole-grain foods has been associated with lower risk of these diseases (20). Whole-grain foods are important sources of various phytochemicals such as dietary fiber, magnesium, tocotrienols, and lignans that can potentially improve cardiovascular health (21). Therefore, refined carbohydrate-rich foods may increase risk of cardiometabolic disease directly by straining the glucose-homeostatic system or indirectly by displacing the consumption of whole-grain foods that may exert protective effects (18, 19, 21).
The contribution of refined high-carbohydrate foods to CVD risk is possibly magnified in Asian populations, who derive a large proportion of their energy from refined grains (2) and may experience exacerbated glycemic responses to such foods than do persons of European ancestry (22). White rice, which is one of the main sources of carbohydrates in a traditional Asian diet, has been associated with higher risk of type 2 diabetes in both Western and Asian populations (23). However, few studies have investigated the associations of different carbohydrate sources with CVD risk in the context of an Asian population, and these studies have yielded discrepant results (24–26). Therefore, we examined the association of total carbohydrate intake, types of carbohydrates, and food sources of carbohydrates with IHD mortality risk in a large cohort of Chinese in Singapore.
SUBJECTS AND METHODS
Study population
The Singapore Chinese Health Study cohort was established to assess the role of lifestyle and dietary factors on risk of cancer and other chronic diseases in Chinese adults in Singapore. From 1993 to 1998, Singapore citizens or permanent residents aged between 45–74 y who were residing at public housing estates (where ∼86% of the Singaporean population lived at the time of recruitment) were interviewed during home visits by using a structured questionnaire that elicited information on height, weight, education, medical history, and lifestyle behaviors including diet, tobacco use, alcohol intake, physical activity, and sleep duration. This cohort comprises 63,257 participants of Chinese ethnicity restricted to the 2 major dialect groups, the Cantonese and the Hokkiens, who originated from the contiguous provinces of Fujian and Guangdong, respectively, in the southern part of China. Details on the study design have been previously published (27). The study has been approved by the institutional review boards of the National University of Singapore and the University of Pittsburgh.
Dietary assessment
Habitual dietary intake over the past 1 y was assessed by using an interview-administered 165-item semiquantitative food-frequency questionnaire (FFQ) at the time of study enrollment. Information on the usual frequency of consumption for each food item was collected by using 8 categories that ranged from never to ≥2 times/d (never or hardly ever, 1 or 2–3 times/mo, 1, 2–3, or 4–6 times/wk, and 1 or ≥2 times/d). Information on the usual serving size (generally 3 options) was also obtained. Serving-size options were either number based for foods that could be readily counted such as bread (≤1, 2, or ≥3 slices) or based on colored photographs of food items on same-sized plates with portions representing the 15th, 50th, and 85th percentiles of the portion size. Intakes of 15 types of fruit were captured by the FFQ, and total daily fruit consumption was calculated as the sum of the servings of all fruit. The FFQ assessed intake of 34 types of fresh vegetables including tomatoes, yellow-orange, cruciferous, and green vegetables, and vegetable juices. For the purposes of our analyses, we excluded the consumption of potatoes and preserved vegetables from the total vegetable consumption. Rice intake was assessed by using 8 line items that included plain rice or porridge, flavored porridges, fried rice, coconut rice dishes, curry rice dishes, chicken rice, and other types of flavored rice. Noodle intake was assessed on the basis of 5 line items and included fried vegetarian noodles, other fried noodles, noodles with a soupy base, noodles with gravy, and dry noodles.
Nutrient intakes for each participant were computed from the Singapore Food Composition database (28). The FFQ has subsequently been compared with a series of 24-h dietary recall interviews collected from 1022 randomly chosen cohort subjects (425 men and 597 women) (28). Correlation coefficients between 24-h diet recalls and the FFQ for Cantonese and Hokkiens were as follows: carbohydrate intake (percentage of energy), 0.43 and 0.51 for men and 0.48 and 0.46 for women; and fiber intake (energy-adjusted residuals), 0.72 and 0.66 for men and 0.67 and 0.65 for women (28). Comparable correlations for carbohydrate-based foods have been reported in other Asian cohorts (26, 29). The difference in mean nutrient intake measured by the FFQ and 24-h recall for most nutrients including carbohydrates and fiber was <10%, which suggested good coverage of carbohydrate-containing foods by the FFQ (28).
Covariate assessment
Data on other covariates including cigarette smoking, alcohol consumption, sleep duration, education level, medical history (physician-diagnosed hypertension, diabetes, heart attack or angina, and cancer), physical activity, reproductive history (only women), and height and weight were also collected by using interview-administered questionnaires. If either height (n = 295; 0.47% of participants) or weight (n = 10,280; 16.3% of participants) data were missing, values were estimated by using linear regression on the nonmissing variable. If both height and weight data were missing (n = 196; 0.31% of participants), the sex-specific median value of height was used, and weight was imputed by using linear regression on height. BMI (in kg/m2) was computed as weight divided by the square of height. Alcohol intake was computed on the basis of the consumption of beer, rice wine, other wines, and hard liquor. Physical activity was assessed by asking participants the amount of time they spent per week on strenuous activities such as jogging, tennis, or moving heavy furniture and on moderate activities such as brisk walking or tai chi over the past year. Sleep duration was assessed by asking participants about the average number of hours slept per day including naps with options that ranged from ≤5 to ≥10 h/d. For women, reproductive history including information on menstrual status was also obtained.
IHD mortality assessment
The cohort is followed up for mortality through regular record linkage with the population-based Singapore Registry of Births and Deaths. For the current analysis, all deaths including the specific cause of death and date of death up to 31 December 2011 were used. IHD death was defined according to the International Classification of Diseases, 9th Revision, codes 410.0–414.9 that were recorded as the primary cause of death in death certificates. As of 31 December 2011, only 47 cohort subjects were lost to follow-up, mainly because of their emigration out of Singapore. Thus, the identification of deaths in Singapore Chinese Health Study participants was virtually complete.
Statistical analyses
We excluded participants who had a previous self-reported history of cancer (n = 864), heart attack or angina (n = 2598), stroke (n = 947), or diabetes (n = 5696). We also excluded participants who had extreme daily energy intakes [<700 or >3700 kcal/d for men (n = 476) and <600 and >3000 kcal/d for women (n = 584); >3 SDs from the mean]. The number of participants excluded from each category was not exclusive. Therefore, the current analyses were based on data from 53,469 participants.
For each participant, we computed the follow-up time in the study from the date of interview at enrollment to the date of death, date of last contact, or 31 December 2011, whichever came first. Carbohydrate, starch, and monosaccharide and disaccharide intakes were expressed as a percentage of total energy intake. With the exception of bread consumption, which was not substantially associated with energy intake, all other dietary variables were adjusted for energy intake by using the residual method (30). We computed sex-specific quintiles for dietary intakes. Distributions of demographic, lifestyle, and dietary characteristics in study participants were compared across different quintiles of carbohydrate intake for men and women, separately. Pairwise Spearman's correlation coefficients were calculated to assess the correlation between total and types of carbohydrates as well as between these carbohydrates and major food sources. The Cox proportional hazards regression was used to compute HRs and 95% CIs of risk of IHD death for different carbohydrate intakes related to the lowest quintile. We tested for a trend by modeling dependent variables such as total carbohydrate as continuous variables in a Cox proportional hazards regression.
We fitted 3 models with different sets of covariates to adjust for potential confounding effects. Besides the primary variable of interest, the basic model included age at recruitment (y), dialect group (Hokkien or Cantonese), year of interview, and energy intake (kcal/d). In our second model, we further adjusted for lifestyle factors including cigarette smoking (never smoker, ex-smoker, current smoker <13 cigarettes/d, and current-smoker ≥13 cigarettes/d), alcohol intake (never drinkers, <5 g/d, and ≥5 g/d), physical activity (0 h/wk moderate and strenuous activity, <4 h/wk of moderate and <2 h/wk of strenuous activity, and ≥4 h/wk of moderate or ≥2 h/wk of strenuous activity), sleep duration (5, 6, 7, 8, and ≥9 h/d), education (none, primary, secondary, and above), BMI (<18.5, 18.5 to <23, 23 to <25, 25 to <27.5, 27.5 to <30, and ≥30), history of hypertension (yes or no), and use of hormone-replacement therapy (HRT) for women (premenopausal, postmenopausal without a history of the use of HRT, and postmenopausal with a history of the use of HRT). In addition to all covariates in models 1 and 2, the final set of models included the following diet-related factors: the ratio of PUFAs to SFAs, cholesterol, and fiber intakes (in quintiles) for the evaluation of associations for carbohydrates, starch, fiber, and monosaccharides and disaccharides. For models that evaluated associations of IHD mortality risk with rice, noodles, fruit, and vegetables, final models included whole-wheat bread intake (0, <1, and ≥1 slice/d), white bread intake (0, <1, and ≥ 1 slice/d), and quintiles of red meat, fish, soy protein, egg, poultry, nonsoy legume, and polyunsaturated fat–to–saturated fat intake ratio, all of which were modeled as ordinal variables. These covariates were selected because they are potential risk factors for IHD on the basis of previous literature.
