Abstract
Objective:
The aim was to evaluate the effect of Er, Cr: YSGG laser and casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on surface micro-hardness of primary tooth enamel.
Materials and Methods:
A total of 30 freshly extracted caries free primary anterior teeth were cleaned and stored in 1% thymol. Teeth were embedded in acrylic resin such that only their buccal surfaces were exposed and were divided into four groups. Group I: Five intact teeth (negative control). The remaining 25 teeth were immersed for 30 min in 1% citric acid for demineralization. Group II: Five demineralized teeth (positive control), Group III: CPP-ACP (GC tooth mousse-GC International, Itabashi-Ku, Tokyo, Japan) application and Group IV: Etching using Er, Cr: YSGG laser + CPP-ACP application. Groups III and IV were subjected to pH cycling for 5 days. Surface micro-hardness of all the teeth was measured using Brinell hardness tester (Fuel Instruments and Engineers Pvt. Ltd.). Data were analyzed using ANOVA.
Results:
Mean surface micro-hardness of Groups I and II were 177.43 kgf/mm2 and 164.86 kgf/mm2, respectively. Group IV showed a higher mean surface micro-hardness (230.68 kgf/mm2) compared with that of Group III (190.28 kgf/mm2). In comparison to all other groups, laser etching prior to CPP-ACP application increased surface micro-hardness significantly (P < 0.001).
Conclusion:
Laser irradiation of primary teeth followed by CPP-ACP application increased surface micro-hardness of enamel.
Keywords: Casein phosphopeptide-amorphous calcium phosphate, erbium, chromium:yttrium, scandium, gallium, garnet laser, primary tooth enamel, re-mineralization surface hardness
INTRODUCTION
The use of lasers in dentistry has increased over the past few years. Lasers have been traditionally classified based on the active medium, e.g. gas, liquid, solid state, or semiconductor diode. Clinically, lasers can be of two types: Soft and hard tissue lasers. The soft tissue lasers available are argon laser, neodymium-doped yttrium aluminum garnet (Nd: YAG) laser and diode laser whereas, erbium-doped yttrium aluminium garnet and erbium, chromium: yttrium, scandium, gallium, garnet (Er, Cr: YSGG) are hard tissue lasers. Lasers can be used in dentistry for tooth whitening, caries removal, cavity preparations and various soft tissue surgeries. Different types of lasers such as ruby laser, argon laser, and Nd: YAG lasers have been used to demonstrate the increased acid resistance of human enamel after laser treatment.[1,2,3] The Er, Cr: YSGG is a newly introduced laser which is proposed for treatment of dental hard tissue. It has a wavelength of 2.79 μm and also shows strong absorption in water (μa = 7000/cm). This laser works by a hydro-kinetic tissue cutting system that uses laser power to energize water. Thus, it can be used for both hard tissue and soft tissue applications.
Re-mineralization and demineralization of tooth enamel is a dynamic process. To restore the natural equilibrium, either re-mineralization must be enhanced, or demineralization must be retarded. Early enamel lesions have a potential for re-mineralization, with an increased resistance to further acid challenge, particularly with the use of enhanced re-mineralization treatments.
In a neutral environment, the hydroxyapatite of enamel is in equilibrium with saliva, which is saturated with calcium and phosphate ions. At or below pH 5.5, H+ ions produced by the bacterial metabolites react preferentially with the phosphate group of the enamel crystals, converting (PO4)2 − ion to (HPO4)2 − ion which, once formed, can no more form the crystal lattice; at the same time H+ ions are buffered. This leads to enamel dissolution, termed as demineralization, which marks the beginning of early enamel caries.[4]
However, the demineralization can be reversed if the acidic pH is neutralized with the availability of sufficient calcium and phosphate ions in the immediate environment. For this purpose re-mineralizing agents such as fluoride gels, varnishes, dentifrices and casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) have been used.
