Abstract
Older adults with mild cognitive impairment (MCI) often have difficulty performing complex instrumental activities of daily living (IADLs), which are critical to independent living. In this study, amnestic multi-domain MCI (N = 29), amnestic single-domain MCI (N = 18), and healthy older participants (N = 47) completed eight scripted IADLs (e.g., cook oatmeal on the stove) in a smart apartment testbed. We developed and experimented with a graded hierarchy of technology-based prompts to investigate both the amount of prompting and type of prompts required to assist individuals with MCI in completing the activities. When task errors occurred, progressive levels of assistance were provided, starting with the lowest level needed to adjust performance. Results showed that the multi-domain MCI group made more errors and required more prompts than the single-domain MCI and healthy older adult groups. Similar to the other two groups, the multi-domain MCI group responded well to the indirect prompts and did not need a higher level of prompting to get back on track successfully with the tasks. Need for prompting assistance was best predicted by verbal memory abilities in multi-domain amnestic MCI. Participants across groups indicated that they perceived the prompting technology to be very helpful.
Keywords: Ecological assessment, Instrumental activities of daily living, Aging, Dementia, Smart environment technologies, Rehabilitation
INTRODUCTION
There will be dramatic growth in the aging population over the next 40 years (Vincent & Velkoff, 2010) combined with shortages in healthcare resources and personnel (Salzhauer, 2005; WHO, 2007). Given the prohibitive costs of traditional clinic-based healthcare and institutionalization, along with older adults’ desire to “age in place” (Eckert, Morgan, & Swamy, 2004), there is a mounting need for the development of in-home assistive technologies to extend the amount of time individuals can live independently in their homes. In recent years, advancements have been made in the development of assistive smart environment technologies geared toward increasing older adults’ functional independence and improving health outcomes and well-being. These technologies include unobtrusive in-home monitoring (Hayes, Pavel, & Kaye, 2004), complex activity recognition (Singla, Cook, & Schmitter-Edgecombe, 2010), and reminder systems (Das, Chen, Seelye, & Cook, 2011; Rudary, Singh, & Pollack, 2004).
As the general population ages, the number of older adults with mild cognitive impairment (MCI) is growing (Dlugaj et al., 2010). MCI has been defined as an intermediate state between normal aging and dementia (Winblad et al., 2004), and is characterized by impairments greater than expected for age in memory and other cognitive abilities with sparing of basic functional abilities. To better characterize the underlying etiology and trajectory of MCI and facilitate earlier and more targeted treatments, the MCI construct has been revised into subtypes (Petersen & Morris, 2005). Amnestic and non-amnestic subtypes are based on whether impairment exists in the memory domain. A further categorization is single- or multi-domain, based on whether impairment exists in only one or multiple cognitive domains. Viewed on a continuum in terms of symptom severity, single-domain MCI might occur in the earliest stages with more cognitive domains becoming affected as the disease progresses.
Despite intact abilities to carry out basic functional tasks, people with MCI often experience difficulty carrying out instrumental activities of daily living (IADLs), which are cognitively complex functional tasks like preparing meals and managing medications and finances (Farias et al., 2006). Activities dependent on memory and executive functions, such as medicine use and financial management, tend to be most difficult for individuals with MCI (Allaire, Gamaldo, Ayotte, Sims, & Whitfield, 2009; Brooks, 2006; Griffith et al., 2010; Schmitter-Edgecombe, Woo, & Greeley, 2009); however, to function independently at home, individuals need to be able to complete these IADLs (Diehl et al., 2005). Studies have shown that individuals with multi-domain MCI have greater impairments in IADLs than those with single domain MCI (Aretouli & Brandt, 2010; Brandt et al., 2009; Gold, 2012), and are also at greater risk for progression to dementia (Aretouli, Okonkwo, Samek, & Brandt, 2011). One significant limitation of most existing studies in this area is the use of self-report or collateral report of IADL functioning, which may be biased or inaccurate. Thus, ecologically valid research methods (e.g., naturalistic observational assessment) are needed to help clarify the frequency and types of IADL difficulties that occur in MCI subtypes to inform development of targeted interventions for IADL impairment.
When individuals with cognitive impairment fail to initiate or complete everyday IADLs, caregivers are often responsible for monitoring IADL completion and providing reminders or prompts. These can be time consuming and burdensome tasks that are often associated with negative effects for the caregiver’s own health (Vitaliano et al., 2005). Technologies that help people with MCI carry out their IADLs by detecting when assistance is needed and delivering reminders or prompts have the potential to reduce caregiver burden and allow individuals to age in place longer. Implementing an IADL monitoring/prompting system as early as possible in the MCI process might improve acceptance, which will be beneficial when cognitive and functional impairment progresses. Although technology-based prompting systems are in development by gerontechnology researchers to assist individuals with cognitive impairment, there has been little research investigating the amount of prompting required or the most useful prompt content for individuals with MCI in attempting complex IADLs. Prompt content can encompass modality (e.g., verbal, multi-modal) and the level of information (e.g., indirect, direct prompts) presented.
Cognitive rehabilitation principles can be applied to the development of smart prompting technologies (Seelye, Schmitter-Edgecombe, Das, & Cook, 2012). For example, graded cue hierarchies that offer increasing levels of directive assistance depending upon an individual’s level of cognitive or functional impairment have been used successfully with Alzheimer’s disease (AD) and pervasive developmental disorders (PDD) populations (Greber, Ziviani, & Rodger, 2007; Mihaildis, Boger, Craig, & Hoey, 2008). Studies have also shown that individuals with less severe impairments respond to less directive prompts (Greber et al., 2007). Offering progressive levels of assistance to individuals having difficulty completing tasks, starting with the lowest level may also be important for maintaining an individual’s sense of autonomy, independence, and positive perceptions of the helper (Nadler, 1997).
