Abstract
Objective:
This in vitro study analyzed the effects of a bleaching treatment containing 35% hydrogen peroxide (HP) with or without calcium on bovine enamel, using the Knoop hardness number (KHN), tristimulus colorimetry (TC), and scanning electron microscopy.
Materials and Methods:
Forty-five specimens were randomly divided into groups (n = 5), which included artificial saliva (negative control [NC]), 35% HP (positive control [PC]), and 35% HP Blue Calcium (HP Blue). The specimens were subjected to three bleaching sessions. During the sessions, the specimens were immersed in artificial saliva at 37°C. Before and after bleaching, KHN tests were conducted using a force of 25 gf for 5 s. TC was performed using the CIE-L*a*b* system and readouts were obtained at the following 4 time points: Before the bleaching treatment; after the first session, the second session, and the third session. The specimens were dehydrated and coated with gold, and the photomicrographs were analyzed in a double-blind manner with a LEO microscope.
Results:
Using one-way analysis of variance and Tukey's test (P < 0.05), a statistically significant difference was identified between the initial and final mean KHNs of the NC and PC groups, while the initial and final mean KHNs were not significantly different in the HP Blue group. The final mean values of ΔE, ΔL, and Δb of the PC and HP Blue groups were significantly higher than the initial values (P < 0.01 for both). The photomicrographs revealed no differences among the groups.
Conclusions:
Therefore, treatment with HP Blue prevented changes in the KHN without reducing the efficacy of bleaching.
Keywords: Color, hydrogen peroxide, microhardness, scanning electron microscopy
INTRODUCTION
The evolutions of bleaching products and improved techniques have made dental whitening the most sought-after esthetic treatment because it is effective, simple, and minimally invasive when compared with other restorative treatments.[1,2]
Bleaching agents containing peroxide have been widely used for the whitening of stained and discolored teeth, both home treatment and in office bleaching and both techniques appear to be effective in treating of the dark teeth.[3,4] Bleaching occurs through an oxidation reaction that results in the dissociation of carbamide peroxide and hydrogen peroxide (HP) molecules, which penetrate the enamel, and consequently the dentine, by diffusion. Complex organic pigment molecules are cleaved into simpler and more hydrophilic molecules through oxidation-reduction reactions mediated by free radicals such as perhydroxyl, which originates from the degradation of HP. Small molecules can then leave the dental structure easily upon contact with water.[5]
Reactive oxygen species are highly reactive, with exceedingly short half-lives and the ability to target a wide range of molecules. Therefore, over a long period of time, the intimate contact between bleaching agents and dental tissues can cause adverse effects to the tissue.[6,7] For example, bleaching treatments have been reported to cause a loss of tissue components,[4,8] leading to surface changes associated with decreased calcium and fluoride content in the enamel.[9,10] Dental enamel is evaluated by the Knoop hardness number (KHN) test, which provides data regarding the loss or gain of components in the enamel. The KHN test is nondestructive in nature, as several indentations can be made in the same specimen without causing changes in the enamel during bleaching. The loss of enamel components is indicated by an increase in the size of the diagonal dental impressions produced by a diamond indenter, whilst those diagonals become smaller upon the gain of components, as it is more difficult for the indenter to penetrate the surface. The manufacturers of bleaching products add different concentrations of fluoride ions (F−) and amorphous calcium phosphate (Ca3 (PO4)2) to the composition of their products to minimize the loss of surface hardness and possible morphological changes caused by the bleaching treatment.[8,9] Theoretically, the addition of fluoride or calcium ions could saturate the bleaching agent and enable the replenishment of these ions through ion exchange, thereby restoring the acid resistance of the enamel while overcoming the adverse effects of bleaching.[11]
Several methods of analysis and quantification have been developed to compare the changes in tooth color before and after bleaching. Colorimetric analysis is an effective method for the evaluation of color changes because it measures the wavelength of the light beam reflected by an object and allows quantification of a parameter that was previously subjective.[8] The evaluation of specimen color is usually performed using the International Commission on Illumination (Commission Internationale de l’Éclairage) standard, wherein the values of the L*, a* and b* coordinates, which refer to the variation in the black and white (luminosity), red-green and blue-yellow scales, respectively, are assessed, thus enabling calculation of the total colorimetric variation (ΔE).[12,13]
The aim of this in vitro study was to determine if the presence of 2% calcium in 35% HP solution prevents a loss in dental hardness integrity and morphology during bleaching without affecting the efficacy of the treatment. The KHN, tristimulus colorimetry (TC) and scanning electron microscopy (SEM) methods were used to fully answer this question.
