Abstract
Objective:
The aim was to evaluate the color and surface roughness of nanoparticle (C1) and nanohybrid (C2) composites after immersion in distilled water, acai juice, grape juice and red wine and repolishing.
Materials and Methods:
After recording the initial surface roughness and color, the specimens were divided into four groups according to the storage solution. The specimens were reassessed after immersion for 1, 2, 4, 8, and 12 weeks and after repolishing.
Results:
The results showed that after 2 weeks, there were statistically significant changes in color of both resins in all groups, with the exception of the specimens stored in distilled water (P > 0.05). Only 12 weeks of immersion in red wine changed the roughness of composite C1 (P = 0.009).
Conclusions:
Red wine produced the greatest color change in nanocomposites, followed by grape juice. Acai juice made the color unacceptable clinically only after 12 weeks. Repolishing reduced the color change in all groups.
Keywords: Color stability, drinks, nanocomposite, surface roughness
INTRODUCTION
With their improved esthetics, physical properties, better bonding systems, curing refinements, and environmental concerns, resin composites are now widely used for the direct restoration of both anterior and posterior teeth rather than amalgam.[1]
Resin composites have been classified according to various characteristics, such as size, content, and filler type, and the physical and mechanical properties of the materials.[2] Nanotechnology, known as molecular nanotechnology or molecular engineering, is the production of functional materials and structures, at a range of 0.1-100 nm, by various physical and chemical methods.[3] A nanohybrid is a hybrid resin composite with nanofiller in a prepolymerized filler form, whereas nanofill is a composite resin that is composed of both nanomers and nanoclusters.[2]
The success of dental restorations depends on their compressive, diametral tensile and flexural strength, wear and fracture resistance, and polish retention.[4] Moreover, the esthetics of restorative materials should mimic the appearance of natural teeth, which is directly related to color matching and color stability.[4] However, when exposed to the oral environment, restorative composites have a tendency to discolor.[5]
Color changes in resins occur as a result of intrinsic and extrinsic factors. Intrinsic factors, such as the resin matrix of composites[6] and incomplete polymerization,[7] have a considerable influence on color stability. This is usually attributed to chemical degeneration of the filler-resin bond and the solubility of the resin matrix.[8] Extrinsic factors such as adsorption or absorption of extrinsic stains, on the other hand, are still a major problem with esthetic restorations.[9] The degree of color change is affected by a number of factors, including water sorption, chemical reactivity, dietary and smoking habits, bad oral hygiene, and surface smoothness of the restoration.[10]
Besides the composition of the materials, the characteristics of the particles and the finishing and polishing procedures have a direct effect on surface smoothness and susceptibility to extrinsic staining.[9,11] Roughening of the surface caused by wear and chemical degradation may also affect the gloss and consequently increase extrinsic staining.[12] Water sorption may cause softening of the resin matrix, degradation of resin, reduction of stain resistance,[13] and changes in translucency.[14]
The longevity and esthetic appearance of tooth-colored dental restorative materials greatly depend on the quality of the finishing and polishing techniques used.[11,15] High-quality finishing and polishing improve both the esthetics and the longevity of composite restorations, whereas rough, poorly polished surfaces contribute to staining, plaque accumulation, gingival irritation, recurrent caries, and discoloration of the restoration.[15]
The aim of this in vitro study was to evaluate the effect of drinks on color stability and surface roughness of nanocomposites. In this context, laboratory tests were performed to assess the performance of nanocomposites in direct contact with different substances that are part of the daily diet. The null hypothesis is that the drinks (red wine, grape juice, and acai juice) have no effect on susceptibility to staining and the surface roughness of nanocomposites resins.
MATERIALS AND METHODS
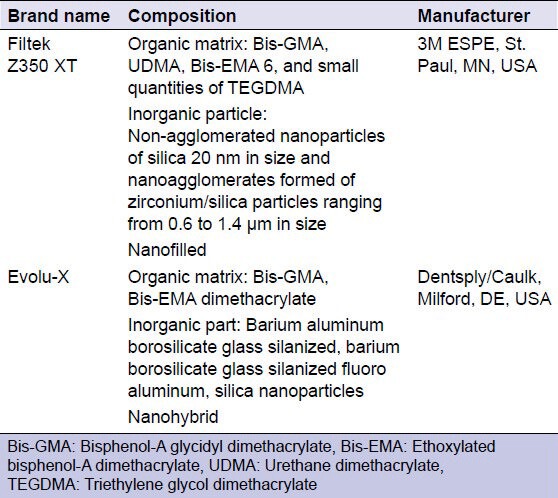
Two commercially available resin composite products, chosen for their different types of filler particles, were used in this study [Table 1].
