Abstract
This study compared pitch ranking, electrode discrimination, and electrically evoked compound action potential (ECAP) spatial excitation patterns for adjacent physical electrodes (PEs) and the corresponding dual electrodes (DEs) for newer-generation Cochlear devices (Cochlear Ltd., Macquarie, New South Wales, Australia). The first goal was to determine whether pitch ranking and electrode discrimination yield similar outcomes for PEs and DEs. The second goal was to determine if the amount of spatial separation among ECAP excitation patterns (separation index, Σ) between adjacent PEs and the PE-DE pairs can predict performance on the psychophysical tasks. Using non-adaptive procedures, 13 subjects completed pitch ranking and electrode discrimination for adjacent PEs and the corresponding PE-DE pairs (DE versus each flanking PE) from the basal, middle, and apical electrode regions. Analysis of d′ scores indicated that pitch-ranking and electrode-discrimination scores were not significantly different, but rather produced similar levels of performance. As expected, accuracy was significantly better for the PE-PE comparison than either PE-DE comparison. Correlations of the psychophysical versus ECAP Σ measures were positive; however, not all test/region correlations were significant across the array. Thus, the ECAP separation index is not sensitive enough to predict performance on behavioral tasks of pitch ranking or electrode discrimination for adjacent PEs or corresponding DEs.
I. INTRODUCTION
Dual-electrode (DE) technology, a capability of the newer-generation Cochlear Corporation devices (24RE/CI422 and CI512; Cochlear Ltd., Macquarie, New South Wales, Australia), is intended to elicit additional pitch percepts along the cochlear implant (CI) array. DE stimulation is accomplished by electrically coupling (shorting together) two adjacent electrodes to create one “electrode.” The assumption is that the electrical field, produced by the DE, will be positioned somewhere between the two physical electrodes (PEs), theoretically eliciting a pitch intermediate to the two PEs. Previous research has shown that performance on psychophysical tasks for DEs can be variable within and across subjects (Busby and Plant, 2005; Busby et al., 2008). Additionally, not all CI recipients can perform tasks of pitch perception with good accuracy; therefore, finding an objective correlate to predict pitch percepts would be useful for the clinical management of pediatric recipients and others who have difficulties with behavioral tasks. In the current study, psychophysical measures of pitch ranking and electrode discrimination were investigated for PEs and the corresponding DE for basal, middle, and apical regions of the electrode array. These results were then compared to electrophysiological measures of spatial separation to determine whether an objective physiological method can be used to predict behavioral measures.
Since the early days of multichannel CIs, it has been of interest to determine whether CI recipients can distinguish different electrodes on the basis of pitch (Townshend et al., 1987). The primary psychophysical measures used to assess pitch perception in CI recipients have been pitch ranking and electrode discrimination (Collins et al., 1997; Zwolan et al., 1997; Collins and Throckmorton, 2000; Dawson et al., 2000; Busby and Plant, 2005; Donaldson et al., 2005; Hughes and Abbas, 2006a; Firszt et al., 2007; Busby et al., 2008). Clinically, these tasks may be used to determine whether particular electrodes should be deactivated from the speech-processor program to improve pitch resolution (especially if two or more electrodes have the same pitch percept, or if there is a pitch reversal). With pitch ranking, recipients are asked to rank the electrodes along a tonotopic pitch scale (i.e., “higher” versus “lower”), while electrode discrimination can reveal whether any perceptual differences can be perceived between electrodes (i.e., “same” or “different”). Formal measures of pitch ranking are usually performed using a two-interval forced-choice (2IFC) procedure in which the subject compares the two stimuli to judge relative pitch. Feedback is typically not provided with pitch ranking due to the subjective nature of the task (i.e., there is no “right” answer because pitch is based on the recipient's own perception). Electrode discrimination is often evaluated using a three-interval forced-choice (3IFC) procedure in which the subject performs an oddity task to pick the interval with the different stimulus. With electrode discrimination of pitch, correct-answer feedback assists the subject in learning which pitch-related percepts are associated with different electrodes. Both 2IFC pitch ranking and 3IFC electrode discrimination were employed in the current study and results were compared with previous studies that used similar methods.
