Abstract
Objective:
Bone marrow (BM) is the most utilized and well-studied source of stem cells. Stem cells from dental tissues have provided an alternate source of mesenchymal stem cells (MSCs). Dental pulp stem cells (DPSCs) have been shown to share a similar pattern of protein expression with BMMSCs in vitro. However, differences have been noted between DPSCs and BMMSCs. This study focuses on variation in expression of stem cell and differentiation markers between DPSCs and BMMSCs.
Materials and Methods:
The two stem cells were isolated and compared for clonogenic potential, growth characteristics, multipotency, and stem cell marker expression. Specifically, the fatty acid binding protein 4, perilipin, alkaline phosphatase and osteonectic gene expression was analyzed by real-time polymerase chain reaction to confirm the capacity for adipogenic and osteogenic differentiation.
Results:
MSCs from these cell sources were similar in their morphology and immune phenotype except for the expression of CD105. Growth curves and colony formation assay revealed proliferation rate of DPSCs was significantly faster than BMMSCs (P < 0.05). DPSCs appeared less able to differentiate into adipogenic lineage, although more able to differentiate into osteogenic lineage.
Conclusion:
Data from the present study indicate how DPSCs are different from BMMSCs though they are a population of MSCs. DPSCs are a novel population of MSCs as observed by their unique expression of differentiation and lineage specific genes. Further microarray analysis could be used to determine, which genes are differentially regulated in BMMSCs and DPSCs to establish uniqueness of each population of MSCs.
Keywords: Bone marrow, comparative analysis, dental pulp, differentiation, mesenchymal stem cells
INTRODUCTION
Mesenchymal stem cells (MSCs) isolated from bone marrow (BM) were originally reported by Friedenstein et al.[1] and later isolated from multiple tissues such as adipose tissue,[2] skin,[3] dental pulp,[4] periodontal ligament,[5] muscle,[6] umbilical cord blood,[7] and placenta.[8] They are characterized as cells with multipotency and thought to be a promising candidate for novel cell-based therapeutic strategies including regenerative medicine. The in vitro multipotency of MSCs may depend on their source and donor,[9,10,11] which suggests that they may behave differently in vivo.[12] Although BM has been considered as main cell source of MSCs, BMMSCs isolation is a highly invasive and painful procedure. The number, proliferative capacity and maximal lifespan of MSCs derived from BM declines with age.[13,14] Therefore, other cell sources of MSCs are being extensively investigated. Dental stem cells have emerged in the recent past as an alternate source of MSCs as they can differentiate into odontoblasts, adipocytes, neuronal-like cells, glial cells, osteoblasts, chondrocytes, melanocytes, myotubes, and endothelial cells.[15,16,17,18]
Dental pulp is a promising source of MSCs, which is obtained from impacted third molars or premolar teeth extracted for orthodontic purposes without harm to the donor. Isolated cells from dental pulp have been described as MSC-like odontogenic precursor cells with high proliferation and an ability to regenerate dentin in an immune compromised host.[4] By comparing the antigenic features of the dental pulp stem cells (DPSCs) and BMMSCs, cDNA microarray studies show that they differ in the expression of only a small number of genes.[19,20] However, DPSCs show higher self-renewal, plasticity, multipotency, and proliferation in vitro.[21] DPSCs have been shown to share a similar pattern of protein expression with BMMSCs in vitro. However, differences have been noted between DPSCs and BMMSCs.[19] Over the recent years, a variety of phenotypic markers including adhesion molecule, lineage antigens, growth factor receptors, cytokine/chemokine receptors, immune-related proteins, etc., on MSC from different origins, have been investigated.[15,19] Conflicting results emphasize the need for gathering more information to complete our understanding of DPSCs phenotype. There have been no systematic comparisons of the phenotypic characteristics in terms of putative stem cell and differentiation markers expressed by the DPSCs and BMMSCs. In this study, MSCs isolated from BM and dental pulp have been compared in terms of their morphology, stem cell marker expression, clonogenic potential, growth curves, and multipotency.
