Abstract
The purpose of this study was to investigate current blood lead (PbB) and zinc protoporphyrin (ZPP) levels in adults presently living in environmentally Pb-contaminated Andean communities, and to compare the findings with the PbB and ZPP levels of Pb-exposed adult cohorts from the same study area tested between 1996 and 2007. Blood samples from 39 adults were measured for PbB and ZPP concentrations. The current mean PbB level (22.7 μg/dl) was significantly lower than the mean (37.9 μg/dl) of the initial 1996 cohort. PbB levels for the 1997, 1998, 2003, and 2006 cohorts were also significantly lower than the levels for the 1996 group. Elevated ZPP/heme ratios of 103.3, 128.4 and 134.2 μmol/mol were not significantly different for the 2006, 2007 and 2012 groups, indicating chronic Pb exposure. While ZPP levels of Andean Ecuadorian Pb-glazing workers have remained elevated, PbB levels declined. Pb exposure of the workers need to be continually monitored.
Keywords: Blood, Lead, Zinc Protoporphyrin, Adults, Occupational
INTRODUCTION
Occupational lead (Pb) exposure remains a global health issue in communities involved in enterprises such as gold mining, Pb smelting, Pb-acid battery manufacturing and battery recycling (Suplido and Choon 2000; Popovic et al. 2005; Rosin 2009; Dounias et al. 2010; Gottesfeld and Pokhrel 2011; Raafatl et al. 2012; Were et al. 2012). This is especially a problem in low-income countries where Pb exposure is unregulated, and monitoring of Pb exposure levels is not conducted on a regular basis by public health authorities. In some areas, both adults and children engage in occupational practices that expose them to Pb and its attendant health hazards. While children are particularly susceptible to Pb poisoning and its associated medical sequelae (Counter et al. 2009b; Buchanan et al. 2011), adults may also develop serious health complications related to Pb exposure. In adults, prolonged or chronic Pb intoxication may induce peripheral, central, and autonomic nervous system abnormalities, as well as nephrological impairment and increased blood pressure (Murata et al. 1993; Fenga et al. 2006; ATSDR 2007; Bleecker et al. 2007). Neurocognitive impairments, such as diminished executive functioning, short-term memory deficits, manual dexterity difficulties, and visual-spatial deficits have been associated with occupational Pb exposure (Meyer-Baron and Seeber 2000; Schwartz et al. 2000). Some investigations indicate that Pb-induced neurocognitive impairments are irreversible, and that past or cumulative Pb exposure is associated with neurodegeneration (Dietrich et al. 2004; Stewart et al. 2006). However, other studies suggest that after removal of the Pb source or initiation of pharmacological intervention, Pb-induced neurocognitive deficits may be reversed (Baker et al. 1985; Chuang et al. 2005; Counter et al. 2009a). In genotoxic studies of adult workers, Pb exposure was also associated with DNA damage, including single and double strand breaks and chromosomal aberrations (Danadevi et al. 2003; Olewińska et al. 2010; Kašuba et al. 2012).
The toxicokinetics of Pb indicate that the metal enters the body through ingestion of Pb-contaminated foods, dust, soil, and through Pb particulates in Pb-polluted air that permeate the alveolar sacs of the lungs. Pb is absorbed by the soft tissues of the body, and may be stored in the bones for years. Bone Pb stores mobilize during pregnancy, breast-feeding or bone injuries, and may lead to the endogenous release of Pb into blood and other soft tissues (ATSDR 2007). In Pb-exposed adults, a PbB level of ≥ 25 μg/dl is currently considered elevated by the U.S. Centers for Disease Control and Prevention (CDC, 2011).
In instances of chronic Pb exposure, protoporphyrin accumulates in the absence of iron (Fe) in hemoglobin (Hb) and attracts zinc as a replacement, forming zinc protoporphyrin (ZPP). Elevated levels of ZPP indicate Pb-induced inhibition of heme biosynthesis. Because elevated ZPP levels lag increased PbB levels by weeks to months, the ZPP/heme ratio serves as a biomarker for chronic Pb exposure. Moreover, the ZPP/heme ratio may indicate prolonged Pb exposure for up to two years (Froom et al. 1998; Martin et al. 2004).
As part of an ongoing health initiative, our team has tracked over a period of years the PbB levels in adults living in Andean villages of Ecuador where Pb glazing of ceramics is the primary livelihood (Counter et al. 1998; 2001; Buchanan et al. 2011). Men, women and children living in these Pb-glazing communities have presented with Pb intoxication and related health complications (Counter and Buchanan 2002; Counter et al. 2004; 2007). There are no Ecuadorian government regulations of this cottage industry, and as a consequence, there is no regular monitoring of the Pb exposure of the workers involved or their families, who may be equally Pb exposed. Because of previously observed elevated PbB levels, Counter et al. (1997; 2003) instituted a Pb-exposure education, prevention and treatment program for the inhabitants of the study area. The purpose of this study was to investigate current PbB and ZPP levels in adults presently living in environmentally Pb-contaminated Andean communities, and to compare the findings with the PbB and ZPP levels of Pb-exposed adult cohorts from the same study area tested between 1996 and 2007.
