Abstract
A BMI cutoff point at the 99th percentile for age and gender or at 40 kg/m2 has been suggested for more aggressive treatment of adolescent obesity. The main objective of this study was to determine the proportion of adolescents eligible for weight loss surgery (WLS) based on various BMI cutoff points. Data was extracted from the electronic medical record database of an urban pediatric ambulatory care center over 4 years. National data were used to calculate BMI percentiles (Centers for Disease Control and Prevention (CDC), 2000). Eligibility for WLS was based on a BMI percentile criterion (≥Cthe adult WLS cutoff point (≥40 kg/m2). The sample consisted of 3,220 adolescents aged 12–17.9 years, of which 53% were female, 55% were of black race, and 17% of Hispanic ethnicity. Overall, 88 (3%) adolescents had a BMI ≥40 kg/m2 and 236 (7%) had a BMI ≥99th percentile (P < 0.001). All adolescents with BMI ≥40 kg/m2 had a BMI ≥99th percentile. A total of 159/2,007 (8%) of 12–14.9-year olds had a BMI ≥99th percentile compared with 77/1,213 (6%) 15–17.9-year olds (P = 0.10), whereas 43/2,007 (2%) of 12–14.9-year olds had a BMI ≥40 kg/m2 compared with 45/1,213 (4%) 15–17.9-year olds (P = 0.003). In summary, a relatively large proportion of adolescents from a diverse urban population would qualify for WLS based on the percentile criterion. Fewer adolescents would be eligible based on the adult WLS criterion, and younger adolescents would be less likely to be eligible for WLS than older adolescents.
INTRODUCTION
Pediatric obesity is a major public health threat (1). Like adults, children and adolescents may have a wide range of associated medical problems including type 2 diabetes mellitus (2), cardiovascular risk factors (3,4), sleep apnea (5), steatohepatitis (6), and pseudotumor cerebri (7) among others, resulting in poor quality of life (8) and decreased life expectancy (9). Of particular concern is the rapidly progressive nature of type 2 diabetes mellitus within the first 5 years of diagnosis (10). As health risk increases with severity of obesity and the presence of major obesity related morbidity, it is imperative that we identify adolescents who may require more aggressive obesity treatment.
Starting at age 18 years old, a BMI ≥40 kg/m2 is used to define Class III obesity (extreme obesity) (11). Class III obesity is associated with higher risk for complications among adults (12,13). As a result, the adult weight loss surgery (WLS) criterion has been defined as a BMI ≥40 kg/m2, or a BMI ≥35 kg/m2 in association with major medical complications of obesity (e.g., cardiovascular disease, type 2 diabetes, sleep apnea) (12,14). WLS is more effective than nonsurgical treatment of more severe forms of obesity in terms of weight loss duration and treatment of comorbid conditions (15–17). Given the lack of studies and medical concerns for the younger patient, the adolescent WLS criterion was initially defined as BMI ≥50 kg/m2, or BMI ≥40 kg/m2 in association with serious medical complications of obesity (type 2 diabetes, sleep apnea, pseudotumor cerebri) (18,19). As in adults, WLS is associated with significant decrease in BMI (20,21) and obesity related comorbidities (22–25). More recently, the Betsy Lehman Pediatric/Adolescent Task Force for WLS stated that there is increasing evidence that the benefits of WLS outweigh the risks in adolescents with severe forms of obesity and major obesity related morbidities (10).