MATERIALS AND METHODS
Human BM aspirates were obtained from the sternums of patients aged between 18 and 25 years with congenital heart diseases, at the Cardiac Surgery Department of Manipal Hospital, Bangalore. Patients with cyanosis, hepatitis, severe organ dysfunction or pulmonary hypertension were excluded. Human dental pulp was obtained from third molars extracted from patients aged between 18 and 25 years, who gave their informed written consent. BM aspirates and dental pulp were obtained in accordance with university regulatory and Local Ethics Committees. BM was obtained during cardiac surgery and obtained as described elsewhere.[9] The puncture site for BM aspiration at the sternum was located in the sternal midline. The trocar with the sharp obturator of an 11-gauge and 10-cm long BM biopsy needle (Bone Marrow Harvest Needle; Angiotech, Gainsville, Florida, USA) was inserted perpendicularly into the skin incision and advanced with gentle force and rotary to-and-fro motion approximately 2 cm into the bone. After the obturator was removed, a heparinized 12-ml syringe (5000 IU heparin-sodium/10 ml BM aspirate [Heparin-Natrium Braun; B. Braun, Melsungen, Germany]) was attached and BM was aspirated.
Bone marrow mononuclear cells were then isolated from the aspirate by density gradient centrifugation (1.073 g/ml, GE Healthcare, Austria) and plated at 1 × 106 cells/cm2 in T25 culture flasks. DPSCs were isolated from teeth as described elsewhere.[4] Briefly, freshly extracted teeth were immediately cracked open, pulp tissue removed, minced into small fragments of 1 mm, and then digested in 3 mg/ml collagenase type I (Gibco-Invitrogen, Carlsbad, CA) for 1 h at 37°C. Both tissues were processed separately, and digested in solution of 2 mg/ml collagenase type I and 4 mg/ml dipase for 1 h at 37°C. The tissue pellet thus obtained was resuspended in Dulbecco's modified Eagle's Medium containing penicillin G (100 U/ml) and streptomycin (100 μg/ml) supplemented with 15% (weight/volume) fetal bovine serum (FBS) and cultured at 37°C in a humidified atmosphere of 5% CO2.[4] The cells were subcultured using 0.25% trypsin and 0.05% EDTA after reaching 80% confluency. Medium was replaced every 3 days and all experiments were performed with passage 3-5 cells.
Flow cytometry
Dental pulp stem cells and BMMSCs were characterized using flow cytometry (FACSCalibur, Becton Dickinson, San Jose, CA). Flow cytometry was done on BD FACSCalibur cytometer and data were processed with CellQuest software (Becton Dickinson Biosciences). A total of 0.5 × 106 DPSCs and BMMSCs were incubated with specific individual monoclonal antibodies, conjugated with fluorescence isothiocyanate (FITC), phycoerythrin (PE) in 250 μl phosphate buffered saline for 30 min in the dark at room temperature. The following cell surface antigens were observed CD29-FITC, CD90-FITC, CD34-FITC, CD105-PE, and CD45-peridium chlorophyll protein complex (BD Pharmingen). CD 29 and CD 90 are known stromal precursor of BM and highly expressed in MSCs.[22,23] CD105 (endoglin) is expressed in MSCs and hematopoietic stem cells. CD 34 and CD 45 are cell surface markers widely used in isolation and identification of hematopoietic stem and progenitor cells. They are expressed exclusively on hematopoietic stem cells.[24] Mouse isotype-matched IgG served as a negative control (BD Pharmingen). 100,000 labeled cells were acquired and analyzed using CellQuest software [Figure 1 (Becton Dickinson)].
Figure 1.
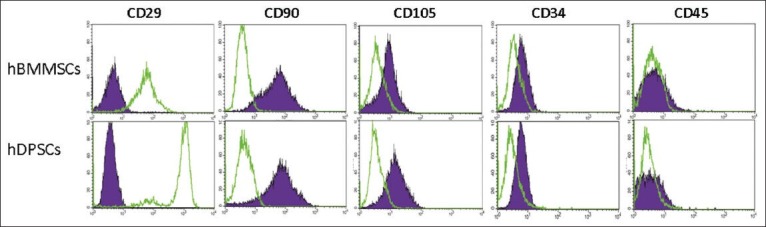
Flow cytometry results of mesenchymal stem cells (MSC) markers CD29, CD90, CD105, CD34 and CD45. Both MSCs were positive for CD29, CD90 (>90%), and negative for the leukocyte common antigen CD45 and hematopoietic lineage marker CD34 (<5%). There is a significant difference in CD105 and CD 29 expression between bone marrow mesenchymal stem cells and dental pulp stem cells (P < 0.001). The purple area represents isotype control IgG expression and green lines depict the marker expression. The results are representative of four independent experiments. Data results correspond to ±standard deviation
Cell growth characteristics
Cultured BMMSCs and DPSCs were seeded into six well plates. Cell numbers were counted and cells were passaged at 3, 6, 9, and 12 days. The cell number was calculated from 3 wells/time point per group and averaged. Growth curves were constructed based on these data [Figure 2c].