Table 1.
The commercial brand name, composition, and manufacturer of the materials used in the study
Forty circular samples were prepared ±2.5 mm thick and ±6 mm in diameter. Uncured resin composite samples were prepared by condensing them into a Teflon ring mold in two increments according to the manufacturer's instructions. After inserting the second increment of material into the mold, a polyester strip was pressed onto the surface of the mold with a glass plate in order to obtain a flat surface without bubble formation; excess material was extruded by pressing a glass plate onto the mold with a 500-g weight on top over the resin composite/matrix ensemble, producing specimens with a smooth flat surface. The weight was removed after 1 min. The specimens were light polymerized for 20 s on each increment using conventional quartz halogen tungsten light (Optilight Plus/Gnatus). The light output of the curing light unit was 600 mW/cm2. The first increment was polymerized on a polyester strip. For the second increment, the distance between the light source and the specimen was standardized using a 1-mm glass slide. The specimens were taken out of the mold immediately after the light-curing cycle and were stored in distilled water for 24 h. The specimens were finished and polished successively with the Sof-Lex system (3M ESPE, St. Paul, MN, USA), color-coded from dark (coarse) to light (superfine): Coarse (5 s), medium (5 s), fine (20 s), and superfine (20 s). Each disk was used twice in a low-speed hand piece; they were rinsed briefly in distilled water (10 s) and dried with paper towels between each grit. The specimens were polished with felt discs (FGM, Joinville, SC, BRA) and Diamond Excel paste (FGM, Joinville, SC, BRA) for 20 s. The specimens were then washed with distilled water for 30 s, dried with paper towels and immersed in distilled water for 24 h at 37°C. The specimens were then treated as follows.
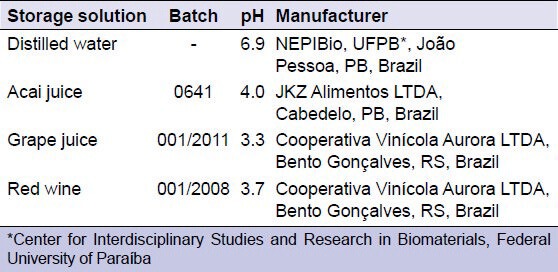
Specimens were randomized to four groups according to the storage solution: (1) Distilled water (control group); (2) acai juice; (3) grape juice; and (4) red wine. The pH of the solutions was verified using a pH meter (PHT; T-1000, TEKNA Ind/Com Ltda, São Bernardo do Campo, SP, Brazil), which indicated that the pH did not change during the period of treatment. All the procedures were carried out by the same operator at room temperature (25°C) and relative humidity of 50%. The test groups and their respective pH values are summarized in Table 2.
Table 2.
Type, batch number, pH, and manufacturers of the immersion solutions
After this treatment, the specimens were evaluated for surface roughness. The average surface roughness (Ra, μm) was measured with a surface profilometer (Surftest SJ-301, Mitutoyo, Japan) using a tracing length of 1.25 mm and a cutoff of 0.25 mm to maximize filtration surface waviness, and a measuring speed of 0.5 mm/s using Tools 301, Mitutoyo, Japan. Each specimen was measured 3 times at different locations and in different directions near the center of the specimen, and the average roughness, Ra, was derived from these readings. The three measurements were taken by rotating the specimen 90° around its center. A calibration block was used periodically to check the performance of the profilometer, and all test procedures were performed by only one operator.
Color values were recorded in sequence using a digital spectrophotometer (Vita Easyshade, Vita Zahnfabrik, Bad Säckingen, Germany). The spectrophotometer measured the tooth color based on the CIEL*a*b* color space system, which allows the color to be determined in three-dimensional space. L* represents the value (lightness or darkness). The a* value is a measure of redness (positive a*) or greenness (negative a*). The b* value is a measure of yellowness (positive b*) or blueness (negative b*). The color difference (ΔE) between the color coordinates was calculated by applying the formula ΔE * = [(ΔL*)2 + (Δa*)2 + (Δb*)2]½ in order to compare values before and after the storage treatment. Three measurements were taken with the active point of the spectrophotometer in the center of each specimen, and thus the instrument automatically averaged the three readings for each specimen, which was then used for the overall data analysis. All the procedures were performed by the same operator.
