Abstract
Context:
Although several previous studies have investigated the association of metabolic syndrome (MS) and insulin resistance (IR) with androgenetic alopecia (AGA), the results have been inconsistent.
Aim:
We attempted to assess the presence of MS and IR in patients with AGA. This may help to detect if AGA can be considered as a clue for underlying serious systemic diseases.
Materials and Methods:
One hundred male patients with stages III-VII AGA, in Hamilton-Norwood classification, and 100 normal, gender- and age-matched control subjects were included. Anthropometric measures, blood pressure, fasting glucose, fasting insulin, high-density lipoprotein cholesterol, and triglycerides were measured for the all participants. The presence of MS and IR was evaluated.
Results:
There were statistically significant differences regarding mean values of body weight (P < 0.001), height (P = 0.002), waist circumference (P < 0.001), body mass index (P < 0.001), systolic (P < 0.001), and diastolic blood pressure (P < 0.001), fasting glucose (P < 0.001), triglycerides (P < 0.001), high-density lipoprotein cholesterol (P < 0.01), fasting insulin (P = 0.02) and homeostasis model assessment of insulin resistance (P < 0.001) between cases and controls. A statistically significant association was found between AGA and MS (P = 0.002) and between AGA and IR (P < 0.001). Multiple logistic regression analysis revealed that waist circumference (>102 cm) was the most significant risk factor for developing MS. It increased the risk of MS by 1.25-folds (95% CI = 1.10-1.42, P < 0.001).
Conclusion:
Our results support the recommendation for assessing MS and IR in all young males with stage III or higher AGA. Early intervention is critical to reduce the risk and complications of cardiovascular disease and type 2 diabetes mellitus later in life.
Keywords: Androgenetic alopecia, cardiovascular disease, insulin resistance, metabolic syndrome
INTRODUCTION
Androgenetic alopecia (AGA) is a disorder characterized by hair loss in genetically predisposed men and women. It requires adequate circulating androgens and a genetic predisposition. It has a polygenetic pattern. Dihydrotestosterone (DHT) binding to androgenic receptors in hair follicles of the scalp triggers the genes accountable for gradual transformation of large terminal follicles to miniature ones. Such miniaturization is observed on the frontotemporal area and vertex in men, and over the crown in women, as these areas are more sensitive to androgen effects.[1]
In 1972, it was first suggested that AGA may be a risk factor for cardiovascular disease (CVD) when Cotton et al.[2] demonstrated an association between the occurrence of CVD and hair loss. Conversely, others indicated that there is no association between CVD and AGA.[3] In many subsequent studies, AGA has been shown to be associated with several diseases, such as insulin resistance (IR),[4] abnormal serum lipid profiles,[5] and obesity.[6]
We were interested in studying the presence of metabolic syndrome (MS) and IR in patients with AGA to detect if AGA can be considered as a clue for underlying serious systemic diseases.
MATERIALS AND METHODS
Ethics
Written consent forms approved by The Committee of Human Rights in Research in Menoufiya University were obtained from studied cases and control subjects. Those were in accordance with the Helsinki Declaration of 1975 (revised in 2000).
Patients
This study represented a case–control study. One hundred male patients with early-onset AGA (before the age of 35 years) who did not have known CVD or glucose metabolism disorder were selected from Dermatology Outpatient Clinic, Menoufiya University Hospital, during the period from March 2011 to March 2012. The Hamilton-Norwood scale[7] was used for grading of AGA, and the all participants were assessed by the same doctor. One hundred, normal, age- and gender-matched volunteers from medical students and working staff at Menoufiya Faculty of Medicine were selected as a control group. Clinical data describing patients’ age, age of onset, and family history of AGA were all documented.
Inclusion criteria
Voluntary participation in the study, normal hepatic and renal functions, normal thyroid and adrenal assessment, normal blood count, and a standard urine analysis.