Earlier studies using lasers in association with fluoride and re-mineralizing agents have on the synergistic effect resulting in re-mineralization.[5,6,7,8,9] There has been contradictory report regarding the cumulative effects of laser and re-mineralizing agents on enamel.[10,11,12,13] Most of these studies have been conducted on permanent teeth and have primarily assessed mineral loss, fluoride content and surface topography of enamel following laser irradiation.[14,15] However, studies on the application of Er, Cr: YSGG laser along with CPP-ACP are lacking. Hence the aim of this study was to evaluate the effect of Er, Cr: YSGG laser and CPP-ACP on surface micro-hardness of primary tooth enamel.
MATERIALS AND METHODS
A total of 30 freshly extracted caries free, retained primary anterior teeth were collected from children visiting the Department of Pedodontics and Preventive Dentistry. Only teeth with no cracks, restorations, and/or developmental lesions were selected. The teeth were cleaned with pumice slurry, polished with rubber cup at slow speed and stored in 1% thymol solution until the study was carried out. The selected teeth were then embedded in acrylic resin blocks in a manner that only the buccal surfaces of the teeth were exposed. These 30 teeth were divided into four groups (Groups I-IV).
Groups I and II consisted of five teeth samples each and formed the negative and positive control, respectively. Groups III and IV consisted of 10 teeth each and formed the experimental groups. No treatment was done for the teeth in Group I (negative control). Teeth samples in Groups II, III and IV were subjected to demineralization in 1% citric acid for 30 min. In Group II, following demineralization no further treatment was carried out (positive control). In Group III, CPP-ACP (GC tooth mousse-GC International, Itabashi-Ku, Tokyo, Japan) was applied on the buccal surface of the demineralized enamel for 30 min. This was repeated at the same time of the day, for a period of 5 days. In Group IV, on the 1st day, the buccal surface of the demineralized enamel was laser etched from for 20 s using Er, Cr: YSGG laser (Waterlase® iPLUS™) in noncontact mode, at a distance of 15 mm with pulse duration of 140 μs at 4 W energy (60% water 40% air), 50 Hz frequency. This was followed by the application of CPP-ACP (GC tooth mousse) for 30 min and repeated, at the same time of the day for a total of 5 days. The teeth in Groups III and IV were then subjected to pH cycling at room temperature. They were stored overnight in artificial saliva at pH 7.[16]
All 30 teeth were tested for surface micro-hardness using Brinell's hardness tester (Fuel Instruments and Engineers Pvt. Ltd.). In this method, a hardened steel ball of 1.58 mm in diameter was pressed onto the buccal surface of the teeth under a load of 60 kg for 20 s and then released. The depth of impression created on the tooth surface was then measured using Brinell microscope (Scientific Technologies). The Brinell hardness number (BHN) was calculated using the following formula:

Data obtained was subjected to statistical analysis using one-way ANOVA and comparison between the groups was done using Mann–Whitney test. Significance was considered at P < 0.05; and P < 0.001 as highly significant.
RESULTS
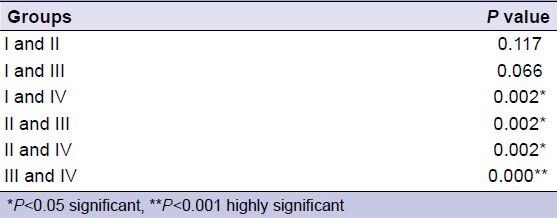
The mean surface micro-hardness (BHN) was highest in Group IV (230.7 kgf/mm2) and lowest in Group II (164.9 kgf/mm2). In comparison to all other groups, Group IV showed significantly higher surface micro-hardness (P < 0.001) [Table 1]. Among the experimental groups, Group IV (laser irradiation and CPP-ACP) showed a significantly higher surface micro-hardness in comparison to Group III (only CPP-ACP) (P < 0.001) [Table 2].
Table 1.
