Abstract
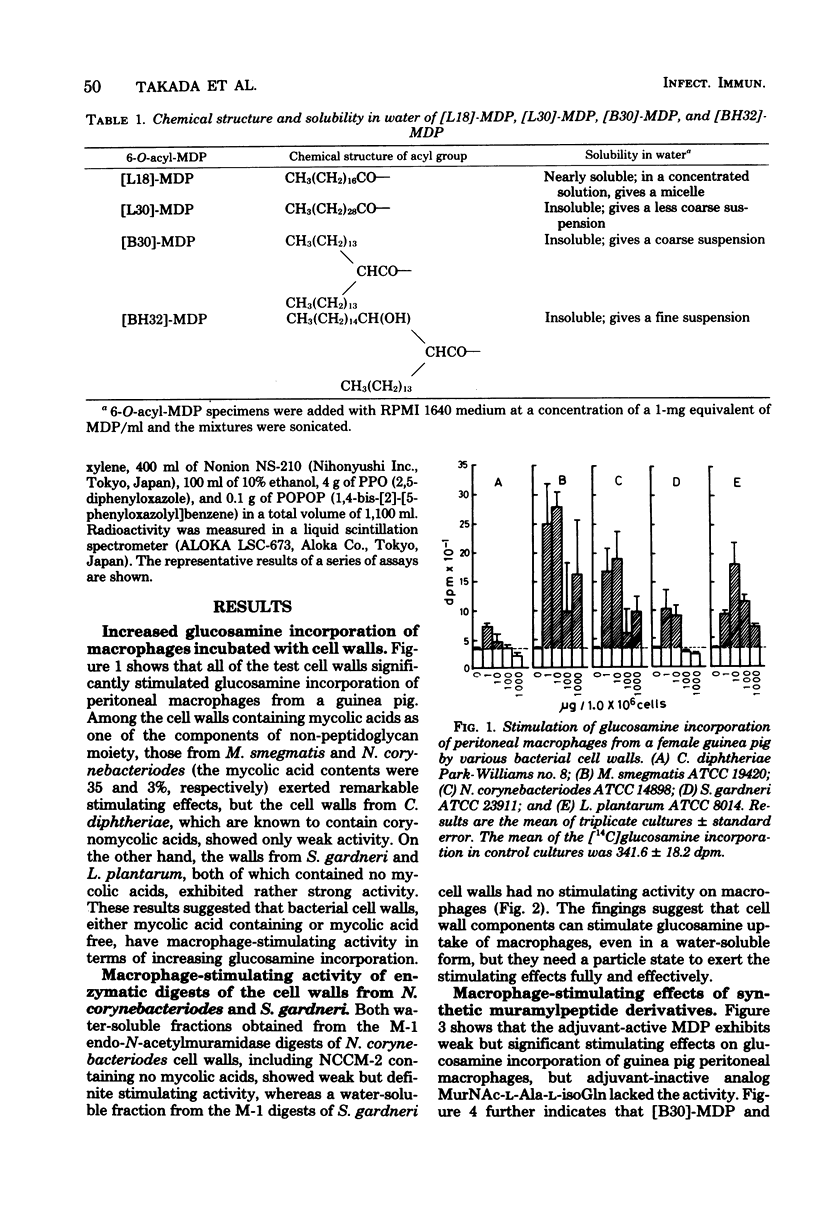
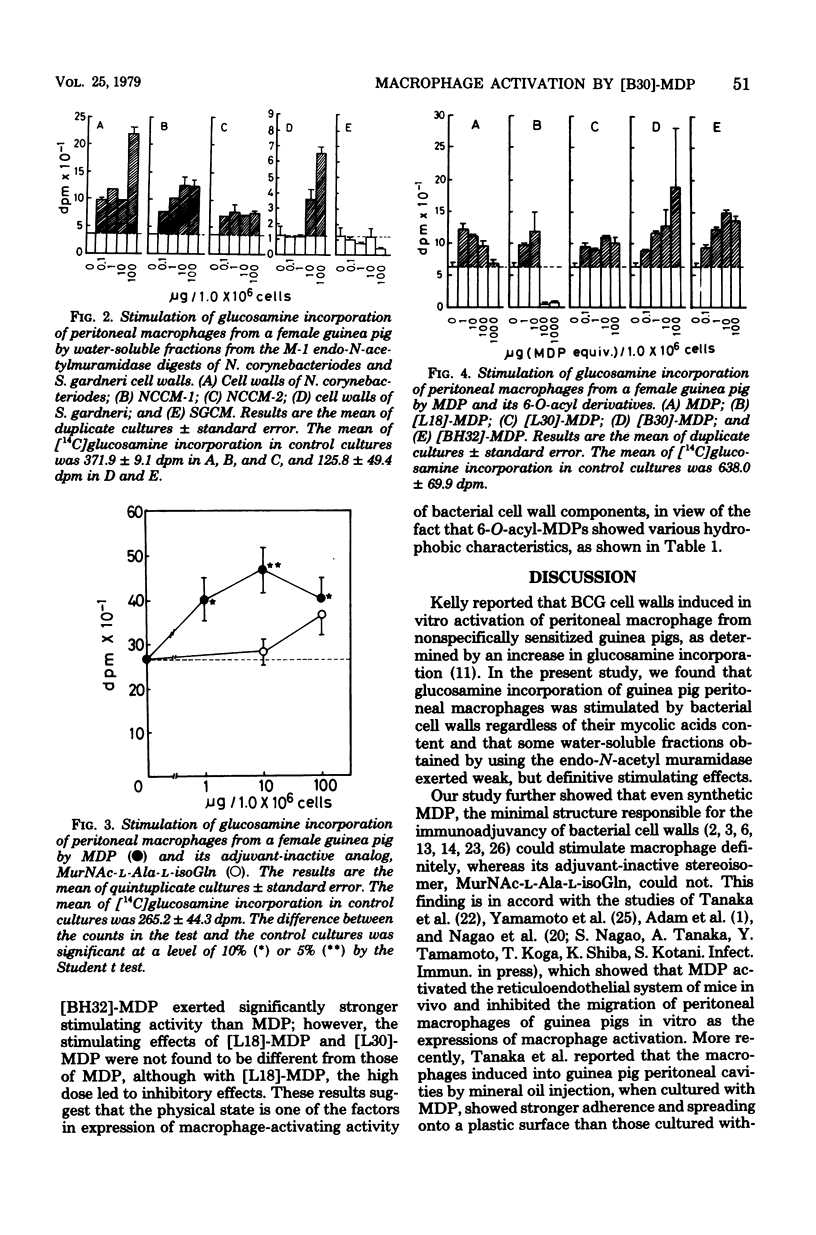
Activation of peritoneal macrophages from guinea pigs by various bacterial cell walls, M-1 endo-N-acetylmuramidase enzymatically digested bacterial cell walls and synthetic muramyl dipeptides was studied in terms of stimulation of [14C] glucosamine incorporation. All test bacterial cell wall preparations significantly increased a [14C]glucosamine uptake by the macrophages. Some of the water-soluble M-1 enzyme digests also exerted stimulating effects on macrophages, although the activity of the digests was found to be weaker than those of original cell walls. Furthermore, an adjuvant-active synthetic MurNAc-L-Ala-D-isoGln (MDP) showed a weak but significant activity, whereas an adjuvant-inactive analog, MurNAc-L-Ala-L-iso-Gln, did not show a significant activity, at least with the dose of 100 microgram. Additional studies with 6-O-acyl derivatives of MDP revealed that 6-O-(2-tetradecylhexadecanoyl)-MDP and 6-O-(3-hydroxy-2-tetradecyl-octadecanoyl)-MDP exhibit stronger macrophage-stimulating effects than MDP. It can be concluded from the above findings that MDP is the essential structure responsible for stimulating the activity of cell walls on guinea pig peritoneal macrophages, but it requires a particle state, which results from an additive character of lipophilicity, to exert the activity fully and effectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Souvannavong V., Lederer E. Non-specific MIF-like activity induced by the synthetic immunoadjuvant: N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP). Biochem Biophys Res Commun. 1978 Nov 29;85(2):684–690. doi: 10.1016/0006-291x(78)91216-0. [DOI] [PubMed] [Google Scholar]