Exclusion criteria
Congenital adrenal hyperplasia, CVD, thyroid disease including subclinical hypothyroidism (thyroid stimulating hormone >5 mIU/L), smoking, Cushing's disease, history of viral hepatitis, cirrhosis or liver failure, renal failure, alcoholics, androgen or antiandrogen therapy, slimming, insulin-sensitizing drugs or insulin treatment, glucocorticoid treatment within the previous 6 months and changes related to body weight within last 3 months.
Every participant was subjected to the following:
Anthropometric and blood pressure measurement
Height measurements were taken twice, with a maximum variation of 0.5 cm, and the average was considered. Weight measurements were done while the participants were using light clothes and no shoes. The body mass index (BMI) was calculated by dividing the weight by the square of height (kg/m2). The participants’ waist circumference (cm) was measured using a nonextendable metric tape, between the 12th costal lower boundary and the iliac crest. It was measured twice, with a maximum variation of 1 cm and the average was calculated. A value ≥102 cm was taken as a recommended cut off point for abdominal obesity.[8] Blood pressure (BP) was measured using a sphygmomanometer after a 20-min rest. The mean value for systolic and diastolic BP was calculated from four readings. Systolic BP ≥ 135 mmHg and diastolic BP ≥ 85 mmHg were recommended as cut off points for hypertension.[8]
Laboratory investigations
Triglycerides (TGs), and high-density lipoprotein cholesterol (HDL-C) were measured in blood samples drawn after a 12-h fast, and plasma was separated immediately by refrigerated centrifugation at 2500-3000 rpm for a period of 10 min. The samples were processed immediately or in the first week following preservation at -20°C. The mean fasting serum insulin and fasting blood sugar (FBS) concentration levels were obtained from blood samples drawn three times after 5-min intervals.
Fasting serum insulin assays were carried out using enzyme-linked immunosorbent assay technique (Calbiotech insulin ELISA kit, Cat No. IS130D; Calbiotech Incorporation, Spring Valley, CA, USA), and fasting plasma glucose levels were measured with C × 5 Synchron Delta Chemistry Autoanalyzer (Beckman, Fullerton, CA, USA). TGs and HDL-C were measured by enzymatic colorimetric method.
TGs value ≥150 mg/dL and HDL-C value ≤40 mg/dL were recommended as cut off points of dyslipidemia. FBS ≥ 110 mg/dL was recommended as cutoff point of impaired fasting glycemia.[8]
Diagnosis of metabolic syndrome
Metabolic syndrome is the combination of hypertension, dyslipidemia, and impaired fasting glycemia. Diagnosis of MS was done based on the National Cholesterol Education Programme (NCEP) Adult Treatment Panel III by the presence of three or more of the following criteria:[8]
(a) waist circumference ≥102 cm in males, (b) TGs value ≥150 mg/dL, (c) HDL-C <40 mg/dL in males, (d) FBS ≥ 110 mg/dL, and (e) BP ≥ 130/85 mmHg.
Diagnosis of insulin resistance
Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) according to the following formula: [fasting insulin level (μIU/mL) × fasting glucose level (mmol/L)/22.5]. A value above 2.7 was considered to indicate IR.[9]
Statistical analysis
Data were collected, tabulated, and statistically analyzed using a personal computer with “(SPSS) version 11” program. Data were statistically described in terms of range, mean ± standard deviation (X ± SD), frequencies (number of cases) and relative frequencies (percentages) when appropriate. Student's t test was used for continuous quantitative parametric variables and Mann-Whitney U test for nonparametric variables. Chi-square test was used for categorical variables. Fisher exact test was used for 2 × 2 tables when expected cell count of >25% of cases was <5. Z-test was used to study the association between two qualitative variables. Logistic regression analysis was used for multivariate determination of parameters affecting MS. Comparisons of data were made with overall α error set at 0.05 (two tailed).