Figure 2.
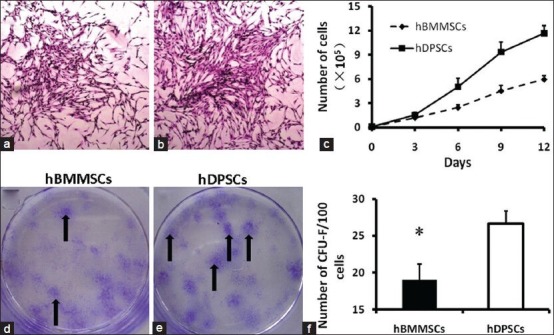
The colony formation capacity and proliferation rates of human dental pulp stem cells (DPSCs) and human bone marrow mesenchymal stem cells (BMMSCs). Representative colonies with the fibroblast-like cells of BMMSCs (a) (×100) and DPSCs (b) (×100), which were visualized by Wright-Giemsa staining (indicated by arrows d and e). (c) Growth curves of BMMSCs and DPSCs at passage 3 are depicted in the graph and it was found that growth curves of DPSCs was higher than BMMSCs. Quantification of colonies after 14 days of culture, shows more number of CFU-F in DPSCs (f)
Colony formation assay
A colony formation assay was performed by seeding cells in a six well plate (100 cells/well) in MesenCult medium (Stem Cell Technologies). After 2 weeks, cells were washed twice, fixed with 70% ethanol for 15 min and stained with 0.5% crystal violet at room temperature. Colonies containing 50 cells or more were counted, and colony efficiency was calculated [Figure 2f].
In vitro differentiation
Osteogenic differentiation
Bone marrow mesenchymal stem cells and DPSCs were seeded in six well plates, cultured to 70% confluence and then incubated in culture medium supplemented with 10-8 dexamethasone, 20 mM β-glycerophosphate, 50 μM ascorbate-2-phosphate for 4 weeks. Cultures were then fixed with 70% ethanol for 15 min and stained for mineralization with 2% alizarin red S [Figure 3]. Control cells were maintained in Iscove's modified Dulbecco's medium supplemented with 15% FBS for the same period. In addition, real-time polymerase chain reaction (PCR) was performed to assess the mRNA level of osteoblast-specific osteonectin and alkaline phosphatase (ALP) in differentiated and control cultures.
Figure 3.
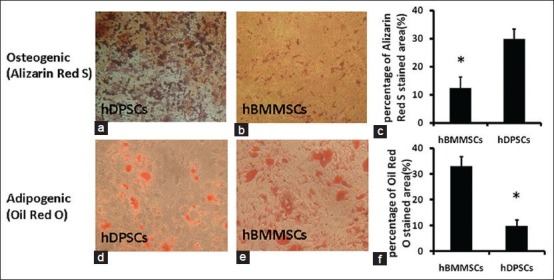
Results of osteogenic and adipogenic differentiation after 4 weeks in vitro Representative photomicrographs of cultured dental pulp stem cells (DPSCs) (a) and bone marrow mesenchymal stem cells (BMMSCs) (b) stained with alizarin red S. The photomicrographs were processed with the same contrast and brightness. Representative micrographs showing oil red-O stained lipid inclusions in cultured DPSCs (d) and BMMSCs (e). Quantitative analysis of alizarin red S and oil red-O stained areas (c and f) demonstrated that the osteogenic potential of DPSCs was stronger compared with that of BMMSCs, whereas the adipogenic potential was weaker than that of BMMSCs
Adipogenic differentiation
To induce adipogenic differentiation, subconfluent (70%) BMMSCs and DPSCs were seeded in six well plates and cultured for 4 weeks in medium supplemented with 0.5 μM isobutyl-methylxanthine, 50 μM indomethacin and 0.5 μM dexamethasone. Adipogenic differentiation was confirmed using oil red-O staining as an indicator of intracellular lipid accumulation. Adipogenic differentiation was further evaluated by real-time PCR analysis of adipocyte specific fatty acid binding protein 4 (FABP4) and perilipin mRNA.