MATERIALS AND METHODS
Specimen preparation
The study protocol was approved by the Ethical Committee for Animal Trials (Belem, Brazil) (No. OD059-12). Bovine incisors (obtained from the Maguari Slaughterhouse, Belem, Brazil) were selected. Each tooth was cross-sectioned at the crown-root boundary along the cementoenamel junction. The crowns were separated and the roots discarded.
The buccal surfaces were sectioned using a double-sided steel disk driven by a low-speed micromotor under water cooling and were smoothed using a diamond tip to form fragments of 9 mm2 in the area and 2 mm in thickness. Fragment sizes were standardized and measured using a digital caliper. The sectioned portion always corresponded to the central area of the coronary buccal surface to ensure enamel prisms with the same inclination.
The specimen surfaces were flattened in an APL-4 polishing machine (AROTEC Ltda, São Paulo, SP, Brazil) equipped with 400-, 600-, 1200- and 2000-grit wet sandpaper. Polishing was subsequently performed using a felt disk associated with a micromotor and diamond polishing slurry.
The specimens were randomly divided into groups (n = 5) and were subjected to different bleaching treatments and experimental tests [Table 1].
Table 1.
Summary of experimental conditions
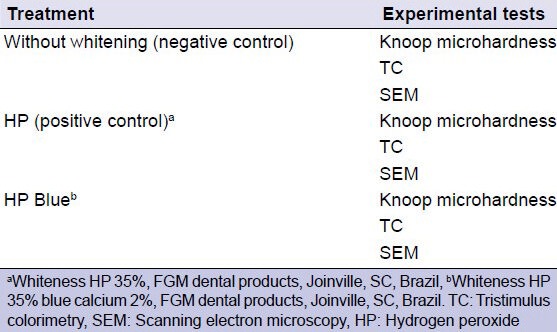
Experimental procedures
The bleaching agents were used according to the manufacturers’ recommendations. Bleaching treatments were performed in three sessions, with an interval of 7 days between each session. The Whiteness HP 35% (HP concentrate, thickener, glycol, and water) was applied 3 times for 15 min/session. The Whiteness Blue Calcium 2% (2% calcium gluconate, HP, thickener, glycol, water, and dye) was applied for 40 min/session. The specimens were stored in artificial saliva (2190 mg sodium bicarbonate, 125 mg magnesium chloride, 820 mg potassium chloride, 10 mg nipagin, 24 mg sorbitol, 1,270 mg potassium phosphate, 441 mg calcium chloride, 4.5 mg sodium fluoride, 100 mg nipasol, 8 mg carboxymethylcellulose, and 1 L distilled water)[14] at 37°C between sessions and 24 h after the last session of the bleaching treatment, and this solution was replaced daily. Specimens from the negative control group were not submitted to any whitening treatment, and the artificial saliva was replaced daily.
Knoop microhardness
Before and after the bleaching treatments, five indentations were made on each specimen with a load of 25 gf for 15 s using a Future Tech Microdurometer (Shimadzu Corporation, Kyoto, Japan). The locations of the indentations were chosen to enable the hypothetical mapping of the entire area of the test specimens, including the lateral left and right ends as well as the top, bottom, and center.
Tristimulus colorimetry
Colorimetric readouts were obtained with a Chroma Meter CR-400 Colorimetry (Tristimulus Colorimeter CR-400, Konica Minolta, NJ) and CIE L*a*b* system. These values enable an object to be located in a three-dimensional color space. The L* axis represents the degree of brightness within a sample and ranges from 0 (black) to 100 (white). The a* plane represents the degree of green/red color (−a* = green and +*a = red), while the b* plane represents the level of blue/yellow (−b* = blue and + b* = yellow).