In the present study, we examined both the number of prompts and the prompt content required when healthy older adults and individuals with single- and multi-domain MCI completed complex IADLs in a smart apartment testbed. Participants performed eight scripted IADLs (e.g., cook oatmeal on the stove) while out of sight experimenters monitored participants’ activity completion via Web camera. When experimenters’ detected errors occurring during activity completion, pre-recorded prompts were delivered using a graded hierarchy. We were particularly interested in learning whether multi-domain MCI participants would require prompting more often and of a higher level than the other groups. The hierarchy of prompts given began with a verbal indirect prompt (e.g., “The oatmeal may burn if the stove is left on”) followed by a verbal direct prompt (e.g., “You can turn the stove off now”) and then a multi-modal prompt (a video appears on the laptop of a person turning the knob on the stove off and the verbal direct prompt is delivered).
Due to the presence of greater cognitive impairments, it was hypothesized that individuals with multi-domain MCI would require more prompts across activities and more steps prompted within activities than single-domain MCI and healthy older adults (Farias et al., 2006). Based on prior research indicating that individuals with less severe cognitive impairments have been found to respond successfully to less directive prompts (Greber et al., 2007), it was expected that individuals with single-domain MCI would respond successfully to less directive prompts when errors were made and not require higher levels of prompting assistance.
We also obtained user feedback to examine how individuals with MCI reacted to the IADL prompting technology. This is important because whether a technology is used correctly or adopted at all is often associated with the user’s perception of it (Brose et al., 2010; Courtney, 2008). In addition, past studies have shown that memory and executive functioning are the cognitive abilities involved in the IADLs that are most difficult for people with MCI (e.g., Allaire et al., 2009; Brooks, 2006; Griffith et al., 2010; Schmitter-Edgecombe et al., 2009). We, therefore, examined relationships between the prompting data and neuropsychological measures of memory and executive functioning.
METHOD
Participants
Participants were 47 community dwelling persons with amnestic MCI (24 female, 23 male) and 47 healthy older adults (OAs; 26 female, 21 male), age 50 or older. Participants were recruited through advertisements, community health and wellness fairs, physician referrals, and from past studies in our laboratory. Recruitment and data collection occurred over a period of 2 years. Participants were first screened by telephone using a medical interview, the Clinical Dementia Rating instrument (Morris, 1993) to assess dementia staging; and the; Telephone Interview of Cognitive Status-modified (Brandt et al., 1993) to assess cognitive status. Those who met initial screening criteria completed two study visits (two participants withdrew after the first study visit). At visit 1, participants underwent a battery of neuropsychological tests. During visit 2, participants completed a variety of complex activities of daily living within an apartment testbed located on the Washington State University campus. These evaluations were scheduled 1 week apart with each testing session lasting approximately 3 hr. Each MCI participant was closely matched with a healthy OA participant in terms of age, sex, and education. Because the MCI and healthy OA samples were recruited and tested during the same time period as part of a larger study (Schmitter-Edgecombe, Parsey, & Cook, 2011), the OA group represents a subsample of 168 tested healthy OAs who best demographically matched the MCI participants.
The testing and medical data gathered for each participant was carefully evaluated by a clinical neuropsychologist to determine whether each participant met clinical criteria for amnestic MCI. Inclusion criteria were consistent with the diagnostic criteria defined by Petersen and colleagues (Petersen et al., 2001; Petersen & Morris, 2005) and with the criteria outlined by the National Institute on Aging-Alzheimer’s Association workgroup (Albert et al., 2011), including: (a) self-report or knowledgeable informant report of subjective memory impairment for at least 6 months; (b) objective evidence of impairment in single or multiple cognitive domains, with scores falling at least 1.5 standard deviations below age-matched norms and in reference to the individuals educational and socioeconomic background; (c) nonfulfillment of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for dementia (American Psychiatric Association, 2000); (d) preserved general cognitive functions as confirmed by a score of 27 or above on the TICS (equivalent to the normality cutoff score of 24 on the Mini Mental Status Exam) (Measso, Cavarzeran, Zappalà, & Lebowitz, 1993); (e) no significant impact of the memory deficit on the participant’s daily activities, as confirmed by a total CDR score of 0.5 which is consistent with a minimal change in the participant’s habits; and (f) absence of severe depression as confirmed by a score < 10 on the Geriatric Depression Scale (GDS) – Short Form (Sheikh & Yesavage, 1986).
The clinical neuropsychological testing data were then further used to categorize the MCI group as amnestic multi-domain MCI (N = 29) (memory, executive, speeded processing, and/or language) or amnestic single-domain MCI (N = 18) (memory). Neuropsychological tests used for diagnosis included the Memory Assessment Scale-List Learning subtest (Williams, 1991), Brief Visual Memory Test (Benedict, 1997), Symbol Digit Modalities Test (Smith, 1991), Number-Letter Sequencing subtest from Wechsler Adult Intelligence Scale-Third Edition (Wechsler, 1997), Boston Naming Test (Ivnik, Malec, Smith, Tangalos, & Petersen, 1996); Letter, Category, and Design Fluency subtests from the Delis-Kaplan Executive Functioning Scale (Delis, Kaplan, & Kramer, 2001), and Trails A and B (Reitan, 1992). When available, collateral medical information, including laboratory and brain imaging results, were reviewed. This protocol was approved by the Institutional Review Board at WSU.
