Abstract
Evaluation of lungs from GalTKO.hCD46 pigs, genetically modified to lack the galactose-α(1,3)-galactose epitope (GalTKO) and to express human CD46, a complement regulatory protein, has not previously been described. Physiologic, hematologic and biochemical parameters during perfusion with heparinized fresh human blood were measured for 33 GalTKO.hCD46, GalTKO (n=16), and wild type pig lungs (n=16), and 12 pig lungs perfused with autologous pig blood. Median GalTKO.hCD46 lung survival was 171 minutes compared to 120 for GalTKO (p=0.27) and 10 for wild type lungs (p<0.001). Complement activation, platelet activation, and histamine elaboration were significantly reduced during the first 2h of perfusion in GalTKO.hCD46 lungs compared to GalTKO (ΔC3a at 120′ 812±230 vs. 1412±1047, p=0.02; ΔCD62P at 120′ 9.8±7.2 vs. 25.4±18.2, p<0.01; Δhistamine at 60′ 97±62 vs. 189±194, p=0.03). We conclude that, in addition to significant down-modulation of complement activation, hCD46 expression in GalTKO lungs diminished platelet and coagulation cascade activation, neutrophil sequestration and histamine release. Because GalTKO.hCD46 lung failure kinetics correlated directly with platelet and neutrophil sequestration, coagulation cascade activation, and a rise in histamine levels within the first hour of perfusion, further progress will likely depend upon improved control of these pathways, by rationally targeted additional modifications to pigs and pharmacologic interventions.
Keywords: lung, xenotransplantation, ex-vivo perfusion, complement, coagulation, platelet activation, leukocyte adhesion, GalTKO.hCD46
Introduction
Major advances have been achieved in survival and function of pig hearts, kidneys and liver xenografts in translational primate models based on genetic modifications to the pig (1–7). The same is true for cell and tissue xenotransplantation (8–10). Pig gene constructs tested to date include removal of the primary carbohydrate target on pig cells, galactose-α(1,3)-galactose, by “knockout” of the α-galactosyl-transferase gene (GalTKO) or introduction of human complement regulatory proteins (hCRPs) such as human membrane cofactor protein (hMCP/hCD46) or human decay-accelerating factor (hDAF/hCD55). With either of these genetic modifications, the incidence of hyperacute rejection (HAR) and early graft failure (EGF) of xenogenic hearts, kidneys and livers is significantly reduced (11–12). In contrast, hCRP or GalTKO- expressing pig lung xenografts remain susceptible to injury (which we term hyperacute lung rejection, or HALR) within minutes to hours after ex vivo perfusion with human blood or and pig-to-baboon models (13–17).
Since GalTKO-transgenic cells are protected from preformed anti-Gal antibodies but can still be recognized by anti-non-Gal antibodies, in vitro experiments have proven that additional expression of hCRP adds further protection from xenogenic cell injury by inhibiting the complement cascade activation, triggered by antibody-antigen binding (18). Recent work from Westall et al. (19) demonstrated that GalTKO lungs expressing multiple genetic modifications (hCD55, hCD59) showed improved and sustained pulmonary function in a xenogenic perfusion model.
Here we test in a large experimental series how the GalTKO.hCD46 phenotype modulates HALR, and begin to reveal the mechanisms underlying GalTKO.hCD46 lung xenograft failure.
Materials and Methods
Animals
Genetically engineered pigs (BW 6–15 kg) lacking the alpha-Gal epitope (GalTKO) with or without human membrane cofactor protein (hCD46) were supplied by Revivicor (Blacksburg, VA). For GalTKO.hCD46 transgenic pigs, a human CD46 minigene construct, containing the endogenous hCD46 promoter and first two introns of genomic DNA fused to hCD46 coding sequence, was used to produce pigs with constitutive high level expression of the human complement inhibitor transgene (20). This hCD46 transgenic pig line was cross-bred with homozygous GalTKO pigs (21) over more than 4 generations to produce a stable GalTKO.hCD46 line.
(The GalTKO lung results reported here were obtained using Revivicor pigs, and do not include previously reported results associated with GalTKO lungs evaluated in collaboration with MGH and Immerge Biotherapeutics Inc. (14–15)).
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine and were conducted in compliance with National Institutes of Health guidelines for the care and use of laboratory animals.
Lung Harvest
Induction of anesthesia and surgical organ dissection was performed as previously described (14,18). Prior to flushing the lungs, 1-benzylimidazole (5 mg/kg BW; Sigma-Aldrich, St. Louis, MO, a thromboxane synthase inhibitor), and synthetic prostaglandin I2 (0.03 mg/kg BW; Flolan; GlaxoSmithKline, Research Triangle Park, NC) were administered intravenously and allowed to circulate for several minutes.
Lung Perfusion
The right and left lungs were separately perfused via the pulmonary artery using side-by-side circuits fashioned from silicon tubing and polyurethane connectors as previously described (13,22). Results associated with a variety of drug interventions to one lung of each pair will be reported separately. Pulmonary artery flow was measured and recorded with a flowmeter (Transonic Systems Inc., Model T206, Ithaca, NY). The Digimed System Integrator (Micro-Med, Louisville, KY) was used to provide a continuous measurement of both PA- and airway pressures via transducers integrated into the perfusion and ventilator circuits, respectively. Pulmonary vascular resistance (PVR) calculation and criteria defining “lung failure” prior to elective experimental termination at 4 hours have previously been described (14).
Perfusate Preparation
Xenogenic perfusions
Approximately 450 ml of type A or O fresh whole blood was collected from two healthy human blood donors into a blood collection bag, containing 64 ml of CPDA-1. Two units (~240 ml each) of type-compatible thawed plasma were added for each unit of blood to obtain an initial perfusate volume of about two liters. Heparin (3 IU/ml blood, Heparin Sodium Injection, Sagent, Schaumburg, IL), calcium chloride (1.3 to 1.6 mg/ml blood, American Regent, Inc., Shirley, NY) to neutralize the CPDA chelating agent, and 8.4% sodium bicarbonate (~0.84 mg/ml blood, target pH of 7.4, Hospira, Inc., Lake Forest, IL) were added to the blood pool. All blood components were pooled and thoroughly mixed before circuit priming. Pre-perfusion hematocrit values were similar in all experimental groups (GalTKO.hCD46 16.3±0.4%; GalTKO 17.0±0.6%; WT 15.9±1.5%).
Autologous perfusions
One pint of blood was collected from the venous circulation by gravity drainage into a clinical blood collection bag over 5–15 minutes, with simultaneous peripheral volume replacement with crystalloid solution to minimize hemodynamic perturbations prior to lung recovery. An equal volume (one pint) of saline was added to dilute the blood to a hematocrit of 16.9±1.6%. Finally, heparin, calcium chloride and HCO3 were added to the perfusate at similar dosages as described for the human blood preparation.
Experimental groups
Human blood was perfused through GalTKO lungs (n=16) and GalTKO.hCD46 lungs (n=33). Historical and contemporaneous experiments using wild type (WT) pig lungs perfused with human blood (n=16, 10 of which were previously reported (14)) and pig lungs perfused with autologous pig blood (n=12, 10 of which were previously reported (14)) are reported for contextual analysis.