The roughness and the color were measured before (baseline) and 1, 2, 4, 8, and 12 weeks after storage in the different liquids, and after repolishing. For each group, the specimens were kept immersed in the respective solutions for 4 h daily over a period of 12 weeks. These solutions were renewed daily and after the immersion period, the specimens were washed and stored in distilled water. At the end of the periods of immersion, the samples were repolished, following the same protocol as that performed at baseline.
The values of the optical properties and surface roughness were tabulated and subjected to statistical analysis (SPSS for Windows, version 13.0; SPSS Inc., Chicago, IL, USA) using the paired t-test, one-way analysis of variance, and the Tukey test and the independent sample t-test (P < 0.05).
RESULTS
For Filtek Z350 XT, when each group was compared over time, acai juice (P = 0.003), grape juice (P = 0.03), and red wine (P = 0.003) presented statistically significant changes in the color of the resin from the 2nd week; distilled water did not show a significant change (P > 0.05). The specimens in distilled water did present differences after 8 weeks of immersion (P = 0.002). A decrease in staining was observed after repolishing in all groups (acai juice, P = 0.001; grape juice, P = 0.000; wine, P = 0.007), except the distilled water group (P > 0.05); the average staining for the distilled water group was much lower than that in the other groups throughout the experiment.
When the Filtek Z350 XT groups were compared at each time point, there was no statistically significant difference between the distilled water group and the acai juice group (P = 0.17), although there are numerical differences in the averages; these groups showed less staining compared with the other groups. There was also no significant difference between the grape juice and red wine groups (P = 0.51), however, they presented a statistically significant difference compared with the distilled water and acai juice groups (P = 0.000), which stained less. After repolishing, there was a decrease in the mean staining at 12 weeks, although the pattern of the differences between the groups remained (P = 0.000) [Table 3]; water and acai juice stained less and grape juice and red wine stained more. After repolishing, the staining was similar to the reading at the 2nd week for the distilled water and acai groups. For grape juice and red wine, the staining pattern was similar to that of the 1st week.
Table 3.
Means and standard deviations for the color difference (ΔE) between the specimens immersed in different beverages
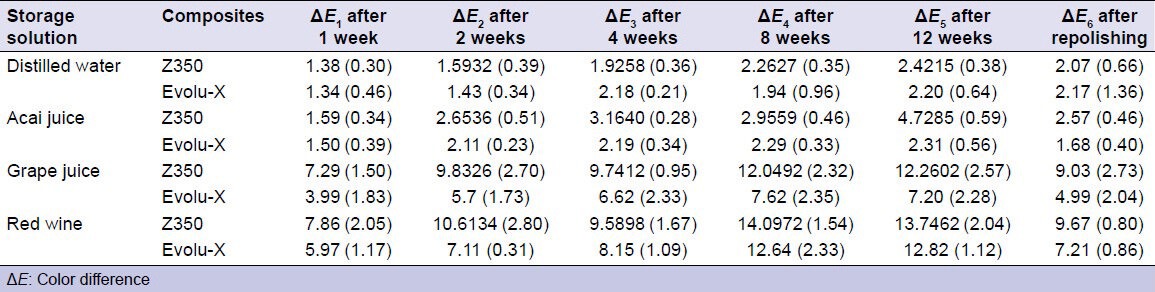
For Evolu-X, acai juice (P = 0.008), grape juice (P = 0.003), and red wine (P = 0.003) presented statistically significant changes in the color of the resin from the 2nd week; there was no change in the control group (P > 0.05). The color for the distilled water group changed after 12 weeks (P = 0.016). There was a decrease in the staining after repolishing for the grape juice (P = 0.001) and red wine groups (P = 0.000), however, the distilled water and acai juice groups did not have a statistically significant decrease in staining (P > 0.05), even though the average value of staining in these groups was much less than in others. There was no significant difference between the grape juice and red wine groups in the first 4 weeks (P > 0.05); however, the difference was statistically significant compared with the distilled water and acai juice groups (P = 0.000). From week 8, the red wine group showed more staining than the grape juice group (P = 0.000). However, there was no significant difference between the distilled water and acai juice groups (P > 0.05) and they showed less staining than the other groups. After repolishing, the mean amount of staining decreased compared with the values at 12 weeks, although the pattern of the differences between the groups remained the same (P = 0.000) [Table 3]; water and acai juice stained less and grape juice and red wine stained more. After repolishing, the staining pattern for the acai juice group was similar to that of the 1st week. For the distilled water, grape juice and red wine groups, the staining pattern was similar to that at the 2nd week.