PROCEDURE
For the project, the entry, living room, dining room, and kitchen of an on-campus apartment testbed were equipped with sensors, three Web cameras, and two laptop computers with speakers, one in the living room and one in the kitchen. An upstairs bedroom was equipped with a computer connected with video feeds displaying live video of the participant in the downstairs of the apartment (see Appendix A). Two experimenters were positioned in the upstairs bedroom to monitor participants during experiments, deliver activity instructions, and initiate delivery of pre-recorded prompts when necessary (see Appendix B).
Participants performed a sequence of eight scripted activities that were non-invasive and indicative of routine everyday behavior. The activities selected included seven IADLs, which are disrupted in MCI and early-stage AD and one basic ADL, which is disrupted in the later stages of AD. The current study was part of a larger study with additional aims, and as such, both more complex (e.g., cook oatmeal on the stove) and less complex (e.g., washing hands, cleaning counters) IADLs were chosen to fit within its parameters. All IADL domains evaluated in the current study are commonly assessed with clinical questionnaire measures and rely on cognitive processes. The eight activities selected were broken down into component steps that were considered necessary for successful completion of the activity (see Appendix C). It is possible that each activity could be conceived of having more or less steps than was conceptualized in this study. However, we aimed to narrow each activity down to the minimum steps necessary for successful completion.
A graded hierarchy of three prompts (e.g., indirect, direct, multimodal) was generated for each step needed to complete the eight IADLs. The total number of prompts possible for a given activity varied by activity and depended on the number of component steps (e.g., cooking activity = 8 steps, total possible 24 prompts). The prompts (e.g., verbal and multi-modal) were pre-recorded on a digital recorder and preprogrammed into the smart environment computer system. When an error in activity completion occurred, an experimenter typed the appropriate prompt code into the computer. The corresponding prompt was instantly delivered over a laptop computer speaker system in the downstairs of the apartment. Examples of errors that occurred were failure to initiate an activity step important for task completion (e.g., did not open a cabinet), incorrect completion of an activity step (e.g., opened incorrect cabinet), and activity steps performed in a sequence that would lead to inaccurate task completion (e.g., returned pitcher of water to refrigerator before pouring glass of water). Timing of prompt delivery was determined by the experimenter’s clinical judgment. For example, after an error occurred, a prompt would be delivered when it was clear to the experimenter that the participant was not getting back on track on his/her own. In some cases, participants were able to self-correct their erroneous action quickly before a prompt was needed.
To obtain the participant’s attention, the laptop computer closest to the participant emitted a loud beep preceding prompt delivery. The prompting sequence began with a verbal indirect prompt designed to gently orient the participant back to the task (e.g., The oatmeal may burn if the stove is left on). The indirect prompt referred to the activity step that needed manipulation or attention without directly saying what needed to be done. If the indirect prompt did not work, it was immediately followed by a verbal direct prompt designed to assist with activity step completion by directly saying what needed to be done (e.g., You can turn the stove off now). If the direct prompt did not work, participants were directed to the nearest computer screen, and then a multimodal prompt was delivered involving a direct verbal prompt along with a visual prompt of a person completing the step of the activity in which the error occurred (e.g., a video clip appears on a computer screen of a person in the apartment turning the knob on the stove to the off position and direct verbal prompt is delivered). The multi-modal prompt video clips were recorded in the same apartment in which the experiments took place. Prompting data for each activity were recorded by experimenters on testing sheets.
Data were coded and entered into an SPSS database and checked for quality assurance. Collapsed variables were computed that collapsed individual activity data across all eight activities, which included:
Total prompts received
Total steps prompted
Total prompts received of each level: 1 = verbal indirect, 2 = verbal direct, and 3 = verbal direct and video
Total activities prompted
Average Experimenter rating of quality of activity completion: 1 = Efficient execution of task, 100% accuracy, no errors, 2 = Task generally executed efficiently and fully completed, accuracy above 75%, 3 = Notable difficulties with efficiency of task execution, task may or may not have been fully completed, accuracy above 50%, 4 = Task not fully completed, accuracy above 25%, 5 = Task barely initiated and not completed, many errors
Average Experimenter rating of amount of assistance needed: 1 = no assistance needed, 2 = Minimal assistance needed, less than ¼ of steps prompted, generally indirect prompts, 3 = Moderate assistance needed, more than ½ of steps prompted, 4 = Much assistance needed, more than ¾ of steps prompted, 5 = Much assistance needed, more than ¾ of steps prompted
Average Participant reported prompt helpfulness, timing, and natural feeling across eight activities: 1 = not at all, 3 = somewhat, 5 = very. Calculated by taking the average ratings of all activities for which prompts were needed.
Average Ratings were calculated by taking the average of the two quality and assistance ratings for the eight activities. Raters were blind to patient diagnosis. The inter-rater reliability for the raters across all activities was found to be in almost perfect agreement, Kappa’s > 0.96, p < .001.
Neuropsychological Measures
To provide information about the MCI and older adults’ memory abilities and executive functioning, the following neuropsychological variables were used for analyses:
Memory Assessment Scale Story Memory subtest [MAS] (Williams, 1991)
This is a short story memory task that consists of immediate recall and 20-min delayed recall trials. The number of 18 story idea units at long-delay recall was used as a measure of verbal memory.