Real-time polymerase chain reaction
Following differentiation, total RNA was extracted using Trizol reagent (Invitrogen). RNA concentration was determined by spectrophotometry. cDNA was synthesized using a reverse transcription (RT) system (Promega) according to the manufacturer's protocol. Quantitative real-time PCR was performed using an ABI PRISM 7000 sequence detection system (Applied Biosystems) with SYBR green (Applied Biosystems). Primers used were as follows: osteonectin forward: 5’-GGC ATC AAG CAG AAG GAT-3’, reverse: 5’- GCA CCG TTA ATG TAT TCA CT-3’, 183 bp; ALP forward: 5’-TAC AAG GTG GTG GGC GGT GAA CGA-3’, reverse: 5’- TGG CGC AGG GGC ACA GCA GAC-3’, 92 bp; FABP4 forward: 5’-ATG GGA TGG AAA ATC AAC CA-3’, reverse: 5’- GTG GAA GTG ACG CCT TTC AT-3’, 87 bp; perilipin forward: 5’-AAA CAG CAT CAG CGT TCC CCA TC-3’, reverse: 5’- AGT GTT GGC AGC AAA TTC CG-3’, 118 bp. Standard curves were generated for each gene including the control (housekeeping) gene. Quantitative real-time PCR was performed and analyzed as described elsewhere.[25] Since, RT-PCR provides the simultaneous measurement of gene expression in many different samples for a limited number of genes, and is especially suitable when only a small number of cells are available it was preferred. In addition, it also enables the quantification of the gene (or transcript) numbers when these are proportional to the starting template concentration.[25] Thus, in this study the RT-PCR was used to quantify the differentiation gene expression in DPSCs and BMMSCs.
Data were presented as the mean ± standard deviation. Comparison of results was performed by one-way analysis of variance. P < 0.05 was considered to be significant.
RESULTS
Isolation, growth curve and colony formation of bone marrow mesenchymal stem cells and dental pulp stem cells
Both BMMSCs [Figure 2a] and DPSCs [Figure 2b] showed a fibroblast-like, elongated, adherent and spindle-shaped morphology under a phase contrast microscope at early passages. However, the cell proliferation rate differed significantly between the two cell sources [Figure 2c]. Representative colonies were visualized by Wright-Giemsa staining indicated by arrows [Figure 2d and e] Growth curves showed that MSCs derived from dental pulp proliferated much faster than those from BM (P < 0.001). Colony formation units [Figure 2f] calculated for BMMSCs (19.00 ± 2.16%) were lower than those of DPSCs (26.67 ± 1.70%) (P < 0.001). The results are representative of four independent experiments. Data results shown correspond to ± standard deviation.
Immunophenotype characterization
Figure 1 shows the immunophenotypic characterization of MSCs from both cell sources using flow cytometry. Regardless of the cell source both BMMSCs and DPSCs expressed CD29 and CD90, which are MSC markers, while weakly expressing the hematopoietic lineage marker CD34 and leukocyte common antigen CD45. Mouse isotype-matched IgG served as a negative control. However, CD105 expression was relatively high in BMMSCs (83.14 ± 1.94%), but weakly expressed in DPSCs (34.54 ± 1.91%), whereas CD29 expression was highly expressed in DPSCs (89.1 ± 1.4%), whereas weak expression in BMMSCs (32.45 ± 1.7%).
Multilineage differentiation
Mesenchymal stem cells from dental pulp and BM differentiated into osteogenic and adipogenic lineages, which was verified by specific staining [Figure 3] and real-time PCR analysis. Quantitative analysis of alizarin red S and oil red-O stained areas [Figure 3c and f] demonstrated that the osteogenic potential of DPSCs was stronger compared with that of BMMSCs, whereas the adipogenic potential was weaker than that of BMMSCs.
Real-time polymerase chain reaction results
The expression of MSC differentiation marker genes (osteogenic: Osteonectin and ALP; adipogenic: FABP4 and perilipin) was confirmed by real-time PCR analysis. Perilipin and particularly FABP4 expression in BMMSCs was significantly higher compared with that in DPSCs [Figure 4]. However, osteonectin and ALP expression was lower than that in DPSCs. Real-time PCR results were consistent with those of specific staining.