Early work investigating pitch ranking and electrode discrimination in CIs was performed by Collins and colleagues (Collins et al., 1994, 1997; Zwolan et al., 1997; Collins and Throckmorton, 2000). Subjects in these studies had the first-generation Cochlear Corporation Nucleus-22 device (Cochlear Ltd., Macquarie, New South Wales, Australia). The Nucleus-22 utilized bipolar stimulation rather than monopolar, which is now more widely used in newer-generation devices. The studies found that data for many of the subjects from the pitch-ranking task were not consistent with the tonotopic pitch structure of the cochlea (for PEs). For example, as the electrodes progressed in a basal to apical fashion along the cochlea, pitch percepts did not decrease in an orderly fashion. The authors did not state whether any electrode region was particularly vulnerable to the tonotopic mismatch for pitch ranking. For electrode discrimination, there was large variability among subjects, ranging from “perfect” discrimination of all intracochlear electrodes to poor performance (Collins et al., 1994, 1997; Zwolan et al., 1997). Discrimination of basal electrodes was poorer than that for apical electrodes, although the difference was not statistically significant. For 10 of the 11 subjects, electrode discrimination yielded more discriminable electrodes than pitch ranking, suggesting subjects may have used dimensions other than pitch to judge which stimulus was different for the discrimination task (Collins et al., 1997). Note, however, that these experiments used loudness-balancing to try to equate loudness across conditions (Collins et al., 1997; Zwolan et al., 1997; Collins and Throckmorton, 2000); with loudness balancing, it is difficult to truly balance every stimulus combination. Therefore, remaining loudness cues (regardless of pitch) could have been one factor influencing task judgments in those studies (Shannon, 1983). From these results, it was hypothesized that the perception of pitch associated with place of stimulation along the cochlea was potentially multidimensional for many CI recipients.
Given the challenges of obtaining reliable pitch-perception data, Hughes and Abbas (2006a) examined the relation between pitch ranking and the electrically evoked compound action potential (ECAP) spread-of-excitation (SOE) patterns for PEs. The ECAP SOE function reflects the neural excitation pattern elicited by a probe electrode resolved using a forward-masking paradigm. It has been shown that neural excitation measured by the ECAP SOE is comparable to that measured psychophysically using a forward-masking paradigm (Cohen et al., 2003; Hughes and Stille, 2008). In Hughes and Abbas (2006a), the width of the SOE function (calculated as 75% of the normalized amplitude) was compared to the slope of the electrode pitch-ranking (2IFC) function in ten subjects. Three subjects showed a moderate positive correlation between the ECAP and pitch-ranking data, three showed a moderate negative correlation, and four demonstrated no clear trend. Thus, it was suggested that the width of the SOE function was not a satisfactory measure to objectively characterize the neural mechanisms that contribute to pitch ranking, likely because it does not take into account the location of the pattern edges or the overall spread of the SOE function.
Reanalyzing the data from Hughes and Abbas (2006a), Hughes (2008) used a different approach to assess the relation between electrode pitch ranking and ECAP SOE. The amount of spatial separation between pairs of SOE functions (rather than the width of individual functions) was compared to pitch ranking for the same PE pairs [see Table I in Hughes and Abbas (2006a) for the PEs tested for each subject]. The amount of spatial separation, termed the separation index (Σ), was quantified as the sum of the absolute values of the difference in normalized ECAP amplitudes across all masker electrodes for the two SOE patterns being compared. As electrode separation was increased (for PEs spaced 1, 2, 4, 5, or 6 electrodes apart), there was greater separation of SOE patterns (larger Σ), which was significantly correlated with greater accuracy in pitch ranking. The current study extends the approach of Hughes (2008) by comparing Σ values to pitch ranking and electrode discrimination of DEs and the flanking PEs. The goal was to determine if the method used for comparison across PEs (Hughes, 2008) is sensitive enough to be applied to DEs.
There is now considerable evidence that simultaneous stimulation of two PEs can elicit intermediate pitch percepts for many CI recipients (e.g., Townshend et al., 1987; Busby and Plant, 2005; Donaldson et al., 2005; Wilson et al., 2005). With the Cochlear Corporation Nucleus 24RE device (Cochlear Ltd., Macquarie, New South Wales, Australia), Busby and Plant (2005) examined pitch ranking for adjacent PE pairs and the corresponding DE in eight recipients. Twenty-one electrode pairs, spanning the electrode array, were tested using monopolar mode and a stimulus-level rove (2IFC). Results indicated tonotopic pitch ranking of the DE between that of the adjacent PEs was significantly above chance performance in 38/50 (76%) of electrode pairs tested. All subjects were able to perceive pitch differences for a PE versus DE pair for at least part of the array. Three of the eight subjects ranked all electrode pairs as perceptually distinct from each other and in the expected tonotopic order. For the remaining subjects, three accurately ranked 6–15 electrode pairs, while two subjects had inconsistent patterns suggestive of pitch reversals.