Sampling regimen
Baseline blood samples were taken after the blood preparation (“pre” sample), and after circulating the blood in the perfusion circuit for at least 3 minutes (time 0 sample). Further samples were collected 5, 15, 30, 60, 120 and 240′ after lung perfusion was initiated. All samples were stored at −70°C.
Lung tissue samples were collected pre-perfusion and 10, 30, 60, 120 and 240′ after perfusion was begun.
Hematologic Analysis
Blood cell counts were enumerated by standard automated techniques (Antech Diagnostics, Rockville, MD) in blood samples collected in ethylenediaminetetraacetic acid (EDTA).
Beta-thromboglobulin, Thrombin, Thromboxane B2 (TXB2), Histamine, and Complement Enzyme-Linked Immunosorbent Assays
Beta-thromboglobulin (βTG) and prothrombin fragments 1+2 (F1+2) were measured by commercial enzyme-linked immunosorbent assay (ELISA) in plasma samples collected in CTAD tubes as previously described (14). EDTA plasma was used to measure histamine (ELISA kit Starfish, Gernusco, Italy) and C3a levels (14). Blood, collected at 3 time points (0′, 15′ and 60′) in EDTA tubes containing 100 μl (at 10 μg/ml) of meclofenamate (Sigma-Aldrich, St. Louis, MO), was analyzed to measure plasma levels of thromboxane (Thromboxane B2 EIA Kit, Cayman Chemical Company, Ann Arbor, MI; Catalog No. 519031).
Measurement of Platelet Activation by flow cytometry
Blood samples were stained by monoclonal antibodies specific for CD41 (as a marker for platelets) (AbD Serotec, Raleigh, NC) and CD62P (expressed by activated platelets) (BD Pharmingen, San Diego, CA) and acquired on a FACSCalibur (BD Biosciences, San Jose, CA). Platelets and platelet aggregates, identified by size and by presence of CD41 staining, were analyzed for expression of CD62P by FACS analysis. Results were expressed as the percentage of CD62P-positive cells among CD41-positive cells.
Measurement of Anti-non-Gal antibody levels by flow cytometry
IgM Antibody levels were measured in plasma samples collected before lung perfusion (“pre” sample) as previously described (14) with modifications.
Histology and Immunochemistry
Lung biopsy specimens obtained during the perfusion and terminally samples were trisected, and processed as previously described (14). Frozen tissue sections from OCT-infused biopsy specimens (10′ biopsies) were assessed by immunohistochemistry with monoclonal antibodies against human C5b-9 C5b-9at 1:50, rabbit anti-von Willebrand’s factor antibody (both Dako, Carpinteria, CA), C4d (Quidel Corporation, San Diego, CA) at 1:50 and CD41 (Immunotech, Marseilles, France) at 1:100. The deposition of platelets and complement in lung tissue was quantified by staining intensity (from 0 to 3), taking into account both the extent (proportion of endothelial surface with detectable staining) and intensity of staining, relative to standard positive and negative “control” sections.
GalTKO.hCD46 group analysis
The GalTKO.hCD46 lung cohorts was arbitrarily divided into three groups according to whether the lungs failed within 120 min (“early”), after 120 but before 240 min (“intermediate”), or did not meet failure criteria at four hours (“surviving” >240 min). The experimental groups was analyzed for complement cascade activation (C3a), thrombin generation (F1+2) and platelet activation (βTG, CD62P).
Statistical analysis
Statistical analysis were performed as previously described (14) using a personal computer with the statistical package SigmaPlot for Windows (Version 11.0).
Results
Graft Survival and Function
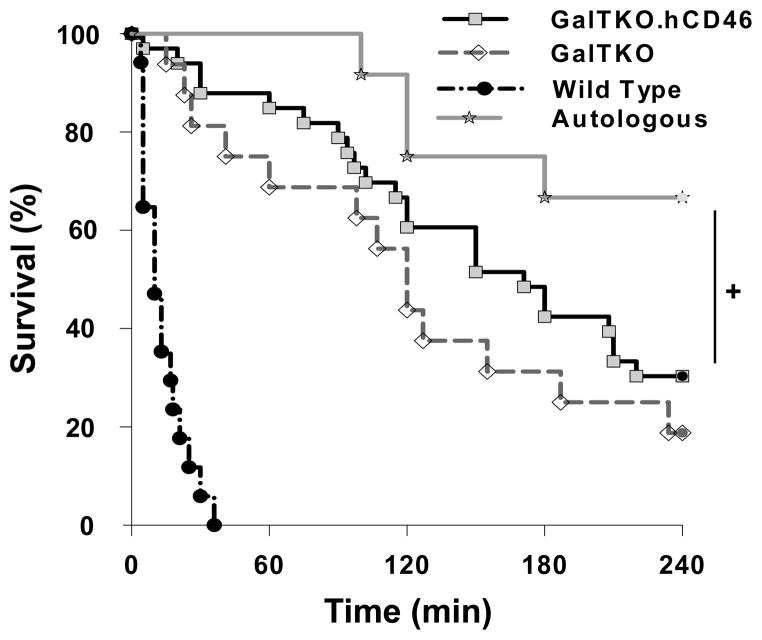
Kaplan Meier survival analysis [Figure 1] shows that 8 of 12 pig lungs perfused with autologous blood reached elective termination after 240 minutes of perfusion. In contrast, as previously reported, all xenogenic-perfused lungs of wild type pigs failed within 36 minutes, in association with markedly elevated pulmonary vascular resistance. GalTKO lungs perfused with human blood survived for a median of 120 minutes (range 15–240 min) whereas organs from GalTKO.hCD46 animals had a median survival time (MST) of 171 minutes (range 5–240 min; p = 0.27 vs. GalTKO). Three (of 16; 19%) of the GalTKO- and ten (of 33; 30%) of the GalTKO.hCD46 transgenic lungs survived to an elective termination. Although 4 of the 12 autologously-perfused lungs failed before reaching the elective termination time point, survival in this cohort was significantly prolonged compared to xenogenetically perfused groups (p = 0.04 vs. GalTKO.hCD46). Gradual loss of pulmonary vascular barrier function, with tracheal edema, was the prevalent lung failure mode in this group. The lung failure mode associated with different lung phenotypes are listed in Table 1.
Figure 1.
Cumulative survival time of ex vivo perfused lungs. Lung “survival time” represents the time of perfusion at which a lung failure endpoint was met, based on the pre-defined criteria. Lung survival was significantly prolonged in GalTKO.hCD46 lungs compared to wild type (p < 0.001) but not compared to GalTKO lungs (p = 0.27). Autologously perfused lungs survived significantly longer than GalTKO.hCD46 transgenic lungs (+ p = 0.04).
Table 1.