Figure 4.
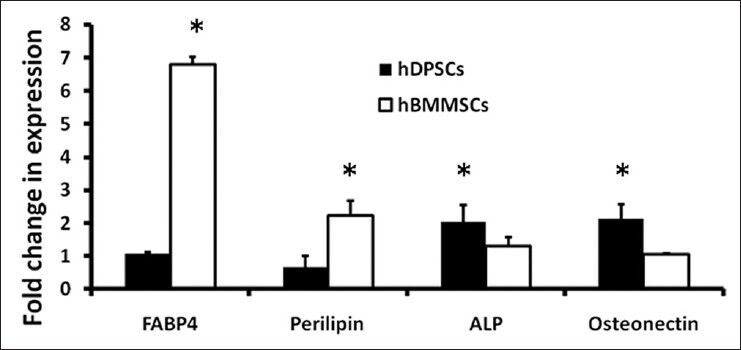
Expression of adipogenic and osteogenic lineage markers. Bone marrow mesenchymal stem cells and dental pulp stem cells were maintained in induction or control medium for 28 days and then assayed for adipogenic and osteogenic specific mRNA levels. Adipogenic differentiation markers fatty acid binding protein 4 and perilipin, and osteogenic differentiation markers alkaline phosphatase and osteonectin were analyzed by real-time polymerase chain reaction
DISCUSSION
In this study, MSCs from BM and dental pulp have been compared in terms of basic MSC characteristics. It has been observed that stem cells from mesenchymal sources exhibit similar characteristics and phenotypes and BMMSCs and DPSCs share a similar pattern of protein and gene expression in vitro.[19] There have been no systemic comparisons in the phenotypic markers and differentiation genes expressed by these two populations of MSCs. Thus, the present study attempts to study the unique phenotypes of these MSCs.
Cells isolated from dental pulp and BM both exhibited typical MSC characteristics including a fibroblastoid morphology, clonogenic potential, multipotency, and expression of a typical set of stem cell surface markers. BMMSCs and DPSCs both highly expressed classic MSC marker proteins CD29 and CD90, while lacking hematopoietic and leukocytic markers. It was observed that there were significant differences in CD105 expression, in contrast to BMMSCs, DPSCs expressed a relatively low level of CD105 [Figure 1]. It has been observed that CD105 expression declines toward the osteogenic differentiation process.[24] However, previous studies report that CD105 expression is similar between DPSCs and BMMSCs.[26,27] CD105 is associated with hematopoiesis and cell migration,[28] and its possible functional importance for stroma and homing capacities needs to be investigated further. It has been observed previously that expression of classical markers such as CD34, CD45, CD105, and CD29 differ between virtually same stem and progenitor cells, which are endothelial or MSCs, when they were obtained from different tissues.[23,29,30]
This finding raises questions whether phenotypic differences are due to the source or is only caused by different isolation and experimental conditions. Different levels of expression of certain stem cell markers will help in understanding the multilineage differentiation potential of these dental stem cells and which population can be utilized for regeneration of specific tissues such as bone, periodontal ligament, cementum, and even neurogenic tissues.
