Abstract
Objective
The goal of this study was to use highly accurate, non-rigid algorithms to locate the position of cochlear implant electrodes and correlate this with audiological performance.
Patients
After obtaining IRB approval, adult, bilateral CI patients were identified and those with pre-operative temporal bone CT scans were asked to return for a post-intervention CT. Sixteen adult patients agreed. Demographics, etiology of deafness, length of auditory deprivation and audiological performance were recorded.
Intervention
Using a non-rigid model of the shape variations of intracochlear anatomy, the location of the basilar membrane was specified in relationship to the electrode array. Number of electrodes within each compartment of the cochlea was correlated with HINT and CNC scores for the known confounding variable, length of deafness.
Main Outcomes
Mann-Whitney tests of differences were used to compare the hearing performance resulting from implants completely in the ST versus those not completely in the ST.
Results
62.5% implants were fully inserted in ST; 34.4% were partially inserted into the ST and 3.1% was fully inserted in SV. Controlling for the known contributing variable of length of auditory deprivation our results show that location of electrodes in relationship to the scala is not predictive of audiological performance.
Conclusions
We have assessed electrode placement and correlated it with audiological outcome. Presence of the electrodes solely in ST was not predictive of outcome. We estimate that is would take analysis of thousands of CI patients data before any valid correlations could be made.
Objectives
Numerous studies have identified significant predictive factors for hearing outcomes in patients with cochlear implants (CI).(1,2,3) These include duration of deafness,(4) level of pre-implant speech recognition,(5) pre/post lingual status, (6) and electrode programming configuration.(7,8) Recipient age does not appear to have an impact on hearing outcomes in elderly candidates.(9)
As studies done by Shepherd et al.(10) reported that the scala tympani (ST), at least from a dimensional standpoint, is the ideal place for electrode placement, a number of recent studies have proposed that electrode location within the SV may be an important determinant of audiological outcome.(11,12,13,14) Skinner et al.(11) and Finley et al.(13) used rigid registration methods which are based on aligning structures from postoperative CTs to preoperative CTs and use of a high resolution cochlear atlas in order to overcome the inability to positively identify the basilar membrane on clinically-applicable temporal bone CT scans. Such rigid registration using a cadaveric model of the cochlea is a good technique if the model can be scaled by stretching, rotating, and skewing to fit the patient’s anatomy. If anatomical differences other than scaling exist – e.g., a more acute basal turn in the patient versus the model – rigid registration imparts error on where the basilar membrane is depicted. Aschendroff relied on post-operative rotational tomography to determine whether the electrodes stayed in ST or crossed into scala vestibuli (SV).(14)
We have developed and applied non-rigid methods to predict the location of the basilar membrane on clinically-applicable CT scans. To do this we created a model of the shape variations of intra-cochlear anatomy using micro-CT scans of 6 cadaveric temporal bones. We have successfully validated its predictive accuracy on cadaver models. (15) The method is semi-automated, dramatically reducing the labor involved in analyzing individual specimens. With validation of this technique, we are able now to accurately locate the position of CI electrodes in relation to the basilar membrane in CI patients who have undergone CT scanning of the temporal bones before and after CI. For first clinical testing we choose to study bilaterally implanted adults such that potential confounding variables, with the exception of length of deafness, would be eliminated.
The goal of this study, then, is to use the non-rigid algorithms we have developed to locate the position of CI electrodes in relationship to the basilar membrane in bilaterally implanted adults and correlate such with audiological performance.
Patients
After obtaining IRB approval, adult, bilateral CI patients were identified and a subset that had pre-operative, temporal bone CT scans were asked to return to our facility to obtain a post-intervention CT. Sixteen adult patients agreed and are included in this report. These patients’ demographics, etiology of deafness, length of auditory deprivation and audiological performance were recorded.