Summary of lung failure mechanisms in GalTKO.hCD46 and control lungs. Lungs from Wild Type pigs failed almost all due to a loss of blood flow through the lungs (elevated PVR) whereas this mechanism is only responsible for about 50% of GalTKO.hCD46 lung failures. The development of trachea edema could be identified as the predominant reason for organ failure within the autologously perfused group.
| Rejection reason | Experimental group n/% of failed lungs |
|||
|---|---|---|---|---|
| GalTKO.hCD46 | GalTKO | Wild type | Autologous | |
| Trachea edema | 7/30% | 2/15% | 2/12% | 3/75% |
| Loss of perfusate | 2/9% | 2/15% | - | 1/25% |
| Loss of blood flow | 12/52% | 7/54% | 15/88% | - |
| Oxygenation failure | 2/9% | 2/15% | - | - |
Pulmonary Vascular Resistance
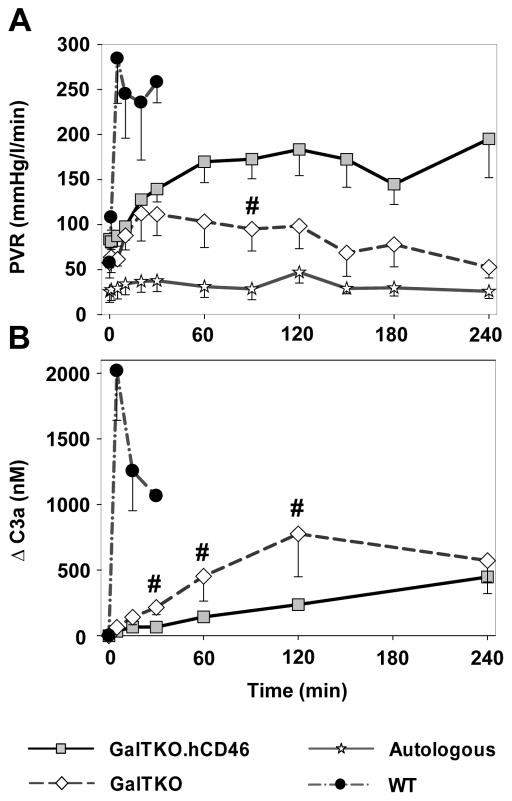
Autologously perfused pig lungs maintained very stable and low values over the time of perfusion whereas xenogenetic perfusion of wild type pig lungs let to a rapid extreme rise in PVR (284±50 mmHg·min/L at 5′) [Figure 2A]. Both GalTKO (+/−hCRP) groups showed an identical moderate rise in PVR to ~110–140 mmHg·min/L within the initial 30 minutes. At the subsequent measurement intervals, GalTKO.hCD46 transgenic lungs exhibited higher PVR values than with GalTKO organs, reaching statistical significance at 90 minutes of perfusion (p = 0.04).
Figure 2.
Pulmonary vascular resistance (PVR) and plasma levels of complement activation byproduct (C3a). (A) PVR expressed as a function of time for control and study groups. Time 0 represents measurements obtained during the first minute of lung perfusion. PVR after 4 hours of perfusion initiation was significantly higher in GalTKO.hCD46 lungs than GalTKO lungs (# p < 0.05 for GalTKO.hCD46 vs. GalTKO). (B) Plasma C3a levels expressed as the amount of complement fragments produced above the pre-perfusion baseline. Complement activation was significantly lower in GalTKO pig lungs expressing hCD46 between 30 and 120 min of perfusion (# p < 0.03 for GalTKO.hCD46 vs. GalTKO). All data are shown as the mean ± SEM of all surviving experiments.
Complement Activation
In contrast to the prolific elaboration of complement noted with wild type xenograft perfusion (ΔC3a at 5′ 2019±377 ng/ml), both GalTKO (63±29, p < 0.001) and GalTKO.hCD46 (33±10, p < 0.001) experimental groups exhibited statistically significantly reduced complement activation. C3a elaboration was not fully inhibited by the additional expression of hCD46, but was significantly reduced at 30, 60 and 120′ time points when compared to the GalTKO group [Figure 2B].
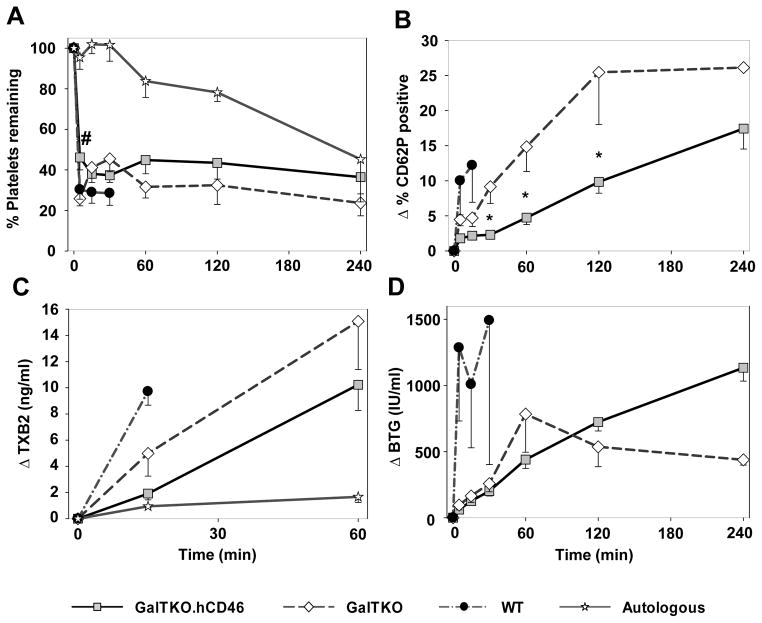
Platelet Sequestration and Activation
Platelet sequestration by GalTKO.hCD46 lungs was only significantly reduced at 5′ when compared to GalTKO pig lung perfusions (% of initial platelets at 5′ 26±4 vs. 46±6, p = 0.02) [Figure 3A]. At later time points (1 to 4 h of perfusion) there was no statistical significant difference in platelet counts between GalTKO.hCD46 and GalTKO experiments. Activation of platelets measured by CD62P-expression on platelets was significantly decreased between 30 min and 2 h in the GalTKO.hCD46 group when compared to the GalTKO group (ΔCD62 at 120′ 10.2±1.6 vs. 25.4±7.4, p < 0.01). In WT experiments, CD62P expression was significantly higher when compared to values of GalTKO.hCD46 lungs [Figure 3B].
Figure 3.
Platelet and coagulation cascade activation profile. (A) Blood platelet counts measured serially by automated counting. Platelet sequestration was expressed as the percentage of platelets remaining in the perfusate at each time point. # at 5 min, p = 0.02 for GalTKO.hCD46 vs. GalTKO. (B) Platelet activation assessed by measuring the proportion of CD41+ platelets expressing CD62P by flow cytometry. * p < 0.01 for GalTKO.hCD46 vs. GalTKO. (C) Platelet activation assessed by plasma Thromboxane B2 levels. (D) Platelet activation assessed by plasma levels of βTG released from platelet granules. All values are shown as the mean ± SEM of surviving experiments.
Plasma thromboxane B2 levels remained lower in the GalTKO.hCD46 than in GalTKO group without reaching statistical significance (1919±483 vs. 4986±1736 pg/mL, p = 0.17) [Figure 3C]. Plasma beta-thromboglobulin (βTG) rose significantly within 5 minutes of perfusion in wild type lungs (ΔβTG (IU/mL) 1286±554 vs. GalTKO 144±36, p = 0.01 vs. GalTKO.hCD46 62±15, p < 0.001). Although the initial rise of βTG level tended to be lower in GalTKO.hCD46 compared to GalTKO lungs during the subsequent hour (at 60′: 440±66 vs. 784±288, p = 0.11), βTG levels after 4 hours of perfusion were significantly lower in the three survivors among the GalTKO group when compared to ten survivors in the GalTKO.hCD46 group (483±54 vs. 1350±133, p < 0.02) [Figure 3D].