RESULTS
Study population
Studied population included 100 male patients with grades III-VII, early onset AGA and 100 normal, gender- and age-matched volunteers. Patients’ ages ranged from 25 to 60 years with a mean ± standard deviation age of 40.09 ± 10.57 years. Controls’ ages ranged from 25 to 59 years with a mean ± standard deviation age of 37.85 ± 9.48 years.
Anthropometric, BP, and laboratory results
There were statistically significant differences regarding mean values of body weight (P < 0.001), height (P = 0.002), waist circumference (P < 0.001), BMI (P < 0.001), systolic (P < 0.001) and diastolic BP (P < 0.001) between cases and controls [Table 1].
Table 1.
Anthropometric measures, BP, FBS, lipid profile, and fasting insulin in patients and controls (values are expressed as mean±SD)
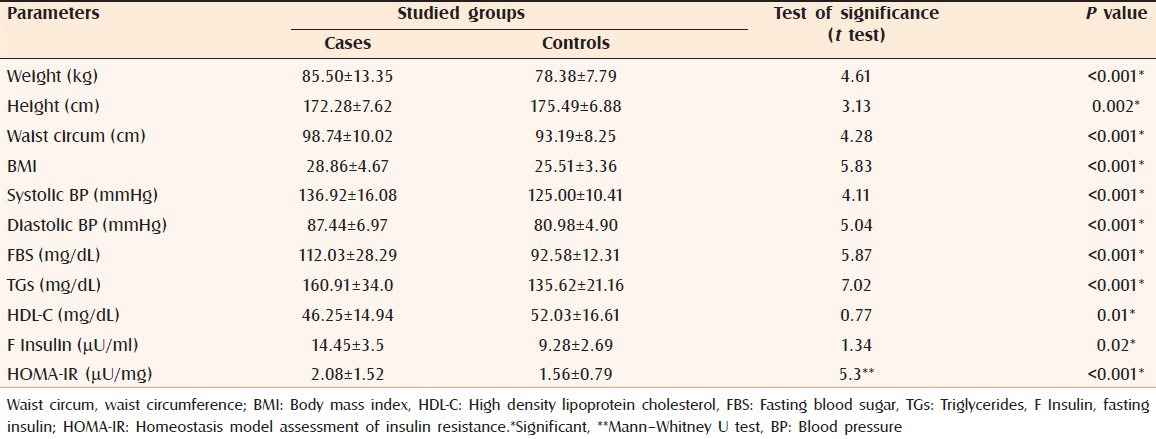
There were statistically significant differences regarding mean values of FBS (P < 0.001), fasting insulin (P = 0.02), TGs (P < 0.001), and HDL-C (P = 0.01) between cases and controls [Table 1].
Evaluation of metabolic syndrome and insulin resistance in studied groups
Mean value of HOMA-IR showed statistically meaningful difference between cases and controls (P < 0.001) [Table 1]. IR was diagnosed in 35 cases and 19 controls with significant difference between both groups (P < 0.001) (data not shown).
Based on diagnostic criteria of MS, it was diagnosed in 39 cases and 19 controls with a significant difference between both groups (P = 0.002) (data not shown).
Twenty-five cases and 10 control subjects had both MS and IR with statistically meaningful difference (P = 0.008) between cases and controls regarding the simultaneous presence of both diseases (data not shown).