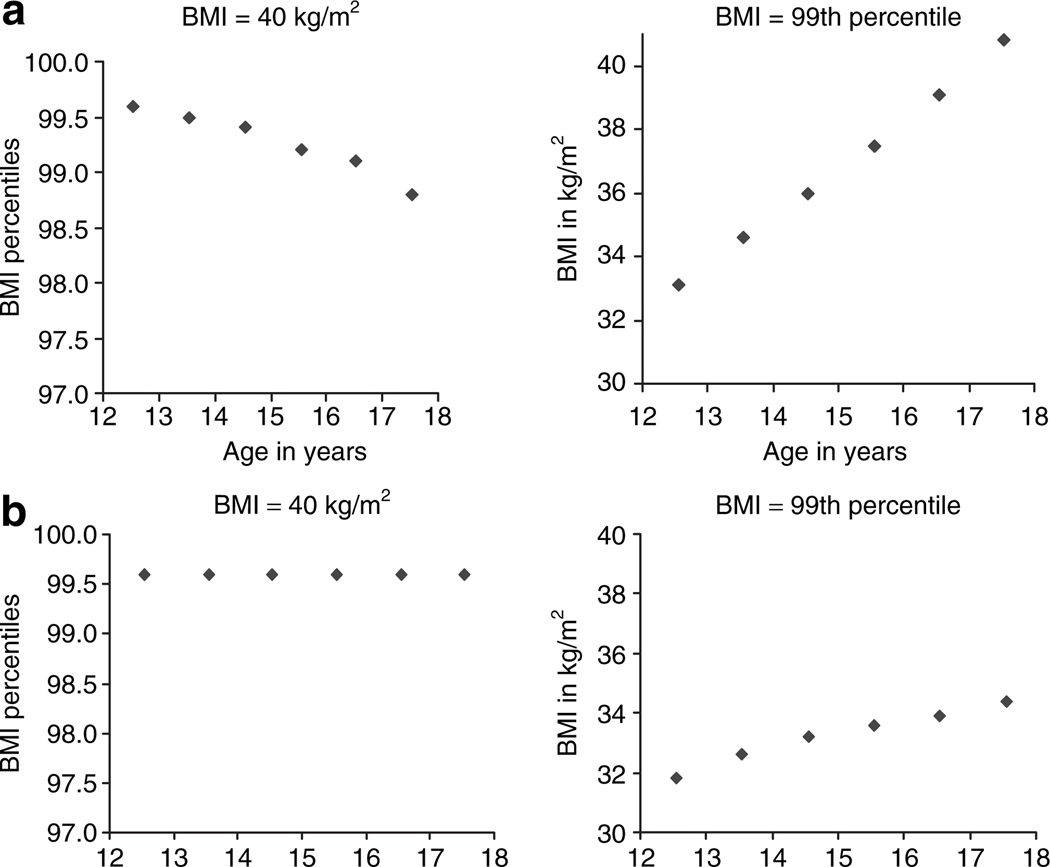
As the percentile values that correspond to the BMI cutoff points used for adult Classes II and III obesity (12) decrease with advancing age among adolescents and the 99th percentile (26) appears to be less conservative at younger ages compared to the more severe forms of adult obesity Classes in a National sample (Figure 1), the Pediatric/Adolescent Task Force for WLS recommends the use of the adult obesity Class III, and Class II with major comorbidity, rather than the BMI percentile criterion to identify potential candidates for WLS (10). Thus, pediatric patients with the highest risk of obesity in adulthood and with major obesity related morbidity would more likely be evaluated for WLS.
Figure 1.
BMI values in percentile or in kg/m2 for cutoff points of severe obesity. (a) Adolescent girls. (b) Adolescent boys. BMI values or percentiles were calculated based on the age at the midpoint of the age category in years (e.g., value at 12.5 years for an individual in the age category 12 (12–12.9) years old) using National growth chart data from the CDC (26); BMI percentile values that correspond to a BMI = 40 kg/m2 are higher than the BMI = 99th percentile and the BMI values that correspond to the 99th percentile are <40 kg/m2, especially among younger adolescents. Adapted from the National growth charts data (26).
Although 4% of children and 7% of minorities in the United States have a BMI ≥99th percentile (27,28), there are no studies that compare the prevalence of adolescents with a BMI ≥99th percentile to adolescents with more severe forms of obesity based on the adult obesity Class system. Therefore, we examined the prevalence of adolescents eligible for WLS based on various BMI cutoff points.