To avoid interference, the specimens were placed on a black background that did not reflect light. Readings were taken before and after each of the three bleaching sessions, with the mean L*, a* and b* values assessed. The values of the color change (ΔE) in the CIE L*a*b* system were calculated using the following formula:
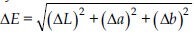 , where in ΔL = L – L0; Δa = a – a0; e Δb = b – b0
, where in ΔL = L – L0; Δa = a – a0; e Δb = b – b0
Scanning electron microscopy
For SEM, the specimens were dried, fixed to aluminum stubs and then sputter-coated with gold-palladium for 120 s. The surface morphology of the enamel was examined with a scanning electron microscope (LEO 1430, Carl Zeiss SMT, Oberkochen, Germany) at a magnification of ×500. Each photomicrograph was evaluated in a double-blind manner by three previously calibrated evaluators.
Statistical analysis
The KHN and colorimetry results were tabulated and statistically analyzed using BioEstat 5.0 software. One-way analysis of variance and Tukey's test (P < 0.05) were applied.
RESULTS
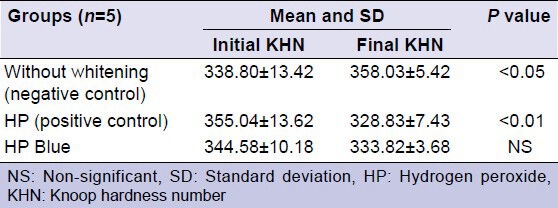
Table 2 shows the initial and final mean KHNs. The without whitening and HP groups showed statistically significant differences between the initial and final mean KHNs (P < 0.05 and P < 0.01, respectively). The initial and final mean KHNs were not significantly different in the HP Blue group.
Table 2.
Means, SD and P values
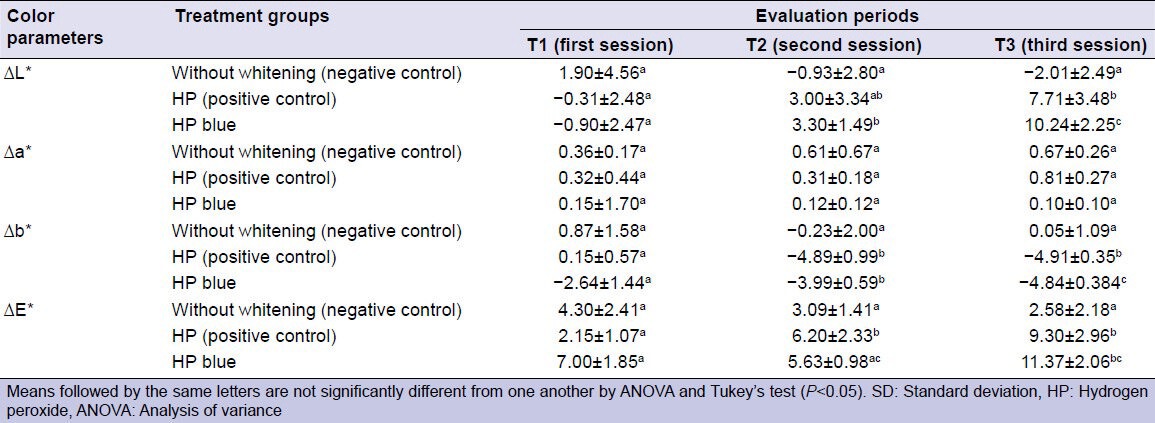
The mean ΔE, ΔL, Δa, and Δb values were also assessed for the groups [Table 3]. The mean Δa was not statistically significant between the initial and final readouts. The final mean ΔE, ΔL, and Δb values were significantly higher than the initial values for the HP (P < 0.01) and HP Blue (P < 0.01) groups.
Table 3.
Color change (ΔE*, ΔL*, Δa* and Δb*) (mean±SD) resulting from the bleaching protocols after each session (T)
The photomicrographs (×500) of the groups demonstrate intact enamel without morphological changes [Figures 1–3].
Figure 1.

Photomicrograph of unbleached specimen (control group)
Figure 3.
Photomicrograph of bleached specimen with 35% hydrogen peroxide (Whiteness HP Blue Calcium 35%)
Figure 2.