Trail Making Test [TMT] (Reitan, 1992)
This test, which involves two forms (TMT-A and TMT-B), is commonly used to examine attention, processing speed, and executive functioning (i.e., sequencing and cognitive flexibility). Part B completion time (seconds) was used as a measure executive functioning, after controlling for processing speed (B-A/A) to isolate the executive component of the task for regression analyses. The TMT-B total completion time was used in the diagnostic battery, without controlling for processing speed.
RESULTS
Demographic and Neuropsychological Testing Data
Table 1 shows the demographic and neuropsychological testing data by group. One-way ANOVAs followed by post hoc Tukey’s HSD contrasts showed that the multi-domain MCI group performed more poorly than the single-domain MCI group and healthy OAs on a brief test measuring global cognitive status, F(2,88) = 8.43, p < .01, and on Trails B (B-A/A), F(2,91) = 13.86, p < .01. Both MCI groups performed more poorly than the healthy OAs on the MAS Story Delayed Recall, F(2,90) = 19.93, p < .01. Although symptom report of depression was minimal overall, the single-domain MCI group reported more depressive symptoms than the healthy OAs, F(2,85) = 4.41, p < .05. The groups did not differ significantly in age, gender, or level of education, p > .05.
Table 1.
Demographic data and mean summary data for multi-domain MCI, single-domain MCI, and healthy older adult groups
| Multiple-MCI (n = 29)
|
Group Single-MCI (n = 18)
|
Healthy OA (n = 47)
|
||||
|---|---|---|---|---|---|---|
| Variable or test | M | SD | M | SD | M | SD |
| Demographics | ||||||
| Age | 71.7 | 8.5 | 70.0 | 8.5 | 70.8 | 8.6 |
| Education (years) | 14.7 | 3.3 | 16.1 | 2.8 | 15.4 | 2.7 |
| GDS | 2.9 | 2.4 | 3.2b | 3.2 | 1.5 | 1.8 |
| Sex (% female) | 52% | 50% | 57% | |||
| Global cognitive status | ||||||
| TICS total score | 31.6ab | 2.6 | 34.4 | 3.0 | 34.1 | 2.8 |
| Memory measure | ||||||
| MAS Story Delayed Recall | 4.8b | 2.6 | 6.2b | 2.4 | 8.3 | 2.3 |
| Executive measure | ||||||
| Trails B (B-A/A) | 130.0ab | 67.3 | 80.2 | 30.8 | 77.5 | 25.3 |
Note. Unless otherwise indicated, mean scores are raw scores. OA = older adult; GDS = Geriatric Depression Scale; TICS = Telephone Interview for Cognitive Status; MAS = Memory Assessment Scale.
Significant difference compared with single-domain MCI group, p < .05.
Significant difference compared with OA group, p < .05.
Prompting Data
Table 2 shows the objective and subjective prompting data by group, collapsed across the eight activities. Nonparametric statistics were used to analyze the prompting data as the variables were not normally distributed.
Table 2.
Group comparison data on prompting variables using independent samples Kruskal-Wallis tests reported as medians (Mdn)
| Variable or test | Multi-MCI (n = 29)
|
Group Single-MCI (n = 18)
|
Healthy OA (n = 47)
|
|||
|---|---|---|---|---|---|---|
| Mdn | SD | Mdn | SD | Mdn | SD | |
| Objective data | ||||||
| Total Prompts | 3ab | 3.8 | 1 | 1.5 | 2 | 1.8 |
| Total Steps Prompted | 2ab | 2.6 | 1 | 1.2 | 1 | 1.2 |
| Average Prompt Level | 1.2 | 0.3 | 1 | 0.2 | 1 | 0.4 |
| Total Activities Prompted | 2ab | 1 | 1 | |||
| Level 1 Prompts Received | 2ab | 1 | 1 | |||
| Level 2 Prompts Received | 1 | 0 | 0 | |||
| Level 3 Prompts Received | 0 | 0 | 0 | |||
| Subjective data-experimenter | ||||||
| Average Activity Quality | 1.9ab | 0.6 | 1.8 | 0.3 | 1.7 | 0.2 |
| Average Assistance | 3ab | 0.8 | 1.7 | 0.5 | 1.7 | 0.7 |
| Subjective data-participant | ||||||
| Helpfulness of Prompts | 4.5 | 1.3 | 4 | 1.1 | 5 | 1.1 |
| Timing of Prompts | 5 | 0.9 | 4 | 1.1 | 5 | 0.7 |
| Natural feeling of Prompts | 3.5 | 1.2 | 3 | 1.2 | 4 | 1.3 |
Notes. OA = older adult. Median data are collapsed across eight activities.
Significant difference compared with single-domain MCI group, p < .05.
Significant difference compared with OA group, p < .05 using Mann-Whitney U tests.
Objective prompting data
Independent samples Kruskal-Wallis tests followed by post hoc Mann Whitney U tests showed that the multi-domain MCI group received more total prompts, H(2) = 8.44, p < .05, and more level 1 prompts, H(2) = 8.07, p < .05, than the single-domain MCI group and healthy OAs (see Table 2). The multi-domain MCI group also required more total activity steps prompted, H(2) = 8.70, p < .05, and more total activities prompted, H(2) = 8.91, p < 05, when compared to the single-domain MCI group and healthy OAs. The groups did not differ significantly in average prompt level required or total level 2 or level 3 prompts received, p’s > .05. Twenty-six multi-domain MCI (90%), 12 single-domain (67%) and 36 (77%) healthy OAs received at least one level 1 prompt. Of participants who received a level 1 prompt, 62% in the multi-domain MCI group, 33% in the single-domain MCI group, and 56% in the healthy OA group progressed to at least one level 2 prompt. The percentage of participants in each group that received a level 2 prompt and progressed to at least one level 3 prompt was 25% (4 people), 0% (0 people), and 35% (7 people) for the multi-domain MCI, single-domain MCI, and healthy OAs, respectively.