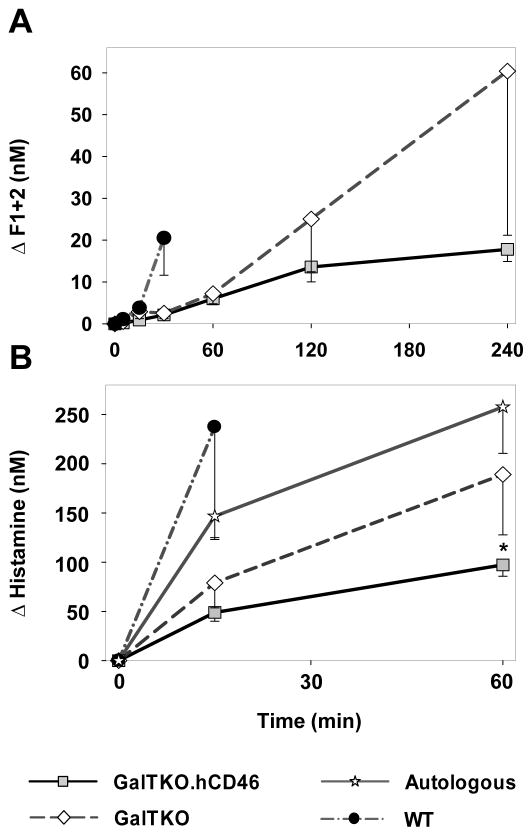
Thrombin Formation
Relative to prolific, rapid thrombin generation with wild-type lungs at 30 min (ΔF1+2 20.5±9 nM), thrombin generation was significantly reduced in association with GalTKO (2.6±1.3, p < 0.01) or GalTKO.hCD46 lungs (2.1±0.4, p < 0.001) [Figure 4A]. Later in the experiment, GalTKO.hCD46 transgenic lungs showed reduced thrombin formation when compared to GalTKO lungs without reaching statistical significance (ΔF1+2 at 240′: 13.6±3.6 vs. 25.1±14.1, p = 0.26).
Figure 4.
(A) Activation of the coagulation cascade was detected by the formation of thrombin measured by plasma levels of fragments F1+2. (B) Release of histamine was measured in blood samples collected during the first hour of perfusion. Histamine levels at 1h of perfusion reached significantly lower values in the GalTKO.hCD46 when compared to GalTKO group (* p < 0.03). All data are expressed as change from the baseline and shown as the mean ± SEM of surviving experiments.
Histamine levels
Histamine, as a marker for mast-cell driven innate immunologic response, showed a rapid increase in WT lungs at the 15′ time point, significantly higher than in GalTKO.hCD46 lung perfusions (237±113 vs.49±9 nM, p < 0.01). Levels in GalTKO experiments were higher than with GalTKO.hCD46, reaching statistical significance after one hour of perfusion (189±61 vs. 97±11 nM, p < 0.03) [Figure 4B]. Interestingly, the porcine blood, collected for the autologous perfusions, showed high histamine levels prior to the start of the perfusions whereas the human blood for the xenogenic perfusions had approximately 10-fold lower levels (96±13 vs. 10±1 nM for GalTKO.hCD46).
Leukocyte Sequestration
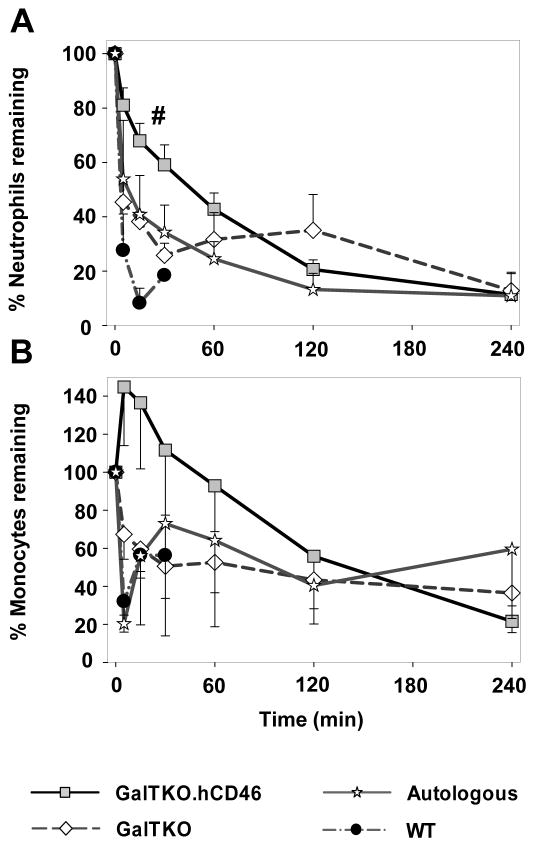
WT-, autologously-perfused- and GalTKO lungs all exhibited immediate neutrophils (PMN) sequestration with 28±13, 54±22 and 45±5 percent of initial neutrophils remaining in the blood after 5′ of perfusion, respectively. During the first half hour of perfusion, neutrophil counts in GalTKO.hCD46-lung perfusions showed significantly higher values when compared to control groups (% remaining at 30′: 59±7 vs. GalTKO 26±4, p < 0.01 vs. WT 7±3, p < 0.01) [Figure 5A]. At later time points there was no statistical difference in PMN sequestration between the groups.
Figure 5.
Neutrophils (A) and monocytes (B) sequestration from circulation. Blood cell numbers measured serially by automated counting are expressed as percent remaining in circulation. Data expressed as change from the baseline and shown as the mean ± SEM of surviving experiments. # p < 0.02 GalTKO.hCD46 vs. GalTKO between 5 and 30 min.
Monocyte counts in GalTKO and WT experiments showed an immediate cell sequestration after perfusion initiation, whereas GalTKO.hCD46 lung perfusions demonstrated a cell number increase at 5′ of perfusion (145±31% of initial monocyte counts) [Figure 5B]. Monocyte numbers showed no difference between the groups after 150′ with about 50% of initial monocytes remaining in the perfusate; the relative contribution of pig and human monocytes was not enumerated in the course of the current work.
Histology and Immunohistochemistry
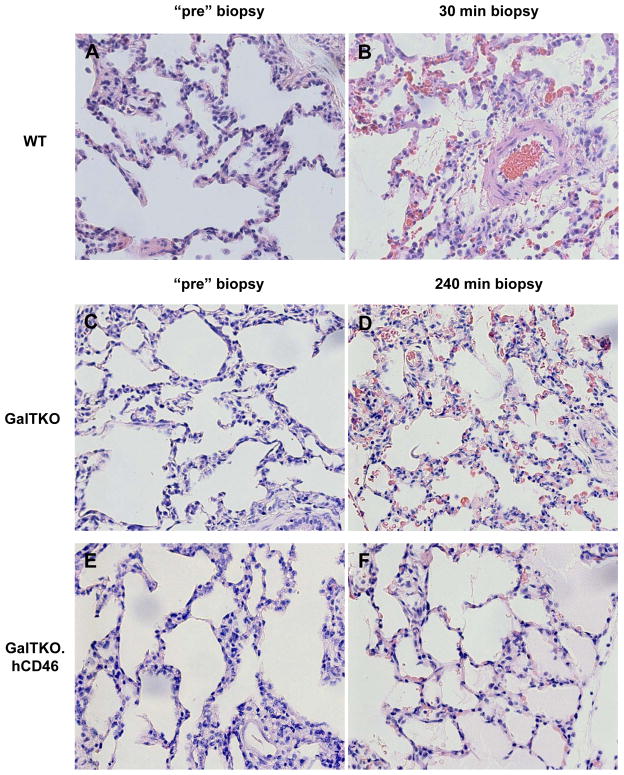
WT lungs exhibited severe interstitial and alveolar hemorrhage that extended to the airway at failure, along with cellular infiltration and prevalent intravascular thrombosis [Figure 6B]. GalTKO lungs showed relatively preserved histology at 60′, but infiltration by polymorphonuclear granulocytes, intravascular thrombosis and hemorrhage were prominent at lung failure [Figure 6D]. Only autologously-perfused and GalTKO.hCD46 lungs that had not met lung failure criteria showed normal microscopic anatomy with air-filled alveoli and thin inter-alveolar septae at an elective termination after 240′ of perfusion [Figure 6F].