Clonogenic potential is considered a characteristic feature of MSCs. A colony formation assay and cell growth curves both demonstrated that DPSCs exhibit a significantly higher proliferation rate than that of BMMSCs and are therefore more appropriate for cell-based therapy in clinical application [Figure 2f]. This result may be due to the developmental state of the respective tissues, because DPSCs were isolated from third molars that are the last permanent teeth to develop and erupt at an earlier stage of development compared with that of BM. In the future, microarray analysis could be used to determine exactly which genes are differentially regulated in BMMSCs and DPSCs. In this study, specific staining and real-time PCR assays were used to demonstrate the multipotency of BMMSCs and DPSCs after culture in induction medium. It was demonstrated that BMMSCs and DPSCs both successfully differentiate into adipocytes and osteoblasts. In contrast to BMMSCs, DPSCs were more restricted in their adipocyte differentiation capacity, while showing a stronger ability for osteoblast differentiation. BMMSC cultures had a greater propensity to differentiate into adipocytes than did DPSC cultures under the same culture conditions [Figure 3]. After differentiation for 4 weeks, BMMSCs showed a more mature adipocyte phenotype by qualitative assessment than did DPSCs. It has been reported that some MSCs in the BM stroma may already be committed to form mature adipocytes in situ[31] and increased adipogenesis correlates with age.[32] However, DPSCs were less able to differentiate toward an adipogenic lineage in our induction medium, which might be related to the ontogenetic age of these cells. In addition, after 4 weeks of differentiation, only a small number of tiny lipid vacuoles were observed in a few DPSCs. Real-time PCR showed that adipogenic-specific markers FABP4 and perilipin were poorly expressed in DPSCs, compared with that in BMMSCs, which was consistent with morphometric assessment. FABP4 is a fatty acid-binding protein characteristically present in adipocytes, and perilipin is also regarded as an adipogenic marker. Tiny lipid vacuoles suggest that differentiation is at its initial stages, and it is highly probable that a longer culture period is necessary for adipogenic differentiation of DPSCs. Further comparative genomic or proteomic approaches are needed to assess MSC differentiation toward adipogenesis. Regarding osteogenic differentiation, the present study showed that DPSCs are capable of producing more mineralized nodules than BMMSCs after osteogenic differentiation. After incubation in induction medium for 4 weeks, cells underwent aggregation and matrix production, which positively stained with the calcium-specific marker alizarin red S. Based on morphological changes, the expression of osteogenic differentiation markers ALP and osteonectin were examined. After osteogenic differentiation of DPSCs, ALP expression increased by 1.59-fold and osteogenic expression increased by 2.04-fold, compared with those of BMMSCs [Figure 4]. This data were consistent with the specific staining observations. It has been demonstrated that ALP is an early marker of osteogenic differentiation, while osteonectin may be considered a marker for terminal differentiation.[33]
Since RT-PCR uses a single housekeeping gene was for normalization, housekeeping gene expression can vary considerably and results are highly dependent on the applied control. It seems reasonable to assume that most genes are regulated and that this will cause significant unpredictable differences in their expression patterns between and even within the same individual. If housekeeping genes are to be used, they must be validated for the specific experimental setup and it is probably necessary to choose more than one.
The successful use of DPSCs in clinical trials for bone repair enforces the notion that DPSCs are appropriate for cell therapy. In addition, further investigation to identify the mechanism of osteoblast differentiation may better explain why different sources of MSCs possess different differentiation potentials. In addition, the third molar is accessible source for collection of DPSCs for tissue engineering and regenerative medicine. Due to various factors such as gender, race, and habitation,[34,35] the wisdom teeth often cannot erupt at the suitable position and remain impacted. In theory, as the last erupted teeth, wisdom teeth have the youngest pulp and thus contain the most undifferentiated cells. Furthermore, their autologous nature will not cause an undesirable immunological response when these cells are used in cell-based therapy in clinical application. Currently, transplantation of MSCs is proposed as a promising approach for regenerative medicine. The effect of cell-based therapy may hinge on selection of an appropriate cell source of MSCs. Countless third molars are harvested and discarded at dental clinics every year, stem cells isolated from dental pulp could be an abundant cell source for regenerative medicine. Further basic research into the characteristic of MSCs is required to understand their role in vivo. This will help investigators choose most appropriate stem cell source for clinical applications in the future.
ACKNOWLEDGMENTS
The author would like to acknowledge Manipal University for supporting this study.
Footnotes
Source of Support: Manipal University.