METHODS AND PROCEDURES
Data source
Data for this study come from the Ambulatory Pediatric Practice at Boston Medical Center (BMC). BMC is a private, not for profit, academic medical center that primarily serves children and adolescents from low-income families and minority communities. With regard to socioeconomic factors, lacking health insurance or having public insurance (Free hospital care, Medicaid, or Medicare) are directly associated with pediatric obesity. More than 50% of BMC patients qualify for Free Care or Medicaid whereas >70% of obese children and adolescents referred to the BMC Pediatric Weight Management Clinic are eligible (29). Approximately 5% of these referrals have a major morbidity related to obesity (2% have type 2 diabetes mellitus, 3% have sleep apnea, but they rarely have a diagnosis of pseudotumor cerebri or severe steatohepatitis).
Definition of obesity and BMI criteria for WLS
We used the most recent BMI classification system recommended in the United States to define obesity status. Pediatric patients with a BMI (weight/height2) ≥85th percentile for age and gender based on the 2000 Centers for Disease Control and Prevention (CDC) growth charts were classified as overweight and those with a BMI ≥95th percentile as obese (26,30,31). Adolescents with a BMI ≥99th percentile for age and gender on the 2000 CDC growth charts and those with a BMI ≥40 kg/m2 were classified as severely obese (27,28). Adolescents with a BMI ≥35 kg/m2 were classified as less severely obese.
Primary and secondary outcomes
The primary outcome was the prevalence of adolescents eligible for WLS according to two BMI cutoff points (≥99th percentile and ≥40 kg/m2) regardless of age (12–17.9 years) and by age group (12–14.9 years and 15–17.9 years). The secondary outcomes included: (i) the adolescents’ characteristics (age, gender, age at skeletal maturity, and race/ethnicity) by BMI category; (ii) the adolescents’ mean weight and its range in each obesity category by age in years, and the adolescents’ potential for BMI loss following WLS; and, (iii) an estimation of the proportion of adolescents who may be eligible for WLS based on the percentage of adolescents with a BMI between 35 and 39.9 kg/m2 and the prevalence of major morbidity related to pediatric obesity.
Procedures and data analysis
De-identified patient data were extracted from BMC’s electronic medical record system (Centricity; GE Medical Systems, Waukesha, WI) using Crystal Reports, a data reporting software program. Variables extracted from the electronic medical record included weight (in pounds and kilograms), height (in inches and centimeters), race and ethnicity, and age (based on date of birth and patient visit). Data were captured for a 4-year period extending from 1 September 2000 to 31 August 2004. Institutional review board approval was obtained at the Boston University School of Medicine.
Data from patients ages 2–18 years old were extracted (n = 14,543 unique patient records) to study prevalence rates of obesity by age, gender and race/ethnicity. Although patients had multiple entries over the 4-year period, data were included only once in the analysis. Data from the first patient record to fall within the 4-year period (2000–2004) were used in the analyses. Data were excluded if they appeared to be erroneous based on values that were implausible (e.g., height of 2 inches). Outliers were defined using the criterion of ±6 s.d. from the sample mean. Among 14,543 patient records, 14,451 weight z-scores were obtained and 14,399 were within range. Height z-scores were obtained from 11,177 patients, and 11,093 were within range. BMI was calculated for those patients who had both weight and height listed (10,993 patients). Of these patients, 10,797 had a BMI z-score within range. There were no significant differences (P > 0.05) between the sample pool extracted from the electronic medical record (n = 14,543) and those available for analyses (n = 10,797) with respect to demographic variables (data not shown).
For this cross-sectional study, the adolescent sample consisted of 3,220 individuals aged 12–17.9 years old who were further divided into groups based on their gender (girls and boys), their race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and others), their age (12–14.9 years and 15–17.9 years), and age at skeletal maturity (15–17.9 years for males and 13–17.9 years for females). z-Scores and percentiles were computed using the methods outlined by the CDC (26). BMI was calculated based on the weight in kilograms divided by the squared height in centimeters.
Descriptive statistical analyses, Pearson χ2-tests, and Fisher Exact tests were used to compare the proportion of adolescents in each BMI category and age group, by gender and race/ethnicity. A two-sided P value at <0.05 was used to define statistical significance. Data were analyzed using SPSS for Windows version 12.0.1 (Chicago, IL).