- Azuma I., Sugimura K., Taniyama T., Yamawaki M., Yamamura Y. Adjuvant activity of mycobacterial fractions: adjuvant activity of synthetic N-acetylmuramyl-dipeptide and the related compounds. Infect Immun. 1976 Jul;14(1):18–27. doi: 10.1128/iai.14.1.18-27.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma I., Sugimura K., Yamamura Y., Kusumoto S., Tarumi Y. Adjuvant activity of synthetic cell-wall peptidoglycan subunits on monoazobenzenearsonate-N-acetyl-L-tyrosine and bacterial alpha-amylase in guinea pigs. Jpn J Microbiol. 1976 Feb;20(1):63–66. doi: 10.1111/j.1348-0421.1976.tb00909.x. [DOI] [PubMed] [Google Scholar]
- Damais C., Parant M., Chedid L., Lefrancier P., Choay J. In vitro spleen cell responsiveness to various analogs of MDP (N-acetylmuramyl-L-alanyl-D-isoglutamine), a synthetic immunoadjuvant, in MDP high-responder mice. Cell Immunol. 1978 Jan;35(1):173–179. doi: 10.1016/0008-8749(78)90137-5. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Elin R. J., Chedid L., Wolff S. M. The pyrogenicity of the synthetic adjuvant muramyl dipeptide and two structural analogues. J Infect Dis. 1978 Dec;138(6):760–767. doi: 10.1093/infdis/138.6.760. [DOI] [PubMed] [Google Scholar]
- Fevrier M., Birrien J. L., Leclerc C., Chedid L., Liacopoulos P. The macrophage, target cell of the synthetic adjuvant muramyl dipeptide. Eur J Immunol. 1978 Aug;8(8):558–562. doi: 10.1002/eji.1830080804. [DOI] [PubMed] [Google Scholar]
- Hammond M. E., Dvorak H. F. Antigen-induced stimulation of glucosamine incorporation by guinea pig peritoneal macrophages in delayed hypersensitivity. J Exp Med. 1972 Dec 1;136(6):1518–1532. doi: 10.1084/jem.136.6.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond M. E., Selvaggio S. S., Dvorak H. F. Antigen-enhanced glucosamine incorporation by peritoneal macrophages in cell-mediated hypersensitivity. I. Studies on biology and mechanism. J Immunol. 1975 Oct;115(4):914–921. [PubMed] [Google Scholar]