The association between metabolic syndrome and clinical criteria of studied cases
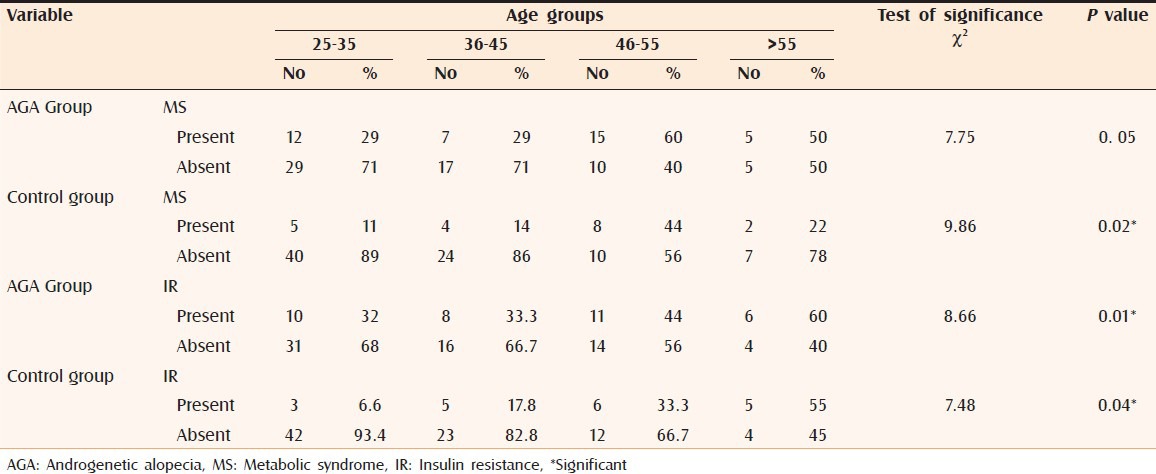
Studying the age-specific prevalence of MS showed that, its occurrence peaked in the age group (46-55 years) in cases and controls [Table 2]. Studying the age-specific prevalence of IR showed that, its occurrence peaked in the age group > 55 years in cases and controls [Table 2].
Table 2.
Age-specific prevalence of MS and IR in the studied groups
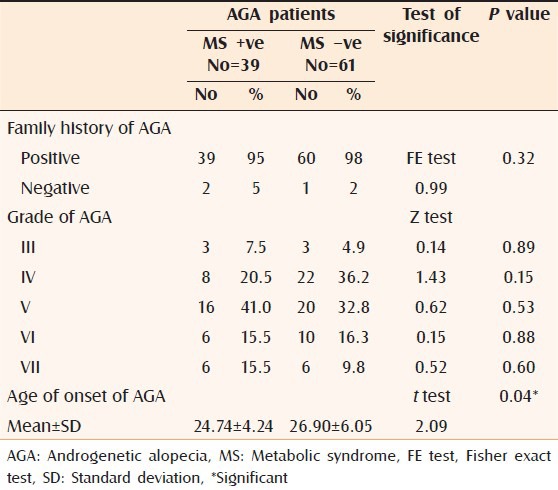
There was a significant difference (P = 0.04) between MS +ve and MS –ve AGA patients regarding the age of onset of AGA; where MS +ve patients tended to have an earlier age of onset. However, the grade and family history of AGA were not significantly different between both groups [Table 3].
Table 3.
Comparison between MS positive and negative AGA cases regarding family history, disease grade and age of onset
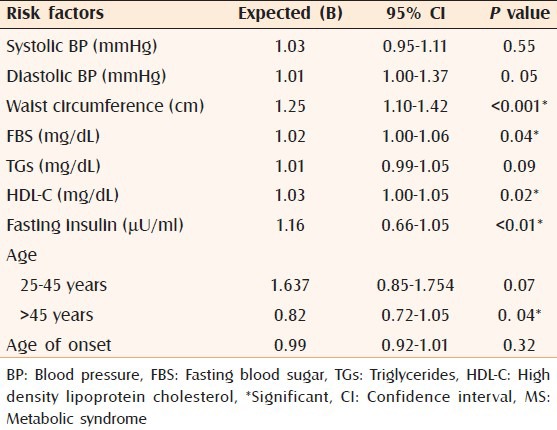
Multiple logistic regression analysis revealed that waist circumference (≥102 cm) increased the risk of MS by 1.25-folds (95% CI = 1.10-1.42, P < 0.001), followed by elevated fasting insulin, which increased the risk by 1.16-folds (95% CI = 0.66-1.05, P < 0.01). Decreased HDL-C (<40 mg/dL) increased the risk by 1.03-folds (95% CI = 1.00-1.05, P = 0.02), FBS (≥110 mg/dL) increased the risk by 1.02-folds (95% CI = 1.00-1.06, P = 0.04), and elevated diastolic BP (≥85 mmHg) increased the risk by 1.01-fold (95% CI = 1.00-1.37, P = 0.05). Age older than 45 years increased the risk of MS by 0.82-fold (95% CI = 0.72-1.05, P = 0.04) [Table 4].