Comparison of surface micro-hardness
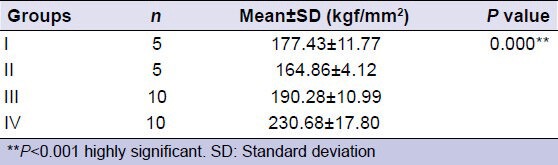
Table 2.
Comparison of surface micro-hardness between each pair of groups
DISCUSSION
The primary dentition is at a much greater risk for caries development than the permanent dentition. The composition of primary enamel is considerably different from that of permanent teeth. The reported mineral content is 81.3-94.2 wt% for primary enamel, with the remainder consisting of water and organic matrix. The time interval from initial surface demineralization to the development of clinically detectable white spot lesions and frank cavitation is comparatively much shorter due to the thin layer of enamel (1 mm) overlying the dentin of primary teeth. This could be the reason that two-thirds of caries in primary teeth occur on smooth surfaces.[17]
Relatively frequent hypomineralized and hypocalcified areas on smooth surfaces of primary teeth may in part account for increased caries susceptibility. In addition, the rapid amelogenesis that must occur during the in utero, perinatal, and early infancy periods may also result in a lessened degree of mineralization and less caries-resistant primary tooth enamel.
Hardness is indicative of the ease of finishing of a structure and its resistance to in service scratching. Finishing or polishing a structure is important for esthetic purposes and scratches can compromise fatigue strength and lead to primitive failure. Hardness of dental fillings is very important because it determines the lifetime of the filling material and its ability to do the required function. In our study, Brinell hardness tester was used as it is a well-established and reliable method of assessing surface hardness.[18]
The use of CPP-ACP as a re-mineralizing agent has been shown to re-mineralize enamel, making it more resistant to acid challenge than normal tooth enamel mineral. The use of laser irradiation for preventing dental caries is based on chemical, physical and crystalline changes induced in enamel due to the heating of the surface.[19]
The present study showed a significant increase in the surface micro-hardness of demineralized enamel followed by the use of CPP-ACP. Protein nanotechnology combines specific phosphoproteins of bovine milk with nanoparticles of ACP. The precise ratio is 144 calcium ions plus 96 phosphate ions and 6 peptides of CPP. The nano-complexes form over a pH range from 5.0 to 9.0. Under neutral and alkaline conditions, the CPPs stabilize calcium and phosphate ions, forming metastable solutions that are supersaturated with respect to the basic calcium phosphate phases. The amount of calcium and phosphate bound by CPP increases as pH rises, reaching the point where the CPP have bound their equivalent weights of calcium and phosphate.[20]
The anti-cariogenic mechanism of CPP-ACP is achieved by the incorporation of the nano-complexes of the ACP into plaque and onto the tooth surface. The CPP have an important role as an ACP carrier localizing the highly soluble calcium phosphate phase at the tooth surface. This localization maintains high concentration gradients of calcium and phosphate ions in the subsurface enamel; thereby facilitating re-mineralization.[12,13,20,21] Increase in surface micro-hardness in both the experimental group is due to more bioavailability of calcium and phosphate in CPP-ACP. Therefore in the present study surface micro-hardness was higher in Group III (demineralized enamel followed by application of CPP-ACP) in comparison to even Group I (intact enamel).