Comparing the two resins, from the 1st week it was found that the Evolu-X specimens immersed in grape juice stained less than the Filtek Z350 XT specimens (P = 0.014). This pattern continued in weeks 2 (P = 0.020), 4 (P = 0.024), 8 (P = 0.017), and 12 (P = 0.011) and after repolishing (P = 0.002). Samples of Evolu-X resin immersed in red wine differed from the Filtek Z350 XT samples after 2 weeks (P = 0.026) and after repolishing (P = 0.002). For the acai juice group, there was a difference between the composites (P = 0.000) after week 12 and after repolishing (P = 0.011).
The Filtek Z350 XT specimens showed a difference in the roughness of the red wine group after 12 weeks of immersion (P = 0.009), but this difference was reversed after repolishing. After 8 weeks of immersion, there was no difference in the roughness of the samples immersed in different media (P > 0.05). After 12 weeks, the samples immersed in red wine had greater roughness than the samples immersed in grape juice (P = 0.005), and this difference was reversed after repolishing [Table 4].
Table 4.
Means and standard deviations for surface roughness measurements for the specimens immersed in different beverages
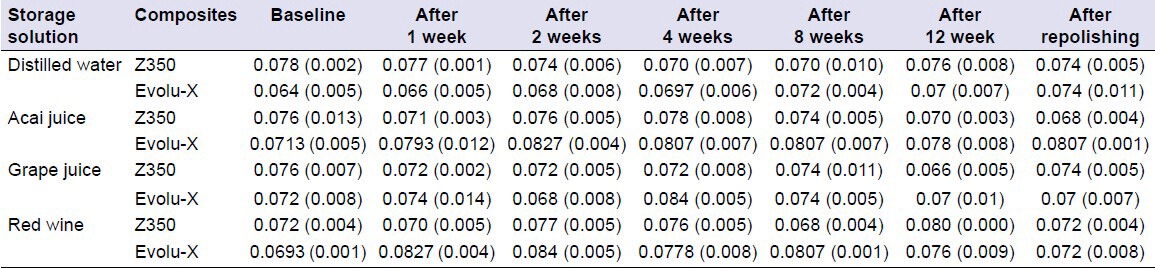
For Evolu-X, there was a statistically significant increase in the roughness of the red wine group from the 1st week (P = 0.03) and the acai juice group from the 2nd week of immersion (P = 0.039). However, repolishing reversed this pattern. After 2 weeks of immersion, there was a difference in roughness when the different groups were compared (P = 0.001). However, there was no significant difference between the distilled water and grape juice groups; the acai juice and red wine groups also did not show a statistically significant difference (P > 0.05).
Comparing the surface roughness of the two resins, Evolu-X had lower roughness than Filtek Z350 XT (initial, P = 0.002; 1 week after, P = 0.08; 12 weeks after, P = 0.025) when immersed in distilled water. In the acai juice group, the roughness of Filtek Z350 XT was lower than Evolu-X after repolishing (P = 0.000). In the grape juice group, Filtek Z350 XT was less rough in the 4th week (P = 0.02). In the red wine group, Filtek Z350 XT was less rough in the 1st week (P = 0.007) and at week 8 (P = 0.000).
DISCUSSION
The null hypothesis was rejected. There were statistical differences on staining susceptibility and the surface roughness of nanocomposites resins.
According to color differences in esthetic restorations, three different intervals were used to distinguish changes in color values: ΔE < 1, imperceptible by the human eye; 1.0 < ΔE < 3.3, appreciated only by a skilled person, clinically acceptable; and ΔE > 3.3, easily observed, not clinically acceptable.[6]
The Filtek Z350 XT and Evolu-X resins immersed in distilled water showed clinically acceptable color changes throughout the study. This observation confirms that water sorption itself did not alter the color of composites to a significant extent because distilled water has no colorant components. However, both the Filtek Z350 XT and Evolu-X resins immersed in distilled water showed statistically significant changes in color in weeks 8 and 12, respectively. This could the result of intrinsic discoloration.[16,17,18]
The Filtek Z350 XT samples immersed in acai juice showed clinically acceptable color changes in the first 8 weeks, but at week 12 the color changes were not clinically acceptable. After repolishing, staining in the Filtek Z350 XT samples was clinically acceptable. The Evolu-X samples immersed in acai juice showed clinically acceptable staining detectable only by a skilled person (1.0 < ΔE <3.3).