Table 4.
Multiple regression analysis of MS components in the studied patients
DISCUSSION
Several previous studies have investigated the association between factors related to MS and AGA. However, the results showed much controversy.[3,4,5] The aim of this study was to assess the presence of MS and IR in patients with AGA to detect if AGA can be considered as a clue for underlying serious systemic disease.
In the present study, there were significant differences between cases and control subjects regarding body weight and waist circumference. Similar results were reported previously by Arias-Santiago et al.[10] regarding waist circumference, although they found a nonsignificant difference in body weight.
Abdominal fat tissue is associated with serious metabolic disorders, such as IR, hyperinsulinemia, hypertension, increased TGs, glucose intolerance, and diabetes mellitus. Some studies have pointed to abdominal fat tissue and waist circumference as independent risk factors for coronary heart disease (CHD).[11]
The current study showed a significant difference between cases and control subjects regarding BMI. This was in line with Matilainen et al.[12] However, Arias-Santiago et al.[10] found that BMI did not differ between AGA cases and normal controls.
In the present study, significant differences between cases and controls regarding mean values of systolic and diastolic BP were detected. Similar results were reported in previous studies.[10,12] A population-based approach reported by Hirsso et al.[13] on AGA patients demonstrated a significant association between AGA and hypertension. A correlation between hypertension and AGA, irrespective of age, has also been demonstrated.[14]
There are androgen-mediated receptors in the arterial wall endothelium. High serum androgen levels in AGA cases[15] contribute to the proliferation of smooth muscle cells in vessels[16] and increase the tendency to hypertension.[17] Another explanation for this association is the binding of androgens to mineralocorticoid receptors, favoring BP increase[18] or increased peripheral sensitivity to androgens despite their normal circulating levels.[19]
In the present study, FBS was significantly higher in cases than in controls. This result conflicted with what was reported by Nabaie et al.[20] and Acibucu et al.[12]
In the current study, mean value of TGs was significantly higher, whereas the mean value of HDL-C was significantly lower in studied cases than in control subjects. Şaşmaz et al.[4] found meaningfully higher levels of serum TGs in AGA group than in normal controls.
Androgens were proved to decrease HDL-C levels in experimental studies.[21] High values of TGs and low values of HDL-C were associated with the transition from atheroma to atherothrombosis. Therefore, investigation and control of lipid profiles in patients with AGA may be important to reduce this risk.[22]
Contrary to our results, Guzzo et al.[23] found no difference in HDL-C and TGs between AGA cases and normal controls. This inconsistency may be partially attributed to the different target populations studied and to the failure to control other confounding factors, such as family history and smoking status.
In the current work, MS was significantly associated with AGA group when compared with control group. A similar finding was previously reported by Acibucu et al.[12] Previous studies reported an increased risk of CAD in AGA cases than in normal controls.[4] Whether this is due to MS or dyslipidemia due to high androgen levels, needs further prospective studies to be proved.
The pathophysiological link between MS and AGA is not yet well understood. IR associated with AGA has previously been reported and might contribute to this association.[5]
In the present study, mean value of fasting serum insulin was significantly higher in AGA cases than in the control group. Similar result was reported by Matilainen et al.[8]
Insulin is found in hair follicles and may play a role in the regulation of androgen metabolism and the hair growth cycle, which are relevant to the loss of scalp hair in male-pattern baldness.[20] Hyperinsulinemia (fasting or postprandial) has been shown to be a risk factor for CHD, accelerates the development of atherosclerosis, and prevent atherosclerotic plaque resorption.[24]
The present work showed that 35% of cases and 19% of controls had IR with significant difference between both groups. Going with that, González-González et al.[25] and Mumcuoglu et al.[26] found a relationship between IR and early baldness. Hirrso et al.[27] demonstrated a reduction in insulin sensitivity in males with AGA. Conversely, Nabaie et al.[20] could not demonstrate a significant difference between AGA cases and control subjects with respect to levels of fasting insulin and IR. Abdel Fattah et al.[28] concluded that there is no true association between AGA and insulin resistance, but their coexistence with MS could contribute to worsening of AGA. Further large-scaled studies are required for more clarification.