Conflict of Interest: None declared
REFERENCES
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47. [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toma JG, Akhavan M, Fernandes KJ, Barnabé-Heider F, Sadikot A, Kaplan DR, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–84. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 6.Bosch P, Musgrave DS, Lee JY, Cummins J, Shuler T, Ghivizzani TC, et al. Osteoprogenitor cells within skeletal muscle. J Orthop Res. 2000;18:933–44. doi: 10.1002/jor.1100180613. [DOI] [PubMed] [Google Scholar]
- 7.Rogers I, Casper RF. Umbilical cord blood stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18:893–908. doi: 10.1016/j.bpobgyn.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Yen BL, Huang HI, Chien CC, Jui HY, Ko BS, Yao M, et al. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. doi: 10.1634/stemcells.2004-0098. [DOI] [PubMed] [Google Scholar]
- 9.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 10.Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006;24:679–85. doi: 10.1634/stemcells.2004-0308. [DOI] [PubMed] [Google Scholar]
- 11.Jin HJ, Park SK, Oh W, Yang YS, Kim SW, Choi SJ. Down-regulation of CD105 is associated with multi-lineage differentiation in human umbilical cord blood-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2009;381:676–81. doi: 10.1016/j.bbrc.2009.02.118. [DOI] [PubMed] [Google Scholar]
- 12.Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–6. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 13.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–26. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Fan M, Chen W, Liu W, Du GQ, Jiang SL, Tian WC, et al. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 2010;13:429–38. doi: 10.1089/rej.2009.0986. [DOI] [PubMed] [Google Scholar]
- 15.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 16.Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S. Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res. 2005;84:907–12. doi: 10.1177/154405910508401007. [DOI] [PubMed] [Google Scholar]
- 17.Chen SC, Marino V, Gronthos S, Bartold PM. Location of putative stem cells in human periodontal ligament. J Periodontal Res. 2006;41:547–53. doi: 10.1111/j.1600-0765.2006.00904.x. [DOI] [PubMed] [Google Scholar]
- 18.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J Endod. 2008;34:166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29:532–9. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y, Fujimoto A, Ito A, Yoshimi R, Ueda M. Cluster analysis and gene expression profiles: A cDNA microarray system-based comparison between human dental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials. 2006;27:3766–81. doi: 10.1016/j.biomaterials.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, et al. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80:836–42. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- 22.Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3:393–6. doi: 10.1080/146532401753277229. [DOI] [PubMed] [Google Scholar]
- 23.Lin NH, Gronthos S, Bartold PM. Stem cells and future periodontal regeneration. Periodontol 2000. 2009;51:239–51. doi: 10.1111/j.1600-0757.2009.00303.x. [DOI] [PubMed] [Google Scholar]
- 24.Janeway CA, Jr, Travers P. Antigen presentation to T lymphocytes. In: Janeway CA, Travers P, Walport M, Shlomchik M, editors. Immunobiology. 6th ed. New York: Garland Science; 2005. pp. 70–7. [Google Scholar]
- 25.Ninomiya Y, Sugahara-Yamashita Y, Nakachi Y, Tokuzawa Y, Okazaki Y, Nishiyama M. Development of a rapid culture method to induce adipocyte differentiation of human bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2010;394:303–8. doi: 10.1016/j.bbrc.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, et al. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605–15. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odabaº S, Sayar F, Güven G, Yanikkaya-Demirel G, Pişkin E. Separation of mesenchymal stem cells with magnetic nanosorbents carrying CD105 and CD73 antibodies in flow-through and batch systems. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;861:74–80. doi: 10.1016/j.jchromb.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Conley BA, Koleva R, Smith JD, Kacer D, Zhang D, Bernabéu C, et al. Endoglin controls cell migration and composition of focal adhesions: Function of the cytosolic domain. J Biol Chem. 2004;279:27440–9. doi: 10.1074/jbc.M312561200. [DOI] [PubMed] [Google Scholar]
- 29.Siggins RW, Zhang P, Welsh D, Lecapitaine NJ, Nelson S. Stem cells, phenotypic inversion, and differentiation. Int J Clin Exp Med. 2008;1:2–21. [PMC free article] [PubMed] [Google Scholar]
- 30.Ponnaiyan D, Bhat KM, Bhat GS. Comparison of immuno-phenotypes of stem cells from human dental pulp and periodontal ligament. Int J Immunopathol Pharmacol. 2012;25:127–34. doi: 10.1177/039463201202500115. [DOI] [PubMed] [Google Scholar]
- 31.Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, et al. Biology of stem cells in human umbilical cord stroma: In situ and in vitro surveys. Stem Cells. 2007;25:319–31. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- 32.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–89. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993;14:424–42. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 34.Kruger E, Thomson WM, Konthasinghe P. Third molar outcomes from age 18 to 26: Findings from a population-based New Zealand longitudinal study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:150–5. doi: 10.1067/moe.2001.115461. [DOI] [PubMed] [Google Scholar]
- 35.Eslaminejad MB, Vahabi S, Shariati M, Nazarian H. In vitro Growth and characterization of stem cells from human dental pulp of deciduous versus permanent teeth. J Dent (Tehran) 2010;7:185–95. [PMC free article] [PubMed] [Google Scholar]