These results indicate that the multi-domain MCI group needed more prompts across activities and more activity steps prompted than the single-domain MCI and healthy OA groups. Importantly, similar to the other two groups, the multi-domain MCI group responded well to the indirect prompts and did not require a higher level of prompt assistance for task completion. The average prompt level required across groups was indirect, indicating that in general, these prompts enabled participants’ to correct critical errors and get back on track.
Subjective prompting data: Experimenter ratings
Independent samples Kruskal-Wallis tests followed by post hoc Mann Whitney U tests showed that the multi-domain MCI group (1.9) received lower activity completion quality ratings than the healthy OA group (1.7), H(2) = 9.36, p < .01, but not the single-domain MCI group (1.8) (see Table 2). Activity quality scores within the 1.5–2 range are within normal limits in terms of ability to complete tasks overall, however, scores that approach 2 suggest a less efficient approach with greater errors. Across all eight activities, the multi-domain MCI group (3.0) received higher assistance ratings than both the single-domain MCI group (1.7) and healthy OAs (1.7), H(2) = 11.86, p < .01. Activity assistance scores in the 1–2 range are likely within normal limits, whereas scores in the 2–3 range (e.g., multi-domain MCI) are likely above expectations in terms of amount of assistance needed.
Subjective prompting data: Participant ratings
Independent samples Kruskal-Wallis tests showed that the groups did not differ significantly in ratings of perceived prompt helpfulness, appropriateness of prompt timing, or natural feeling of prompts, p’s > .05. Across groups, prompts were perceived as very helpful (Mdn = 4–5), very appropriately timed (Mdn = 4–5), and somewhat natural (Mdn = 3–4). These results indicate that both MCI groups responded as positively to the prompting technology as the healthy OAs. Although the prompting technology was perceived as only somewhat natural, it was rated as very helpful and appropriately timed by all groups.
Regression Analysis
Hierarchical multiple regression analysis was conducted to identify whether measures of verbal memory (MAS Long Delay Story Recall) and executive functioning (Trails B-A/A) could predict total amount of prompting received for the multi-domain or single-domain MCI groups. To reduce the number of predictor variables included in the regression analyses, we first examined Spearman’s correlations between the prompting outcome measure and age, education, and depression. Age was the only demographic variable that significantly correlated with the prompting outcome measure and it was entered in the first block of the regression. The cognitive predictor variables (verbal memory/MAS and executive functioning/Trails B-A/A) were then simultaneously entered in the second step of the regression analysis to determine if they held any unique and predictive value for the outcome measure. There was no significant multicollinearity among predictor variables, as the Variance Inflation Factors for each variable were less than 1.6.
For the multi-domain MCI group, the MAS story delay recall score was a significant unique cognitive predictor, B = −.53, t = −3.28, p < .01, whereas the Trails B score (B−A/A) was not, B = −.26, t = −1.44, p = .16. For the single-domain MCI group, there were no significant unique cognitive predictors for total prompts received [Trails B score (B−A/A), B = .26, t = .79, p = .44, MAS story delay recall score, B = .05, t = .21, p = .84]. Overall, results indicate that the total number of prompts received was best predicted by verbal memory abilities in multi-domain MCI, whereas this association was not present for single-domain MCI. The two MCI groups did not significantly differ on the MAS story delay recall test, and, therefore, regression findings should be interpreted with caution.
DISCUSSION
In this study, amnestic multi-domain MCI, amnestic single-domain MCI, and healthy older participants performed scripted IADLs in a naturalistic setting. We used a graded-hierarchy of technology-based prompts to investigate both the amount of prompting and prompt content required to assist individuals with MCI with everyday activity completion. Results showed that individuals with multi-domain MCI required more total prompts during activity completion and received higher experimenter assistance ratings than individuals with single-domain MCI and healthy OAs. Consistent with previous research that has used questionnaire measures (Aretouli & Brandt, 2010), these findings suggest that in a real-world environment, individuals with multi-domain MCI have greater difficulty accurately and efficiently completing complex IADLs than individuals with single-domain MCI.
Across objective prompting measures, the single-domain MCI group looked similar to the healthy OA group. It is possible that our small sample of single-domain MCI participants relative to healthy older adults could have played a role in this finding. Although not significantly different than the healthy OAs, the single-domain MCI group received activity assistance and quality ratings intermediate between the multi-domain MCI group and healthy older adults. This finding is consistent with the conceptualization of MCI on a continuum of cognitive and functional impairment severity. Many healthy older adults made IADL errors and needed prompts (i.e., n = 36; 77%), and of the healthy older adults who received a level 1 prompt, 56% needed at least one level 2 prompt. IADL errors and need for prompts in our healthy OAs could reflect normal age-related changes in everyday functioning, which would be consistent with prior studies (Lafortune & Balestat, 2007; Schmitter-Edgecombe et al., 2011). Other explanations for this finding include unfamiliarity with the novel study environment or the study tasks themselves.