IR plays a pathogenetic role in the miniaturization of hair follicles. Vasoactive substances associated with endothelial dysfunction in IR lead to microcirculatory disturbance, perifollicular vasoconstriction, and proliferation of smooth muscle cells in the vascular wall. This condition leads to microvascular insufficiency, local-tissue hypoxia, and progressive miniaturization of hair follicles.[12] Hyperinsulinemia play a role in local androgen production, whether de novo from cholesterol or by locally converting testosterone to DHT.[29] DHT inhibits adenyl cyclase activity, and is able to curtail the anagen cycle and could be responsible for the miniaturization of follicles in AGA.[30]
In addition, IR leads to the generation of inflammatory mediators and endothelial dysfunction.[8] Insulin increases the release of nitric oxide (NO) from the endothelium at the physiological levels. An increased risk of atherosclerosis in IR cases is thought to be related to the loss of insulin's effects on NO expression.[31]
Analysis of age-specific prevalence of MS in AGA patients was in favor of the age group from 46 to 55 years. Su and Chen[32] reported a nearly similar outcome. The age-specific prevalence of IR was in favor of age group > 55 years. This agreed with previous reports, that mentioned increased occurrence of IR with advancing age.[5]
Our results showed that MS-positive cases tended to have an early-onset AGA, an observation previously reported.[33] This finding may support the hypothesis that early AGA could be a clinical marker of IR.[8] Whether IR induces or promotes AGA needs to be clarified by further studies. Therefore, cases with early-onset AGA should be assessed for components of MS and IR for early detection and control of cardiovascular risk factors.
In the present study, waist circumference was the most significant risk factor for developing MS. Su and Chen[32] reported that the most significant association was observed between HDL-C level and AGA in their studied population. Conversely, Yi et al. reported nonsignificant association between all components of MS and AGA.[33]
In summary, MS is significantly associated with AGA particularly, early-onset alopecia. IR is mostly the underlying pathologic mechanism. More prospective studies are required in order to objectively clarify whether the increased risk of CHD in AGA can be attributed to dyslipidemia due to androgens, IR alone, or MS due to IR. Early-onset AGA patients should be closely followed-up in the long term, particularly for CHD. These data may raise awareness in susceptible individuals that lifestyle changes (weight control, exercise, and eating meals with a low glycemic index) in early life can reduce the risk of CHD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Rebora A. Pathogenesis of androgenetic alopecia. J Am Acad Dermatol. 2004;50:777–9. doi: 10.1016/j.jaad.2003.11.073. [DOI] [PubMed] [Google Scholar]
- 2.Cotton SG, Nixon JM, Carpenter RG, Evans DW. Factors discriminating men with coronary heart disease from healthy controls. Br Heart J. 1972;34:458–64. doi: 10.1136/hrt.34.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis JA, Stebbing M, Harrap SB. Male pattern baldness is not associated with established cardiovascular risk factors in the general population. Clin Sci. 2001;100:401–4. [PubMed] [Google Scholar]