METHODS
Participants and Location
The participants in this field study reside at an altitude of approximately 2,850 m in Andean communities in La Victoria, Pujilí, Ecuador. Blood samples were obtained from 39 adults living in the study area, and examined for Pb and ZPP concentrations. The participants consisted of 25 females and 14 males, aged 17 to 77 years (mean age: 39.9; SD: 15.1, median: 40) from a homogenous population of essentially the same low socioeconomic and education status, with similar Pb-glazing occupational practices, and identical environmental Pb-exposure pathways. The participants were enrolled in this study on the basis of their availability for medical examination during the period of the medical team’s visit to the study area. There was no bias in the selection of participants. No participants were refused medical examination by the field research team. The PbB levels obtained for the 39 participants were compared with results obtained previously by the authors on 145 participants in the same study area who were assessed between 1996 and 2007. The PbB levels of the 145 participants tested previously have been reported in earlier studies (Counter et al. 2001; 2007). The source of Pb exposure in the study area is discarded automobile storage batteries from which entire families, men women, and children extract Pb to use in the glazing of ceramics, particularly roof tiles as part of a local Pb glazing backyard industry. The primary routes of Pb exposure for the adults in the study area are ingestion of Pb-contaminated food, dust and soil, and the inhalation of air-borne particulates from the heavily Pb-contaminated smoke produced by the Pb glazing kilns. The Pb glazing process and the environmental contamination of the study area are described in greater detail elsewhere (Counter et al. 2000).
Informed consent was obtained from all adults prior to testing. This study was approved by the Human Studies Committee (Comité de Bioética) of Universidad San Francisco de Quito. The study was conducted under the auspicies of Universidad San Francisco de Quito Colegio Ciencias de la Salud, Escuela de Medicina in Quito, Ecuador. The results of this investigation were presented to the participants in the study, who were counseled regarding their Pb exposure levels, and referred to local health officials for medical intervention as needed.
Pb Concentration in Blood
Samples of 2–4 ml of whole blood were drawn by venipuncture from 39 participants following meticulous cleaning of the skin using swabs containing isopropanol. The samples were collected from the antecubital vein and kept in 4-mlVacutainer collection tubes with Li-heparin. All whole blood samples were stored in a refrigerated container. The blood samples were later analyzed for Pb concentration by graphite furnace atomic absorption spectroscopy with Zeeman background correction (Perkins Elmer 5000, Zeeman HGA-500 spectrophotometer, Norwalk, Connecticut) at the Department of Laboratory Medicine, Boston Children’s Hospital.
ZPP Concentration in Blood
ZPP/heme ratio was measured directly using a hematofluorometer (ProtoFluor-z, Helena Laboratories, Inc., Beaumont, TX), which presents results in μmol/mol heme. Cells placed on a slide were exposed to a beam of light from a Halogen lamp that projected a beam at 415 nm, the optimal absorption level for hemoglobin. Control samples (Kaulson Laboratories, West Caldwell, NJ) were run at low, medium and high levels. ZPP whole blood (ZPP WB) values were presented in μg/dl with an assumed hematocrit of 35%. The normal reference ranges used were 30–70 μmol ZPP/mol heme, and 15–36 μg ZPP/dl whole blood.
Statistical Analysis
The mean, standard deviation, range, and median were calculated for PbB concentration, ZPP/heme ratio and ZPP whole blood level obtained on the participants in the study. Because some of the PbB and ZPP data were significantly skewed (≥ than 2 standard errors of skewness), non-parametric statistics were used in the data analysis. Differences between groups were analyzed by the Mann-Whitney U test. The association between PbB level and ZPP/heme ratio was analyzed by Spearman rho correlation analysis. All Z and p values reported for the Mann Whitney U test and the Spearman correlation coefficient are tied values. An alpha level of ≤ 0.05 was accepted as an indication of statistical significance.