RESULTS
The sample consisted of 3,220 adolescents aged 12–17.9 years, of which 53% were female, 55% were of black race, and 16% of Hispanic ethnicity. Approximately 43% of the adolescents were overweight (BMI ≥ 85th percentile) and 24% were obese (BMI ≥ 95th percentile). Overall, 88 (3%) adolescents had a BMI ≥40 kg/m2 and 236 (7%) had a BMI ≥99th percentile, P < 0.001 (Table 1). A total of 159/2,007 (8%) of 12–14.9-year olds had a BMI ≥99th percentile compared with 77/1,213 (6%) 15–17.9-year olds (P =0.10), whereas 43/2,007 (2%) of 12–14.9-year olds had a BMI ≥40 kg/m2 compared with 45/1,213 (4%) 15–17.9-year olds (P = 0.008).
Table 1.
Adolescents characteristics by more severe categories of BMI
| BMI ≥ 99th percentile | BMI ≥ 40 (kg/m2) | 35 ≤ BMI <40 (kg/m2) | ||
|---|---|---|---|---|
| n | n (%) | n (%) | n (%) | |
| Total sample | 3,220 | 236 (7) | 88 (3) | 142 (4) |
| Age group | ||||
| 12–14.9 years | 2,007 | 159 (8) | 43 (2) | 77 (4) |
| 15–17.9 years | 1,213 | 77 (6) | 45 (4) | 65 (5) |
| Gender | ||||
| Male | 1,540 | 113 (7) | 27 (2) | 48 (3) |
| Female | 1,680 | 123 (7) | 61 (4) | 94 (6) |
| Age at skeletal maturity | ||||
| Males | ||||
| 12–14.9 years | 1,008 | 73 (7) | 13 (1) | 26 (3) |
| 15–17.9 years | 532 | 40 (8) | 14 (3) | 22 (4) |
| Females | ||||
| 12–12.9 years | 336 | 31 (9) | 3 (1) | 20 (6) |
| 13–17.9 years | 1,344 | 92 (7) | 58 (4) | 74 (6) |
| Race/ethnicity | ||||
| Non-Hispanic white | 230 | 15 (7) | 8 (3) | 11 (5) |
| Non-Hispanic black | 1,774 | 155 (9) | 54 (3) | 94 (5) |
| Hispanic | 522 | 30 (6) | 8 (2) | 15 (3) |
| Others | 694 | 36 (5) | 18 (3) | 22 (3) |
All adolescents (88/88) with BMI ≥40 kg/m2 had a BMI ≥99th percentile. Among those with a BMI ≥99th percentile, the percentage of adolescents with a BMI ≥40 kg/m2 increased with age from 11% (6/56) adolescents in the very young age group (12–12.9 years) to 46% (82/180) as they aged (13–17.9 years), and 27% (43/159) in the younger group (12–14.9 years) compared to 58% (45/77) in the older group (15–17.9 years). There was no significant difference between adolescent boys and girls with a BMI ≥99th percentile compared to those below the 99th percentile (7% vs. 7%, P = 0.99, Table 1). However, there were gender differences among adolescents with a BMI ≥40 kg/m2: the percentage of adolescent girls with BMI ≥40 kg/m2 (4% vs. 2%, P =0.002) and with a BMI 35–39.9 kg/m2 (6% vs. 3%, P < 0.001) were significantly higher compared to adolescent boys (Table 1). There was no significant difference in the proportion of adolescents who had a BMI ≥40 kg/m2 and age at or above the age of skeletal maturity by gender (3% (14/532) adolescent boys vs. 4% (58/1,344) adolescents girls, P > 0.05). However, 3% (14/532) of the adolescents boys were at or above the age of skeletal maturity (15–17.9 years) compared to 1% (13/1,008) below the skeletal maturity age (12–14.9 years, P = 0.06) whereas 4% (58/1,344) of the adolescent girls were at or above the age of skeletal maturity (13–17.9 years) compared to 1% (3/336) of the adolescent girls below the age of skeletal maturity (12–12.9 years), P = 0.002. Although we found no significant differences in the proportion of adolescents in each of the more severe categories of obesity by ethnicity, P > 0.05 (Table 1), black adolescents were more likely than non-black adolescents to have a BMI ≥99th percentile (9 % vs. 6%, P = 0.001) but not a BMI ≥40 kg/m2 (3% vs. 2%, P = 0.23).