Intervention
C T s canning was done using a Xoran XCAT flat-panel volume computerized tomography (fpVCT) machine (Xoran Technologies, Ann Arbor, MI). Following fpVCT scanning, our non-rigid, model-based algorithm was used to predict basilar membrane location in reference to the CI electrode array. (Details of this method can be found online).(16) Our recently reported results (15) show high correlation between such predictions and histopathological analysis. Hearing in Noise (HINT) and Consonant-Noun-Consonant (CNC) scores were recorded and correlations between location of electrode and audiological scores were made as described below.
Main Outcome Measures
Due to the skewed nature of the hearing performance data, values were summarized using means, medians, and 25th–75th inter-quartile ranges which represent the middle 50% of the values in a given distribution. Mann-Whitney tests of differences were used to compare the hearing performance resulting from implants completely in the ST versus those not completely in the ST. Measures of association were made using Spearman rank correlations. Finally, analysis of covariance (ANCOVA) procedures were conducted to test for hearing performance differences between the sets of implants that controlled for length of auditory deprivation in the ear receiving the implant (covariate). The hearing and deprivations duration values were rank transformed to meet the assumptions of ANCOVA.
Results
All three FDA approved CI manufacturers were represented in the study. Twenty of the 32 implants (62.5%) were fully inserted in ST. Figure 1 shows a CT scan and corresponding reconstructed image of patient 1 where the implant is fully inserted in ST. Eleven of the 32 (34.375%) were partially inserted into ST and partially in SV. All the implants that crossed form ST to SV did so at approximately 180 degree. Figure 2 shows a CT scan and reconstructed image of patient 3 where the implant is crossing from ST to SV. One implant (3.125%) was fully inserted in SV.
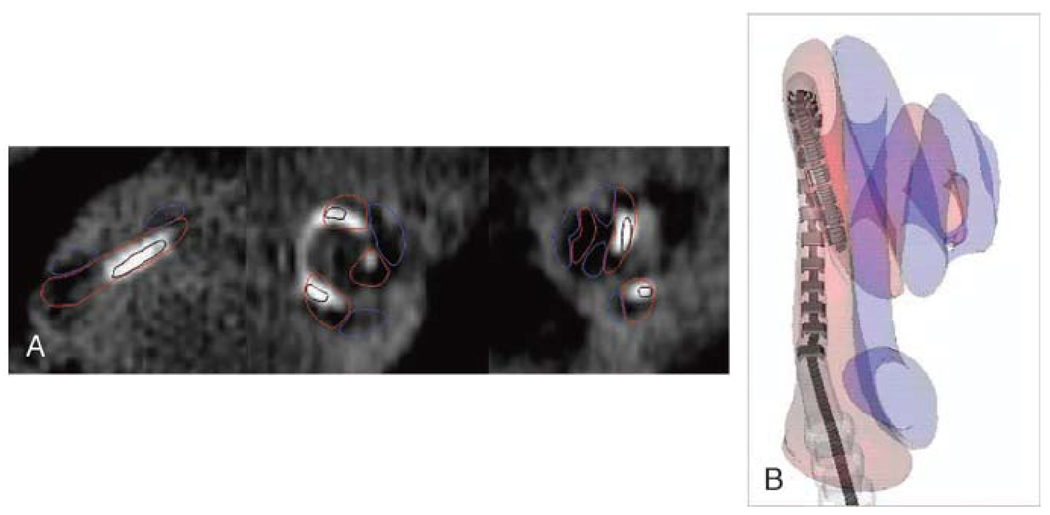
Figure 1.
A. Post-op CT of a patient with full electrode insertion in ST. Note that ST is contoured by red and SV contoured in blue.
Figure 1B. Reconstructed CT in the same patient with full ST insertion. (ST shadowed in red; SV shawdowed in blue).