RESULTS
Pb and ZPP Concentrations
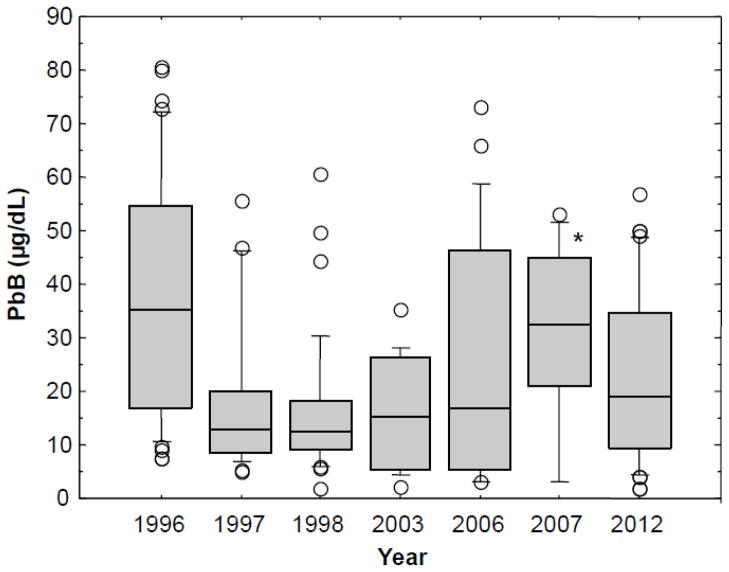
The PbB levels for the current participants (2012 cohort) were compared to previous PbB data obtained by the authors over a period of years, beginning in 1996, to track the PbB levels at specific intervals between 1996 and 2012 in order to examine more closely changes in Pb exposure. The results are shown in the box plots of Figure 1, which compare the PbB distributions for adults tested in the same study area in the years 1996, 1997, 1998, 2003, 2006, 2007 and 2012. The mean PbB level of 22.7 μg/dl (SD: 16.3; median: 19; range: 2–57) for the 2012 cohort was significantly lower than the mean PbB level of 37.9 μg/dl (SD: 22.8; median: 35.3; range: 7.4–80.7) found in the 1996 group, indicating that the overall Pb exposure in the adult population has diminished over time. However, the PbB levels for the 2012 study group was not significantly different from the 1997, 1998, 2003, 2006 or the 2007 cohorts. These findings indicated that after the initial reduction observed in the 1997 cohort, the PbB levels remained relatively stable.
Figure 1.
Box plots showing blood lead (PbB) levels over time for the years 1996 (N = 41), 1997 (N = 25), 1998 (N = 32), 2003 (N = 14), 2006 (N = 23), 2007 (N = 10) and 2012 (N = 39) for cohorts of Andean Ecuadorian adults living in Pb-glazing communities. No PbB tests were performed in the intervening years not listed in the figure. The boxes contain individual PbB levels between the 25th and 75th percentiles. The horizontal lines inside the boxes represent the 50th percentile. The small horizontal lines above the boxes represent the 90th percentile, and the small horizontal lines below the boxes represent the 10th percentile. The individual data points represent cases above the 90th percentile and below the 10th percentile. *The interpretation of the box plot for the 2007 group should be made with caution because of the small number of participants (N =10).
The mean PbB level of 18.2 μg/dl (median: 12.8) for the 1997 cohort was significantly lower than the mean PbB level of 37.9 μg/dl (median: 35.3) for 1996 study group. Similarly, the mean PbB level of 16.1 μg/dl (median: 12.5) for the 1998 group was significantly lower than the mean PbB level for 1996 cohort. The mean PbB level of 16.1 μg/dl (median: 15.4) for the 2003 cohort, and the mean PbB level of 25.8 μg/dl (median: 17) for the 2006 cohort were both significantly lower than the PbB level for the 1996 cohort. The mean PbB level of 30.1 μg/dl (median: 32.5) for the 2007 group was not significantly different from the 1996 cohort. The interpretation of the statistical analysis for the 2007 group needs to be made with caution because of the small number of participants (N =10). The differences among the 1997, 1998, and 2003 groups were not statistically significant. Data in Figure 1 further show an overall decline in PbB levels from 1996 to 2003. The subsequent rise in PbB level from 2003 to 2007 was statistically significant. Although the current (2012) mean PbB level of 22.7 μg/dl (median: 19) tended to be lower than the PbB level of the cohort tested in 2007 (mean: 30.1 μg/dl; median: 32.5), the difference was not statistically significant.
For further comparative analysis, the results were examined by the use of percentile data, which are shown in Table 1. The % of participants who presented with PbB levels ≥ 25 μg/dl (the U.S. CDC’s reference level for adults) for the years 1996, 1997, 1998, 2003, 2006, 2007 and 2012 are shown in Table 2. Data in Table 2 indicate that the % of participants with elevated PbB levels ≥ 25 μg/dl was highest in the 1996 and 2007 cohorts.
Table 1.