The use of laser irradiation for preventing dental caries is based on chemical, physical and crystalline changes induced in enamel due to the heating of the surface. High energy laser irradiation of enamel alone, at a specific wavelength has been shown to cause re-mineralization. Various explanations have been proposed for the same. One theory states that laser irradiation decreases enamel permeability due to the physical fusion of the enamel surface microstructure. Another theory has focused on a combination of reduced enamel permeability with reduced solubility promoted by melting, fusion, and recrystallization of enamel crystallites, which seals the enamel surface.[22] Reduction in enamel solubility could be due to ultra-structural changes in the crystallography of enamel. There is a reduction of water and carbonate content, an increase in the hydroxyl ion contents, formation of pyrophosphates, and decomposition of proteins.[23] At a temperature ranging from 650°C to 1100°C, products in enamel that leads to decrease the solubility are formed, which in turn depends on the calcium-phosphate ratio. At 1100°C, there is the formation of new crystalline phases tetra calcium diphosphate monoxide (alpha tri-calcium phosphate and beta-phases), which is less soluble, has less carbonate content and hence less resistant to demineralization.[24]
Lasers have shown to induce surface changes in enamel that vary from crazing, crating and exfoliation of the enamel. Similarly in our study a marked surface roughness of enamel was observed following laser irradiation.[25]
Laser irradiation followed by application of CPP-ACP allowed for incorporation of calcium nano-complexes on to the tooth surfaces. These numerous nano-clusters of calcium deposits on the tooth surface could act as a reservoir to replenish the soluble calcium and phosphate ions that have diffused into the subsurface enamel. In this manner application of CPP-ACP enhances the action of lasers to inhibit the demineralization.[5]
Hence in the present study the combined action of laser and CPP-ACP resulted in significant higher surface micro-hardness as compared to application of CPP-ACP alone.
In children with initial white spots lesions, prevention through laser irradiation prior to the daily application of CPP-ACP will be more effective as it results a superior enamel re-mineralization and will also be less anxiety-provoking.
CONCLUSION
The surface micro-hardness of primary tooth enamel was significantly higher when Er, Cr: YSGG laser irradiation was done prior to CPP-ACP application.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Korytnicki D, Mayer MP, Daronch M, Singer Jda M, Grande RH. Effects of Nd: YAG laser on enamel microhardness and dental plaque composition: An in situ study. Photomed Laser Surg. 2006;24:59–63. doi: 10.1089/pho.2006.24.59. [DOI] [PubMed] [Google Scholar]
- 2.Hossain M, Nakamura Y, Kimura Y, Yamada Y, Kawanaka T, Matsumoto K. Effect of pulsed Nd: YAG laser irradiation on acid demineralization of enamel and dentin. J Clin Laser Med Surg. 2001;19:105–8. doi: 10.1089/104454701750285421. [DOI] [PubMed] [Google Scholar]
- 3.Tsai CL, Lin YT, Huang ST, Chang HW. In vitro acid resistance of CO2 and Nd-YAG laser-treated human tooth enamel. Caries Res. 2002;36:423–9. doi: 10.1159/000066538. [DOI] [PubMed] [Google Scholar]
- 4.Lata S, Varghese NO, Varughese JM. Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: An in vitro comparative evaluation. J Conserv Dent. 2010;13:42–6. doi: 10.4103/0972-0707.62634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niazy AM, Ehab AH. Synergistic caries inhibitory effect of a remanerializing agent and CO2 laser on human enamel and root dentin. Cairo Dent J. 2009;25:415–24. [Google Scholar]
- 6.Hicks MJ, Flaitz CM, Westerman GH, Blankenau RJ, Powell GL, Berg JH. Enamel caries initiation and progression following low fluence (energy) argon laser and fluoride treatment. J Clin Pediatr Dent. 1995;20:9–13. [PubMed] [Google Scholar]