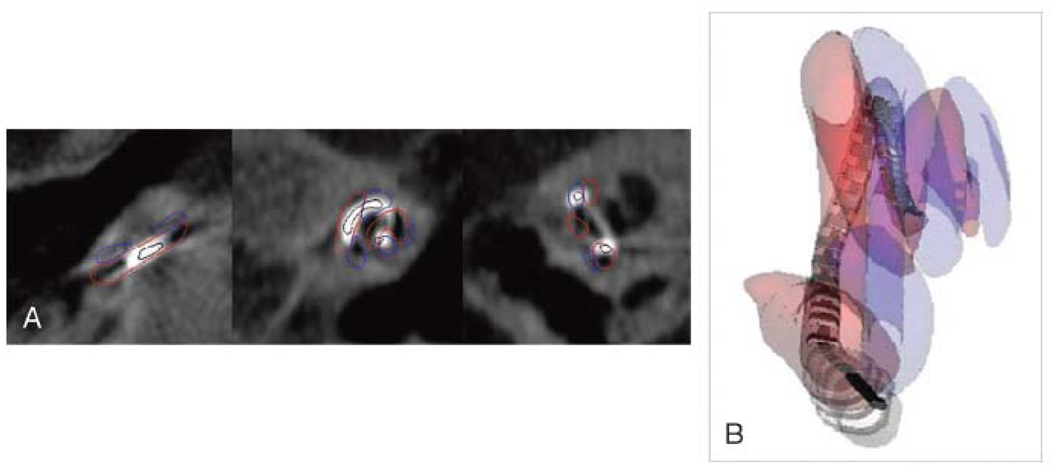
Figure 2.
A. Post-op CT of a patient with the electrode crossing from ST to SV. (ST contoured in red; SV contoured in blue).
Figure 2B. Reconstructed CT in the same patient with the electrodes starting in ST and then crossing to SV at approximately 180 degrees. (ST-red, SV-blue).
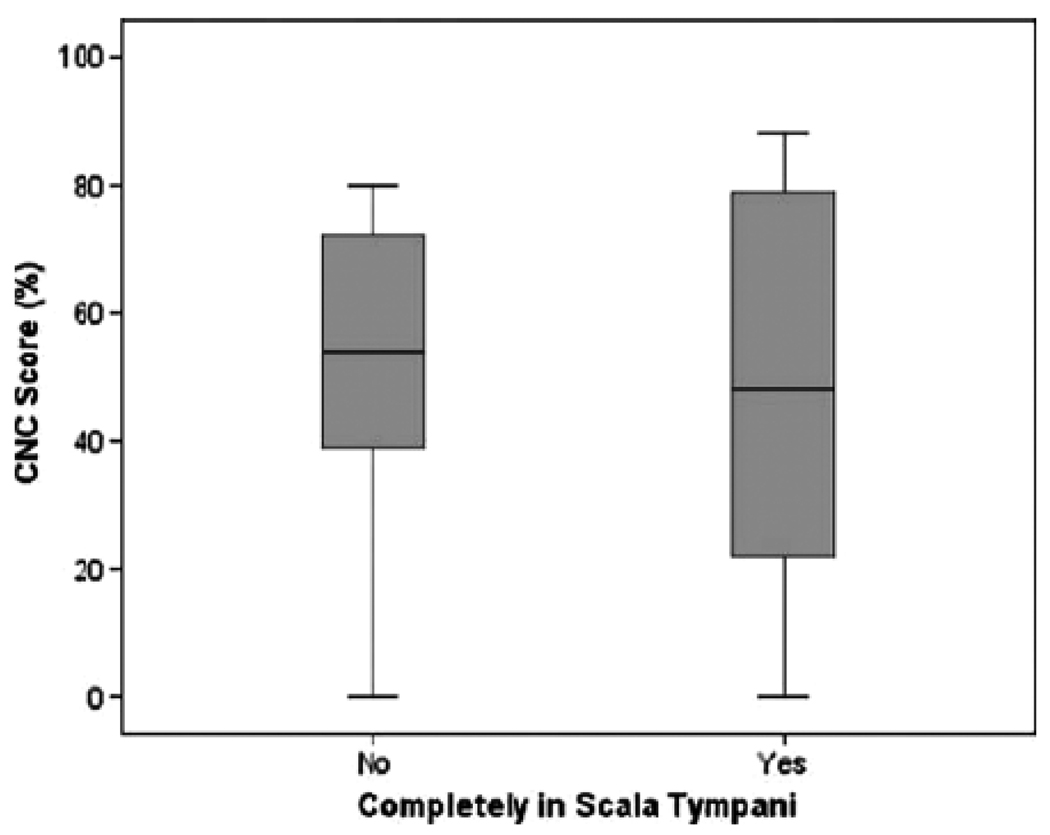
Results are summarized in Tables 1 and 2 (online supplement) where Table 1 includes patients who were simultaneously implanted (n=9) and Table 2 included patients who were sequentially implanted (n=7). Figure 3 graphically shows the results comparing CNC scores in the group with the electrodes fully inserted in ST versus the group where the electrodes cross from ST to SV and figure 4 (online supplement) shows the HINT scores in these groups. The distributions of hearing performance were subjectively similar for the two groups and differences were not statistically significant (Mann-Whitney tests, HINT score: Z=0.117, p=0.907, CNC score: Z=0.370, p=0.711). While it appeared that auditory deprivation had been slightly longer for the group with implants partially inserted in ST versus those fully inserted in ST, the difference was not statistically significant (Mann-Whitney test: Z=1.426, p=0.154). The association of auditory deprivation with hearing performance as measured by the HINT was at a meaningful, albeit not statistically significant, level in this sample (rs=−0.309, p=0.097). The association of length of auditory deprivation with CNC score was substantially smaller (rs=−0.129, p=0.498). When length of auditory deprivation was included as a covariate in the analysis of the differences in hearing between the two groups, there were no statistically significant changes in the primary conclusions (HINT: F(df=1,27)=0.110, p=0.742; CNC: F(df=1,27)=0.003, p=0.959). This is shown in Table 3 (online supplement).