Both resins immersed in grape juice and red wine showed clinically unacceptable color changes from week 1. Even after repolishing, both resins showed ΔE > 3.3 for grape juice and red wine; when these color changes are easily observed, they are not clinically acceptable and replacement of restorations is required.[14]
Many studies have demonstrated that resin-based composites are susceptible to staining by common beverages, especially red wine and grape juice. In our study, red wine caused the highest discoloration of the restorative materials, followed by grape juice, acai juice, and distilled water. Foods that are rich in anthocyanins, such as blueberries, red grapes, red wine, and acai juice have a strong color. Anthocyanins are water-soluble vacuolar pigments that may appear red, purple, or blue, according to the pH.[19] It has been reported previously that alcohol causes some degradation on the surface properties of resin composites. A rougher, degraded surface provides an extensive surface area for adsorption of pigments, thereby leading to more staining.[11,20] In addition, it is likely that the lower pH of the grape juice affected the surface of the composite resin, increasing absorption of pigment.[14]
Throughout the study, Evolu-X resin showed less staining at the various times and in different immersion media compared with Filtek Z350 XT. The susceptibility of the resins to staining may be attributed to the composition of the materials and the characteristics of the particles.[9,11] The hydrophilicity and degree of water sorption of a resin matrix could affect the staining susceptibility of resin composites. If the resin composite can absorb water, then it can also absorb other fluids, which results in discoloration.[10] Water sorption occurs mainly by direct absorption into the resin matrix, whereas glass filler particles do not absorb water into the bulk of the material, but can adsorb water onto the surface. Water sorption of the resin composite may decrease the life of the restoration by expanding and plasticizing the resin component, hydrolyzing the silane, and causing the formation of microcracks. These microcracks or interfacial gaps at the interface between filler and matrix, allow stains to penetrate and cause discoloration.[21]
In this study, the advantages of nanofills did not seem to render them more stain resistant as described in other studies.[22,23,24] However, studies have shown that nanofill resins stain more than nanohybrid resins[25] and microhybrid resins.[9,25,26,27] The type of resin matrix used can be a major contributor to the discoloration of the resin composite. It was found that water uptake in bisphenol-A glycidyl dimethacrylate-based resins increased from 3% to 6% as the proportion of triethylene glycol dimethacrylate (TEGDMA) increased from 0% to 1%, respectively.[28] This study demonstrated that Filtek Z350 XT, which includes TEGDMA, was the most susceptible to staining. Evolu-X does not include TEGDMA in its monomer matrix.[16]
Low pH and alcohol may affect the surface integrity of composite resins. Absorption of alcohol molecules contained in beverages into the resin matrix could result in softening of the surface of the composite.[27] This explains the change in surface roughness of the resin when immersed in red wine.[29]
Despite the statistically significant increase in roughness at some points during this study, studies by Berger et al.[30] and Antonson et al.[31] showed that the average surface roughness of a resin nanoparticulate after polishing using the Sof-Lex system was 0.1 and 0.08, respectively. Yeh et al.[32] showed that the surface roughness of a nanohybrid resin when immersed in distilled water was 0.1. Thus, with the knowledge that roughness values between 0.06 and 0.1 are within the standard range for these composites, the change in roughness observed in this study does not seem to be relevant.
There is a hypothesis that staining occurs in the most superficial layer of the composite.[33] Thus, repolishing can possibly removing discoloration, and prevent premature replacement of the restoration. In the current study, repolishing reduced the discoloration caused by immersion in colored beverages, but was not sufficient to remove the discoloration. These results are in contrast to the results obtained by Anfe et al.[25] They concluded that staining caused by coffee and red wine was superficial and 20-μm wear was sufficient to remove the discoloration.
The solutions tested in this study do not represent all the substances to which restorative materials may be exposed in the oral environment, therefore additional studies are necessary to investigate the color stability of composite resin-based materials.[14]
CONCLUSION
According to study, it was concluded that:
Colored drinks promoted color change of Filtek Z350 XT and Evolu-X composites during the study
Both Filtek Z350 XT and Evolu-X resins showed clinically unacceptable color change after immersion in grape juice and red wine, even after repolishing, indicating a need for replacement of the restoration
Repolishing was effective in reducing the color change of the composites under study, although always to a clinically acceptable level
In general, Evolu-X composite showed less color change than Filtek Z350 XT composite in the period evaluated and after repolishing
The increase in roughness was reversed after repolishing.