Means and percentiles for blood lead (PbB) levels obtained for cohorts of lead-exposed Ecuadorian Andean adults during the years 1996, 1997, 1998, 2003, 2006, 2007 and 2012. Standard deviation values are shown in parentheses below the mean values.
| PbB Level Monitoring Year | |||||||
|---|---|---|---|---|---|---|---|
| 1996 (n 41) | 1997 (n 25) | 1998 (n 32) | 2003 (n 14) | 2006 (n 23) | 2007 (n 10) | 2012 (n 39) | |
| Mean | 37.9 (22.8) | 18.2 (14.0) | 16.1 (13.0) | 16.1 (10.3) | 25.8 (22.7) | 30.1 (17.4) | 22.7 (16.3) |
| 10th Percentile | 10.6 | 6.9 | 5.8 | 4.3 | 3.0 | 3.0 | 4.4 |
| 25th Percentile | 16.8 | 8.4 | 9.2 | 5.4 | 5.2 | 21.0 | 9.2 |
| 50th Percentile | 35.3 | 12.8 | 12.5 | 15.4 | 17.0 | 32.5 | 19.0 |
| 75th Percentile | 54.5 | 20.1 | 18.1 | 26.1 | 46.2 | 45.0 | 34.6 |
| 90th Percentile | 72.3 | 46.4 | 30.4 | 28.2 | 58.8 | 51.5 | 48.6 |
Table 2.
Percentage of Ecuadorian Andean adults with blood lead (PbB) levels ≥ 25 μg/dL* for the years 1996, 1997, 1998, 2003, 2006, 2007 and 2012.
| Year | 1996 | 1997 | 1998 | 2003 | 2006 | 2007 | 2012 |
| Percentage | 63.4 | 20.0 | 9.4 | 28.6 | 47.8 | 70.0 | 41.0 |
A PbB level of ≥ 25 μg/dL is defined by the U.S. Centers for Disease Control and Prevention (CDC) as elevated in adults.22
The difference in PbB levels between males and females for the aggregate cohorts tested from 1996 to 2007 was compared with the PbB levels for males and females in the 2012 study group. The mean PbB level for the combined 1996–2007 cohorts of 30.5 μg/dl (SD: 22.7; median: 19.6; range: 2.1–79.9) for the males (N = 42) was not significantly different from the mean PbB level of 22.9 μg/dl (SD: 18.6; median: 17; range: 2–80.7) for the 103 females. Similarly, the mean PbB level of 29.3 μg/dl (SD: 18.4; median: 29.5; range: 2–57) for the males tested in 2012 was not significantly different from the mean PbB level of 19 μg/dl (SD: 14; median: 15; range: 2–50) for the females examined in 2012.
The current ZPP levels (2012) were compared with ZPP levels obtained on cohorts of adults tested in 2006 and in 2007. These ZPP results are displayed in the box plots of Figure 2A. For comparative purposes, the PbB levels for the same period are shown in Figure 2B. The mean ZPP/heme ratio for the 2012 cohort was 134.2 μmol/mol (SD 106.3; median: 80; range: 28– 377), and the mean ZPP WB level was 61.6 μg/dl (SD 47.6; median: 38; range: 18–171). These mean values were higher than the normal reference range for ZPP/heme (30–70 μmol/mol), and for ZPP WB (15–36 μg/dl). The mean ZPP/heme ratio of 134.2 μmol/mol observed in the 2012 study group was not significantly different from the mean of 128.4 μmol/mol (SD 112.5; median: 85; range: 41–470) measured in the study area in the 2006 cohort, nor significantly different from the mean of 103.3 μmol/mol (SD: 49.7; median: 79; range: 53–188) found in the 2007 cohort. Similarly, the PbB levels shown in Figure 2B for the 2006, 2007 and 2012 cohorts were not significantly different from each other.
Figure 2.
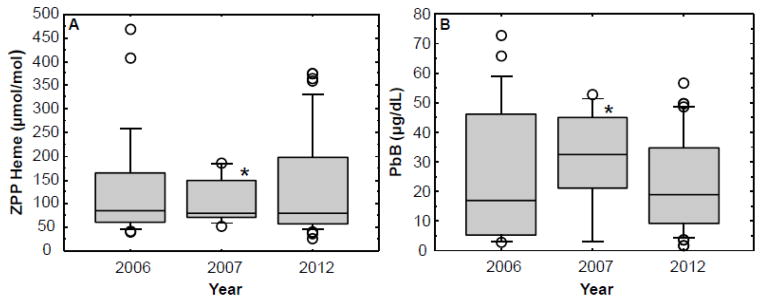
Box plots comparing zinc protoporphyrin/heme ratios (ZPP) (A) and blood lead (PbB) levels (B) observed over three years (2006, 2007 and 2012) in adults living in Pb-glazing communities in the Andes Mountains of Ecuador. See Figure 1 legend for a description of the box plots. *The interpretation of the box plot for the 2007 group should be made with caution because of the small number of participants (N =10).