These data indicate that across single- and multiple domain amnestic MCI, the fundamental need for prompts was related to degree of cognitive impairment, but the level of prompting was not. Participants who had impairments in memory and at least one other cognitive domain made more errors and needed to be cued more often that there was a problem. Once cued, however, these individuals possessed the cognitive abilities to respond to feedback, repair the problem and get back on track successfully, similar to their less impaired counterparts. Regression analyses suggested that impaired memory abilities likely play a significant role in interfering with the everyday task completion of the multi-domain amnestic MCI group and increased the need for prompting assistance to get back on track.
In general, across groups there was a relatively equal need to advance to direct level prompts, and there was a very minimal need to advance to multi-modal level prompts. Anecdotally, participants reported that they liked how indirect prompts gave them the autonomy to figure out how to get back on track on their own. Offering the minimum amount of assistance needed respects individuals’ adult status and might also lead to less dependency (Nadler, 1997). Importantly, these results indicate that prompting can be minimal and verbal and yet provide adequate assistance for individuals with both single- and multi-domain MCI.
User feedback from participants in each group indicated that in general they perceived the prompting technology to be very helpful and appropriately timed. Previous studies have shown that everyday devices are generally complex and can be difficult for individuals with MCI to use (Seelye, Howieson, Wild, Sauceda, & Kaye, 2009). In the present study, we implemented a prompting technology within a smart environment that provided assistance when needed while requiring no direct user operation or feedback. Because of these unique features, barriers to successful use associated with commonplace technology aids might have been mitigated.
Limitations of the present study include a relatively homogenous sample of predominately Caucasian, highly educated community dwelling volunteers with low levels of self-reported depression, which limit generalizability of our findings. A less-educated sample might be less familiar or comfortable with using technology in general, and, therefore, might not respond as positively as our well-educated sample. Reactions to this type of technology by less-educated samples should be examined in future studies. In the present study, an experimenter was needed to monitor activity completion and initiate prompt delivery. Future studies are needed to examine whether intelligent computer algorithms can be trained to monitor complex activity performance and provide fully automated prompting assistance when needed.
Although this study was set in a naturalistic environment, it still differs from a normal home. For instance, there were no distractions, interruptions, or family members present in the environment, and participants performed one activity at a time after receiving instructions (e.g., no multi-tasking or activity switching). The structured and scripted nature of the study environment might have been less demanding on executive abilities that support complex IADLs in real-world environments, and less sensitive overall to capturing subtle IADL difficulties that might occur in a more typical home setting. On the other hand, it is also possible that the novelty of the study environment and knowledge of being monitored for activity errors might have increased cognitive demands on participants. To address these issues, future naturalistic assessment studies should involve multiple tasks occurring at once and be performed in environments designed closest to individuals’ actual homes to examine generalizability of findings across settings.
MCI diagnosis was made according to standard research criteria; however, a limitation exists in that participants could be classified as impaired in a specific domain based on one impaired test score. Given that individual variability often exists within cognitive domains and that stability of MCI diagnosis is variable, future studies might benefit from incorporating a comprehensive diagnostic approach for MCI, which requires at least two impaired test scores within a cognitive domain for an MCI classification (Jak et al., 2009). Another limitation of the current study is that some of the less difficult activities chosen for the present study were not sensitive enough to detect subtle IADL impairment in individuals with MCI. More difficult and complex activities, such as medication and finance management should be used in future studies. Findings from the regression analyses were limited by study sample size and to the specific neuropsychological measures chosen as the predictor variables to represent the cognitive constructs of memory and executive functioning.
Naturalistic assessment of everyday functioning and response to technology-based prompting in healthy aging and MCI are novel approaches to enhance our understanding of the earliest IADL impairments that occur in these populations. A more in-depth understanding of subtypes of IADL errors commonly made and cognitive abilities involved for amnestic and non-amnestic MCI subgroups will be critical in informing the development of the most useful IADL prompts for each subgroup. An important goal for future longitudinal research will be to examine whether early, subtle functional changes and response to prompting in normal aging and MCI might relate to early cognitive and cortical changes and be indicative of incident cognitive and functional decline.
Acknowledgments
This study was partially supported by grants from the Life Science Discovery Fund of Washington State; NIBIB (Grant R01 EB009675); and NSF (Grant DGE-0900781) to M.S.E. and D.J.C. We thank Chad Sanders and Jennifer Walker for their assistance in coordinating data collection. We also thank Amie Smith and other members of the Aging and Dementia laboratory for their help in collecting, scoring, coding, and entering the data. We thank Barnan Das, Parisa Rashidi, and other members of the CASAS laboratory for their help in developing and maintaining the prompting and smart environment technologies. Finally, we are grateful to Diane B. Howieson, Ph.D., Dennis Dyck, Ph.D., and David Marcus, Ph.D., whose comments greatly strengthened the manuscript.
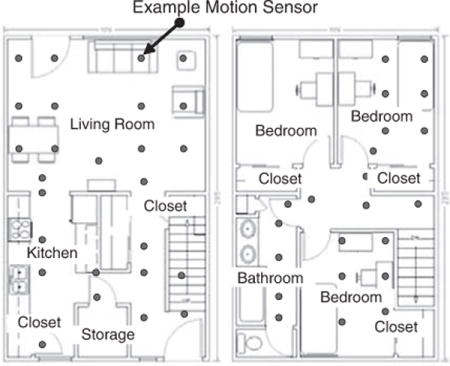
APPENDIX A: Smart Apartment Layout
APPENDIX B: Eight Activity Instructions
“This new set of activities will not be as well defined as the prior set of activities. If you experience difficulty completing any of the activities, you will hear a beep which will be followed by an audio cue. The tone may also be followed by the statement ‘move to the computer screen’. If this occurs, please move to the closest computer screen. One of the computer screens is located here on the bookshelf next to the TV (show participant where this computer screen is), the other computer screen is located on the kitchen counter next to the microwave (show participant where this computer screen is). Once you move to the computer screen, you will see a visual cue on the computer screen. Please do not initiate each activity until I have completed the instructions and said begin.”