Correlations between Schoenfeld's residuals and time were not significant (P ≥ 0.05) for most models indicating that the proportional hazards assumptions were not violated. Only for rice and starch models were these correlations significant (P = 0.02). However, when we stratified the follow-up time by 10 y, we observed similar associations across strata, which indicated that these deviations did not materially affect the results. We tested for interactions by sex, BMI, (overweight status according to Asian criteria of ≥23 or <23 kg/m2), and age (median age: ≥55 and <55 y) by including a multiplicative term with sex, BMI, or age as a binary variable and the consumption of carbohydrates and its sources as continuous variables in fully adjusted models. We also evaluated effects of substituting one serving of rice for other foods such as noodles, vegetables, fruit, or whole-wheat breads by using the method described by Bernstein et al (31). We did this analysis by simultaneously including the consumption of the foods of interest as continuous variables in the multivariate model. We defined one serving as 100 g rice, noodles, or vegetables, 90 g fish, red meat, or poultry. 12.5 g soy protein, 80 g legumes, and 53 g eggs on the basis of the Singapore Health Promotion Board serving-size guide (32). The exponential of the difference between coefficients was used to estimate the RR of substitution, and the covariance between the 2 food groups was used to derive the SE and 95% CI.
In sensitivity analyses, we excluded people who had an event within the first 5 y of follow-up to account for a potential bias because of preclinical disease at enrollment. We used PASW (SPSS) software (version 19.0; IBM, SPPS Inc), and 2-sided P values <0.05 were considered statistically significant.
RESULTS
During the 804,433 person-years of follow-up, from 1993 to 2011, we noted 1660 deaths from IHD including 1022 deaths in men (29.93 deaths/10,000 person-years) and 638 deaths in women (13.78 deaths/10,000 person-years). Both men and women who had higher carbohydrate intake were more likely to be older, hypertensive, and alcohol abstainers and have a lower education level (Table 1). In men, higher carbohydrate intake was also associated with smoking, and in women, higher carbohydrate intake was associated with being physically inactive and postmenopausal without a history of HRT use.
TABLE 1.
Baseline characteristics according to quintiles of carbohydrate intake in men and women
| Men |
Women |
|||||
| Quintile 1 (low) | Quintile 3 | Quintile 5 (high) | Quintile 1 (low) | Quintile 3 | Quintile 5 (high) | |
| n | 4688 | 4572 | 5068 | 6005 | 6122 | 5626 |
| Median carbohydrate intake (% of energy) | 49.6 | 59.2 | 68.8 | 50.0 | 59.2 | 68.5 |
| Age at interview (y) | 55.0 ± 7.61 | 56.2 ± 7.9 | 57.3 ± 8.0 | 53.8 ± 7.4 | 55.7 ± 7.8 | 58.0 ± 8.2 |
| BMI (kg/m2) | 22.9 ± 3.3 | 22.8 ± 3.1 | 22.8 ± 3.2 | 23.2 ± 3.4 | 23.1 ± 3.2 | 23.1 ± 3.3 |
| Sleep duration (h/d) | 7.0 ± 1.1 | 7.0 ± 1.1 | 7.1 ± 1.1 | 7.0 ± 1.1 | 7.0 ± 1.1 | 6.9 ± 1.1 |
| Hypertension [n (%)] | 840 (18) | 940 (21) | 1050 (21) | 1134 (19) | 1225 (20) | 1170 (21) |
| Alcohol consumption [n (%)] | ||||||
| 0 g | 2396 (51) | 3126 (68) | 4047 (80) | 5123 (85) | 5570 (91) | 5298 (94) |
| >0 but <5 g | 887 (19) | 914 (20) | 791 (16) | 653 (11) | 448 (7) | 294 (5) |
| ≥5 g | 1405 (30) | 532 (12) | 230 (5) | 229 (4) | 104 (2) | 34 (1) |
| Educational level [n (%)] | ||||||
| No formal education | 440 (9) | 452 (10) | 662 (13) | 1844 (31) | 2333 (38) | 2744 (49) |
| Primary school education | 2213 (47) | 2342 (51) | 2804 (55) | 2363 (39) | 2453 (40) | 2111 (38) |
| Secondary/A levels/university | 2035 (43) | 1778 (39) | 1602 (32) | 1798 (30) | 1336 (22) | 771 (14) |
| Smoking status [n (%)] | ||||||
| Nonsmoker | 1779 (38) | 2008 (44) | 2243 (44) | 5535 (92) | 5660 (92) | 5083 (90) |
| Ex-smoker | 854 (18) | 933 (20) | 1091 (22) | 125 (2) | 118 (2) | 131 (2) |
| Current smoker (1–12 cigarettes/d) | 571 (12) | 599 (13) | 685 (14) | 234 (4) | 225 (4) | 283 (5) |
| Current smoker (≥13 cigarettes/d) | 1484 (32) | 1032 (23) | 1049 (21) | 111 (2) | 119 (2) | 129 (2) |
| Dialect group [n (%)] | ||||||
| Cantonese | 2013 (43) | 2062 (45) | 2328 (46) | 2892 (48) | 2896 (47) | 2727 (48) |
| Hokkien | 2675 (57) | 2510 (55) | 2740 (54) | 3113 (52) | 3226 (53) | 2899 (52) |
| Leisure physical activity [n (%)] | ||||||
| No moderate or vigorous activity | 3226 (69) | 3010 (66) | 3495 (69) | 4532 (75) | 4709 (77) | 4501 (80) |
| <2 h/wk vigorous or <4 h/wk moderate | 774 (17) | 869 (19) | 878 (17) | 897 (15) | 865 (14) | 675 (12) |
| ≥2 h/wk vigorous or ≥4 h/wk moderate | 688 (15) | 693 (15) | 695 (14) | 576 (10) | 548 (9) | 450 (8) |
| Menopausal status and HRT2 use [n (%)] | ||||||
| Premenopausal | — | — | — | 2296 (38) | 1868 (31) | 1217 (22) |
| Postmenopausal, no HRT | — | — | — | 3353 (56) | 3966 (65) | 4184 (74) |
| Postmenopausal, HRT | — | — | — | 355 (6) | 287 (5) | 224 (4) |
Mean ± SD (all such values).
HRT, hormone-replacement therapy.
Total carbohydrate intake was inversely correlated to intakes of protein, total fat, meat, fish, and soy protein (Table 2). Furthermore, total carbohydrate intake was highly correlated with starch and rice intakes, but not with intakes of monosaccharides and disaccharides or fiber. Fiber intake was directly correlated with intake of monosaccharides and disaccharides, fruit, and vegetables.
TABLE 2.
