INTRODUCTION
Wounds and skin are colonized by bacteria and currently there is a lack of evidence that the presence of colonizing bacteria impedes wound healing. In a systematic review of antimicrobials, including antiseptics on chronic wounds[1] In another systematic review that looked for effects of using tap water in comparison to distilled water or boiled water or normal saline for cleansing of wound found no difference in infection or healing rates, while using any of them.[2]
DEBRIDEMENT
Necrotic tissue left in the ulcer contributes to reduced host resistance to infections because it acts like a foreign body. In this area, there is usually a high concentration of harmful proteases and bacteria that can inhibit wound healing. Skin debridement consists of removing nonviable, nonbleeding slough.
A chronic wound has to be converted bydebridement to an acute wound and hence that itcan proceed through the normal healing phases. Removal of necrotic and devitalized tissue can be achieved through mechanical, autolytic, or enzymatic, and biological debridement.[3]
SURGICAL DEBRIDEMENT
It is carried out using a curette and scissors where chronic wound is converted into an acute wound. Neutrophils and macrophages, which are recruited to the area secrete growth factors and phagocytize the bacteria and nonviable tissue.
Mechanical debridement should be undertaken by the expert with the surgical skills (evidence level C).[4]
AUTOLYTIC DEBRIDEMENT
This is the gentle separation of slough from the wound bed that occurs slowly in a moist wound environment. It is accomplished by placing an occlusive dressing on a wound and allowing proteases within the wound space to liquefy the necrotic tissue and useful in patients with bleeding tendencies where surgical debridement is not feasible.
ENZYMATIC DEBRIDEMENT
Consists of application of topical protease preparation that targets the fibrin and collagen of necrotic tissue. These include papain urea preparations; Papain obtained from papaya contains protease and urea are a protein denaturant. Collagenase derived from Clostridium histolyticum.
BIODEBRIDEMENT
This is achieved by using maggots. They are the larvae of a species of fly (Lucilia sericata) also known as “green bottle” blow fly, which selectively debride the dead tissue and tissue debris by secreting collagenase and mixture of other enzymes.
TOPICAL ANTIMICROBIALS AND ANTISEPTICS
In chronic wounds, reduction of certain microbial species, such as anaerobic bacteria in order to limit undesirable odors or perhaps mixed communities of four or more bacterial species that impede healing, use of topical antibiotics may be justified (evidence level C).[5]
Various studies on dressings incorporating antibiotics and antiseptics are reviewed, but no single consensus for any particular topical agent could be made. This is partly due to the different mechanism and spectrum of action of the antimicrobials. The most frequently used topical antimicrobials in wound care practice are chlorhexidine, iodine, silver containing products, mupriocin and fucidic acid. In the past, acetic acid, honey, hydrogen peroxide, sodium hypochlorite, potassium permanganate, and proflavine have been used.
CHLORHEXIDINE IMPREGNATED DRESSINGS
In a recent review of human studies has demonstrated that it is associated with few adverse effects on healing.[6] Despite reports of decreased bacterial counts, increased healing rates, and lack of toxicity, it is concluded that at present, there is insufficient data to assess safety and efficacy, and that further clinical trials are required before the use of chlorhexidine on open wounds is either recommended or condemned.
Iodine
It is available as povidone-iodine and second generation dextranomer and cadexomer. In one study, healing rates of chronic venous leg ulcers, each treated with one of three topical agents (silver sulfadiazine and chlorhexidine digluconate) were compared to untreated control ulcers in each respective patient. All agents were seen to reduce bacterial load resulting in slight improvements in healing rates and times, but povidone-iodine yielded statistically significant better outcome. Furthermore, histological assessment indicated a lack of cytotoxicity because povidone-iodine induced less change in microvessels and dendrocytes.[7] In addition, a report of the ability of iodine released from a dressing to modulate the secretion of cytokines by human macrophages in vitro has provided another justification of its role in promoting healing.[8]
It reduces bacterial load, decreases infection rates and promotes healing (evidence level C).[7,8]
Cadexomer iodine
It leads to reduction of methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa with evidence from clinical reports of efficacy in stimulating healing (evidence level C).[9] Its lack of toxicity for human fibroblasts in vitro suggests low potential of toxicity for chronic wounds in vivo (evidence level D).[10]
Silver sulfadiazine
At present, human studies with silver containing dressings are rather limited, yet many trials provide encouraging results.[11] In an uncontrolled, prospective study of a series of chronic wounds treated with an ionized nanocrystalline silver dressing demonstrated improved clinical parameters together with decreased surface wound bioburden, but unchanged deep tissue loads. The implication was that surface flora contributed more significantly to delayed healing than deeper flora (evidence level D).[12]
MUPIROCIN
A systematic review identified one small randomized controlled trials (RCT) (n = 30) of patients with leg ulcer, which compared topical mupirocin with placebo, in addition to standard compression for all. There was no significant difference between groups in rates of complete healing, or eradication of Gram-positive bacteria.[13] There is insufficient evidence on which to base a recommendation for mupirocin.