- 4.Sasmaz S, Senol M, Ozcan A, Dogan G, Tuncer C, Akyol O, et al. The risk of coronary heart disease in men with androgenetic alopecia. J Eur Acad Dermatol Venereol. 1999;12:123–5. [PubMed] [Google Scholar]
- 5.Matilainen V, Koskela P, Keinanen-Kiukaanniemi S. Early androgenetic alopecia as a marker of insulin resistance. Lancet. 2000;356:1165–6. doi: 10.1016/S0140-6736(00)02763-X. [DOI] [PubMed] [Google Scholar]
- 6.6 Hirsso P, Rajala U, Hiltunen L, Jokelainen J, Keinänen-Kiukaanniemi S, Näyhä S. Obesity and low-grade inflammation among young Finnish men with early-onset alopecia. Dermatol. 2007;214:125–9. doi: 10.1159/000098570. [DOI] [PubMed] [Google Scholar]
- 7.Norwood OT. Male pattern baldness: Classification and incidence. South Med J. 1975;68:1359–65. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.Arias-Santiago S, Gutiérrez-Salmerón MT, Castellote-Caballero L, Buendía-Eisman A, Naranjo-Sintes R. Androgenetic alopecia and cardiovascular risk factors in men and women: A comparative study. J Am Acad Dermatol. 2010;63:420–9. doi: 10.1016/j.jaad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Acibucu F, Kayatas M, Candan F. The association of insulin resistance and metabolic syndrome in early androgenetic alopecia. Singapore Med J. 2010;51:931–6. [PubMed] [Google Scholar]
- 12.Matilainen V, Laakso M, Hirsso P, Koskela P, Rajala U, Keinänen-Kiukaanniemi S. Hair loss, insulin resistance, and heredity in middle-aged women. A population-based study. J Cardiovasc Risk. 2003;10:227–31. doi: 10.1097/01.hjr.0000070200.72977.c6. [DOI] [PubMed] [Google Scholar]
- 13.Hirsso P, Laakso M, Matilainen V, Hiltunen L, Rajala U, Jokelainen J, et al. Association of insulin resistance-linked diseases and hair loss in elderly men. Finnish population-based study. Cent Eur J Public Health. 2006;14:78–81. doi: 10.21101/cejph.b0045. [DOI] [PubMed] [Google Scholar]
- 14.Ahouansou S, Le Toumelin P, Crickx B, Descamps V. Association of androgenetic alopecia and hypertension. Eur J Dermatol. 2007;17:220–7. doi: 10.1684/ejd.2007.0152. [DOI] [PubMed] [Google Scholar]
- 15.Hibberts NA, Howell AE, Randall VA. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J Endocrinol. 1998;156:59–65. doi: 10.1677/joe.0.1560059. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto R, Morimoto I, Morita E, Sugimoto H, Ito Y, Eto S. Androgen receptors, 5 alpha-reductase activity and androgen-dependent proliferation of vascular smooth muscle cells. J Steroid Biochem Mol Biol. 1994;50:169–74. doi: 10.1016/0960-0760(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 17.Sheridan PJ, McGill HC, Jr, Aufdemorte TB, Triplett RG, Holt RG. Heart contains receptors for dihydrotestosterone but not testosterone: Possible role in the sex differential in coronary heart disease. Anat Rec. 1989;223:414–9. doi: 10.1002/ar.1092230410. [DOI] [PubMed] [Google Scholar]
- 18.Weyrich AS, Rejeski WJ, Brubaker PH, Parks JS. The effect of testosterone on lipids and eicosanoids in cynomolgus monkey. Med Sci Sports Ex. 1992;24:333–8. [PubMed] [Google Scholar]
- 19.Arias-Santiago S, Gutiérrez-Salmerón MT, Buendía-Eisman A, Girón-Prieto MS, Naranjo-Sintes R. Hypertension and aldosterone levels in women with early onset androgenetic alopecia. Br J Dermatol. 2010;162:786–9. doi: 10.1111/j.1365-2133.2009.09588.x. [DOI] [PubMed] [Google Scholar]