Figure 6.
Pre-perfusion biopsies of Wild type (A), GalTKO (C) and GalTKO.hCD46 (E) show intact lung tissue. GalTKO.hCD46 lung tissue showed well preserved microscopic anatomy with air-filled alveoli and thin inter-alveolar septae and only little hemorrhage after 4 hours of xenogenic perfusion (F) whereas GalTKO biopsies (from a still functioning lung) exhibited infiltration by polymorphonuclear granulocytes, intravascular thrombosis and diffuse hemorrhage at the same time point (D). Wild type lung biopsies showed these findings already after 30 minutes of perfusion (B). H&E, 40X
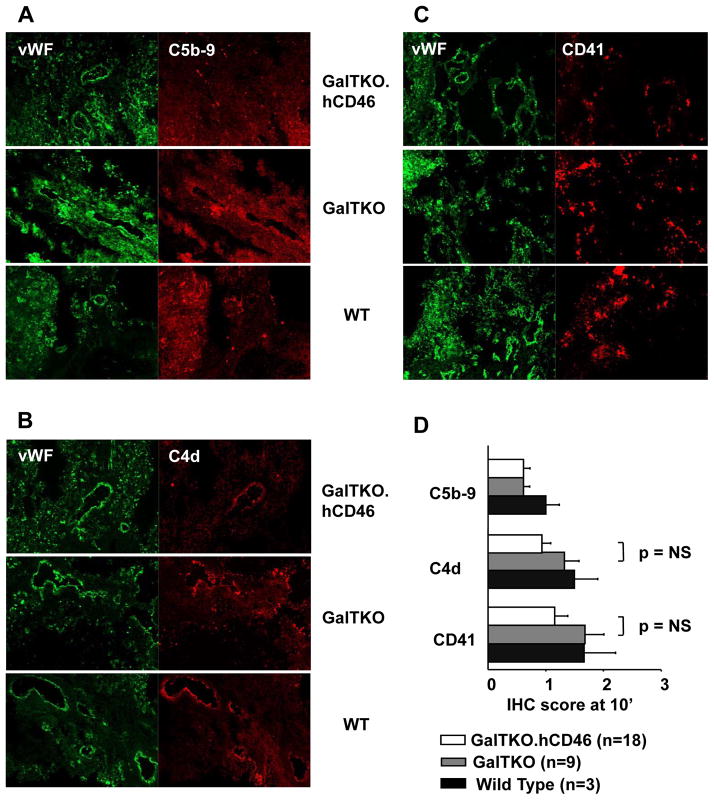
Classical pathway complement activation fragment C4d deposition in 10′ biopsy samples showed the lowest scores in GalTKO.hCD46 tissue (0.94±0.15 vs. GalTKO 1.3±0.25, p = 0.15). C5b-9 membrane attack complex scores were very similar in GalTKO.hCD46 and GalTKO lungs but showed slightly lower values than measured in WT lungs (GalTKO.hCD46 0.61±0.11 vs. WT 1.0±0.24, p = 0.25). CD41 staining, as a marker for platelet deposition, tended toward reduced intensity in GalTKO.hCD46 tissue (1.16±0.23 vs. GalTKO 1.68±0.32, p = 0.19) [Figure 7A–D].
Figure 7.
Two-color immunochemistry analysis of tissues from GalTKO.hCD46 and control perfused pig lungs. Pig lung tissue collected at identical time points (10 min after initiation of perfusion) was stained for von Willebrand Factor (green), identifying endothelium, and C5b-9 (A), C4d (B) and CD41 (C) (red). (D) Immunochemistry findings scored as the extent of tissue deposition as indicated in methods. GalTKO.hCD46 lungs showed non-significantly reduced C4d (0.9±0.1 vs. GalTKO 1.3±0.2, p = 0.15) and CD41 deposition (1.1±0.2 vs. GalTKO 1.7±0.3, p = 0.19 vs. GalTKO) in lung biopsies collected at 10 min when compared to GalTKO or Wild Type lungs. C5b-9 IHC-score was similar in GalTKO.hCD46 and GalTKO lungs.
Anti-non-Gal antibody levels
Pre-perfusion anti-non-Gal IgM antibody levels were comparable in the perfusate used for GalTKO and GalTKO.hCD46 lung perfusions (GalTKO 17.3±6.3 vs. GalTKO.hCD46 15.6±6.9, p = 0.39). Blood used for WT lung perfusions showed significantly lower anti-non-Gal antibody levels (9.2±2.1, vs. GalTKO.hCD46 p < 0.01). In neither group did the individual preformed antibody level correlate to survival time. C3a levels at 15′ and 30′ correlated in the GalTKO group to the level of anti-non-Gal antibody (at 15′ r = 0.62, p < 0.02). This correlation was not significant in the GalTKO.hCD46 group (at 15′ r = 0.28, p = 0.16).
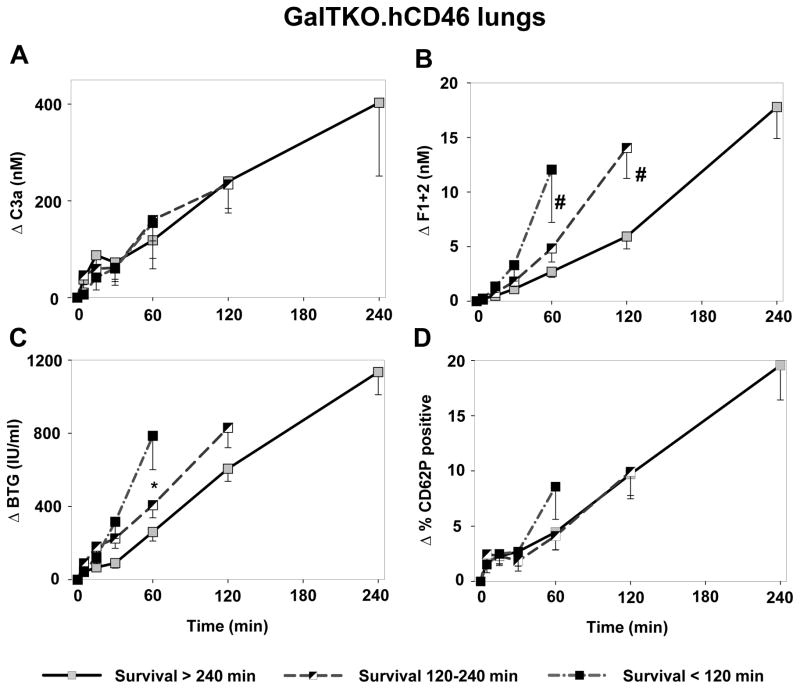
GalTKO.hCD46 group analysis
No difference in the kinetics of complement cascade activation (as ΔC3a) was seen in association with early lung failure relative to longer-surviving lungs [Figure 8A]. Thrombin generation (F1+2 at 60′ 2.7±0.5 vs.12.1±4.8, p < 0.02; at 120′ 5.9±1.1 vs. 14.0±1.8, p < 0.02) [Figure 8B] as well as platelet activation (βTG at 60′ 261±50 vs. 786±85, p < 0.02) [Figure 8C] was significantly lower in association with longer-surviving lungs when compared to lungs with short or intermediate survival times. CD62P-expression on platelets did not differ between groups [Figure 8D].