The mean ZPP/heme ratio for the males in the 2012 study group was 146.9 μmol/mol (SD: 109.9; median: 111; range: 41– 377), and the mean for the females was 127.2 μmol/mol (SD: 105.8; median: 77; range: 28– 376). The difference in the ZPP/heme ratio between males and females was not statistically significant. Spearman rho correlation analyses showed significant associations between ZPP/heme ratio and PbB levels for the 2006 cohort, the 2007 cohort, and the current 2012 group. When the 2006, 2007 and 2012 groups were combined (N = 72), a significant correlation coefficient of .687 between ZPP/heme ratio and PbB level was obtained.
The mean ZPP WB for the 2012 study group was 61.6 μg/dl (SD: 47.6; median: 38; range: 18 – 171), compared with a mean of 58 μg/dl (SD: 50.9; median: 39; range: 19–213) for the 2006 cohort, and a mean of 46.7 μg/dl (SD: 22.6; median: 35.5; range: 24– 85 μg/dl) for the 2007 cohort. The differences between the ZPP WB levels of the 2006, 2007 and the 2012 cohorts were not statistically significant. The mean ZPP WB for the males was 66.5 μg/dl (SD: 49.9; median: 50; range: 19– 171), and for the females, 58.8 μg/dl (SD: 47.1; median: 36; range: 18– 170). The difference in ZPP WB between males and females was not statistically significant. Further statistical analysis was not performed on the ZPP WB data since the ZPP/heme ratio is essentially a reflection of the ZPP WB data, as indicated by a near perfect correlation coefficient of .953 between ZPP/heme and ZPP WB.
Figure 3A, B shows a comparative sample of matched (same) individuals tested previously (between 1996 and 2007) for PbB and ZPP/heme levels in the same Pb-contaminated communities that comprised the study area, and who were re-examined in 2012. For PbB comparisons (Figure 3A), 19 matched cases are presented, and for the ZPP/heme comparisons (Figure 3B), 8 matched individual participants are presented. The individual data points shown in Figure 3 illustrate that while the PbB and ZPP levels declined for some participants in the current test period, other individual’s PbB and ZPP levels were generally unchanged. One participant exhibited a marked increase in the current PbB level.
Figure 3.
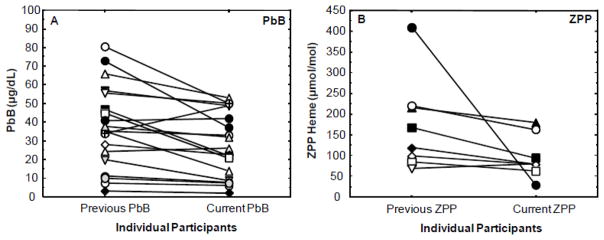
Comparison of previous (1996–2007) blood lead (PbB) levels (A) and zinc protoporphyrin/heme (ZPP) ratios (B) with current (2012) PbB and ZPP/heme levels in matched (same) individual Andean Ecuadorian adults living in Pb-glazing communities. For PbB comparisons (A), 19 matched cases are presented, and for the ZPP/heme comparisons (B) 8 matched individual participants are presented.
DISCUSSION
This study investigated the current levels of Pb exposure as indicated by PbB and ZPP levels in Andean adults living in communities with a history of environmental Pb contamination from a local Pb-glazing cottage industry, and compared the findings with previous PbB and ZPP levels observed in inhabitants of the same study area. Previous investigations in the study area by the authors showed high levels of Pb poisoning in both children and adults. The results of this study showed that the overall PbB levels in adults decreased from a mean PbB level of 37.9 μg/dl in a 1996 cohort to 22.7 μg/dl in 2012. The 22.7 μg/dl for the 2012 cohort is less than the 25μg/dl reference level for elevated PbB in adult workers in the U.S., and the 31μg/dl (1.5 μmol/L) reference level used in Canada (Saskatchewan Ministry of Labour Relations and Workplace Safety 2013). If the U.S. Occupational Safety and Health Administration’s (OSHA) criterion of removing a worker from further Pb exposure when the PbB level is equal to or greater than 50–60 μg/dl is used (Tak et al. 2008), then only three of the participants in the 2012 cohort of the current study would require removal from the work site. However, according to the CDC, the reference level of ≥ 25 μg/dl for Pb-exposed adults underestimates the adverse health effects of occupational Pb exposure, because Pb toxicity may occur as low as 5 μg/dl. Further, the U.S. Healthy People 2020 Public Health Initiative has as one of its objectives reducing the prevalence of adults with PbB level > 10 μg/dl (CDC 2011).