RETAPAMULIN
Retapamulin, in the class of pleuromutilin antibiotics acts by inhibiting bacterial protein synthesis by interacting at a site on the 50S ribosome subunit of bacterial ribosome. In 664 isolates of S. aureus, including many with high levels of resistance to mupirocin and fusidic acid and 448 (73%) MRSA isolates, retapamulin demonstrated excellent in vitro activity.[14] More clinical studies of retapamulin in the treatment of resistant S. aureus are needed.
Dressings
A moist environment to accelerate wound healing is very important to accelerate wound healing. This can be accomplished by frequent application of saline dressings over the wound, which helps to keep the wound surface moist, debrides the wound and removes the surface bacteria.
Recent research on wound care has resulted in increased use of interactive and active dressings rather than passive dressings that cover and absorb. Active dressings stimulate growth factors, which are important in the healing cascade. Moist occlusive dressings control the exudates, while epithelial cell migration is encouraged. Eschar is liquefied, and fibrin is managed through wound fluid rich in leukocytes. These dressings are also believed to provide symptomatic relief such as decreased pain and pruritus. Chronic ulcer management requires the use of the wound dressings that provide the optimal “moist” environment. Dressing should be simple, low or nonadherent, low cost and acceptable to the patient[15] (evidence level A).
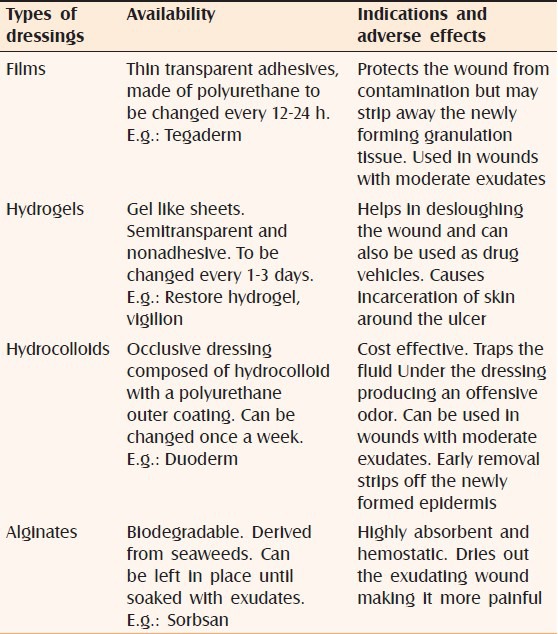
The various dressings its advantages and disadvantages[16] are elaborated in Table 1. In the two systematic reviews, many RCT's are identified comparing various dressings and topical agents in patients of venous ulcers, but no single consensus can be drawn in favor of any particular dressing material[17] (evidence level C).
Table 1.
Types of dressings
Periwound protection
The skin surrounding the ulcer can be damaged due to excess moisture, wound fluid proteases and adhesives present in the dressings.[18] Barrier creams and ointments are available, which protects the skin around the ulcer.