The mean weight and range of adolescents age 12–12.9 years with BMI ≥99th percentile was 90.9 (54.8–115.9) kg and those with BMI ≥40 kg/m2 was 110 (104.3–115.9) kg while the mean weight and range of adolescents age 17–17.9 years with BMI ≥ 99th percentile was 121.2 (101.2–177.0) kg and those with BMI ≥40 kg/m2 was 130.2 (109.7–177.0) (Table 2). Based on a theoretical BMI loss of 30%, the average BMI loss 1–2 years after surgery in this study sample would be of ~13 kg/m2 reaching ~30 kg/m2 among mature boys and girls (at or above their skeletal maturation age). We also estimated that ~7 (5% of 142) adolescents may be eligible for WLS based on 142 adolescents with a BMI between 35 and 39.9 kg/m2 (Table 1) and the prevalence of major morbidity related to obesity (5%). Therefore, we expect that over a period of 4 years, 88 adolescents with BMI ≥40 kg/m2 and 7 adolescents with BMI 35–39.9 kg/m2 and major comorbidities may be eligible for WLS (total of 95 eligible adolescents) as opposed to 236 adolescents with BMI ≥99th percentile. Based on the updated guidelines for pediatric/adolescents WLS, we expect that ~3% (95/3,220) or 24 adolescents will be eligible for WLS at the BMC Pediatric Ambulatory Care Center each year.
Table 2.
Adolescent mean weight and range according to their BMI category and age (n = 3,220)
| BMI ≥ 99th percentile | BMI ≥ 40 kg/m2 | 35 ≤ BMI < 40 kg/m2 | ||
|---|---|---|---|---|
| Age (years) | n | Mean weight (range) | Mean weight (range) | Mean weight (range) |
| 12.0–12.9 | 703 | 90.9 (54.8–115.9) | 110.0 (104.3–115.9) | 93.6 (71.2–106.8) |
| 13.0–13.9 | 673 | 101.2 (72.6–133.7) | 111.5 (74.8–133.7) | 100.9 (85.3–125.6) |
| 14.0–14.9 | 631 | 109.2 (83.2–152.4) | 115.9 (89.1–152.4) | 103.5 (88.5–126.6) |
| 15.0–15.9 | 548 | 117.1 (90.9–145.3) | 125.7 (101.4–145.3) | 103.7 (82.3–135.6) |
| 16.0–16.9 | 440 | 118.8 (79.4–161.7) | 122.1 (96.7–161.7) | 106.2 (85.3–140.6) |
| 17.0–17.9 | 225 | 121.2 (101.2–177.0) | 130.2 (109.7–177.0) | 102.6 (86.2–117.7) |
DISCUSSION
In this urban ambulatory care center serving mainly low-income, minority populations, we found that ~7% of adolescents have a BMI at or above the 99th percentile. In contrast, Class III obesity is less prevalent among younger adolescents (2% of the 12–14.9 years vs. 4% of the 15–17.9 years) and adolescent boys (2% male vs. 4% female). That data are consistent with previous data on prevalence of adolescents with a BMI above the 99th percentile in NHANES (28) and the Bogalusa study (27). To our knowledge, this is the first study to compare prevalence rates of WLS eligibility among adolescents using two distinct BMI criteria, a percentile criterion and the adult obesity Class system.
One concern related to using the CDC growth chart’s 99th percentile cutoff point to define more severe forms of obesity among adolescents is that an adolescent may be offered WLS although s/he may not have required such surgery had s/he waited until adulthood. In our sample study, 20% (48/236) of the adolescents with a BMI ≥99th percentile also had a BMI<35 kg/m2 and 63% (148/236) had a BMI <40 kg/m2. This high percentage of adolescents without Class II or III obesity among adolescents age 12–17.9 years with BMI ≥99th percentile is consistent with findings in the Bogalusa study: 12 % of children aged 5–14 years old with a BMI ≥99th percentile had a BMI <35 kg/m2 as young adults and 35% had a BMI <40 kg/m2 (27). Given the secular trend in severe obesity that particularly affects minorities (28), an unacceptably large number of adolescents would qualify for WLS based on the percentile criterion.