The maximum PbB level observed in the adult participants living in the Pb-contaminated study area in 1996 was approximately 81 μg/dl, compared to 57 μg/dl in the current cohort, suggesting that there are fewer adults currently in the study area with severe Pb intoxication. When the PbB data were tracked on a yearly basis, it was found that after an initial decline from 1996 to 1997, the PbB levels remained relatively constant from 1997 to 2006. There was, however, a noticeable increase in PbB levels in the 2007 cohort, but this may be a function of the small number of participants in this cohort. The significant rise in PbB levels from 2003 to 2007 may reflect an associated escalation in Pb-glazing activities in the study area during this period, and thus greater occupational Pb exposure. The reduction in PbB levels from 2007 to 2012, although not statistically significant, suggests a declining trend in Pb-glazing activities in the study area. Moreover, fewer Pb acid storage battery casings were observed in the study area during the current investigation, which is consistent with the declining trend in Pb-glazing activities. The lower PbB levels observed in 2012 appear to be associated with the introduction of new Pb-baking kiln technology in the study area, as well as current observance of the principles of our previously introduced Pb-exposure education and prevention program (Counter et al. 1997; 2003). The authors have previously demonstrated empirically that the Pb-exposure education and prevention program has been effective in reducing PbB levels in children in the study area (Counter et al, 2003). The observation that PbB levels of men and women living in the study area were not significantly different is not an unexpected finding, since historically both men and women in these Andean communities actively engage in Pb-glazing activities that place them at equal risk for Pb exposure.
ZPP levels, an indication of deleterious Pb effects on the erythrocytic enzyme ferrochelatase, were obtained on cohorts of men and women in the study area in 2006, 2007 and 2012. The ZPP levels of the participants were markedly elevated for each year, indicating chronic Pb exposure in adults residing and working in the study area. In 2006, 56.5% of the adults examined had ZPP/heme levels above the normal range; in 2007, 80% of the participants were above the normal range; and in 2012, 56.4% of the adults had ZZP/heme levels above the normal range. These results indicate that a majority of the adults continue to show evidence of prolonged Pb exposure with adverse effects on the hematological system, as evidenced by the elevated ZPP/heme levels over a period of several years.
In conclusion, the current elevated PbB levels observed in Andean Ecuadorian adults living in communities with high Pb-contamination from a Pb-glazing cottage industry continues to pose a significant health risk. It should be noted that this study was conducted in the field, with its attendant challenges and constraints, including logistics of field testing, observation of local cultural customs, participants’ work schedules and availability, which in some years accounted for the relatively small number of participants examined. Although these factors may impose certain limitations on the interpretation of data, the findings of this study clearly demonstrate the patterns of Pb exposure during the years of monitoring. This study did not examine potential confounders, such as smoking, or alcohol consumption, since these habits were not found to be prevalent among the participants in the study areas, and because this study focused on tracking or monitoring the changes in PbB and ZPP levels over a period of years. There was no indication of confounding factors, including biomedical variables that would impact the PbB and ZPP levels observed in this study. Further investigation in the study area is necessary to determine the possible adverse neurological, neurocognitive and other medical outcomes associated with the Pb exposure found in this population.
The participants in this study were informed of their Pb exposure and counseled regarding the health implications of their PbB levels. The participants with elevated PbB levels were referred to local health authorities for medical intervention where appropriate. In addition, a community-wide meeting, which included Pb-glazing workers and their families, regional health authorities, and representatives from the Ecuadorian Ministry of Health was held in La Victoria Centro to further apprise the community of the hazards of Pb exposure, and re-emphasize prevention of Pb poisoning. It is important that the PbB levels of the adult Pb-glazing workers and their families be continually monitored and managed to reduce the health risks of Pb exposure. Specific prevention and management procedures have been proposed in earlier reports (Counter et al. 1997; 2000; Kosnett et al. 2007).
Acknowledgments
The authors thank Universidad San Francisco de Quito Colegio Ciencias de la Salud, Escuela de Medicina in Quito, Ecuador for continued support of this project. We thank Dr. Gonzalo Mantilla, Dean of the College of Health Sciences, Universidad San Francisco de Quito Medical School, for ongoing support and excellent advice. We thank Gladys Pacheco, nurse at the Subcentro de Salud, La Victoria, Ecuador for assistance. The Minister of Public Health of Ecuador, Carina Vance Mafla, is thanked for her consultation and Ministry of Public Health staff support. The authors are grateful to Dr. Merilee Grindle and Monica Tesoriero of the David Rockefeller Center for Latin American Studies at Harvard University for support of this project. We thank Dr. Jeremy Bloxham, Dean of Science at Harvard University; Harvard Biological Laboratories; Harvard University Health Services; and the Eunice Kennedy Shriver Center/University of Massachusetts Medical School for support. LHB is supported in part by NIH grant P30 HD04147.