Photomicrograph of bleached specimen with 35% hydrogen peroxide (Whiteness HP 35%)
DISCUSSION
In laboratory studies, bovine teeth are accepted as surrogates for human teeth because the teeth of both species have similar physical and chemical characteristics.[15,16] The use of bovine teeth for research provides some advantages, including the high availability and the ease with which the specimen surface size can be standardized. In addition, euthanizing the animals at a certain time enables control of age and storage length of the teeth.[15]
The analysis of hardness has been the most widely used method to evaluate the effects of HP on dental enamel.[4] A decrease in hardness is typically related to the loss of mineral content caused by the clearing process.[17] Mineralization agents have been incorporated into bleaching gels to reduce or avoid adverse effects, such as morphological changes, on dental[17,18] and to provide decreased enamel solubility and sensitivity through the mineral deposition of crystals in the enamel.[19]
Hydrogen peroxide in high concentration causes demineralization of enamel, although this change is not considered clinically significant.[20,21] Previous studies have reported that the addition of calcium ions and fluoride promoted the saturation of the bleaching gel, allowing its incorporation in apatite enamel, increasing resistance to demineralization, reducing the adverse effects caused by HP.[8,22,23]
The pH of bleaching agents may cause deleterious effects on tooth structure.[24] Both bleaching materials used in this study have an initial pH of 7.0. According to the manufacturer, the Whiteness HP Blue Calcium maintains this pH during the period of stay of the product on the tooth enamel, however, the pH Whiteness HP 35% decreases to 5.5, may have favored the reduction KHN of specimens. Sun et al.[24] in their study have reported that high concentration of bleaching agents to acidic pH were effective in whitening, however, caused a decrease of KHN and morphological changes.
During bleaching, enamel demineralization is associated with the mechanism of oxidation, the pH of the peroxides and with some components of the bleaching agents.[23] The formation of free radicals occurs during the oxidation of peroxide.[25] These free radicals act nonspecifically and are able to degrade both the organic and inorganic matrix of the substrate, causing enamel loss and affecting the ionic balance that would enable the deposition of calcium on the enamel surface.[23]
CIE L*a*b* is a three-dimensional color system and the most commonly-used index for examining the efficacy of bleaching agents.[26] Some studies have reported that the color change is most affected by the parameters of L* and b*, where L* values increase and b* values tend to decrease.[18] Conversely, the values of the a* coordinate are considered to affect the bleaching process the least.[26,27] The results obtained here for ΔE, ΔL, and Δ b demonstrated that both bleaching treatments were effective, and that the addition of calcium to the bleaching agent didn't affect the effectiveness of the whitening. The changes in these parameters most likely resulted from the removal of pigments through the activity of the bleaching agents.[28]
The morphological analysis by SEM identified no differences between the bleached and control groups. The preservation of surface morphology could be explained by the presence of artificial saliva, which prevented the enamel demineralization during bleaching.[29] The effect of saliva on the loss of calcium and phosphorus (Ca-P) elemental contents after bleaching with 35% HP and reported that saliva increased the Ca-P ratio. Owing to its remineralization and buffering activities, saliva played a key role in the recovery of the tooth enamel structure and promoted the dilution of substances deleterious to tooth structure.[29]
New clinical studies should be developed in situ and in vivo to evaluate the buffering action of human saliva, due to bicarbonate and phosphate systems, containing inorganic electrolytes, such as phosphorus, calcium and fluoride, as well as enzymes and bacteria, which are unlikely to be reproduced in the laboratory.[30]
CONCLUSIONS
According to the methodology used, this study found that adding 2% calcium to 35% HP was able to prevent changes in enamel hardness and morphology without reducing bleaching efficacy.
ACKNOWLEDGMENTS
The authors would like to thank FAPESPA (Fundação Amazônia Paraense de Amaro a Pesquisa) for financing of research.