Figure 8.
Analysis of complement and coagulation cascade and platelet activation within the GalTKO.hCD46 group. Perfusions were divided in experiments where lung failure occurred before 2 hours of perfusion (Survival < 120 min), between 2 and 4 hours (Survival 120–240 min) and experiments that were terminated electively at 4 hours (Survival > 240 min). (A) C3a levels in the groups were similar. There was no correlation between survival time and complement cascade activation. (B) Lungs that failed before the 2 hour time point and lungs that failed between 2 and 4 hours of perfusion showed significantly higher thrombin generation (# p < 0.02) when compared to surviving lungs at 1h and 2 h time points, respectively. (C) BTG levels at 1 h were significantly reduced in surviving lungs when compared to lungs failing before 2 hours (* p < 0.01). (D) P-Selectin expression was identical in lungs surviving longer than 2 hours of perfusion. Experiments being terminated before the 2 hour mark showed increased P-Selectin values.
Discussion
Previously published studies have demonstrated that wild type, hCRP, and GalTKO xenograft injury can be triggered or accelerated by anti-pig antibody (13–14,25–26), and implicate a combination of complement activation, coagulation cascade activation, and cellular adhesion and activation (27–29). The genetic modifications of adding a human complement pathway regulatory protein on the WT pig background or GalTKO alone led to a reduction of complement cascade activation and “survival” prolongation (14). Residual lung injury was attributed in part to binding of classical pathway complement activation triggered by anti-pig antibody (30–31) or anti-non-Gal antibody (14).
Membrane cofactor protein (CD46) catalyzes the degradation of C3b and C4b by Factor I, thereby inhibiting downstream complement pathway constituents (23–24) and should thus reduce GalTKO organ injury caused by the complement system. In support of this hypothesis, we find in our study that GalTKO lungs that additionally express human CD46 induced lower C3a generation in the first 2 hours of perfusion relative to GalTKO lungs.
We infer that the binding of preformed non-Gal antibodies to GalTKO xenograft, activating leukocytes and endothelial cells as well as the complement cascade, is presumably responsible for acute organ injury. The correlation between preformed non-GalT-antibody-levels and lung survival in the GalTKO, but not in the GalTKO.hCD46 group, suggests that the additional complement regulation by overexpression of hCD46 is effective to inhibit, if not prevent, lung injury caused by this mechanism.
Expression of hCD46 on GalTKO lungs modulates platelet activation, as CD41 deposition in the lung and CD62 expression on circulating platelets, and tends to lower thrombin generation. Since expression of human complement regulatory proteins is not thought to directly inhibit clotting cascade amplification or the adhesion and activation of platelets, we infer that these are indirect effects through reduced complement-driven tissue and cell injury. For example, reduced complement activation may result in less vWF expression on endothelium and diminished vWF secretion into the blood and on circulating human platelets. Although the precise mechanism(s) is not defined by our studies to date, here we show that expression of hCRP on GalTKO organs confers anti-inflammatory, anti-coagulant, and anti-thrombotic effects that extend beyond the targeted pathway.
White blood cell and platelet sequestration is observable in all groups, but is greatly accelerated during pig lung perfusion by human blood. Platelet counts in autologous perfusions showed low-level sequestration with about 80% of initial platelets remaining in the perfusate after 2 hours of perfusion, illustrating the artifactual effects associated with the ex vivo lung perfusion model. In contrast, more than 50% of initial thrombocytes are sequestered within the first 15 minutes in xenogenic perfusions, demonstrating a drastic reaction to xenogenic stimuli. Neutrophil sequestration is associated with ischemia-reperfusion injury of allografts (32–33). We find a neutrophil sequestration in our autologously perfused lungs that is quantitatively similar to that observed in the GalTKO or WT lung perfusions. Interestingly, hCD46 expression on GalTKO led to a significant delay in neutrophil (and monocyte) sequestration when compared to other xenogenically- and autologously perfused experimental groups. We provisionally infer that complement activation plays an important role in cell sequestration in this early perfusion phase, as previously reported (34), and that it can be partly controlled by reduced complement activation seen in the context of genetic “overexpression” of complement regulatory molecules.
The significant rise in PVR in WT perfusions reflects one clinically important physiological consequence of the xenogenic immunologic reaction and the associated rapidly progressive lung injury. GalTKO and GalTKO.hCD46 lung perfusions generally do not exhibit as extreme PVR elevation. Although average PVR values among “survivors” in the GalTKO group remained lower than in surviving GalTKO.hCD46 lungs, this result is biased by the relatively low PVRs in 3 GalTKO lungs that survived to four hours. PVR data analysis of the GalTKO lungs revealed that several lungs were censored that just reached the PVR rejection criteria, reducing the average PVR for that group at subsequent time points. In contrast, a higher proportion of GalTKO.hCD46 organs remained in the “still-functioning” group by PVR (and all other) survival criteria, albeit with persistently high PVR, leading to the relatively elevated PVR values for the group.
Preliminary analysis suggests that “failure” of 4 out of 12 autologously-perfused lungs prior to elective termination was associated with surprisingly elevated pre-perfusion histamine-levels, which may adversely affect vascular barrier function.
GalTKO lungs expressing hCD46 exhibited better control of the complement cascade activation, as expected, but did not exhibit significantly prolonged survival compared to GalTKO lungs that do not express additional hCRP. As a hypothesis-generating strategy to begin to explain this observation, we compared biochemical indices of inflammation between three groups of GalTKP.CD46 lungs cohorted according to “survival time” of the lung. The thrombin generation and platelet activation (βTG), but not complement activation, were significantly lower in association with longer-surviving lungs. We infer that the activation of platelets and the coagulation cascade in “non-surviving” lungs contributed importantly to the organ injury and determined outcome and survival time. In contrast, increased early complement activation (as C3a) was directly correlated with earlier graft failure for GalTKO lungs [Figure S1A]. GalTKO lungs that survived until the elective termination also showed a strong trend to reduced βTG levels when compared to earlier failing lungs [Figure S1C], suggesting to us that platelet activation is an important factor contributing to GalTKO lung injury, as for GalTKO.hCD46 lungs in our current report. Thus this post-hoc analysis of intra-group variability in outcome, comparing groups cohorted by arbitrarily selected survival duration intervals, and putative mechanistic indices of specific pathways associated with lung xenograft injury revealed expected (GalTKO) and hypothesis-generating (GalTKO.hCD46) data regarding what pathways remain important to progress toward clinically useful lung xenograft performance.