Table 1.
Patients Simultaneously Implanted (n=9)
| Implant Type | Sex | Side | Age | DOS | Etiology | Duration | HINT | CNC | Electrodes |
|---|---|---|---|---|---|---|---|---|---|
| Nucleus | Male | Right | 10/3/47 | 7/23/08 | Noise | 8 y | 99% | 80% | Full ST |
| Nucleus | Male | Left | 10/3/47 | 7/23/08 | Noise | 8 y | 99% | 80% | Full ST |
| Nucleus | Male | Right | 2/26/30 | 8/26/02 | Noise | 6 y | 66% | 20% | Full ST |
| Nucleus | Male | Left | 2/26/30 | 8/26/02 | Noise | 6 y | 74% | 28% | Full ST |
| Nucleus | Female | Right | 12/29/55 | 12/5/08 | Unknown | 6 y | 98% | 88% | Full ST |
| Nucleus | Female | Left | 12/29/55 | 12/5/08 | Unknown | 6 y | 100% | 88% | Full ST |
| Nucleus | Female | Right | 7/29/47 | 3/27/09 | Unknown | unknown | 95% | 74% | Partial ST/SV |
| Nucleus | Female | Left | 7/29/47 | 3/27/09 | Unknown | unknown | 42% | 18% | Full ST |
| Nucleus | Female | Right | 5/8/31 | 4/23/09 | Unknown | 9 y | 44% | 46% | Full ST |
| Nucleus | Female | Left | 5/8/31 | 4/23/09 | Unknown | 9 y | 66% | 44% | Partial ST/SV |
| ABC | Male | Right | 2/22/49 | 5/10/07 | poisone | 37 y | 80% | 60% | Full SV |
| ABC | Male | Left | 2/2249 | 5/10/07 | poisone | 37 y | 86% | 58% | Partial ST/SV |
| ABC | Female | Right | 12/8/68 | 7/19/07 | R A | 5 y | 88% | 40% | Full ST |
| ABC | Female | Left | 12/8/68 | 7/19/07 | RA | 5 y | 90% | 40% | Partial ST/SV |
| MedEl | Male | Right | 7/14/37 | 2/6/09 | Unknown | 3 y | 13% | 0% | Full ST |
| MedEl | Male | Left | 7/14/37 | 2/6/09 | Unknown | 3 y | 99% | 50% | Full ST |
| ABC | Male | Right | 1/23/33 | 10/31/07 | Unkown | 5 y | 0% | 0% | Partial ST/SV |
| ABC | Male | Left | 1/23/33 | 10/31/07 | Unkown | 5 y | 0% | 0% | Full ST |
Table 2.