The authors are gratefully acknowledged FGM (Joinville, SC, Brazil) for providing some materials used in this study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Dietschi D, Benbachir N, Krejci I. In vitro colorimetric evaluation of the efficacy of home bleaching and over-the-counter bleaching products. Quintessence Int. 2010;41:505–16. [PubMed] [Google Scholar]
- 2.Kim YS, Kwon HK, Kim BI. Effect of nano-carbonate apatite to prevent re-stain after dental bleaching in vitro. J Dent. 2011;39:636–42. doi: 10.1016/j.jdent.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Moghadam FV, Majidinia S, Chasteen J, Havamnasiri M. The degree of color change, rebound effect and sensitivity of bleached teeth associated with at-home and power bleaching techniques: A randomized clinical trial. Eur J Dent. 2013;7:405–11. doi: 10.4103/1305-7456.120655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joiner A. The bleaching of teeth: A review of the literature. J Dent. 2006;34:412–9. doi: 10.1016/j.jdent.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Francci C, Marson FC, Briso AL, Gomes MN. Clareamento dental – Técnicas e conceitos atuais (Dental bleaching – Current concepts and techniques) Rev Assoc Paul Cir Dent. 2010;1:78–89. [Google Scholar]
- 6.Hannig C, Lindner D, Attin T. Efficacy and tolerability of two home bleaching systems having different peroxide delivery. Clin Oral Investig. 2007;11:321–9. doi: 10.1007/s00784-007-0128-x. [DOI] [PubMed] [Google Scholar]
- 7.Tredwin CJ, Naik S, Lewis NJ, Scully C. Hydrogen peroxide tooth-whitening (bleaching) products: Review of adverse effects and safety issues. Br Dent J. 2006;200:371–6. doi: 10.1038/sj.bdj.4813423. [DOI] [PubMed] [Google Scholar]
- 8.Giannini M, Silva AP, Cavalli V, Leme AF. Effect of carbamide peroxide-based bleaching agents containing fluoride or calcium on tensile strength of human enamel. J Appl Oral Sci. 2006;14:82–7. doi: 10.1590/S1678-77572006000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borges AB, Yui KC, D’Avila TC, Takahashi CL, Torres CR, Borges AL. Influence of remineralizing gels on bleached enamel microhardness in different time intervals. Oper Dent. 2010;35:180–6. doi: 10.2341/09-117-L. [DOI] [PubMed] [Google Scholar]
- 10.Fu B, Hoth-Hannig W, Hannig M. Effects of dental bleaching on micro-and nano-morphological alterations of the enamel surface. Am J Dent. 2007;20:35–40. [PubMed] [Google Scholar]
- 11.Bistey T, Nagy IP, Simó A, Hegedus C. In vitro FT-IR study of the effects of hydrogen peroxide on superficial tooth enamel. J Dent. 2007;35:325–30. doi: 10.1016/j.jdent.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Ma X, Li R, Sa Y, Liang S, Sun L, Jiang T, et al. Separate contribution of enamel and dentine to overall tooth colour change in tooth bleaching. J Dent. 2011;39:739–45. doi: 10.1016/j.jdent.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Sa Y, Chen D, Liu Y, Wen W, Xu M, Jiang T, et al. Effects of two in-office bleaching agents with different pH values on enamel surface structure and color: An in situ vs. in vitro study. J Dent. 2012;(40 Suppl 1):e26–34. doi: 10.1016/j.jdent.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima S, Yoshie M, Sano H, Bahar A. Effect of a test dentifrice containing nano-sized calcium carbonate on remineralization of enamel lesions in vitro. J Oral Sci. 2009;51:69–77. doi: 10.2334/josnusd.51.69. [DOI] [PubMed] [Google Scholar]
- 15.Leandro GA, Attia ML, Cavalli V, do Rego MA, Liporoni PC. Effects of 10% carbamide peroxide treatment and sodium fluoride therapies on human enamel surface microhardness. Gen Dent. 2008;56:274–7. [PubMed] [Google Scholar]
- 16.Zanet CG, Fava M, Alves LA. In vitro evaluation of the microhardness of bovine enamel exposed to acid solutions after bleaching. Braz Oral Res. 2011;25:562–7. doi: 10.1590/s1806-83242011000600015. [DOI] [PubMed] [Google Scholar]