Spearman's correlations between carbohydrate intakes and nutrient and food intakes1
| Carbohydrates |
Starch |
Monosaccharides and disaccharides |
Dietary fiber |
|||||
| M | F | M | F | M | F | M | F | |
| Carbohydrates | 1.00 | 1.00 | 0.76 | 0.70 | — | — | — | — |
| Starch | 0.76 | 0.70 | 1.00 | 1.00 | −0.62 | −0.63 | −0.34 | −0.43 |
| Monosaccharides and disaccharides | — | — | −0.62 | −0.63 | 1.00 | 1.00 | 0.48 | 0.53 |
| Dietary fiber | — | — | −0.34 | −0.43 | 0.48 | 0.53 | 1.00 | 1.00 |
| Protein | −0.73 | −0.81 | −0.51 | −0.55 | — | — | — | — |
| Total fat | −0.84 | −0.93 | −0.71 | −0.78 | — | — | 0.19 | 0.17 |
| Cholesterol | −0.61 | −0.61 | −0.45 | −0.41 | — | — | — | −0.18 |
| P:S2 | — | — | — | — | — | — | 0.25 | 0.20 |
| Rice | 0.70 | 0.68 | 0.91 | 0.90 | −0.54 | −0.50 | −0.45 | −0.50 |
| Noodles | −0.31 | −0.32 | −0.20 | −0.16 | — | — | — | — |
| Vegetables | −0.27 | −0.34 | −0.31 | −0.39 | — | — | 0.46 | 0.41 |
| Fruit | — | — | −0.28 | −0.39 | 0.55 | 0.63 | 0.77 | 0.74 |
| Red meat | −0.51 | −0.45 | −0.31 | −0.21 | — | −0.17 | −0.19 | −0.24 |
| Soy protein | −0.30 | −0.38 | −0.33 | −0.39 | — | — | 0.32 | 0.23 |
| Fish | −0.42 | −0.45 | −0.30 | −0.28 | — | — | — | — |
| Poultry | −0.40 | −0.38 | −0.25 | −0.22 | — | — | — | −0.17 |
| Eggs | −0.26 | −0.23 | −0.19 | — | — | — | — | — |
| Legumes | — | — | — | — | 0.18 | — | 0.21 | — |
| White bread3 | — | — | — | — | — | — | — | — |
| Whole-wheat bread3 | — | — | — | — | — | — | 0.34 | 0.31 |
Only correlations with an absolute r ≥ 0.15 are shown for the purpose of simplicity. All displayed correlations were significant at P ≤ 0.05. Spearman's correlations were computed by using energy-adjusted variables.
P:S, ratio of PUFA to SFA.
Correlations were obtained by using partial correlations with adjustment for total energy intake.
Total carbohydrate intake was not significantly associated with IHD mortality in age-adjusted, lifestyle-adjusted, and dietary intake–adjusted models in either men or women (Table 3). To examine risk of participants with very high carbohydrate intake, we examined IHD risk across deciles of carbohydrate intake in fully adjusted models. We observed similar RR estimates [men: HR, 0.93 (95% CI: 0.67, 1.30); women: HR, 1.08 (95% CI: 0.71, 1.65)] for the highest (men: 71.73% of energy from carbohydrates; women: 70.77% of energy from carbohydrates) compared with lowest deciles (men: 47.05%; women: 47.40%) of carbohydrate intake. When we examined the association between IHD mortality and types of carbohydrates, we observed differences in associations according to sex. Higher starch intake was significantly associated with higher risk of IHD death in women but not men (P-interaction by sex = 0.005).
TABLE 3.
HRs (95% CIs) of ischemic heart disease mortality across quintiles of carbohydrates, starch, monosaccharides and disaccharides, and dietary fiber1
| Quintiles of intake |
|||||||
| 1 (low) | 2 | 3 | 4 | 5 (high) | P-trend | Per 5% of energy2 | |
| Carbohydrates | |||||||
| Men (n) | 4688 | 4560 | 4572 | 4613 | 5068 | — | — |
| Median (range) intake (% of energy) | 49.64 (28.14, 53.04) | 55.33 (53.04, 57.32) | 59.18 (57.32, 61.03) | 63.05 (61.03, 65.39) | 68.80 (65.39, 86.14) | — | — |
| Age-adjusted HR3 | 1.00 | 0.94 (0.77, 1.15) | 0.82 (0.67, 1.01) | 0.82 (0.67, 1.00) | 0.84 (0.69, 1.02) | 0.19 | 0.97 (0.93, 1.01) |
| Multivariable HR4 | 1.00 | 0.95 (0.78, 1.16) | 0.82 (0.67, 1.01) | 0.82 (0.67, 1.00) | 0.83 (0.68, 1.02) | 0.19 | 0.97 (0.93, 1.01) |
| Additional adjustment for diet5 | 1.00 | 0.98 (0.80, 1.20) | 0.86 (0.69, 1.07) | 0.85 (0.68, 1.07) | 0.82 (0.64, 1.05) | 0.36 | 0.97 (0.92, 1.03) |
| Women (n) | 6005 | 6134 | 6122 | 6081 | 5626 | — | — |
| Median (range) intake (% of energy) | 50.01 (29.17, 53.04) | 55.38 (53.04, 57.32) | 59.17 (57.32, 61.03) | 63.01 (61.03, 65.39) | 68.47 (65.39, 83.10) | — | — |
| Age-adjusted HR3 | 1.00 | 0.92 (0.69, 1.21) | 0.92 (0.70, 1.21) | 1.13 (0.87, 1.46) | 1.03 (0.79, 1.34) | 0.34 | 1.03 (0.97, 1.09) |
| Multivariable HR4 | 1.00 | 0.91 (0.69, 1.21) | 0.91 (0.69, 1.20) | 1.10 (0.85, 1.43) | 1.01 (0.78, 1.31) | 0.47 | 1.02 (0.96, 1.08) |
| Additional adjustment for diet5 | 1.00 | 0.96 (0.72, 1.29) | 0.99 (0.74, 1.33) | 1.22 (0.91, 1.63) | 1.17 (0.85, 1.61) | 0.12 | 1.06 (0.99, 1.14) |
| Starch | |||||||
| Men (n) | 4128 | 4470 | 4601 | 4760 | 5542 | — | — |
| Median (range) intake (% of energy) | 30.40 (4.41, 33.80) | 36.58 (33.80, 39.03) | 41.47 (39.04, 43.88) | 46.65 (43.88, 49.82) | 54.70 (49.83, 83.00) | — | — |
| Age-adjusted HR3 | 1.00 | 1.01 (0.81, 1.26) | 1.09 (0.88, 1.35) | 1.08 (0.88, 1.34) | 1.14 (0.93, 1.39) | 0.14 | 1.02 (0.99, 1.06) |
| Multivariable HR4 | 1.00 | 1.01 (0.81, 1.26) | 1.05 (0.85, 1.31) | 1.05 (0.84, 1.31) | 1.09 (0.88, 1.34) | 0.30 | 1.02 (0.98, 1.05) |
| Additional adjustment for diet5 | 1.00 | 1.04 (0.83, 1.30) | 1.11 (0.88, 1.39) | 1.13 (0.89, 1.43) | 1.17 (0.91, 1.51) | 0.16 | 1.03 (0.99, 1.08) |
| Women (n) | 6565 | 6224 | 6093 | 5934 | 5152 | — | — |
| Median (range) intake (% of energy) | 30.01 (5.97, 33.80) | 36.61 (33.81, 39.04) | 41.45 (39.04, 43.87) | 46.51 (43.88, 49.82) | 54.21 (49.82, 80.62) | — | — |
| Age-adjusted HR3 | 1.00 | 1.29 (0.95, 1.76) | 1.34 (0.99, 1.81) | 1.68 (1.26, 2.25) | 1.76 (1.32, 2.36) | <0.01 | 1.09 (1.05, 1.14) |
| Multivariable HR4 | 1.00 | 1.24 (0.91, 1.70) | 1.24 (0.91, 1.68) | 1.57 (1.17, 2.10) | 1.61 (1.20, 2.16) | <0.01 | 1.08 (1.03, 1.13) |
| Additional adjustment for diet5 | 1.00 | 1.25 (0.91, 1.72) | 1.24 (0.91, 1.71) | 1.56 (1.14, 2.15) | 1.60 (1.13, 2.26) | 0.01 | 1.08 (1.02, 1.14) |
| Monosaccharides and disaccharides6 | |||||||
| Men (n) | 5224 | 4962 | 4740 | 4542 | 4033 | — | — |
| Median (range) intake (% of energy) | 7.28 (0, 9.20) | 10.68 (9.20, 12.05) | 13.37 (12.05, 14.79) | 16.39 (14.79, 18.41) | 21.34 (18.41, 50.43) | — | — |