Patients Sequentially Implant (n=7)
| Implant Type | Sex | Side | Age | DOS | Etiology | Duration | HINT | CNC | Electrodes |
|---|---|---|---|---|---|---|---|---|---|
| Nucleus | Female | Right | 2/13/81 | 10/19/05 | ECMO | 1 y | 94% | 70% | Partial ST/SV |
| Nucleus | Female | Left | 2/13/81 | 6/28/06 | ECMO | 1 y | 100% | 86% | Full ST |
| Nucleus | Female | Right | 8/22/80 | 8/1/05 | genetic | 24 y | 87% | 20% | Partial ST/SV |
| Nucleus | Female | Left | 8/22/80 | 7/19/07 | genetic | 20 y | 90% | 20% | Full ST |
| Nucleus | Male | Right | 3/11/67 | 3/1/98 | Cogan | 7 y | 94% | 78% | Full ST |
| Nucleus | Male | Left | 3/11/67 | 7/14/08 | Cogan | 10 y | 96% | 76% | Partial ST/SV |
| Nucleus | Female | Right | 8/13/65 | 12/17/02 | Genetic | 32 y | 63% | 24% | Full ST |
| Nucleus | Female | Left | 8/13/65 | 11/24/08 | Genetic | 40 y | 43% | 30% | Full ST |
| Nucleus | Female | Right | 12/3/69 | 9/1/04 | Unknown | 21 y | 75% | 38% | Partial ST/SV |
| Nucleus | Female | Left | 12/3/69 | 1/20/10 | Unknown | 35 y | 95% | 60% | Partial ST/SV |
| ABC | Male | Right | 9/17/67 | 2/28/07 | Ototoxic | 39 y | 22% | 0% | Full ST |
| ABC | Male | Left | 9/17/67 | 9/18/08 | Ototoxic | 40 y | 11% | 0% | Partial ST/SV |
| ABC | Female | Right | 7/20/67 | 1/5/05 | Genetic | 3 y | 94% | 58% | Full ST |
| ABC | Female | Left | 7/20/67 | 8/30/07 | Genetic | 5 y | 92% | 68% | Full ST |
Figure 3.
Comparison of CNC word scores for bilaterally implanted patients with complete versus incomplete ST insertion. The data is shown as a horizontal median value with 25–75th percentiles shown in the gray box and complete data shown between the whiskered lines.
Table 3.
Primary Conclusions
| HINT % | Mean | Median | IQR* |
|---|---|---|---|
| Completely in scala tymp | 71.2 | 89.9 | 44.8 – 98.8 |
| Not inserted completely | 72.9 | 86.5 | 68.3 – 94.8 |
| CNC % | |||
| Completely in scala tymp | 46.6 | 48.0 | 21.0 – 79.5 |
| Not inserted completely | 49.2 | 54.0 | 38.5 – 73.0 |
| Auditory deprivation (years) | |||
| Completely in scala tymp | 11.3 | 6.0 | 5.0 – 10.0 |
| Not inserted completely | 20.1 | 21.0 | 5.0 – 37.0 |
(F(df=1,27)=0.110, p=0.742; CNC: F(df=1,27)=0.003, p=0.959)
25th-75th inter-quartile range, representing the middle 50% of the values
Conclusion
Intracochlear position of the cochlear implant has been shown in previous studies to correlate with audiological outcome. Skinner et al.(11) presented 15 patients implanted with Advanced Bionics devices and determined that the position of electrodes within the SV is negatively correlated with audiological performance. Aschendroff et al.(12) used post operative rotational tomography to determine the position of cochlear implant electrodes with respect to the basilar membrane. Results of speech tests demonstrated best results after insertion in ST with poorer results after dislocation from ST to SV. Finley et al.(13) continued the work of Skinner and demonstrated in an additional 14 patients with Advanced Bionics implants that poorer audiological outcomes could be predicted by number of electrodes in SV, depth of electrode insertion, and age at time of insertion.(13)
Herein, our group reports use of a novel, proprietary, automated, non-rigid algorithm to predict cochlear implant electrode location in relationship to the basilar membrane. The accuracy of three dimensional reconstructions produced with this software were verified using anatomic micro dissections, demonstrating that this method is highly precise and poised for clinical application.( 15) Reviewing the placement of electrodes for bilaterally implanted adults, we found that over 1/3 cross the basilar membrane and that this occurs universally at 180 degrees from the insertion site. Controlling for the known contributing variable of length of auditory deprivation and using a linear modeling approach to account for the impact of type of surgery (sequential versus simultaneous), results from analysis of the 32 implants show that location of CI electrode in relationship to the basilar membrane (ST versus SV) is not predictive of audiological performance.