APPENDIX C: Eight Activities Descriptions and Steps
| Activity | Description | Steps |
|---|---|---|
| Household chore: Change light bulb | Change a light bulb making sure to select the correct wattage light bulb from the torage drawer. |
|
| Hygiene: Wash hands (Basic ADL) | Wash hands in the kitchen sink choosing correct soap and using towel to dry. |
|
| Household chore: Clean kitchen countertops | Use soap and a sponge to wash kitchen countertops |
|
| Telephone use: Use telephone and phonebook | Look up a specified number in the yellow pages of a phone book, operate a telephone, call the number, and write down a message, press a button to repeat the message if necessary. |
|
| Household chore: Sort and fold laundry | Fold and sort a basket that is full of laundry for a man, woman, and small child. |
|
| Meal preparation: Cook oatmeal on the stove | Boil water on the stove and cook oatmeal according to the recorded directions, which also includes the addition of brown sugar and raisins |
|
| Organization: File mail into mail organizer | Sort and organize bill statements correctly into filing drawer |
|
| Hobby: Give instructions how to play a card game | Retrieve a deck of playing cards, set up a chosen card game, and tell experimenter how to play the card game |
|
Footnotes
No conflicts of interest exist.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaire JC, Gamaldo A, Ayotte BJ, Sims R, Whitfield K. Mild cognitive impairment and objective instrumental everyday functioning: The everyday cognition battery memory test. [Research Support, N.I.H., Extramural] Journal of the American Geriatric Society. 2009;57(1):120–125. doi: 10.1111/j.1532-5415.2008.02054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press, Inc; 2000. Text Revision. [Google Scholar]
- Aretouli E, Brandt J. Everyday functioning in mild cognitive impairment and its relationship with executive cognition. [Research Support, N.I.H., Extramural] International Journal of Geriatric Psychiatry. 2010;25(3):224–233. doi: 10.1002/gps.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aretouli E, Okonkwo OC, Samek J, Brandt J. The fate of the 0.5s: Predictors of 2-year outcome in mild cognitive impairment. [Research Support, N.I.H., Extramural] Journal of the International Neuropsychological Society. 2011;17(2):277–288. doi: 10.1017/S1355617710001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict RHB. Brief visuospatial memory test – revised. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- Brandt J, Aretouli E, Neijstrom E, Samek J, Manning K, Albert MS, Bandeen-Roche K. Selectivity of executive function deficits in mild cognitive impairment. [Research Support, N.I.H., Extramural] Neuropsychology. 2009;23(5):607–618. doi: 10.1037/a0015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Welsh KA, Breitner JC, Folstein MF, Helms M, Christian JC. Hereditary influences on cognitive functioning in older men. A study of 4000 twin pairs. [Research Support, U.S. Gov’t, P.H.S.] Archives of Neurology. 1993;50(6):599–603. doi: 10.1001/archneur.1993.00540060039014. [DOI] [PubMed] [Google Scholar]
- Brooks BL. The relationship between executive functioning and independent living skills in mild cognitive impairment and mild dementia. 8-B. Vol. 66. University of Calgary, Dissertation Abstracts International: Section B: The Sciences and Engineering; 2006. p. 4473. [Google Scholar]
- Brose SW, Weber DJ, Salatin BA, Grindle GG, Wang H, Vazquez JJ, Cooper RA. The role of assistive robotics in the lives of persons with disability. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Review] American Journal of Physical Medicine Rehabilitation. 2010;89(6):509–521. doi: 10.1097/PHM.0b013e3181cf569b. [DOI] [PubMed] [Google Scholar]
- Courtney KL. Privacy and senior willingness to adopt smart home information technology in residential care facilities. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Methods of Information in Medicine. 2008;47(1):76–81. doi: 10.3414/me9104. [DOI] [PubMed] [Google Scholar]
- Das B, Chen C, Seelye A, Cook D. An automated prompting system for smart environments. Paper presented at the 9th International Conference on Smart Homes and Health Telematics; Montreal. 2011. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan executive function system: Examiner’s manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Diehl M, Marsiske M, Horgas AL, Rosenberg A, Saczynski JS, Willis SL. The revised observed tasks of daily living: A performance-based assessment of everyday problem solving in older adults. Journal of Applied Gerontology. 2005;24(3):211–230. doi: 10.1177/0733464804273772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlugaj M, Weimar C, Wege N, Verde PE, Gerwig M, Dragano N, Siegrist J. Prevalence of mild cognitive impairment and its subtypes in the Heinz Nixdorf Recall study cohort. [Research Support, Non-U.S. Gov’t] Dementia and Geriatric Cognitive Disorders. 2010;30(4):362–373. doi: 10.1159/000320988. [DOI] [PubMed] [Google Scholar]
- Eckert JK, Morgan LA, Swamy N. Preferences for receipt of care among community-dwelling adults. Journal of Aging & Social Policy. 2004;16(2):49–65. doi: 10.1300/J031v16n02_04. [DOI] [PubMed] [Google Scholar]
- Farias ST, Mungas D, Reed BR, Harvey DH, Cahn-Weiner D, Decarli C. MCI is associated with deficits in everyday living. Alzheimer’s disease and Associated Disorders. 2006;20(4):217–223. doi: 10.1097/01.wad.0000213849.51495.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DA. An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. [Research Support, Non-U.S. Gov’t Review] Journal of Clinical & Experimental Neuropsychology. 2012;34(1):11–34. doi: 10.1080/13803395.2011.614598. [DOI] [PubMed] [Google Scholar]