| Age-adjusted HR3 | 1.00 | 0.81 (0.68, 0.97) | 0.76 (0.63, 0.91) | 0.83 (0.70, 1.00) | 0.64 (0.52, 0.79) | <0.01 | 0.90 (0.85, 0.95) |
| Multivariable HR4 | 1.00 | 0.82 (0.69, 0.98) | 0.79 (0.66, 0.95) | 0.86 (0.72, 1.04) | 0.67 (0.54, 0.82) | <0.01 | 0.91 (0.86, 0.96) |
| Additional adjustment for diet5 | 1.00 | 0.82 (0.68, 0.98) | 0.78 (0.64, 0.94) | 0.84 (0.68, 1.02) | 0.64 (0.50, 0.81) | <0.01 | 0.90 (0.84, 0.96) |
| Women (n) | 5469 | 5732 | 5954 | 6152 | 6661 | — | — |
| Median (range) intake (% of energy) | 7.20 (0, 9.20) | 10.71 (9.20, 12.05) | 13.40 (12.05, 14.79) | 16.41 (14.79, 18.41) | 21.61 (18.41, 49.11) | — | — |
| Age-adjusted HR3 | 1.00 | 0.94 (0.76, 1.17) | 0.71 (0.56, 0.90) | 0.71 (0.55, 0.90) | 0.70 (0.55, 0.90) | <0.01 | 0.86 (0.80, 0.92) |
| Multivariable HR4 | 1.00 | 0.96 (0.77, 1.20) | 0.73 (0.57, 0.93) | 0.74 (0.58, 0.95) | 0.75 (0.58, 0.96) | <0.01 | 0.87 (0.81, 0.94) |
| Additional adjustment for diet5 | 1.00 | 1.03 (0.82, 1.29) | 0.82 (0.64, 1.06) | 0.88 (0.68, 1.14) | 0.95 (0.72, 1.27) | 0.08 | 0.93 (0.86, 1.01) |
| Dietary fiber | |||||||
| Men (n) | 7097 | 4765 | 4131 | 3802 | 3706 | — | — |
| Median (range) intake (g/d) | 7.83 (0, 9.46) | 10.40 (9.46, 11.29) | 12.13 (11.30, 13.09) | 14.18 (13.09, 15.59) | 17.77 (15.59, 50.68) | — | — |
| Age-adjusted HR3 | 1.00 | 0.72 (0.60, 0.87) | 0.85 (0.71, 1.02) | 0.78 (0.64, 0.94) | 0.74 (0.61, 0.90) | <0.01 | 0.82 (0.72, 0.93) |
| Multivariable HR4 | 1.00 | 0.78 (0.65, 0.93) | 0.96 (0.80, 1.15) | 0.90 (0.74, 1.09) | 0.88 (0.72, 1.08) | 0.34 | 0.94 (0.82, 1.07) |
| Additional adjustment for diet5 | 1.00 | 0.78 (0.65, 0.93) | 0.96 (0.80, 1.16) | 0.91 (0.74, 1.10) | 0.89 (0.72, 1.10) | 0.39 | 0.94 (0.82, 1.08) |
| Women (n) | 3596 | 5929 | 6563 | 6892 | 6988 | — | — |
| Median (range) intake (g/d) | 8.50 (1.54, 9.46) | 10.46 (9.46, 11.30) | 12.15 (11.30, 13.09) | 14.18 (13.09, 15.59) | 17.81 (15.59, 49.00) | — | — |
| Age-adjusted HR3 | 1.00 | 0.90 (0.71, 1.15) | 0.76 (0.59, 0.98) | 0.63 (0.49, 0.82) | 0.56 (0.42, 0.74) | <0.01 | 0.67 (0.57, 0.78) |
| Multivariable HR4 | 1.00 | 0.95 (0.74, 1.21) | 0.81 (0.63, 1.04) | 0.69 (0.53, 0.90) | 0.64 (0.48, 0.84) | <0.01 | 0.72 (0.61, 0.85) |
| Additional adjustment for diet5 | 1.00 | 0.91 (0.72, 1.17) | 0.78 (0.61, 1.01) | 0.66 (0.51, 0.86) | 0.62 (0.46, 0.82) | <0.01 | 0.71 (0.60, 0.84) |
Data were analyzed by using Cox proportional hazards regression. P-interactions by sex in fully adjusted models were as follows: carbohydrates, 0.066; starch, 0.005; fiber, 0.001; and monosaccharides and disaccharides, 0.106.
Dietary fiber was modeled as 5 g/1000 kcal.
Adjusted for age, year of interview, father's dialect, and total energy intake.
Additional adjustment for cigarette smoking, alcohol consumption, physical activity, sleep duration, education level, BMI, history of hypertension, and, for women only, menopausal status and hormone-replacement therapy use.
Multivariable model with additional adjustment for dietary cholesterol, ratio of polyunsaturated to saturated fat, and fiber intake.
Persons with negative values for monosaccharide and disaccharide consumption were given a consumption value of 0 (n = 22).
Dietary monosaccharides and disaccharides and fiber were inversely associated with IHD mortality in both men and women in age-adjusted models (Table 3). However, in women, the association between monosaccharides and disaccharides and IHD mortality was substantially weakened after adjustment for dietary fiber. In men, monosaccharide and disaccharide intake remained inversely associated with IHD mortality after adjustment for lifestyle and dietary covariates. For dietary fiber, adjustment for smoking and hypertension weakened the association with IHD mortality risk in men, whereas in women, this association persisted in fully adjusted models (HR: 0.62; 95% CI: 0.46, 0.82; P-trend < 0.01, P-interaction < 0.01). In sensitivity analyses, we observed similar findings when we used insoluble nonstarch polysaccharides or soluble nonstarch polysaccharides (data not shown).
We also evaluated major food sources of carbohydrates and fiber. The 3 main sources of carbohydrates in this population were rice, noodles, and bread, which contributed 40.7%, 8.2% and 7.2%, respectively, to carbohydrate intake. Of primary contributors to dietary fiber intake were fruit and fruit juices (27.6%), vegetables (21.0%), and bread (10.5%). After adjustment for multiple covariates, we observed significantly higher IHD mortality risk with higher noodle consumption in both men and women (Table 4). Each daily serving increment in noodle intake was associated with 30% higher risk of IHD mortality in men and 38% higher risk of IHD mortality in women. When we examined the association between different types of noodles (dry, soupy, and fried) and risk of IHD mortality, we observed higher risk with all noodle subtypes including dry noodles [women: HR, 1.92 (95% CI: 1.04, 3.53) per daily serving increment (P-trend = 0.04); men: HR, 1.47 (95% CI: 0.95, 2.28) (P-trend 0.09)], soupy noodles [women: 1.24 (95% CI: 0.65, 2.37) (P-trend = 0.04); men, HR: 1.60 (95% CI: 1.02, 2.49) (P-trend = 0.04)], and fried noodles [women, HR: 1.43 (95% CI: 0.82, 2.48) (P-trend = 0.21); men, HR: 1.31 (95% CI: 0.89, 1.93) (P-trend = 0.17)]. Because noodle dishes were an important source of sodium intake for this population and contributed 9.5% to total estimated dietary sodium intake, we conducted sensitivity analyses to examine if the association between noodle consumption and IHD risk might have been mediated by sodium. Results were not materially altered when we adjusted for sodium intake. Noodle consumption was also not substantially associated with the consumption of Western fast foods (men: r = 0.03; women: r = 0.05), and adjustment for these foods did not materially alter results.
TABLE 4.