We know from surgical experience that patients who have revision cochlear implant surgery can have very good audiological outcomes. At the time of revision surgery the cochlea is often filled with soft tissue as a pseudo-capsule has formed around the electrode array obliterating anatomical distinctions between ST and SV. Combining such clinical observations with the data presented herein, we propose that electrode array position - in reference to the basilar membrane - does not affect audiological performance. Note that we propose this only for traditional CI surgery and not hearing preservation/hybrid surgery.
A strategy for further testing of this hypothesis would be to conduct an equivalence trial of randomly selected patients with implants fully inserted in the ST and another randomly selected sample with implants that are at least partially outside the ST. A parallel group study designed to test the equivalence of average CNC scores (i.e., true difference of 0%) would require 1850 patients per group to achieve 90% statistical power in testing equivalence limits within ≤ ±5% performance (using one-sided alpha level of .05). Given all of the same assumptions, groups of 1455 patients each would achieve 80% statistical power to test the proposed equivalence limits of a mean difference of ≤ ±5% performance. While this is a large task, (a) it is necessary to determine if intracochlear position matters and (b) it can be accomplished using automated software such as that developed by our group.
Acknowledgements
The project described was supported by Award Number R01DC008408 from the National Institute on Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health.
Work in this project was also supported in part by a research contract with Cochlear Corporation who provided electrodes and financial support of the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Robert F. Labadie is a consultant for Cochlear Corporation.
The software described herein has been copywritten. Authors Jack H. Noble, Benoit M. Dawant and Robert F. Labadie may benefit financially from future licensing agreements.
Contributor Information
George B. Wanna, Email: george.wanna@vanderbilt.edu, Assistant Professor of Otolaryngology-Head and Neck Surgery Department of Otolaryngology-Head and Neck Surgery 7209 Medical Center East, South Tower Vanderbilt University Medical Center Nashville, TN 37232-8605 (917) 340-5060.
Jack H. Noble, Email: jack.h.noble@vanderbilt.edu, Department of Electrical Engineering and Computer Science Vanderbilt University, Nashville, TN.
Theodore R. McRrackan, Email: theodore.mccracken@vanderbilt.edu, Department of Otolaryngology-Head and Neck Surgery Vanderbilt University Medical Center, Nashville, TN.
Benoit M. Dawant, Email: benoit.dawant@vanderbilt.edu, Department of Electrical Engineering and Computer Science Vanderbilt University, Nashville, TN.
Mary S. Dietrich, Email: mary.dietrich@Vanderbilt.Edu, Research Associate Professor, School of Medicine Vanderbilt University, Nashville, TN.
Linsey Watkins, Email: linsey.d.watkins@vanderbilt.edu, Vanderbilt University Medical Center Bill Wilkerson Center, Nashville, TN.
Alejandro Rivas, Email: alejandro.rivas@vanderbilt.edu, Department of Otolaryngology-Head and Neck Surgery Vanderbilt University Medical Center, Nashville, TN.