- 17.Borges BC, de Vasconselos AA, Cunha AG, Pinheiro FH, Machado CT, dos Santos AJ. Preliminary clinical reports of a novel night-guard tooth bleaching technique modified by casein phosphopeptide-amorphous calcium phosphate (CCP-ACP) Eur J Esthet Dent. 2011;6:446–53. [PubMed] [Google Scholar]
- 18.de Vasconcelos AA, Cunha AG, Borges BC, Vitoriano Jde O, Alves-Júnior C, Machado CT, et al. Enamel properties after tooth bleaching with hydrogen/carbamide peroxides in association with a CPP-ACP paste. Acta Odontol Scand. 2012;70:337–43. doi: 10.3109/00016357.2011.654261. [DOI] [PubMed] [Google Scholar]
- 19.Gladwell J, Simmons D, Wright JT. Remineralization potential of a fluoridated carbamide peroxide whitening gel. J Esthet Restor Dent. 2006;18:206–12. doi: 10.1111/j.1708-8240.2006.00021_1.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee KH, Kim HI, Kim KH, Kwon YH. Mineral loss from bovine enamel by a 30% hydrogen peroxide solution. J Oral Rehabil. 2006;33:229–33. doi: 10.1111/j.1365-2842.2004.01360.x. [DOI] [PubMed] [Google Scholar]
- 21.Al-Salehi SK, Wood DJ, Hatton PV. The effect of 24h non-stop hydrogen peroxide concentration on bovine enamel and dentine mineral content and microhardness. J Dent. 2007;35:845–50. doi: 10.1016/j.jdent.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Borges AB, Samezima LY, Fonseca LP, Yui KC, Borges AL, Torres CR. Influence of potentially remineralizing agents on bleached enamel microhardness. Oper Dent. 2009;34:593–7. doi: 10.2341/08-081-L. [DOI] [PubMed] [Google Scholar]
- 23.Borges BC, Pinheiro MH, Feitosa DA, Correia TC, Braz R, Montes MA, et al. Preliminary study of a novel in-office bleaching therapy modified with a casein phosphopeptide-amorphous calcium phosphate. Microsc Res Tech. 2012;75:1571–5. doi: 10.1002/jemt.22102. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Liang S, Sa Y, Wang Z, Ma X, Jiang T, et al. Surface alteration of human tooth enamel subjected to acidic and neutral 30% hydrogen peroxide. J Dent. 2011;39:686–92. doi: 10.1016/j.jdent.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Cavalli V, Giannini M, Carvalho RM. Effect of carbamide peroxide bleaching agents on tensile strength of human enamel. Dent Mater. 2004;20:733–9. doi: 10.1016/j.dental.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Gomes MN, Francci C, Medeiros IS, De Godoy Froes Salgado NR, Riehl H, Marasca JM, et al. Effect of light irradiation on tooth whitening: Enamel microhardness and color change. J Esthet Restor Dent. 2009;21:387–94. doi: 10.1111/j.1708-8240.2009.00295.x. [DOI] [PubMed] [Google Scholar]
- 27.Westland S. Review of the CIE system of colorimetry and its use in dentistry. J Esthet Restor Dent. 2003;(15 Suppl 1):S5–12. doi: 10.1111/j.1708-8240.2003.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 28.Meireles SS, Fontes ST, Coimbra LA, Della Bona Á, Demarco FF. Effectiveness of different carbamide peroxide concentrations used for tooth bleaching: An in vitro study. J Appl Oral Sci. 2012;20:186–91. doi: 10.1590/S1678-77572012000200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abreu DE DR, Sasaki RT, Amaral FL, Flório FM, Basting RT. Effect of home-use and in-office bleaching agents containing hydrogen peroxide associated with amorphous calcium phosphate on enamel microhardness and surface roughness. J Esthet Restor Dent. 2011;23:158–68. doi: 10.1111/j.1708-8240.2010.00394.x. [DOI] [PubMed] [Google Scholar]
- 30.Tam LE, Bahrami P, Oguienko O, Limeback H. Effect of 10% and 15% carbamide peroxide on fracture toughness of human dentin in situ. Oper Dent. 2013;38:142–50. doi: 10.2341/12-127-C. [DOI] [PubMed] [Google Scholar]