One limitation to our study is that we did not also study CD46 modification by itself. Thus, we have not formally excluded the possibility that the hCD46 construct used here might alone account for the observed effects. Previous work shows that hCD46 (hMCP) or hCD55 (hDAF) (17–18,35) exhibit incomplete protection, similar to the GalTKO results reported here. Based on two decades of work by us and many others, the GalTKO modification is widely viewed by scientists and regulators as an essential component of the pig genetic background needed for proceeding to clinical application most safely, while minimizing recipient immunosuppressive requirements. Repeating the extensive prior work by us and others with hCRP transgenic pig lungs was judged scientifically unnecessary to address our primary objectives: to determine whether hCD46 protects GalTKO lung from complement-driven injury; and to describe its influence on other HALR mechanisms.
In conclusion, GalTKO.hCD46 lungs exhibit biochemically significant and physiologically important protection from antibody- and complement-mediated HALR. Our data and analysis support our working hypothesis is that coagulation- and platelet-driven mechanisms are the principle mediators of PVR elevation and injury of GalTKO.hCD46 lungs in our model, although additional roles for residual complement activation, pulmonary intravascular macrophage activation, and human leukocytes (neutrophils, monocytes, NK cells) remain to be formally evaluated. We predict that efficient control of one or more among several known molecular incompatibilities regulating coagulation and platelet and leukocyte adhesion (37–44) will prove necessary and sufficient to prevent these well-described HALR phenomena, and yield consistent “survival” to four hours in this model, as well as life-supporting function of pig lungs in baboons and, eventually, in man.
Supplementary Material
List of non-standardized abbreviations
- βTG
β-thromboglobulin
- CD62P
P-selectin
- EGF
early graft failure
- ELISA
enzyme-linked immunosorbent assay
- F1+2
Prothrombin fragments 1 + 2
- FACS
Fluorescence-activated cell sorting
- GalTKO
α1,3-galactosyl transferase knockout
- HAR
hyperacute rejection
- HALR
hyperacute lung rejection
- hCD46
human membrane cofactor protein, hMCP
- hCRP
human complement regulatory protein
- hDAF
human decay-accelerating factor, hCD55
- hMCP
human membrane cofactor protein, hCD46
- MST
Median survival time
- OCT
optimal cutting temperature
- PMN
polymorphonuclear leukocyte
- PVR
pulmonary vascular resistance
- vWF
von Willebrand Factor
- WT
Wild type
Footnotes
Disclosure
Dr. Pierson serves on the Scientific Advisory Board of Revivicor.
Author contributions
LB: concept/design, data analysis/interpretation, statistics, drafting article
TS: concept/design, data analysis/interpretation, approval of article
TZ: concept/design, approval of article
ER: concept/design, approval of article
AR: data analysis/interpretation, approval of article
CA: concept/design, approval of article
AL: concept/design, approval of article
XC: concept/design, approval of article
ES: concept/design, approval of article
GB: concept/design, approval of article
AN: concept/design, approval of article
CP: Providing of genetically modified pigs, approval of article
DA: Providing of genetically modified pigs, critical revision of article
AMA: concept/design, data analysis/interpretation, statistics, critical revision of article
RNP: concept/design, data analysis/interpretation, statistics, critical revision of article
References
- 1.Pierson RN, 3rd, Dorling A, Ayares D, Rees MA, Seebach JD, Fishman JA, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16(5):263–80. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper DK, Hara H, Ezzelarab M, Bottino R, Trucco M, Phelps C, et al. The potential of genetically-engineered pigs in providing an alternative source of organs and cells for transplantation. J Biomed Res. 2013;27(4):249–53. doi: 10.7555/JBR.27.20130063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne GW, McGregor CG. Cardiac xenotransplantation: progress and challenges. Curr Opin Organ Transplant. 2012;17(2):148–54. doi: 10.1097/MOT.0b013e3283509120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RF, Jr, Thomas ML, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12(3):763–71. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griesemer AD, Hirakata A, Shimizu A, Moran S, Tena A, Iwaki H, et al. Results of gal-knockout porcine thymokidney xenografts. Am J Transplant. 2009;9(12):2669–78. doi: 10.1111/j.1600-6143.2009.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekser B, Echeverri GJ, Hassett AC, Yazer MH, Long C, Meyer M, et al. Hepatic function after genetically engineered pig liver transplantation in baboons. Transplantation. 2010;90(5):483–93. doi: 10.1097/TP.0b013e3181e98d51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim K, Schuetz C, Elias N, Veillette GR, Wamala I, Varma M, et al. Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation. 2012;19(4):256–64. doi: 10.1111/j.1399-3089.2012.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Windt DJ, Bottino R, Kumar G, Wijkstrom M, Hara H, Ezzelarab M, et al. Clinical islet xenotransplantation: how close are we? Diabetes. 2012;61(12):3046–55. doi: 10.2337/db12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manji RA, Menkis AH, Ekser B, Cooper DK. Porcine bioprosthetic heart valves: The next generation. Am Heart J. 2012;164(2):177–85. doi: 10.1016/j.ahj.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 10.McGregor CG, Carpentier A, Lila N, Logan JS, Byrne GW. Cardiac xenotransplantation technology provides materials for improved bioprosthetic heart valves. J Thorac Cardiovasc Surg. 2011;141(1):269–75. doi: 10.1016/j.jtcvs.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 11.Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RF, Jr, Thomas ML, et al. Pig heart B-cell depletion extends the survival of GTKO.hCD46Tg xenografts in baboons for up to 8 months. Am J Transplant. 2012;12(3):763–71. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, Lanteri MB, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11(2):171–83. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder C, Allan JS, Nguyen BN, Wu G, Zhang T, Azimzadeh AM, et al. Hyperacute rejection is attenuated in GalT knockout swine lungs perfused ex vivo with human blood. Transplant Proc. 2005;37(1):512–3. doi: 10.1016/j.transproceed.2004.12.133. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen BN, Azimzadeh AM, Schroeder C, Buddensick T, Zhang T, Laaris A, et al. Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection. Xenotransplantation. 2011;18(2):94–107. doi: 10.1111/j.1399-3089.2011.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen BN, Azimzadeh AM, Zhang T, Wu G, Schuurman HJ, Sachs DH, et al. Life-supporting function of genetically modified swine lungs in baboons. J Thorac Cardiovasc Surg. 2007;133(5):1354–63. doi: 10.1016/j.jtcvs.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 16.Bush EL, Barbas AS, Holzknecht ZE, Byrne GW, McGregor CG, Parker W, et al. Coagulopathy in a-galactosyl transferase knockout pulmonary xenotransplants. Xenotransplantation. 2011;18(1):6–13. doi: 10.1111/j.1399-3089.2011.00621.x. [DOI] [PubMed] [Google Scholar]