References
- ATSDR. Toxicological profile for lead. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 2007. [Google Scholar]
- Baker EL, White RF, Pothier LJ, Berkey CS, Dinse GE, Travers PH, Harley JP, Feldman RG. Occupational lead neurotoxicity: improvement in behavioural effects after reduction of exposure. Br J Ind Med. 1985;42:507–516. doi: 10.1136/oem.42.8.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker ML, Ford DP, Vaughan CG, Walsh KS, Lindgren KN. The association of lead exposure and motor performance mediated by cerebral white matter change. Neurotoxicology. 2007;28:318–323. doi: 10.1016/j.neuro.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Buchanan LH, Counter SA, Ortega F. Environmental lead exposure and otoacoustic emissions in Andean children. J Toxicol Environ Health A. 2011;74:1280–1293. doi: 10.1080/15287394.2011.587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Adult blood lead epidemiology and surveillance—United States, 2008–2009. Morbidity and Mortality Weekly Report. 2011;60:841–845. [PubMed] [Google Scholar]
- Chuang HY, Chao KY, Tsai SY. Reversible neurobehavioral performance with reductions in blood lead levels--a prospective study on lead workers. Neurotoxicol Teratol. 2005;27:497–504. doi: 10.1016/j.ntt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH. Neuro-ototoxicity in Andean Adults with chronic lead and noise exposure. J Occup Environ Med. 2002;44:30–38. doi: 10.1097/00043764-200201000-00006. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Laurell G, Ortega F. Field screening of blood lead levels in remote Andean villages. Neurotoxicology. 1998;19:871–877. [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F. Current pediatric and maternal lead levels in blood and breast milk in Andean inhabitants of a lead-glazing enclave. J Occup Environ Med. 2004;46:967–973. doi: 10.1097/01.jom.0000137712.21963.76. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F. Gender differences in blood lead and hemoglobin levels in Andean adults with chronic Pb exposure. Int J Occup Environ Health. 2001;7:113–118. doi: 10.1179/107735201800339551. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F. Lead concentrations in maternal blood and breast milk and pediatric blood of Andean villagers: 2006 follow-up investigation. J Occup Environ Med. 2007;49:302–309. doi: 10.1097/jom.0b013e31803225b0. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F. Neurophysiologic and neurocognitive case profiles of Andean patients with chronic environmental lead poisoning. J Toxicol Environ Health A. 2009a;72:1150–1159. doi: 10.1080/15287390903091772. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F. Neurocognitive screening of lead-Exposed Andean adolescents and young adults. J Toxicol Environ Health A. 2009b;72:625–632. doi: 10.1080/15287390902769410. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F, Amarisiriwardena C, Hu H. Environmental lead contamination and pediatric lead intoxication in an Ecuadorian Andean Village. Int J Occup Environ Health. 2000;6:169–176. doi: 10.1179/oeh.2000.6.3.169. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH, Ortega F, Laurell G. Normal auditory brainstem and cochlear function in extreme pediatric plumbism. J Neurol Sci. 1997;152:85–92. doi: 10.1016/s0022-510x(97)00149-4. [DOI] [PubMed] [Google Scholar]
- Counter SA, Ortega F, Shannon MW, Buchanan LH. Succimer (meso-2,3-dimercaptosuccinic acid (DMSA) treatment of Andean children with environmental lead exposure. Int J Occup Environ Health. 2003;9:164–168. doi: 10.1179/oeh.2003.9.2.164. [DOI] [PubMed] [Google Scholar]
- Danadevi K, Rozati R, Saleha Banu B, Hanumanth Rao P, Grover P. DNA damage in workers exposed to lead using comet assay. Toxicology. 2003;187:183–193. doi: 10.1016/s0300-483x(03)00054-4. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Ware JH, Salganik M, Radcliffe J, Rogan WJ, Rhoads GG, Fay ME, Davoli CT, Denckla MB, Bornschein RL, Schwarz D, Dockery DW, Adubato S, Jones RL Treatment of Lead-Exposed Children Clinical Trial Group. Effect of chelation therapy on the neuropsychological and behavioral development of lead-exposed children after school entry. Pediatrics. 2004;114:19–26. doi: 10.1542/peds.114.1.19. [DOI] [PubMed] [Google Scholar]