We estimated that 3% of all adolescents may have Class III obesity (2.7%), or Class II obesity and major morbidity related to obesity (0.2%), that would require a WLS assessment at BMC. This corresponds to ~24 adolescents per year at BMC. As a comparison, it has been estimated that adolescent WLS in the United States has increased fivefold, from 51 to 282, between 1997 and 2003 (ref. 32). This survey used data from 2 to 3 million hospital discharge records per year (sampling increased from 22 to 36 states during that study period) and showed that the average number of adolescent WLS per hospital was ≤4 annually (32). No hospital reported >12 WLS among adolescents in a year. However, between the early 1990s and 2003, the adult WLS nationwide rose from ~16,000 to >140,000 a year (33) and >2,700 gastric bypass operations were carried out in 2003 compared to <150 in 1996 in Massachusetts alone (34). Although adolescent WLS represents only 0.7% of all WLS performed in 2003 (ref. 32), adolescent WLS is increasing in the United States and is estimated to have reached several thousands a year (35). Therefore, the use of the updated guidelines from the Betsy Lehman Pediatric/Adolescent Task force for WLS may be associated with a dramatic increase in the number of adolescent WLS performed in Massachusetts, especially in urban settings.
The timing of WLS among obese adolescents is especially controversial because of the rapid neuroendocrine, skeletal and psychological maturation occurring during this period of life. Although bone age is more advanced during the entire period of pubertal development, growth spurt is lower in the obese adolescent compared to the nonobese adolescent and final height at age 18 years is similar regardless of obesity status (36). The difference between chronological and skeletal maturity (bone age) is ~1 year lower among obese adolescents (37) and varies by race/ethnicity (38). In the general population, the linear growth spurt typically occurs prior to Tanner IV and skeletal maturity is attained by age 13 in girls and 15 in boys. Thus, using Tanner stage (at least Tanner Stage IV) and/or skeletal maturity (>95% the adult potential height on X-ray) will limit WLS to children above age 12 years old.
Another distinctive characteristic of the BMC data set compared to the National data on adolescent WLS is the patients’ socioeconomic background. The pediatric population assessed for weight management at BMC is predominantly a minority population (78%), while the population in the recent National study of WLS (32) was predominantly white (68%). Approximately 78–82% of obese adolescents in the recently surveyed hospitals had private insurance (32), compared to 20–30% of pediatric patients at the BMC Pediatric Weight Management Program. This difference in insurance status raises concerns about access to WLS among underserved populations.
Our study has several strengths and limitations. The strengths include the large sample size, consisting of 3,220 urban adolescents aged 12–17.9 years old. Although the overall sample size was large and included an appropriate sample of black adolescents compared to other prevalence study samples (27,28), there were relatively few severely obese adolescents who were Hispanic or non-Hispanic white. The weight and height measurements were taken in a clinical setting rather than measured in duplicates or triplicates. Skeletal maturity was estimated using chronological age rather than a measure on a wrist X-ray. Although BMI is considered the best tool to screen for body fat in adolescents (39), BMI does not distinguish between overfat and overweight. BMI may overestimate body fat in individuals who have higher lean body mass and underestimate body fat in individuals who have lower lean body mass. We have not conducted validation studies to estimate the amount of fat among adolescents by categories of BMI; however, it has been demonstrated that body fat is associated with higher BMI, especially above the 99th percentile (27,40).
In summary, the prevalence of extreme obesity based on the percentile criterion is relatively high for this patient population (7%). A BMI ≥40 kg/m2 is a more conservative approach to WLS (3%), especially among younger, compared to older adolescents (2% vs. 4%). The estimated percent of adolescents with BMI 35–39.9 kg/m2 and at least one major comorbidity is relatively small (0.2%). Thus, adolescents with the highest risk of obesity in adulthood and with major comorbidities would more likely be evaluated for WLS. Tracking studies of BMI, body fat, and major morbidities related to obesity by age and severity of obesity are needed.