HRs (95% CIs) of ischemic heart disease mortality across quintiles of major food sources of carbohydrate and fiber1
| Quintiles of intake |
||||||||
| 1 (low) | 2 | 3 | 4 | 5 (high) | P-trend | Per serving increase (d) | ||
| Rice | ||||||||
| Men (n) | 4148 | 3941 | 4286 | 4415 | 6711 | — | — | |
| Median (range) intake (servings/d) | 2.35 (0, 2.97) | 3.40 (2.97, 3.75) | 4.10 (3.75, 4.43) | 4.80 (4.43, 5.24) | 6.74 (5.24, 10.26) | — | — | |
| Age-adjusted HR2 | 1.00 | 0.98 (0.78, 1.23) | 0.96 (0.77, 1.20) | 1.00 (0.81, 1.25) | 1.07 (0.88, 1.31) | 0.40 | 1.02 (0.98, 1.05) | |
| Multivariable HR3 | 1.00 | 0.95 (0.75, 1.19) | 0.92 (0.74, 1.15) | 0.95 (0.76, 1.19) | 1.00 (0.82, 1.22) | 0.84 | 1.00 (0.97, 1.04) | |
| Additional adjustment for diet4 | 1.00 | 0.96 (0.76, 1.20) | 0.95 (0.75, 1.20) | 0.98 (0.77, 1.25) | 1.02 (0.79, 1.31) | 0.97 | 1.00 (0.95, 1.06) | |
| Women (n) | 6545 | 6753 | 6408 | 6279 | 3983 | — | — | |
| Median (range) intake (servings/d) | 2.40 (0, 2.97) | 3.39 (2.97, 3.75) | 4.08 (3.75, 4.43) | 4.80 (4.43, 5.24) | 5.77 (5.24, 9.68) | — | — | |
| Age-adjusted HR2 | 1.00 | 1.14 (0.85, 1.54) | 1.40 (1.05, 1.87) | 1.28 (0.96, 1.72) | 1.55 (1.15, 2.08) | <0.01 | 1.09 (1.03, 1.16) | |
| Multivariable HR3 | 1.00 | 1.11 (0.82, 1.50) | 1.31 (0.98, 1.76) | 1.22 (0.91, 1.64) | 1.42 (1.06, 1.91) | 0.03 | 1.07 (1.01, 1.14) | |
| Additional adjustment for diet4 | 1.00 | 1.07 (0.79, 1.45) | 1.20 (0.88, 1.63) | 1.07 (0.77, 1.48) | 1.10 (0.77, 1.58) | 0.99 | 1.00 (0.92, 1.08) | |
| Noodles | ||||||||
| Men (n) | 5905 | 4348 | 4106 | 4344 | 4798 | — | — | |
| Median (range) intake (servings/d) | 0.11 (0, 0.24) | 0.31 (0.24, 0.39) | 0.47 (0.39, 0.55) | 0.66 (0.55, 0.81) | 1.08 (0.81, 4.41) | — | — | |
| Age-adjusted HR2 | 1.00 | 0.99 (0.81, 1.20) | 1.10 (0.90, 1.34) | 1.21 (1.00, 1.47) | 1.40 (1.17, 1.69) | <0.01 | 1.38 (1.20, 1.59) | |
| Multivariable HR3 | 1.00 | 0.96 (0.79, 1.17) | 1.06 (0.87, 1.29) | 1.16 (0.95, 1.41) | 1.28 (1.07, 1.55) | <0.01 | 1.28 (1.11, 1.47) | |
| Additional adjustment for diet4 | 1.00 | 0.96 (0.79, 1.18) | 1.07 (0.87, 1.31) | 1.19 (0.96, 1.46) | 1.32 (1.07, 1.62) | <0.01 | 1.30 (1.11, 1.53) | |
| Women (n) | 4788 | 6346 | 6588 | 6350 | 5896 | — | — | |
| Median (range) intake (servings/d) | 0.15 (0, 0.24) | 0.32 (0.24, 0.39) | 0.46 (0.39, 0.55) | 0.65 (0.55, 0.81) | 1.07 (0.81, 4.20) | — | — | |
| Age-adjusted HR2 | 1.00 | 1.03 (0.79, 1.34) | 0.98 (0.74, 1.28) | 1.20 (0.92, 1.57) | 1.50 (1.15, 1.96) | <0.01 | 1.48 (1.21, 1.82) | |
| Multivariable HR3 | 1.00 | 1.04 (0.80, 1.35) | 0.96 (0.73, 1.27) | 1.15 (0.88, 1.51) | 1.39 (1.07, 1.82) | <0.01 | 1.38 (1.12, 1.69) | |
| Additional adjustment for diet4 | 1.00 | 1.01 (0.77, 1.33) | 0.95 (0.71, 1.27) | 1.14 (0.86, 1.53) | 1.38 (1.02, 1.85) | 0.01 | 1.38 (1.09, 1.75) | |
| Vegetables | ||||||||
| Men (n) | 6897 | 4914 | 4140 | 3873 | 3677 | — | — | |
| Median (range) intake (servings/d) | 0.45 (0, 0.62) | 0.72 (0.62, 0.81) | 0.90 (0.81, 1.01) | 1.13 (1.01, 1.29) | 1.57 (1.29, 8.72) | — | — | |
| Age-adjusted HR2 | 1.00 | 0.88 (0.74, 1.04) | 0.77 (0.63, 0.92) | 0.85 (0.70, 1.03) | 0.76 (0.62, 0.94) | 0.01 | 0.82 (0.71, 0.95) | |
| Multivariable HR3 | 1.00 | 0.92 (0.78, 1.09) | 0.82 (0.68, 0.99) | 0.89 (0.74, 1.08) | 0.83 (0.67, 1.03) | 0.06 | 0.87 (0.75, 1.00) | |
| Additional adjustment for diet4 | 1.00 | 0.92 (0.78, 1.10) | 0.80 (0.66, 0.98) | 0.87 (0.71, 1.07) | 0.84 (0.67, 1.05) | 0.11 | 0.88 (0.75, 1.03) | |
| Women (n) | 3796 | 5780 | 6554 | 6821 | 7017 | — | — | |
| Median (range) intake (servings/d) | 0.51 (0, 0.62) | 0.72 (0.62, 0.81) | 0.91 (0.81, 1.01) | 1.13 (1.01, 1.29) | 1.58 (1.29, 10.40) | — | — | |
| Age-adjusted HR2 | 1.00 | 0.88 (0.69, 1.12) | 0.77 (0.61, 0.99) | 0.76 (0.59, 0.98) | 0.59 (0.45, 0.79) | <0.01 | 0.67 (0.55, 0.82) | |
| Multivariable HR3 | 1.00 | 0.90 (0.71, 1.15) | 0.80 (0.62, 1.02) | 0.80 (0.62, 1.03) | 0.62 (0.47, 0.82) | <0.01 | 0.69 (0.57, 0.85) | |
| Additional adjustment for diet4 | 1.00 | 0.93 (0.73, 1.20) | 0.84 (0.65, 1.08) | 0.86 (0.66, 1.12) | 0.69 (0.51, 0.93) | 0.01 | 0.75 (0.60, 0.93) | |
| Fruit | ||||||||
| Men (n) | 6189 | 4517 | 4218 | 4200 | 4377 | — | — | |
| Median (range) intake (servings/d) | 0.26 (0, 0.56) | 0.73 (0.56, 0.92) | 1.10 (0.92, 1.31) | 1.56 (1.31, 1.91) | 2.54 (1.91, 12.93) | — | — | |
| Age-adjusted HR2 | 1.00 | 0.75 (0.63, 0.90) | 0.68 (0.56, 0.83) | 0.76 (0.63, 0.91) | 0.69 (0.57, 0.83) | <0.01 | 0.86 (0.80, 0.92) | |
| Multivariable HR3 | 1.00 | 0.79 (0.66, 0.95) | 0.76 (0.62, 0.92) | 0.84 (0.69, 1.02) | 0.78 (0.64, 0.95) | <0.01 | 0.90 (0.84, 0.97) | |
| Additional adjustment for diet4 | 1.00 | 0.81 (0.67, 0.97) | 0.78 (0.64, 0.95) | 0.88 (0.72, 1.08) | 0.84 (0.68, 1.04) | 0.04 | 0.92 (0.85, 1.00) | |
| Women (n) | 4504 | 6177 | 6476 | 6494 | 6317 | — | — | |
| Median (range) intake (servings/d) | 0.37 (0, 0.56) | 0.75 (0.56, 0.92) | 1.10 (0.92, 1.31) | 1.55 (1.31, 1.91) | 2.53 (1.91, 11.94) | — | — | |
| Age-adjusted HR2 | 1.00 | 0.77 (0.62, 0.97) | 0.74 (0.58, 0.94) | 0.65 (0.51, 0.84) | 0.56 (0.42, 0.73) | <0.01 | 0.77 (0.69, 0.86) | |
| Multivariable HR3 | 1.00 | 0.82 (0.65, 1.03) | 0.81 (0.63, 1.03) | 0.73 (0.56, 0.94) | 0.62 (0.47, 0.83) | <0.01 | 0.81 (0.73, 0.90) | |
| Additional adjustment for diet4 | 1.00 | 0.85 (0.67, 1.07) | 0.87 (0.68, 1.11) | 0.79 (0.61, 1.04) | 0.71 (0.52, 0.95) | <0.01 | 0.85 (0.76, 0.95) | |
| Bread | 0 | 0 to <1 | ≥1 | — | — | — | — | |
| White bread | ||||||||
| Men (n) | 5281 | 12,894 | 5326 | — | — | — | — | |
| Median (range) intake (slices/d) | 0 (0, 0) | 0.33 (0.02, 0.66) | 1.00 (1.00, 4.00) | — | — | — | — | |
| Age-adjusted HR2 | 1.00 | 1.12 (0.96, 1.31) | 1.03 (0.86, 1.24) | — | — | 0.03 | 0.88 (0.78, 0.99) | |
| Multivariable HR3 | 1.00 | 1.10 (0.94, 1.29) | 1.09 (0.90, 1.31) | — | — | 0.18 | 0.92 (0.81, 1.04) | |
| Additional adjustment for diet4 | 1.00 | 1.09 (0.92, 1.29) | 1.12 (0.90, 1.39) | — | — | 0.20 | 0.91 (0.79, 1.05) | |
| Women (n) | 6498 | 17,260 | 6210 | — | — | — | — | |
| Median (range) intake (slices/d) | 0 (0, 0) | 0.33 (0.02, 0.66) | 1.00 (1.00, 4.00) | — | — | — | — | |
| Age-adjusted HR2 | 1.00 | 1.01 (0.84, 1.23) | 0.90 (0.71, 1.14) | — | — | 0.28 | 0.91 (0.76, 1.08) | |
| Multivariable HR3 | 1.00 | 1.00 (0.83, 1.21) | 0.89 (0.70, 1.13) | — | — | 0.30 | 0.91 (0.76, 1.09) | |
| Additional adjustment for diet4 | 1.00 | 0.91 (0.74, 1.11) | 0.79 (0.60, 1.04) | — | — | 0.11 | 0.84 (0.68, 1.04) | |
| Whole-wheat bread | — | — | — | — | ||||
| Men (n) | 18,348 | 4071 | 1082 | — | — | — | — | |
| Median (range) intake (slices/d) | 0 (0, 0) | 0.33 (0.02, 0.66) | 1.00 (1.00, 4.00) | — | — | — | — | |