- 17.Pöling J, Oezkur M, Kogge K, Mengel M, Niemann H, Winkler M. Hyperacute rejection in ex vivo-perfused porcine lungs transgenic for human complement regulatory proteins. Transpl Int. 2006;19(3):225–32. doi: 10.1111/j.1432-2277.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- 18.Hara H, Long C, Lin YJ, Tai HC, Ezzelarab M, Ayares D, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21(12):1163–74. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 19.Westall GP, Levvey BJ, Salvaris E, Gooi J, Marasco S, Rosenfeldt F, et al. Sustained function of genetically modified porcine lungs in an ex vivo model of pulmonary xenotransplantation. J Heart Lung Transplant. 2013;2498(13):01341–7. doi: 10.1016/j.healun.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, Lanteri MB, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–83. doi: 10.1046/j.1399-3089.2003.00103.x. [DOI] [PubMed] [Google Scholar]
- 21.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2004;299(5605):411–4. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdorf L, Azimzadeh AM, Pierson RN., 3rd Xenogeneic lung transplantation models. Methods Mol Biol. 2012;885:169–89. doi: 10.1007/978-1-61779-845-0_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seya T, Atkinson JP. Functional properties of membrane cofactor protein of complement. Biochem J. 1989;264(2):581–8. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barilla-LaBarca ML, Liszewski MK, Lambris JD, Hourcade D, Atkinson JP. Role of membrane cofactor protein (CD46) in regulation of C4b and C3b deposited on cells. J Immunol. 2002;168(12):6298–304. doi: 10.4049/jimmunol.168.12.6298. [DOI] [PubMed] [Google Scholar]
- 25.Dean PG, Cohen AJ, Dalla Valle H, Grande JP, Diamond LE, Byrne GW, et al. The effect of anti-alphaGal antibody removal with immunoabsorption and splenectomy on CD46 transgenic kidney xenograft survival. Transplantation. 2002;74(11):1596–603. doi: 10.1016/s0041-1345(00)02223-5. [DOI] [PubMed] [Google Scholar]
- 26.Pierson RN., 3rd Antibody-mediated xenograft injury: mechanisms and protective strategies. Transpl Immunol. 2009;21(2):65–9. doi: 10.1016/j.trim.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaca JG, Lesher A, Aksoy O, Ruggeri ZM, Parker W, Davis RD. The role of the porcine von Willebrand factor: baboon platelet interactions in pulmonary xenotransplantation. Transplantation. 2002;74(11):1596–603. doi: 10.1097/00007890-200212150-00018. [DOI] [PubMed] [Google Scholar]
- 28.Gaca JG, Lesher A, Aksoy O, Gonzalez-Stawinski GV, Platt JL, Lawson JH, et al. Disseminated intravascular coagulation in association with pig-to-primate pulmonary xenotransplantation. Transplantation. 2002;73(11):1717–23. doi: 10.1097/00007890-200206150-00005. [DOI] [PubMed] [Google Scholar]
- 29.Gaca JG, Appel JZ, 3rd, Lukes JG, Gonzalez-Stawinski GV, Lesher A, Palestrant D, et al. Effect of an anti-C5a monoclonal antibody indicates a prominent role for anaphylatoxin in pulmonary xenograft dysfunction. Transplantation. 2006;81(12):1686–94. doi: 10.1097/01.tp.0000226063.36325.02. [DOI] [PubMed] [Google Scholar]
- 30.Schröder C, Pfeiffer S, Wu G, Zorn GL, 3rd, Ding L, Allen C, Harrison RA, White DJ, Azimzadeh AM, Pierson RN., 3rd Effect of complement fragment 1 esterase inhibition on survival of human decay-accelerating factor pig lungs perfused with human blood. J Heart Lung Transplant. 2003;22(12):1365–75. doi: 10.1016/s1053-2498(03)00026-3. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer S, Zorn GL, 3rd, Blair KS, Farley SM, Wu G, Schuurman HJ, et al. Hyperacute lung rejection in the pig-to-human model 4: evidence for complement and antibody independent mechanisms. Transplantation. 2005;79(6):662–71. doi: 10.1097/01.tp.0000148922.32358.bf. [DOI] [PubMed] [Google Scholar]
- 32.Byrne JG, Karavas AN, Elhalabi A, Cohn LH. Myocardial neutrophil sequestration during reperfusion of the transplanted rabbit heart. J Heart Lung Transplant. 2000;19(8):786–91. doi: 10.1016/s1053-2498(00)00130-3. [DOI] [PubMed] [Google Scholar]
- 33.Pinsky DJ. The vascular biology of heart and lung preservation for transplantation. Thromb Haemost. 1995;74(1):58–65. [PubMed] [Google Scholar]
- 34.Mulligan MS, Paulson JC, De Frees S, Zheng ZL, Lowe JB, Ward PA. Protective effects of oligosaccharides in P-selectin-dependent lung injury. Nature. 1993;364(6433):149–51. doi: 10.1038/364149a0. [DOI] [PubMed] [Google Scholar]
- 35.Brandl U, Michel S, Erhardt M, Brenner P, Burdorf L, Jöckle H, et al. Transgenic animals in experimental xenotransplantation models: orthotopic heart transplantation in the pig-to-baboon model. Transplant Proc. 2007;39(2):577–8. doi: 10.1016/j.transproceed.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Mañez R, Lopez-Pelaez E, Centeno A, Herrera JM, Juffe A, Domenech N, et al. Transgenic expression in pig hearts of both human decay-accelerating factor and human membrane cofactor protein does not provide an additional benefit to that of human decay-accelerating factor alone in pig-to-baboon xenotransplantation. Transplantation. 2004;78(6):930–3. doi: 10.1097/01.tp.0000133309.82387.8c. [DOI] [PubMed] [Google Scholar]
- 37.Cooper DK, Ekser B, Burlak C, Ezzelarab M, Hara H, Paris L, et al. Clinical lung xenotransplantation--what donor genetic modifications may be necessary? Xenotransplantation. 2012;19(3):144–58. doi: 10.1111/j.1399-3089.2012.00708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulte am Esch J, 2nd, Rogiers X, Robson SC. Molecular incompatibilities in hemostasis between swine and men--impact on xenografting. Ann Transplant. 2001;6(3):12–6. [PubMed] [Google Scholar]
- 39.Cowan PJ, Roussel JC, d’Apice AJ. The vascular and coagulation issues in xenotransplantation. Curr Opin Organ Transplant. 2009;14(2):161–7. doi: 10.1097/mot.0b013e3283279591. [DOI] [PubMed] [Google Scholar]
- 40.Roussel JC, Moran CJ, Salvaris EJ, Nandurkar HH, d’Apice AJ, Cowan PJ. Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. 2008;8(6):1101–12. doi: 10.1111/j.1600-6143.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- 41.Cowan PJ. Coagulation and the xenograft endothelium. Xenotransplantation. 2007;14(1):7–12. doi: 10.1111/j.1399-3089.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim YT, Lee HJ, Lee SW, Kim JY, Wi HC, Park SJ, et al. Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15(1):27–35. doi: 10.1111/j.1399-3089.2008.00445.x. [DOI] [PubMed] [Google Scholar]
- 43.Kim HK, Kim JE, Wi HC, Lee SW, Kim JY, Kang HJ, et al. Aurintricarboxylic acid inhibits endothelial activation, complement activation, and von Willebrand factor secretion in vitro and attenuates hyperacute rejection in an ex vivo model of pig-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15(4):246–56. doi: 10.1111/j.1399-3089.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim HK, Kim JE, Park CM, Kim YT, Han KS, Cho HI. Aurintricarboxylic acid upregulates the thrombomodulin expression of endothelial cells and peripheral blood monocytes. Blood Coagul Fibrinolysis. 2008;19(6):489–94. doi: 10.1097/MBC.0b013e3282f2b5c1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.