ACKNOWLEDGMENT
The authors would like to acknowledge the financial support from the National Council for Scientific and Technological Development (CNPq, Brazil).
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Lee I, Chang J, Ferracane J. Slumping resistance and viscoelasticity prior to setting of dental composites. Dent Mater. 2008;24:1586–93. doi: 10.1016/j.dental.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Senawongse P, Pongprueksa P. Surface roughness of nanofill and nanohybrid resin composites after polishing and brushing. J Esthet Restor Dent. 2007;19:265–73. doi: 10.1111/j.1708-8240.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 3.Mitra SB, Wu D, Holmes BN. An application of nanotechnology in advanced dental materials. J Am Dent Assoc. 2003;134:1382–90. doi: 10.14219/jada.archive.2003.0054. [DOI] [PubMed] [Google Scholar]
- 4.Choi MS, Lee YK, Lim BS, Rhee SH, Yang HC. Changes in surface characteristics of dental resin composites after polishing. J Mater Sci Mater Med. 2005;16:347–53. doi: 10.1007/s10856-005-0634-9. [DOI] [PubMed] [Google Scholar]
- 5.Uchida H, Vaidyanathan J, Viswanadhan T, Vaidyanathan TK. Color stability of dental composites as a function of shade. J Prosthet Dent. 1998;79:372–7. doi: 10.1016/s0022-3913(98)70147-7. [DOI] [PubMed] [Google Scholar]
- 6.Vichi A, Ferrari M, Davidson CL. Color and opacity variations in three different resin-based composite products after water aging. Dent Mater. 2004;20:530–4. doi: 10.1016/j.dental.2002.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Janda R, Roulet JF, Kaminsky M, Steffin G, Latta M. Color stability of resin matrix restorative materials as a function of the method of light activation. Eur J Oral Sci. 2004;112:280–5. doi: 10.1111/j.1600-0722.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 8.Iazzetti G, Burgess JO, Gardiner D, Ripps A. Color stability of fluoride-containing restorative materials. Oper Dent. 2000;25:520–5. [PubMed] [Google Scholar]
- 9.Patel SB, Gordan VV, Barrett AA, Shen C. The effect of surface finishing and storage solutions on the color stability of resin-based composites. J Am Dent Assoc. 2004;135:587–94. doi: 10.14219/jada.archive.2004.0246. [DOI] [PubMed] [Google Scholar]
- 10.Bagheri R, Burrow MF, Tyas M. Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J Dent. 2005;33:389–98. doi: 10.1016/j.jdent.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Reis AF, Giannini M, Lovadino JR, Ambrosano GM. Effects of various finishing systems on the surface roughness and staining susceptibility of packable composite resins. Dent Mater. 2003;19:12–8. doi: 10.1016/s0109-5641(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 12.van Groeningen G, Jongebloed W, Arends J. Composite degradation in vivo. Dent Mater. 1986;2:225–7. doi: 10.1016/S0109-5641(86)80018-5. [DOI] [PubMed] [Google Scholar]
- 13.Shah MB, Ferracane JL, Kruzic JJ. R-curve behavior and toughening mechanisms of resin-based dental composites: Effects of hydration and post-cure heat treatment. Dent Mater. 2009;25:760–70. doi: 10.1016/j.dental.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Fontes ST, Fernández MR, de Moura CM, Meireles SS. Color stability of a nanofill composite: Effect of different immersion media. J Appl Oral Sci. 2009;17:388–91. doi: 10.1590/S1678-77572009000500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Başeren M. Surface roughness of nanofill and nanohybrid composite resin and ormocer-based tooth-colored restorative materials after several finishing and polishing procedures. J Biomater Appl. 2004;19:121–34. doi: 10.1177/0885328204044011. [DOI] [PubMed] [Google Scholar]