| Age-adjusted HR2 | 1.00 | 0.83 (0.70, 0.99) | 0.72 (0.53, 0.98) | — | — | 0.03 | 0.78 (0.63, 0.98) | |
| Multivariable HR3 | 1.00 | 0.90 (0.75, 1.07) | 0.83 (0.60, 1.13) | — | — | 0.31 | 0.89 (0.71, 1.11) | |
| Additional adjustment for diet4 | 1.00 | 0.93 (0.77, 1.12) | 0.94 (0.66, 1.33) | 0.95 | 0.99 (0.78, 1.27) | |||
| Women (n) | 22,384 | 6245 | 1339 | — | — | — | — | |
| Median (range) intake (slices/d) | 0 (0, 0) | 0.33 (0.02, 0.66) | 1.00 (1.00, 4.00) | — | — | — | — | |
| Age-adjusted HR2 | 1.00 | 0.83 (0.67, 1.03) | 0.48 (0.29, 0.80) | — | — | <0.01 | 0.53 (0.36, 0.78) | |
| Multivariable HR3 | 1.00 | 0.90 (0.72, 1.13) | 0.53 (0.31, 0.88) | — | — | 0.01 | 0.60 (0.40, 0.88) | |
| Additional adjustment for diet4 | 1.00 | 0.93 (0.74, 1.17) | 0.51 (0.30, 0.89) | — | — | 0.01 | 0.58 (0.38, 0.89) | |
Data were analyzed by using Cox proportional hazards regression. P-interactions by sex in fully adjusted models were as follows: rice, 0.018; noodles, 0.709; vegetables, 0.017; fruit, 0.019; white bread, 0.954; and whole-wheat bread, 0.085.
Adjusted for age, year of interview, father's dialect, and total energy intake.
Additional adjustment for cigarette smoking, alcohol consumption, physical activity, sleep duration, education level, BMI, history of hypertension, and, for women only, menopausal status and hormone-replacement therapy use.
Multivariable model with additional adjustment for the ratio of polyunsaturated to saturated fat and consumption of rice, noodles, vegetables, fruit, fish, red meat, poultry, eggs, legumes, soy protein, white bread, and whole-wheat bread.
Rice consumption was not significantly associated with IHD mortality in men but was associated with significantly higher risk of IHD death in age- and lifestyle-adjusted models in women (Table 4). However, this association in women was attenuated after adjustment for dietary factors such as fruit and vegetable consumption and was not statistically significant in fully adjusted models.
For both women and men, vegetable and fruit consumption was associated with lower risk of IHD death in the fully adjusted model. Each serving increment in vegetable consumption was associated with significant 25% lower IHD mortality risk in women and nonsignificant 12% lower IHD mortality risk in men (P-interaction = 0.02). Each serving increment in fruit consumption was significantly associated with 15% lower IHD mortality risk in women and 8% lower IHD mortality risk in men (P-interaction = 0.02). To better understand associations between monosaccharide and disaccharide intake and IHD mortality, we also examined the association for the following food sources other than fruit: dairy products and sweets (including candy, sugar, and jam). In contrast to fruit consumption, the consumption of dairy [men, HR: 1.00 (95% CI: 0.93, 1.07) per daily serving increment (P-trend = 0.42); women, HR: 1.04 (95% CI: 0.96, 1.12) (P-trend = 0.33)] and consumption of sweets [men, HR: 0.92 (95% CI: 0.42, 2.01) (P-trend = 0.79); women, HR: 0.97 (95% CI: 0.32, 2.99) (P-trend = 0.95)] were not substantially associated with risk of IHD death.
We also examined possible effect modifications by age and BMI for the association between dietary intakes and IHD mortality (see supplemental Table 1 under “Supplemental data” in the online issue ). We observed a significant interaction of age with fiber, vegetables, and fruit in relation to IHD risk in men (all P-interaction ≤ 0.04). In younger men, fiber [HR: 0.58 (95% CI 0.34, 0.98) for highest compared with lowest quintiles; P-trend 0.05], vegetables [HR: 0.52 (95% CI: 0.29, 0.92); P-trend = 0.11], and fruit [HR: 0.58 (95% CI 0.34, 0.98); P-trend = 0.02] were associated with lower IHD risk similar to associations observed in women. In contrast, fiber, vegetables, and fruit intakes were not substantially associated with IHD risk in older men. We also observed an effect modification by BMI for rice and noodle consumption, with stronger direct associations observed between noodles and IHD risk in leaner women and between rice and IHD risk in overweight women (all P-interaction ≤ 0.02).
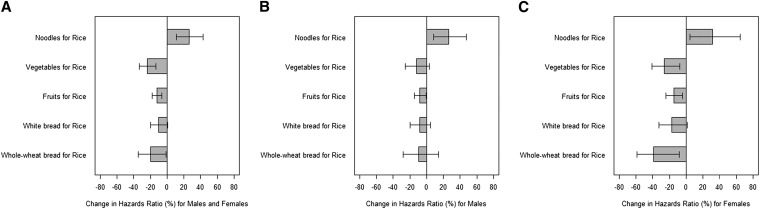
Because real-life food choices often involve the selection of one type of food over another, we modeled effects of food replacement on IHD mortality risks. We showed that the substitution of one daily serving of rice with noodles was associated with 26.11% higher risk of IHD mortality (95% CI: 10.98, 43.30%) (Figure 1A). In contrast, the substitution of one serving of rice with one serving of vegetables (−23.81%; 95% CI: −33.12% to −13.20%), fruit (−11.94%; 95% CI: −17.49%, −6.00%), or whole-wheat bread (−19.46%; 95% CI: −34.28%, −1.29%) was associated with a lower IHD mortality. For this substitution analysis, similar associations were observed for men and women (Figure 1, B and C). Because the energy provided by one serving of fruit is lower than that provided by one serving of rice or noodles, we conducted a sensitivity analyses in which we calculated risk estimates by using isocaloric servings of rice and fruit (with the assumption that one serving of rice was 100 kcal, and one serving of fruit was 50 kcal). We observed consistent, albeit stronger, associations of the replacement of 100 kcal rice with 100 kcal fruit and IHD mortality [all: −20.1% (95% CI: −29.2%, −9.8%); women: −27.4% (95% CI: −41.5%, −9.9%); men: −15.8% (95% CI: −27.3%, −2.4%)].
FIGURE 1.
Estimated percentage change in risk of ischemic heart disease mortality associated with the substitution of one serving of rice for one serving of other commonly consumed foods. Data are shown for all participants (A; n = 53,469), men (B; n = 23,501), and women (C; n = 29,968). Data were analyzed by using Cox proportional hazard regression, and substitution effects were estimated by using a published method (31).
DISCUSSION
In this large cohort of Chinese men and women with a mean follow-up of 15 y, we observed no association between total carbohydrate intake and mortality from IHD despite the substantial variation in intakes with nearly 70% of energy derived from carbohydrates for participants in the highest quintile compared with 50% of energy derived from carbohydrates for participants in the bottom quintile. In contrast, fiber intake was associated with lower risk of IHD death, particularly in women. When we examined food sources of carbohydrates, we observed that noodle consumption was associated with higher risk and fruit and vegetable consumption with lower risk of IHD death.