- Greber C, Ziviani J, Rodger S. The four quadrant model of facilitated learning: A clinically based action research project. Australian Occupational Therapy Journal. 2007;54:149–152. doi: 10.1111/j.1440-1630.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- Griffith HR, Stewart CC, Stoeckel LE, Okonkwo OC, den Hollander JA, Martin RC, Marson DC. Magnetic resonance imaging volume of the angular gyri predicts financial skill deficits in people with amnestic mild cognitive impairment. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Journal of the American Geriatric Society. 2010;58(2):265–274. doi: 10.1111/j.1532-5415.2009.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TL, Pavel M, Kaye JA. An unobtrusive in-home monitoring system for detection of key motor changes preceding cognitive decline. Conference Proceedings in IEEE Engineering in Medicine & Biology Society. 2004;4:2480–2483. doi: 10.1109/IEMBS.2004.1403715.. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC. Neuropsychological testing norms above age 55: COWAT, BNT, MAE TOKEN, WRAT-R Reading, AMNART, Stroop, TMT, and JLO. The Clinical Neuropsychologist. 1996;10:262–278. [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S.] American Journal of Geriatric Psychiatry. 2009;17(5):368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafortune G, Balestat G. Trends in severe disability among elderly people: Assessing the evidence in 12 OECD Countries and the future implications. OECD Health Working Papers No. 26 2007 [Google Scholar]
- Measso G, Cavarzeran F, Zappalà G, Lebowitz BD. The Mini-Mental State Examination: Normative study of an Italian random sample. Developmental Neuropsychology. 1993;9:77–85. [Google Scholar]
- Mihaildis A, Boger J, Craig T, Hoey J. The coach prompting system to assist older adults with dementia through handwashing: An efficacy study. Biomedical Geriatrics. 2008;8(1):28–46. doi: 10.1186/1471-2318-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. [Research Support, U.S. Gov’t, P.H.S.] Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Nadler A. Personality and help seeking: Autonomous versus dependent seeking of help. In: Pierce GR, Lakey B, Sarason IG, Sarason BR, editors. Sourcebook of social support and personality. New York, NY: US Plenum Press; 1997. pp. 379–407. [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Winblad B. Current concepts in mild cognitive impairment. [Research Support, Non-U.S. Gov’t Review] Archives of Neurology. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. [Comment Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S.Review] Archives of Neurology. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. discussion 1167. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail making test: Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- Rudary M, Singh S, Pollack M. Adaptive cognitive orthotics: Combining reinforcement learning and constraint-based temporal reasoning. Paper presented at the The twenty-first international conference on Machine learning; Banff, Alberta. 2004. [Google Scholar]
- Salzhauer A. Is there a patient in the house? : Harvard Business Review 2005 [Google Scholar]
- Schmitter-Edgecombe M, Parsey C, Cook DJ. Cognitive correlates of functional performance in older adults: Comparison of self-report, direct observation, and performance-based measures. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] Journal of the International Neuropsychological Society. 2011;17(5):853–864. doi: 10.1017/S1355617711000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Woo E, Greeley D. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23:168–177. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- Seelye AM, Howieson DB, Wild K, Sauceda LR, Kaye JA. Living well with MCI: Behavioral interventions for older adults with mild cognitive impairment. In: Brougham R, editor. New Directions in Aging Research: Health and Cognition. New York: Nova Science Publishers, Inc; 2009. pp. 57–74. [Google Scholar]
- Seelye AM, Schmitter-Edgecombe M, Das B, Cook D. Application of cognitive rehabilitation theory to the development of smart prompting technologies. IEEE: Reviews in Biomedical Engineering. 2012;5:29–44. doi: 10.1109/RBME.2012.2196691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: A guide to assessment and intervention. New York: The Haworth Press; 1986. pp. 165–173. [Google Scholar]
- Singla G, Cook C, Schmitter-Edgecombe M. Recognizing independent and joint activities among multiple residents in smart environments. Ambient Intelligence and Humanized Computing Journal. 2010;1(1):57–63. doi: 10.1007/s12652-009-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Symbol digit modalities test. Los Angeles, CA: Western Psychological Services; 1991. [Google Scholar]
- Vincent G, Velkoff V. The next four decades – the older population in the United States: 2010 to 2050. Washington, DC: US Census Bureau; 2010. [Google Scholar]
- Vitaliano PP, Echeverria D, Yi J, Phillips PEM, Young H, Siegler IC. Psychophysiological mediators of caregiver stress and differential cognitive decline. Psychology and Aging. 2005;20(3):402–411. doi: 10.1037/0882-7974.20.3.402. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence test-third edition. New York, NY: The Psychological Corporation; 1997. [Google Scholar]
- WHO. Investing in the health workforce enables stronger health systems, Fact sheet. Belgrade, Copenhagen: WHO; 2007. [Google Scholar]
- Williams JM. Memory assessment scales professional manual. Odessa, FL: Psychological Assessment Resources, Inc; 1991. [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Petersen RC. Mild cognitive impairment – beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]