- 16.Barutcigil Ç, Yıldız M. Intrinsic and extrinsic discoloration of dimethacrylate and silorane based composites. J Dent. 2012;(40 Suppl 1):e57–63. doi: 10.1016/j.jdent.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Aguiar FH, Georgetto MH, Soares GP, Catelan A, Dos Santos PH, Ambrosano GM, et al. Effect of different light-curing modes on degree of conversion, staining susceptibility and stain's retention using different beverages in a nanofilled composite resin. J Esthet Restor Dent. 2011;23:106–14. doi: 10.1111/j.1708-8240.2011.00406.x. [DOI] [PubMed] [Google Scholar]
- 18.Luiz BK, Quintella CM, Friedrich LA, Silva EB, Veiga W, Prates LH, et al. Effect of drinks on the surface properties of dental resin composites. Poly Test. 2007;26:855–61. [Google Scholar]
- 19.Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. Fast access of some grape pigments to the brain. J Agric Food Chem. 2005;53:7029–34. doi: 10.1021/jf050565k. [DOI] [PubMed] [Google Scholar]
- 20.Azer SS, Hague AL, Johnston WM. Effect of bleaching on tooth discolouration from food colourant in vitro. J Dent. 2011;(39 Suppl 3):e52–6. doi: 10.1016/j.jdent.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Mair LH. Staining of in vivo subsurface degradation in dental composites with silver nitrate. J Dent Res. 1991;70:215–20. doi: 10.1177/00220345910700031201. [DOI] [PubMed] [Google Scholar]
- 22.Topcu FT, Sahinkesen G, Yamanel K, Erdemir U, Oktay EA, Ersahan S. Influence of different drinks on the colour stability of dental resin composites. Eur J Dent. 2009;3:50–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Pan X, Lin Y, Li Q, Hussain M, Wang Y. Effects of carbamide peroxide on the staining susceptibility of tooth-colored restorative materials. Oper Dent. 2009;34:72–82. doi: 10.2341/08-42. [DOI] [PubMed] [Google Scholar]
- 24.Rao YM, Srilakshmi V, Vinayagam KK, Narayanan LL. An evaluation of the color stability of tooth-colored restorative materials after bleaching using CIELAB color technique. Indian J Dent Res. 2009;20:60–4. doi: 10.4103/0970-9290.49071. [DOI] [PubMed] [Google Scholar]
- 25.Anfe TE, Agra CM, Vieira GF. Evaluation of the possibility of removing staining by repolishing composite resins submitted to artificial aging. J Esthet Restor Dent. 2011;23:260–7. doi: 10.1111/j.1708-8240.2011.00435.x. [DOI] [PubMed] [Google Scholar]
- 26.Ertaş E, Güler AU, Yücel AC, Köprülü H, Güler E. Color stability of resin composites after immersion in different drinks. Dent Mater J. 2006;25:371–6. [PubMed] [Google Scholar]
- 27.Villalta P, Lu H, Okte Z, Garcia-Godoy F, Powers JM. Effects of staining and bleaching on color change of dental composite resins. J Prosthet Dent. 2006;95:137–42. doi: 10.1016/j.prosdent.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Kalachandra S, Turner DT. Water sorption of polymethacrylate networks: Bis-GMA/TEGDM copolymers. J Biomed Mater Res. 1987;21:329–38. doi: 10.1002/jbm.820210306. [DOI] [PubMed] [Google Scholar]
- 29.Lepri CP, Palma-Dibb RG. Surface roughness and color change of a composite: Influence of beverages and brushing. Dent Mater J. 2012;31:689–96. doi: 10.4012/dmj.2012-063. [DOI] [PubMed] [Google Scholar]
- 30.Berger SB, Palialol AR, Cavalli V, Giannini M. Surface roughness and staining susceptibility of composite resins after finishing and polishing. J Esthet Restor Dent. 2011;23:34–43. doi: 10.1111/j.1708-8240.2010.00376.x. [DOI] [PubMed] [Google Scholar]
- 31.Antonson SA, Yazici AR, Kilinc E, Antonson DE, Hardigan PC. Comparison of different finishing/polishing systems on surface roughness and gloss of resin composites. J Dent. 2011;(39 Suppl 1):e9–17. doi: 10.1016/j.jdent.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Yeh ST, Wang HT, Liao HY, Su SL, Chang CC, Kao HC, et al. The roughness, microhardness, and surface analysis of nanocomposites after application of topical fluoride gels. Dent Mater. 2011;27:187–96. doi: 10.1016/j.dental.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Commission Internationale De L’Eclairage. Paris: Bureau Central de la CIE; 1978. Recommendations on Uniform Colour Spaces, Colour Difference Equations and Psychometric Colour Terms. [Google Scholar]