Results for rice consumption in relation to IHD mortality in our study were complex. High rice consumption was associated with higher risk of IHD death in women after adjustment for nondietary covariates. However, this association largely disappeared after adjustment for other foods. In this study population, in which rice is a major part of the diet, it may be difficult to disentangle effects of high rice consumption per se from effects of foods typically consumed along with rice in mixed dishes that are typical of the Chinese diet in Singapore (28). The substitution analysis provided consistent results for men and women that indicated that the replacement of rice with noodles was associated with higher risk of IHD death, whereas the replacement of rice with fruit, vegetables, or whole-wheat bread was associated with lower risk of IHD death.
Our findings of a lack of association between total carbohydrate intake and IHD mortality were consistent with evidence from studies in Europe and United States in which carbohydrate intake was not associated with risk of CVD (7, 11) or cardiovascular mortality (10, 12). In contrast, Italian women who were in the highest quartile of carbohydrate intake had 2-fold higher risk of IHD than that of women in the lowest quartile (9), whereas for men, these associations were not significant. The type of carbohydrate appeared to be an important factor because this association was only observed with carbohydrate consumption from high-GI foods (GI >57) such as bread, sugar, pizza, and rice (9).
We observed an association between high starch intake and higher IHD mortality in women but not men. These results may reflect detrimental metabolic effects of a high–glycemic load diet, which have been associated with higher risk of IHD events in women (16, 17). However, starch intake in our population was correlated with the consumption of a variety of foods that may affect risk of IHD. Although we adjusted for several relevant dietary factors, we could not fully distinguish between physiologic effects of starch and other aspects of dietary patterns associated with high starch intakes that may have differed for men and women.
High rice consumption has been associated with cardiovascular risk factors such as insulin resistance (33), lower HDL cholesterol (33), higher fasting triglycerides (33), and higher risk of type 2 diabetes (23). However, in a Japanese study, high rice intake was associated with lower risk of IHD death in men although this result was not observed in women (26). Because of the high rice consumption and large contrasts in consumption within that study, a key issue is what other foods rice displaced.
It is unlikely that the association between noodle consumption and higher risk of IHD in our study was solely the result of the carbohydrate composition of noodles because rice was a more-important contributor to starch (57%) and carbohydrate (41%) intakes than were noodles (11% and 8%, respectively). In Singapore, noodle-dishes are often prepared with lard or palm oil and tend to contain high amounts of salt, which may contribute to higher IHD risk. Indeed, the composition of many of these dishes with regard to saturated fat, cholesterol, fiber, and sodium contents is comparable to Western fast foods (see supplemental Table 2 under “Supplemental data” in the online issue). Data on health effects of noodle consumption are limited. In Korean studies, dietary patterns characterized by high noodle consumption were associated with lower fiber intake, a higher plasma cholesterol concentration, and a higher waist circumference (34, 35). In contrast, noodle consumption was not associated with type 2 diabetes risk in a Japanese cohort, which may have been related to differences in the composition of noodle dishes (eg, whole-grain buckwheat noodles in Japan) (29).
Our findings of inverse associations of dietary fiber and its primary food sources (ie, fruit and vegetables) with IHD risk were consistent with those observed in cohorts in Western countries (36, 37) and Japan (38–40). For example, in a meta-analysis of prospective studies from the Unites States and Finland, each portion increment was associated with 7% lower IHD risk for fruit consumption and 11% lower IHD risk for vegetable consumption (41). We observed similar estimates for men and stronger associations for women. Dietary fiber can potentially reduce IHD risk through multiple mechanisms including an improved plasma lipoprotein profile, decreased insulin resistance, and reduced blood pressure (42, 43). Apart from dietary fiber, fruit and vegetables have other compounds such as vitamins, minerals, such as potassium, and phenolic compounds that may exert beneficial effects on the cardiovascular system (42).
Intakes of monosaccharides and disaccharides in our cohort were highly correlated with fruit consumption, and this result may largely explain the inverse association of monosaccharides and disaccharides and IHD mortality. Intakes of other sources of monosaccharides and disaccharides such as dairy, jam, sugars, and candy were much lower and not substantially associated with IHD mortality in our study.
Although we observed some differences in the association between carbohydrate sources and IHD risk by age and BMI status, these differences were inconsistent and may have been chance findings. Therefore, additional studies are required to confirm the observed interactions.
Strengths of this study included its prospective design, large size, long follow-up period, and detailed information on dietary exposures and potential confounders obtained by in-person interviews at enrollment. The prospective study design with a minimal loss to follow-up reduced the likelihood of a recall bias or bias that was attributable to a loss to follow-up. Our results were not materially altered after the exclusion of the first 5 y follow-up, which suggested a limited bias because of undiagnosed IHD at baseline. However, our findings have to be interpreted within the context of limitations that are common to many nutritional epidemiologic studies. Because of the observational nature of the study, we could not exclude the possibility that residual confounding from unmeasured or imperfectly measured potential confounders contributed to observed associations. Adjustment for multiple confounders may be considered a subjective exercise; however, we also present results from simpler models to aid in the transparency and interpretation of our findings. It is inevitable that studied dietary factors would have been measured with some error. Because of the prospective design, this measurement error was likely to be nondifferential with respect to death status and would have weakened rather than strengthened observed associations. A modest agreement between dietary estimates as measured by the FFQ and 24-h food recalls in our study is a well-recognized challenge in nutrition research, and the agreement in our study was similar to estimates from other Western and Asian studies (26, 29, 44). In addition to limitations of the FFQ, a modest agreement with the reference instrument may have been partly attributed to differences in the time period assessed by using these 2 methods (45) and the measurement error that affected the reference instrument (46). Dietary intakes were recorded only at baseline. Thus, changes in dietary intakes during the study may have led to a nondifferential misclassification, which could have potentially underestimated the diet-risk association. Results from cohort studies with repeated dietary assessments have suggested that accounting for dietary changes over time improves precision but does not substantially alter the nature of the association with risk of IHD (47). Although we separately asked about whole-wheat and white breads, we did not distinguish between whole-grain and refined types of rice and noodles in our dietary questionnaire because, until recently, the consumption of brown rice and whole-grain noodles was not common in Singapore. We also did not have accurate data on dietary sodium intake and, therefore, were unable to examine with confidence whether observed associations were partly mediated by sodium. Because of these limitations, replications of these findings in other Asian populations are important.
In conclusion, taken together, these findings suggest that diets high in carbohydrates are associated with neither lower nor higher risk of IHD mortality in Chinese adults. However, our results support recommendations to replace refined rice and noodles with fiber-rich foods such as fruit, vegetables, and whole-grain products. Data on noodle consumption and CVD has been sparse, and our observation that the consumption of noodle dishes is associated with higher risk of IHD death warrants more attention for the nutritional quality of these dishes in Asian populations. More broadly, our findings on noodles and the poor nutritional composition of commonly consumed noodle dishes in Singapore highlight that, in addition to Western fast foods (48), unhealthy versions of popular local foods may contribute to higher IHD risk in Asian populations. Our study suggests that emphasizing diets high in fruit, vegetables, and whole grains and improving the nutritional quality of noodle-based dishes may contribute to lowering IHD mortality in Chinese populations.
Supplementary Material
Acknowledgments
We are grateful to Siew-Hong Low of the National University of Singapore for her supervision of the fieldwork of the Singapore Chinese Health Study and Kazuko Arakawa and Renwei Wang for the development and maintenance of the cohort-study database. We also thank the Ministry of Health in Singapore for assistance with the identification of outcomes via database linkages. Finally, we acknowledge Mimi C Yu, who is the founding, long-standing principal investigator of the Singapore Chinese Health Study.
The authors’ responsibilities were as follows—SAR: had primary responsibility for writing the manuscript; HK and CC: analyzed data; RMvD: developed the analytical plan, co-wrote and reviewed the manuscript, and directed the work; and all authors: reviewed and edited the manuscript and approved the final version of the manuscript. None of the authors declared a conflict of interest.
Footnotes
Abbreviations used: CVD, cardiovascular disease; FFQ, food-frequency questionnaire; GI, glycemic index; HRT, hormone-replacement therapy; IHD, ischemic heart disease.