ACKNOWLEDGMENTS
C.M.L. received grant support from the American Society for Clinical Nutrition (Physician Nutrition Specialist Award), the Loomis, Sayles & Company, L.P., and the New Balance Foundation. We are very thankful for the contribution of Howard Bauchner in the review of the manuscript with support from his 5 K24 HD042489.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Cali AM, Caprio S. Prediabetes and type 2 diabetes in youth: an emerging epidemic disease? Curr Opin Endocrinol Diabetes Obes. 2008;15:123–127. doi: 10.1097/MED.0b013e3282f57251. [DOI] [PubMed] [Google Scholar]
- 3.Berenson GS, Srnivasan SR. Bogalusa Heart Study Group. Cardiovascular risk factors in youth with implications for aging: the Bogalusa Heart Study. Neurobiol Aging. 2005;26:303–307. doi: 10.1016/j.neurobiolaging.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Freedman DS, Patel DA, Srinivasan SR, et al. The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. Int J Obes (Lond) 2008;32:749–756. doi: 10.1038/sj.ijo.0803798. [DOI] [PubMed] [Google Scholar]
- 5.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. 2007;92:205–208. doi: 10.1136/adc.2006.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 7.Mercille G, Ospina LH. Pediatric idiopathic intracranial hypertension: a review. Pediatr Rev. 2007;28:e77–e86. doi: 10.1542/pir.28-11-e77. [DOI] [PubMed] [Google Scholar]
- 8.Williams J, Wake M, Hesketh K, Maher E, Waters E. Health-related quality of life of overweight and obese children. JAMA. 2005;293:70–76. doi: 10.1001/jama.293.1.70. [DOI] [PubMed] [Google Scholar]
- 9.Engeland A, Bjørge T, Tverdal A, Søgaard AJ. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology. 2004;15:79–85. doi: 10.1097/01.ede.0000100148.40711.59. [DOI] [PubMed] [Google Scholar]
- 10.Pratt J, Lenders C, Dionne E, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity. doi: 10.1038/oby.2008.577. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Technical Report Series. Geneva: World Health Organization; 1995. WHO Expert Committee on Physical Status: the Use and Interpretation of Anthropometry; p. 854. [PubMed] [Google Scholar]
- 12.NHLBI Expert Panel on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6(Suppl 2):S51–S209. [PubMed] [Google Scholar]
- 13.Strawbridge WJ, Wallhagen MI, Shema SJ. New NHLBI clinical guidelines for obesity and overweight: will they promote health? Am J Public Health. 2000;90:340–343. doi: 10.2105/ajph.90.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–961. [PubMed] [Google Scholar]
- 15.Buchwald H, Avidor Y, Braunald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 16.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 17.Dixon JB, O’Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 18.Inge TH, Krebs NF, Garcia VF, et al. Bariatric surgery for severely overweight adolescents: concerns and recommendations. Pediatrics. 2004;114:217–223. doi: 10.1542/peds.114.1.217. [DOI] [PubMed] [Google Scholar]
- 19.Apovian CM, Baker C, Ludwig DS, et al. Best practice guidelines in pediatric/adolescent weight loss surgery. Obes Res. 2005;13:274–282. doi: 10.1038/oby.2005.37. [DOI] [PubMed] [Google Scholar]
- 20.Inge TH, Zeller MH, Lawson ML, Daniels SR. A critical appraisal of evidence supporting a bariatric surgical approach to weight management for adolescents. J Pediatr. 2005;147:10–19. doi: 10.1016/j.jpeds.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Angrisani L, Favretti F, Furbetta F, et al. Obese teenagers treated by Lap-Band System: the Italian experience. Surgery. 2005;138:877–881. doi: 10.1016/j.surg.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Nadler EP, Youn HA, Ren CJ, Fielding GA. An update on 73 US obese pediatric patients treated with laparoscopic adjustable gastric banding: comorbidity resolution and compliance data. J Pediatr Surg. 2008;43:141–146. doi: 10.1016/j.jpedsurg.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 23.Holterman AX, Browne A, Dillard BE, 3rd, et al. Short-term outcome in the first 10 morbidly obese adolescent patients in the FDA-approved trial for laparoscopic adjustable gastric banding. J Pediatr Gastroenterol Nutr. 2007;45:465–473. doi: 10.1097/MPG.0b013e318063eef6. [DOI] [PubMed] [Google Scholar]
- 24.Kalra M, Inge T, Garcia V, et al. Obstructive sleep apnea in extremely overweight adolescents undergoing bariatric surgery. Obes Res. 2005;13:1175–1179. doi: 10.1038/oby.2005.139. [DOI] [PubMed] [Google Scholar]
- 25.Sugerman HJ, Sugerman EL, DeMaria EJ, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102–107. doi: 10.1016/S1091-255X(02)00125-7. discussion 107–108. [DOI] [PubMed] [Google Scholar]
- 26.CDC National Center for Health Statistics. [Accessed 31 May 2007];CDC growth charts: United States. Percentile Datafile with LMS values. 2000 < http://www.cdc.gov/>.