Theodore A. Schuman, Email: theodore.schuman@vanderbilt.edu, Department of Otolaryngology-Head and Neck Surgery Vanderbilt University Medical Center, Nashville, TN.
Robert F. Labadie, Email: robert.labadie@vanderbilt.edu, Associate Professor of Otolaryngology-Head and Neck Surgery Department of Otolaryngology-Head and Neck Surgery Vanderbilt University Medical Center, Nashville, TN.
References
- 1.Friedland DR, Venick HS, Niparko JK. Choice of ear for cochlear implantation: the effect of history and residual hearing on predicted postoperative performance. Otol Neurotol. 2003;24:582Y9. doi: 10.1097/00129492-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Summerfield AQ, Marshall DH. Preoperative predictors of outcomes from cochlear implantation in adults: Performance and quality of life. Ann Otol Rhinol Laryngol. 1995;(Suppl. 166):105–108. [PubMed] [Google Scholar]
- 3.Shipp DB, Nedzelski JM. Prognostic indicators of speech recognition performance n adult cochlear implant users. Ann Otol Rhinol Laryngol. 1995;(suppl.166):194–196. [PubMed] [Google Scholar]
- 4.Blamey P, Arndt P, Bergeron F, et al. Factors affecting auditory performance of post linguistically deaf adults using cochlear implants. Audiol Neurotol. 1996;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein JT, Parkinson WS, Tyler RS, et al. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol. 1999;20:445–452. [PubMed] [Google Scholar]
- 6.Tong YC, Busby PA, Clark GM. Perceptual studies on cochlear implant patients with early onset of profound hearing impairment prior to normal development of auditory, speech, and language skills. J Acoust Soc Am. 1988;84 doi: 10.1121/1.396664. [DOI] [PubMed] [Google Scholar]
- 7.Pfingst BE, Franck KH, Xu L, et al. Effects of electrode configuration and place of stimulation on speech perception with cochlear prostheses. J Assoc Res Otolaryngol. 2001;2:87Y103. doi: 10.1007/s101620010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mens LH, Berenstein CK. Speech perception with mono- and quadrupolar electrode configurations: a crossover study. Otol Neurotol. 2005;26:957Y64. doi: 10.1097/01.mao.0000185060.74339.9d. [DOI] [PubMed] [Google Scholar]
- 9.Leung J, Wang NY, Yeagle JD, et al. Predictive models for cochlear implantation in elderly candidates. Arch Otolaryngol Head Neck Surg. 2005;131:1049Y54. doi: 10.1001/archotol.131.12.1049. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd RK, Hatsushika S, Clark GM. Electrical stimulation of the auditory nerve: The effect of electrode position on neural excitation. Hearing Research. 1993;66:108–122. doi: 10.1016/0378-5955(93)90265-3. [DOI] [PubMed] [Google Scholar]
- 11.Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of Advanced Bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol. 2007;116(Suppl 197)(4):1–24. [PubMed] [Google Scholar]
- 12.Aschendorff A, Kromeier J, Klenzer T, et al. Quality control after insertion of the Nucleus Contour and Contour Advance electrode in adults. Ear & Hearing. 2007;28:75S–79S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 13.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29:920–928. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aschendorff A, Kubalek R, Turowski B, et al. Quality control after cochlear implant surgery by means of rotational tomography. Otol Neurotol. 2005;26:34Y7. doi: 10.1097/00129492-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Schuman T, Noble J, Wright G, Wanna G, Dawant B, Labadie R. Anatomic verification of a novel, non-rigid registration method for precise intrascalar localization of cochlear implant electrodes in adult human temporal bones using portable computerized tomography. Laryngoscope. 2010 doi: 10.1002/lary.21104. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble J, Rutherford R, Labadie RF, Majdani O, Dawant BM. Modeling and segmentation of intra-cochlear anatomy in conventional CT; Progress in Biomedical Optics and Imaging - Proceedings of SPIE Conf. on Medical Imaging; 2010. p. 7632. 763202. [Google Scholar]