REFERENCES
- 1.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet 2010;375:408–18. [DOI] [PubMed] [Google Scholar]
- 2.Mohan V, Radhika G, Vijayalakshmi P, Sudha V. Can the diabetes/cardiovascular disease epidemic in India be explained, at least in part, by excess refined grain (rice) intake? Indian J Med Res 2010;131:369–72. [PubMed] [Google Scholar]
- 3.Zhou BF, Stamler J, Dennis B, Moag-Stahlberg A, Okuda N, Robertson C, Zhao L, Chan Q, Elliott P. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens 2003;17:623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gadgil MD, Appel LJ, Yeung E, Anderson CA, Sacks FM, Miller ER., 3rd The effects of carbohydrate, unsaturated fat, and protein intake on measures of insulin sensitivity: results from the OmniHeart trial. Diabetes Care 2013;36:1132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005;294:2455–64. [DOI] [PubMed] [Google Scholar]
- 6.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 1992;12:911–9. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Willett WC, Stampfer MJ, Hu FB, Franz M, Sampson L, Hennekens CH, Manson JE. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr 2000;71:1455–61. [DOI] [PubMed] [Google Scholar]
- 8.Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ 2012;344:e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieri S, Krogh V, Berrino F, Evangelista A, Agnoli C, Brighenti F, Pellegrini N, Palli D, Masala G, Sacerdote C, et al. Dietary glycemic load and index and risk of coronary heart disease in a large Italian cohort: the EPICOR study. Arch Intern Med 2010;170:640–7. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson LM, Winkvist A, Eliasson M, Jansson JH, Hallmans G, Johansson I, Lindahl B, Lenner P, Van Guelpen B. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur J Clin Nutr 2012;66:694–700. [DOI] [PubMed] [Google Scholar]
- 11.Beulens JW, de Bruijne LM, Stolk RP, Peeters PH, Bots ML, Grobbee DE, van der Schouw YT. High dietary glycemic load and glycemic index increase risk of cardiovascular disease among middle-aged women: a population-based follow-up study. J Am Coll Cardiol 2007;50:14–21. [DOI] [PubMed] [Google Scholar]
- 12.Lagiou P, Sandin S, Weiderpass E, Lagiou A, Mucci L, Trichopoulos D, Adami HO. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J Intern Med 2007;261:366–74. [DOI] [PubMed] [Google Scholar]
- 13.Sjögren P, Becker W, Warensjo E, Olsson E, Byberg L, Gustafsson IB, Karlstrom B, Cederholm T. Mediterranean and carbohydrate-restricted diets and mortality among elderly men: a cohort study in Sweden. Am J Clin Nutr 2010;92:967–74. [DOI] [PubMed] [Google Scholar]
- 14.Trichopoulou A, Psaltopoulou T, Orfanos P, Hsieh CC, Trichopoulos D. Low-carbohydrate-high-protein diet and long-term survival in a general population cohort. Eur J Clin Nutr 2007;61:575–81. [DOI] [PubMed] [Google Scholar]
- 15.Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr 2001;73:61–7. [DOI] [PubMed] [Google Scholar]
- 16.Mirrahimi A, de Souza RJ, Chiavaroli L, Sievenpiper JL, Beyene J, Hanley AJ, Augustin LS, Kendall CW, Jenkins DJ. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta-analysis of prospective cohorts. J Am Heart Assoc 2012;1:e000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan J, Song Y, Wang Y, Hui R, Zhang W. Dietary glycemic index, glycemic load, and risk of coronary heart disease, stroke, and stroke mortality: a systematic review with meta-analysis. PLoS ONE 2012;7:e52182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffen LM, Jacobs DR, Jr, Stevens J, Shahar E, Carithers T, Folsom AR. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:383–90. [DOI] [PubMed] [Google Scholar]
- 19.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr 2012;142:1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slavin JL, Jacobs D, Marquart L, Wiemer K. The role of whole grains in disease prevention. J Am Diet Assoc 2001;101:780–5. [DOI] [PubMed] [Google Scholar]
- 22.Kataoka M, Venn BJ, Williams SM, Te Morenga LA, Heemels IM, Mann JI. Glycaemic responses to glucose and rice in people of Chinese and European ethnicity. Diabet Med 2013;30:e101–7. [DOI] [PubMed] [Google Scholar]
- 23.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ 2012;344:e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang W, Lee AH, Binns CW. White rice-based food consumption and ischemic stroke risk: a case-control study in southern China. J Stroke Cerebrovasc Dis 2010;19:480–4. [DOI] [PubMed] [Google Scholar]
- 25.Oba S, Nagata C, Nakamura K, Fujii K, Kawachi T, Takatsuka N, Shimizu H. Dietary glycemic index, glycemic load, and intake of carbohydrate and rice in relation to risk of mortality from stroke and its subtypes in Japanese men and women. Metabolism 2010;59:1574–82. [DOI] [PubMed] [Google Scholar]
- 26.Eshak ES, Iso H, Date C, Yamagishi K, Kikuchi S, Watanabe Y, Wada Y, Tamakoshi A. Rice intake is associated with reduced risk of mortality from cardiovascular disease in Japanese men but not women. J Nutr 2011;141:595–602. [DOI] [PubMed] [Google Scholar]
- 27.Yuan JM, Stram DO, Arakawa K, Lee HP, Yu MC. Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev 2003;12:890–8. [PubMed] [Google Scholar]
- 28.Hankin JH, Stram DO, Arakawa K, Park S, Low SH, Lee HP, Yu MC. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer 2001;39:187–95. [DOI] [PubMed] [Google Scholar]
- 29.Nanri A, Mizoue T, Noda M, Takahashi Y, Kato M, Inoue M, Tsugane S. Rice intake and type 2 diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr 2010;92:1468–77. [DOI] [PubMed] [Google Scholar]
- 30.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr . 1997;65(4 Suppl):1220S–8S; discussion 1229S–31S. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 33.Radhika G, Van Dam RM, Sudha V, Ganesan A, Mohan V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57). Metabolism 2009;58:675–81. [DOI] [PubMed] [Google Scholar]
- 34.Lee JE, Kim JH, Son SJ, Ahn Y, Lee J, Park C, Lee L, Erickson KL, Jung IK. Dietary pattern classifications with nutrient intake and health-risk factors in Korean men. Nutrition 2011;27:26–33. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Lee JE, Jung IK. Dietary pattern classifications and the association with general obesity and abdominal obesity in Korean women. J Acad Nutr Diet 2012;112:1550–9. [DOI] [PubMed] [Google Scholar]
- 36.Pereira MA, O'Reilly E, Augustsson K, Fraser GE, Goldbourt U, Heitmann BL, Hallmans G, Knekt P, Liu S, Pietinen P, et al. Dietary fiber and risk of coronary heart disease: a pooled analysis of cohort studies. Arch Intern Med 2004;164:370–6. [DOI] [PubMed] [Google Scholar]
- 37.Chuang SC, Norat T, Murphy N, Olsen A, Tjonneland A, Overvad K, Boutron-Ruault MC, Perquier F, Dartois L, Kaaks R, et al. Fiber intake and total and cause-specific mortality in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 2012;96:164–74. [DOI] [PubMed] [Google Scholar]
- 38.Takachi R, Inoue M, Ishihara J, Kurahashi N, Iwasaki M, Sasazuki S, Iso H, Tsubono Y, Tsugane S. Fruit and vegetable intake and risk of total cancer and cardiovascular disease: Japan Public Health Center-Based Prospective Study. Am J Epidemiol 2008;167:59–70. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura K, Nagata C, Oba S, Takatsuka N, Shimizu H. Fruit and vegetable intake and mortality from cardiovascular disease are inversely associated in Japanese women but not in men. J Nutr 2008;138:1129–34. [DOI] [PubMed] [Google Scholar]
- 40.Eshak ES, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Wakai K, Tamakoshi A. Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J Nutr 2010;140:1445–53. [DOI] [PubMed] [Google Scholar]
- 41.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr 2006;136:2588–93. [DOI] [PubMed] [Google Scholar]
- 42.Slavin JL, Lloyd B. Health benefits of fruit and vegetables. Adv Nutr 2012;3:506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Streppel MT, Arends LR, van 't Veer P, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med 2005;165:150–6. [DOI] [PubMed] [Google Scholar]
- 44.Willett WC, Lenart E. Reproducibility and validity of food frequency questionnaires In: Willett WC, ed. 3rd ed. Oxford, United Kingdom: Oxford University Press, 2012:96–141.
- 45.Willett WC. Commentary: flawed study designs are not salvaged by large samples. Int J Epidemiol 2008;37:987–9. [DOI] [PubMed] [Google Scholar]
- 46.Baranowski T. 24-Hour recall and diet record methods. Willett WC, ed. Nutritional epidemiology. 3rd ed Oxford, United Kingdom: Oxford University Press, 2012:49–69. [Google Scholar]
- 47.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–40. [DOI] [PubMed] [Google Scholar]
- 48.Odegaard AO, Koh WP, Yuan JM, Gross MD, Pereira MA. Western-style fast food intake and cardiometabolic risk in an Eastern country. Circulation 2012;126:182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.