- 7.Hicks MJ, Westerman GH, Flaitz CM, Blankenau RJ, Powell GL, Berg JH. Effects of argon laser irradiation and acidulated phosphate fluoride on root caries. Am J Dent. 1995;8:10–4. [PubMed] [Google Scholar]
- 8.Banda NR, Vanaja Reddy G, Shashikiran ND. Evaluation of primary tooth enamel surface morphology and microhardness after Nd: YAG laser irradiation and APF gel treatment – An in vitro study. J Clin Pediatr Dent. 2011;35:377–82. doi: 10.17796/jcpd.35.4.8550556gp6r5xt6t. [DOI] [PubMed] [Google Scholar]
- 9.Azevedo DT, Faraoni-Romano JJ, Derceli Jdos R, Palma-Dibb RG. Effect of Nd: YAG laser combined with fluoride on the prevention of primary tooth enamel demineralization. Braz Dent J. 2012;23:104–9. doi: 10.1590/s0103-64402012000200003. [DOI] [PubMed] [Google Scholar]
- 10.Featherstone JD, Nobre dos Santos M, Fried D. Effect of a new carbon dioxide laser and fluoride on occlusal caries progression in dental enamel. Lasers Dent. 2002;4610:132–9. [Google Scholar]
- 11.Apel C, Birker L, Meister J, Weiss C, Gutknecht N. The caries-preventive potential of subablative Er: YAG and Er: YSGG laser radiation in an intraoral model: A pilot study. Photomed Laser Surg. 2004;22:312–7. doi: 10.1089/pho.2004.22.312. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds EC, Cai F, Shen P, Walker GD. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J Dent Res. 2003;82:206–11. doi: 10.1177/154405910308200311. [DOI] [PubMed] [Google Scholar]
- 13.Lennon AM, Pfeffer M, Buchalla W, Becker K, Lennon S, Attin T. Effect of a casein/calcium phosphate-containing tooth cream and fluoride on enamel erosion in vitro. Caries Res. 2006;40:154–7. doi: 10.1159/000091063. [DOI] [PubMed] [Google Scholar]
- 14.Cecchini RC, Zezell DM, de Oliveira E, de Freitas PM, Eduardo Cde P. Effect of Er: YAG laser on enamel acid resistance: Morphological and atomic spectrometry analysis. Lasers Surg Med. 2005;37:366–72. doi: 10.1002/lsm.20247. [DOI] [PubMed] [Google Scholar]
- 15.Esteves-Oliveira M, Pasaporti C, Heussen N, Eduardo CP, Lampert F, Apel C. Rehardening of acid-softened enamel and prevention of enamel softening through CO2 laser irradiation. J Dent. 2011;39:414–21. doi: 10.1016/j.jdent.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Karlinsey RL, Mackey AC, Blanken DD, Schwandt CS. Remineralization of eroded enamel lesions by simulated saliva in vitro. Open Dent J. 2012;6:170–6. doi: 10.2174/1874210601206010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newbrun E. 3rd ed. Chicago, Illinois: Quintessence Publishing Co., Inc; 1989. Cariology. [Google Scholar]
- 18.Craig RG, Powes JM. 11th ed. St. Louis: Mosby; 2002. Restorative Dental Materials. [Google Scholar]
- 19.Zezell DM, Ana PA, Albero FG, Cury JA, Bach-Mann L. Effect of infrared lasers on chemical and crystalline properties of enamel. Caries Res. 2009b;43:192. [Google Scholar]
- 20.Rose RK. Effects of an anticariogenic casein phosphopeptide on calcium diffusion in streptococcal model dental plaques. Arch Oral Biol. 2000;45:569–75. doi: 10.1016/s0003-9969(00)00017-0. [DOI] [PubMed] [Google Scholar]
- 21.Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2001;80:2066–70. doi: 10.1177/00220345010800120801. [DOI] [PubMed] [Google Scholar]
- 22.Ana P, Bachmann L, Zezell MD. Lasers effects on enamel for caries prevention. Laser Phys. 2006;16:865–75. [Google Scholar]
- 23.Featherstone JD, Nelson DG. Laser effects on dental hard tissues. Adv Dent Res. 1987;1:21–6. doi: 10.1177/08959374870010010701. [DOI] [PubMed] [Google Scholar]
- 24.Bachmann L, Craievich AF, Zezell DM. Crystalline structure of dental enamel after Ho: YLF laser irradiation. Arch Oral Biol. 2004;49:923–9. doi: 10.1016/j.archoralbio.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Westerman GH, Hicks MJ, Flaitz CM, Powell GL, Blankenau RJ. Surface morphology of sound enamel after argon laser irradiation: An in vitro scanning electron microscopic study. J Clin Pediatr Dent. 1996;21:55–9. [PubMed] [Google Scholar]