Vaseline petroleum jelly: Protects the periwound skin, but interferes with dressing adherence
Unna's paste: It is a preparation containing zinc oxide 5 parts, gelatin 5 parts, boric acid 1part, glycerin 8 parts and water 6 parts with ichthyol 1 or 2 parts. It protects the skin around the wound, has an anti-inflammatory effect and promotes healing. It can also interfere with dressing adherence.[19]
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.O’Meara S, Cullum N, Majid M, Sheldon T. Systematic reviews of wound care management: (3) Antimicrobial agents for chronic wounds; (4) diabetic foot ulceration. Health Technol Assess. 2000;4:1–237. [PubMed] [Google Scholar]
- 2.Fernandez R, Griffiths R, Ussia C. The Cochrane Library. No. 4. Chichester: John Wiley and Sons Ltd; 2003. Water for wound cleansing. [Google Scholar]
- 3.Attinger CE, Janis JE, Steinberg J, Schwartz J, Al-Attar A, Couch K. Clinical approach to wounds: Débridement and wound bed preparation including the use of dressings and wound-healing adjuvants. Plast Reconstr Surg. 2006;117:72S–109. doi: 10.1097/01.prs.0000225470.42514.8f. [DOI] [PubMed] [Google Scholar]
- 4.Kunimoto BT. Management and prevention of venous leg ulcers: A literature-guided approach. (44-9).Ostomy Wound Manage. 2001;47:36–42. [PubMed] [Google Scholar]
- 5.Bowler PG, Davies BJ. The microbiology of acute and chronic wounds. Wounds. 1999;11:72–8. [Google Scholar]
- 6.Drosou A, Falabella A, Kirsner RS. Antiseptics on wounds: an area of controversy. Wounds. 2003;15:149–66. [Google Scholar]
- 7.Fumal I, Braham C, Paquet P, Piérard-Franchimont C, Piérard GE. The beneficial toxicity paradox of antimicrobials in leg ulcer healing impaired by a polymicrobial flora: A proof-of-concept study. Dermatology. 2002;204(Suppl 1):70–4. doi: 10.1159/000057729. [DOI] [PubMed] [Google Scholar]
- 8.Moore K, Thomas A, Harding KG. Iodine released from the wound dressing Iodosorb modulates the secretion of cytokines by human macrophages responding to bacterial lipopolysaccharide. Int J Biochem Cell Biol. 1997;29:163–71. doi: 10.1016/s1357-2725(96)00128-8. [DOI] [PubMed] [Google Scholar]
- 9.Mertz PM, Oliveira-Gandia MF, Davis SC. The evaluation of a cadexomer iodine wound dressing on methicillin resistant Staphylococcus aureus (mrsa) in acute wounds. Dermatol surg. 1999;25:89–93. doi: 10.1046/j.1524-4725.1999.08055.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou LH, Nahm WK, Badiavas E, Yufit T, Falanga V. Slow release iodine preparation and wound healing: In vitro effects consistent with lack of in vivo toxicity in human chronic wounds. Br J Dermatol. 2002;146:365–74. doi: 10.1046/j.1365-2133.2002.04605.x. [DOI] [PubMed] [Google Scholar]
- 11.Tebbe B, Orfanos CE. Therapy of leg ulcers and decubitus ulcers with a xero-dressing: modern wound dressings with antibacterial activity. H+G Brand (Special Edition) 1996;71:11–3. [Google Scholar]
- 12.Sibbald RG, Browne AC, Coutts P, Queen D. Screening evaluation of an ionized nanocrystalline silver dressing in chronic wound care. Ostomy Wound Manage. 2001;47:38–43. [PubMed] [Google Scholar]
- 13.O’Meara S, Al-Kurdi D, Ovington LG. The Cochrane Library. Chichester: John Wiley and Sons Ltd; 2010. Antibiotics and antiseptics for venous leg ulcers. [DOI] [PubMed] [Google Scholar]
- 14.Woodford N, Afzal-Shah M, Warner M, Livermore DM. In vitro activity of retapamulin against Staphylococcus aureus isolates resistant to fusidic acid and mupirocin. J Antimicrob Chemother. 2008;62:766–8. doi: 10.1093/jac/dkn266. [DOI] [PubMed] [Google Scholar]
- 15.Bradley M, Cullum N, Sheldon T. The debridement of chronic wounds: A systematic review. (1-78).Health Technol Assess. 1999;3:iii–iv. [PubMed] [Google Scholar]
- 16.Paddle-Ledinek JE, Nasa Z, Cleland HJ. Effect of different wound dressings on cell viability and proliferation. Plast Reconstr Surg. 2006;117:110S–8. doi: 10.1097/01.prs.0000225439.39352.ce. [DOI] [PubMed] [Google Scholar]
- 17.Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008;58:185–206. doi: 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 18.Bishop SM, Walker M, Rogers AA, Chen WY. Importance of moisture balance at the wound-dressing interface. J Wound Care. 2003;12:125–8. doi: 10.12968/jowc.2003.12.4.26484. [DOI] [PubMed] [Google Scholar]
- 19.Agren MS. Studies on zinc in wound healing. Acta Derm Venereol Suppl (Stockh) 1990;154:1–36. [PubMed] [Google Scholar]