- Juy D., Chedid L. Comparison between macrophage activation and enhancement of nonspecific resistance to tumors by mycobacterial immunoadjuvants. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4105–4109. doi: 10.1073/pnas.72.10.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. T. Activation of guinea pig macrophages by cell walls of Mycobacterium bovis, strain BCG. Cell Immunol. 1976 Oct;26(2):254–263. doi: 10.1016/0008-8749(76)90369-5. [DOI] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Shimono T., Morisaki I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. 1975 Jun;18(2):105–111. [PubMed] [Google Scholar]
- Nagao S., Tanaka A., Yamamoto Y., Koga T., Onoue K., Shiba T., Kusumoto K., Kotani S. Inhibition of macrophage migration by muramyl peptides. Infect Immun. 1979 May;24(2):308–312. doi: 10.1128/iai.24.2.308-312.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Kotani S., Kusumoto S., Tarumi Y., Ikenaka K. Mitogenic activity of adjuvant-active N-acetylmuramyl-L-alanyl-D-isoglutamine and its analogues. Biken J. 1977 Jun;20(2):81–85. [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Saito R., Kotani S., Kusumoto S., Shiba T. Correlation of stereochemically specific structure in muramyl dipeptide between macrophage activation and adjuvant activity. Biochem Biophys Res Commun. 1977 Jul 25;77(2):621–627. doi: 10.1016/s0006-291x(77)80024-7. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Saito R., Sugiyama K., Morisaki I., Kotani S. Adjuvant activity of synthetic N-acetylmuramyl peptides in rats. Infect Immun. 1977 Jan;15(1):332–334. doi: 10.1128/iai.15.1.332-334.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M., Rosenstreich D. L., Oppenheim J. J. Activation of guinea pig macrophages by bacterial lipopolysaccharide requires bone marrow-derived lymphocytes. J Immunol. 1975 Jan;114(1 Pt 2):388–393. [PubMed] [Google Scholar]
- Yamamoto Y., Nagao S., Tanaka A., Koga T., Onoue K. Inhibition of macrophage migration by synthetic muramyl dipeptide. Biochem Biophys Res Commun. 1978 Feb 28;80(4):923–928. doi: 10.1016/0006-291x(78)91333-5. [DOI] [PubMed] [Google Scholar]