- 20.Nabaie L, Kavand S, Robati RM, Sarrafi-Rad N, Kavand S, Shahgholi L, et al. Androgenic alopecia and insulin resistance: Are they really related? Clin Exp Dermatol. 2009;34:694–7. doi: 10.1111/j.1365-2230.2008.03118.x. [DOI] [PubMed] [Google Scholar]
- 21.Greger NG, Insull W, Jr, Probstfield JL, Keenan BS. High density lipoprotein response to 5-alpha-dihydrotestosterone and testosterone in Macaca fascicularis: A hormone-responsive primate model for the study of atherosclerosis. Metabolism. 1990;39:919–24. doi: 10.1016/0026-0495(90)90301-r. [DOI] [PubMed] [Google Scholar]
- 22.Sharrett AR, Sorlie PD, Chambless LE, Folsom AR, Hutchinson RG, Heiss G, et al. Relative importance of various risk factors for asymptomatic carotid atherosclerosis versus coronary heart disease incidence: The Atherosclerosis Risk in Communities Study. J Am Epidemiol. 1999;149:843–52. doi: 10.1093/oxfordjournals.aje.a009900. [DOI] [PubMed] [Google Scholar]
- 23.Guzzo CA, Margolis DJ, Johnson J. Lipid profiles, alopecia, and coronary disease: Any relationship? Dermatol Surg. 1996;22:481. doi: 10.1111/j.1524-4725.1996.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Torres A, Melón J, Muñoz FJ. Insulin stimulates collagen synthesis in vascular smooth muscle cells from elderly patients. Gerontology. 1998;44:144–8. doi: 10.1159/000021998. [DOI] [PubMed] [Google Scholar]
- 25.González-González JG, Mancillas-Adame LG, Fernández-Reyes M, Gómez-Flores M, Lavalle-González FJ, Ocampo-Candiani J, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol (Oxf) 2009;71:494–9. doi: 10.1111/j.1365-2265.2008.03508.x. [DOI] [PubMed] [Google Scholar]
- 26.Mumcuoglu C, Ekmekci TR, Ucak S. The investigation of insulin resistance and metabolic syndrome in male patients with early-onset androgenetic alopecia. Eur J Dermatol. 2011;21:79–82. doi: 10.1684/ejd.2010.1193. [DOI] [PubMed] [Google Scholar]
- 27.Hirsso P, Rajala U, Hiltunen L, Laakso M, Koskela P, Härkönen P, et al. Association of low-insulin sensitivity measured by quantitative insulin sensitivity check index with hair loss in 55-year-old men. A Finnish population-based study. Diabetes Obes Metab. 2006;8:466–86. doi: 10.1111/j.1463-1326.2005.00520.x. [DOI] [PubMed] [Google Scholar]
- 28.Abdel Fattah NS, Darwish YW. Androgenetic alopecia and insulin resistance: Are they truly associated? Int J Dermatol. 2011;50:417–22. doi: 10.1111/j.1365-4632.2010.04677.x. [DOI] [PubMed] [Google Scholar]
- 29.Horton R, Pasupuletti V, Antonipillai I. Androgen induction of steroid 5a-reductase may be mediated via insulin-like growth factor 1. Endocrinology. 1993;133:447–51. doi: 10.1210/endo.133.2.8344190. [DOI] [PubMed] [Google Scholar]
- 30.Yip J, Mattock MB, Morocutti A, Sethi M, Trevisan R, Viberti G. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;342:883–7. doi: 10.1016/0140-6736(93)91943-g. [DOI] [PubMed] [Google Scholar]
- 31.Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691–8. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 32.Su LH, Chen TH. Association of androgenetic alopecia with metabolic syndrome in men: A community-based survey. Br J Dermatol. 2010;163:371–7. doi: 10.1111/j.1365-2133.2010.09816.x. [DOI] [PubMed] [Google Scholar]
- 33.Yi SM, Son SW, Lee KG, Kim SH, Lee SK, Cho ER, et al. Gender-specific association of androgenetic alopecia with metabolic syndrome in a middle-aged Korean population. Br J Dermatol. 2012;167:306–3. doi: 10.1111/j.1365-2133.2012.10978.x. [DOI] [PubMed] [Google Scholar]