- Dounias G, Rachiotis G, Hadjichristodoulou C. Acute lead intoxication in a female battery worker: Diagnosis and management. J Occup Med Toxicol. 2010;5:19. doi: 10.1186/1745-6673-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenga C, Cacciola A, Martino LB, Calderaro SR, Di Nola C, Verzera A, Trimarchi G, Germanò D. Relationship of blood lead levels to blood pressure in exhaust battery storage workers. Ind Health. 2006;44:304–309. doi: 10.2486/indhealth.44.304. [DOI] [PubMed] [Google Scholar]
- Froom P, Kristal-Boneh E, Benbassat J, Ashkanazi R, Ribak J. Predictive value of determinations of zinc protoporphyrin for increased blood lead concentrations. Clin Chem. 1998;44:1283–1288. [PubMed] [Google Scholar]
- Gottesfeld P, Pokhrel AK. Review: Lead exposure in battery manufacturing and recycling in developing countries and among children in nearby communities. J Occup Environ Hyg. 2011;8:520–532. doi: 10.1080/15459624.2011.601710. [DOI] [PubMed] [Google Scholar]
- Kašuba V, Rozgaj R, Milić M, Zelježić D, Kopjar N, Pizent A, Kljaković-Gašpić Z, Jazbec BSA. Evaluation of genotoxic effects of lead in pottery-glaze workers using micronucleus assay, alkaline comet assay and DNA diffusion assay. Int Arch Occup Environ Health. 2012;85:807–818. doi: 10.1007/s00420-011-0726-4. [DOI] [PubMed] [Google Scholar]
- Kosnett MJ, Wedeen RP, Rothenberg SJ, Hipkins KL, Materna BL, Schwartz BS, Hu H, Woolf A. Recommendations for medical management of adult lead exposure. Environ Health Perspect. 2007;115:463–471. doi: 10.1289/ehp.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CJ, Werntz CL, III, Ducatman AM. The interpretation of zinc protoporphyrin changes in lead intoxication: A case report and review of the literature. Occup Med. 2004;54:587–591. doi: 10.1093/occmed/kqh123. [DOI] [PubMed] [Google Scholar]
- Meyer-Baron M, Seeber A. A meta-analysis for neurobehavioural results due to occupational lead exposure with blood lead concentrations <70 microg/100 ml. Arch Toxicol. 2000;73:510–518. doi: 10.1007/s002040050002. [DOI] [PubMed] [Google Scholar]
- Murata K, Araki S, Yokoyama K, Uchida E, Fujimura Y. Assessment of central, peripheral, and autonomic nervous system functions in lead workers: neuroelectrophysiological studies. Environ Res. 1993;61:323–336. doi: 10.1006/enrs.1993.1077. [DOI] [PubMed] [Google Scholar]
- Olewińska E, Kasperczyk A, Kapka L, Kozłowska A, Pawlas N, Dobrakowski M, Birkner E, Kasperczyk S. Level of DNA damage in lead-exposed workers. Ann Agric Environ Med. 2010;17:231–236. [PubMed] [Google Scholar]
- Popovic M, McNeill FE, Chettle DR, Webber CE, Lee CV, Kaye WE. Impact of occupational exposure on lead levels in women. Environ Health Perspect. 2005;113:478–484. doi: 10.1289/ehp.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raafat BM, Hassan NS, Aziz SW. Bone mineral density (BMD) and osteoporosis risk factor in Egyptian male and female battery manufacturing workers. Toxicol Ind Health. 2012;28:245–252. doi: 10.1177/0748233711410912. [DOI] [PubMed] [Google Scholar]
- Rosin A. The long-term consequences of exposure to lead. Isr Med Assoc J. 2009;11:689–694. [PubMed] [Google Scholar]
- Saskatchewan Ministry of Labour Relations and Workplace Safety. Occupational Lead Exposure: Information for Physicians. Retrieved May 2013: http://www.lrws.gov.sk.ca/occupational-lead-exposure-information-physicians.
- Schwartz BS, Stewart WF, Bolla KI, Simon PD, Bandeen-Roche K, Gordon PB, Links JM, Todd AC. Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology. 2000;55:1144–1150. doi: 10.1212/wnl.55.8.1144. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, Wu X, Todd AC, Shi W, Bassett S, Youssem D. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology. 2006;66:1476–1484. doi: 10.1212/01.wnl.0000216138.69777.15. [DOI] [PubMed] [Google Scholar]
- Suplido ML, Choon NO. Lead exposure among small-scale battery recyclers, automobile radiator mechanics and their children in Manila, the Philippines. Environ Res. 2000;82:231–238. doi: 10.1006/enrs.1999.4024. [DOI] [PubMed] [Google Scholar]
- Tak S, Roscoe RJ, Alarcon W, Ju J, Sestito JP, Sussell AL, Calvert GM. Characteristics of US workers whose blood lead levels trigger the medical removal protection provision, and conformity with biological monitoring requirements, 2003–2005. Am J Ind Med. 2008;51:691–700. doi: 10.1002/ajim.20603. [DOI] [PubMed] [Google Scholar]
- Were FH, Kamau GN, Shiundu PM, Wafula GA, Moturi CM. Air and blood lead levels in lead acid battery recycling and manufacturing plants in Kenya. J Occup Environ Hyg. 2012;9:340–344. doi: 10.1080/15459624.2012.673458. [DOI] [PubMed] [Google Scholar]