- 27.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150:12–17. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity (Silver Spring) 2006;14:301–308. doi: 10.1038/oby.2006.39. [DOI] [PubMed] [Google Scholar]
- 29.Lenders C, Meyers A, Oh H. A clinical guide to pediatric ambulatory weight management. In: Apovian C, Lenders C, editors. A Clinical Guide for Management of Overweight and Obese Children and Adults. Boca Raton, FL: CRC press; 2006. pp. 197–238. [Google Scholar]
- 30.Koplan JF, Liverman CT, Kraak VA, editors. Preventing Childhood Obesity: Health in the Balance. Washington, DC: Institute of Medicine. The National Academy Press; 2004. Committee on prevention of obesity in children and youth. [Google Scholar]
- 31.Barlow SE. Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 32.Schilling PL, Davis MM, Albanese CT, Dutta S, Morton J. National trends in adolescent bariatric surgical procedures and implications for surgical centers of excellence. J Am Coll Surg. 2008;206:1–12. doi: 10.1016/j.jamcollsurg.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Steinbrook R. Surgery for severe obesity. NEJM. 2004;350:1075–1079. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 34.Lehman Center Weight Loss Surgery Expert Panel. Commonwealth of Massachusetts Betsy Lehman Center for Patient Safety and Medical Error Reduction Expert Panel on Weight Loss Surgery: executive report. Obes Res. doi: 10.1038/oby.2005.30. in press. [DOI] [PubMed] [Google Scholar]
- 35.Tsai WS, Inge TH, Burd RS. Bariatric surgery in adolescents: recent national trends in use and in-hospital out come. Arch Pediatr Adolesc Med. 2007;161:217–221. doi: 10.1001/archpedi.161.3.217. [DOI] [PubMed] [Google Scholar]
- 36.De Simone M, Farello G, Palumbo M, et al. Growth charts, growth velocity and bone development in childhood obesity. Int J Obes Relat Metab Disord. 1995;19:851–857. [PubMed] [Google Scholar]
- 37.Akridge M, Hilgers KK, Silveira AM, et al. Childhood obesity and skeletal maturation assessed with Fishman’s hand-wrist analysis. Am J Orthod Dentofacial Orthop. 2007;132:185–190. doi: 10.1016/j.ajodo.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 38.Ontell FK, Ivanovic M, Ablin DS, Barlow TW. Bone age in children of diverse ethnicity. Am J Roentgenol. 1996;167:1395–1398. doi: 10.2214/ajr.167.6.8956565. [DOI] [PubMed] [Google Scholar]
- 39.Dietz WH, Robinson TN. Use of the body mass index (BMI) as a measure of overweight in children and adolescents. J Pediatr. 1998;132:191–193. doi: 10.1016/s0022-3476(98)70426-3. [DOI] [PubMed] [Google Scholar]
- 40.Freedman DS, Wang J, Thornton JC, et al. Racial/ethnic differences in body fatness among children and adolescents. Obesity (Silver Spring) 2008;16:1105–1111. doi: 10.1038/oby.2008.30. [DOI] [PubMed] [Google Scholar]