In a follow-up study of nine subjects with the Nucleus 24RE device (two from Busby and Plant, 2005), Busby et al. (2008) compared DE pitch ranking to the ECAP SOE function. Subjects were able to pitch rank 60/81 (74.1%) of the adjacent PEs and the corresponding DE in the expected tonotopic order. As in Busby and Plant (2005), results showed variable performance with some subjects able to accurately pitch rank the adjacent PEs and corresponding DE in the expected order, while others showed evidence of pitch reversals. Of particular interest to the current study, the PE and DE ECAP SOE functions were comparable in shape. For example, the widths of the SOE functions were similar, and there was an expected tonotopic arrangement of the PEs and DEs at the peak and sides of the SOE functions. However, the study found no correlation between the ordering of electrodes at the peak or sides of the SOE function with pitch-ranking results, indicating the width of the SOE function was not a predictor of behavioral perception of DEs. Since the Busby et al. (2008) study, other studies have confirmed that SOE patterns for DEs fall approximately half-way between the excitation patterns for adjacent PEs, indicating distinct neural activation patterns for DEs (Saoji et al., 2009; Hughes and Goulson, 2011; Snel-Bongers et al., 2012; Hughes et al., 2013). Hughes et al. (2013) examined the ECAP separation index (Σ) for DEs and flanking PEs. Results showed greater spatial separation for adjacent PEs versus DE-PE comparisons. Thus, electrophysiological measures can capture aspects of neural separation for DE versus PE stimulation. This study extends Hughes et al. (2013) by comparing the electrophysiological data to accuracy of pitch ranking and electrode discrimination for DEs versus adjacent PEs.
The first goal of the present study was to determine whether the two primary methods used to assess pitch perception (pitch ranking and electrode discrimination) yield similar outcomes for PEs and corresponding DEs. Previous studies with older technology have suggested the two tasks do not produce the same levels of performance (Collins et al., 1994, 1997; Zwolan et al., 1997; Collins and Throckmorton, 2000). Further, only pitch ranking has been explored for DEs versus PEs using newer-generation Cochlear Corporation devices (Busby and Plant, 2005; Busby et al., 2008); to our knowledge, this study is the first to evaluate electrode discrimination for DEs versus PEs. The second goal was to compare the outcomes of pitch ranking and electrode discrimination to ECAP Σ values to determine if ECAP measures can predict performance on the behavioral tasks, and whether potential predictions differ for pitch ranking versus electrode discrimination. It was hypothesized that electrode discrimination would yield better levels of performance than pitch ranking because subjects can learn from perceptual cues (pitch or otherwise) associated with receiving correct-answer feedback, which is not provided in a pitch-ranking task. Second, we hypothesize that accuracy in both the pitch-ranking and electrode-discrimination tasks will be positively correlated with the degree of spatial separation between ECAP SOE functions (i.e., larger Σ values). Having an objective correlate to predict behavioral pitch measures would be time efficient and clinically useful for pediatric CI recipients or others who have difficulty with pitch-related tasks.
II. METHODS
A. Subjects
Data were obtained for 13 ears of 12 subjects (11 adults, 1 teen). For the teenage subject, data were collected for both ears, designated as F10 (right) and F11 (left). Nine subjects were postlingually deafened; three were prelingually deafened (F4, F10/F11, F15). Eight ears were implanted with the Cochlear Corporation Nucleus Freedom 24RE(CA), and five with the CI512 (Cochlear Ltd., Macquarie, New South Wales, Australia). The 24RE(CA) and CI512 devices contain the same receiver/stimulator electronics and Contour Advance electrode array. Demographic details are listed in Table I. Subjects N1, N6, and F10/F11 (both ears) underwent explantation/re-implantation of a previous device before participation in this study; previous devices were Freedom 24RE (N1, N6), Advanced Bionics (AB) HiRes 90K (F10) (Valencia, CA), and AB Clarion 1.2 (F11). In Table I, age at CI and years post-CI were calculated using the original device, indicating total time with electrical stimulation. Time post new CI for N1 was 1 year, 4 months; N6 was 4 months; F10 was 2 years, 2 months; and F11 was 1 year, 7 months. With one exception, subjects had no experience with virtual-channel stimulation before the study. The right ear (F10) for subject F10/F11 utilized the Fidelity 120 speech processing strategy (which employs current steering) with the HiRes 90K device for an eight-month time period approximately two years before re-implantation and subsequent participation in this study. Each ear had a full insertion of the electrode array. This study was approved by the Boys Town National Research Hospital Institutional Review Board under Protocol 03-07-XP.
TABLE I.
Demographic information for participating subjects. Percent-correct scores for consonant-nucleus-consonant (CNC) words (Peterson and Lehiste, 1962) and AzBio sentences in quiet (Spahr et al., 2012) were collected as part of a previous research study from the laboratory (using the subject's everyday processor and program) or the subject's clinical file. All tests were performed with the CI alone in the auditory-only condition using recorded stimuli at 60 dB sound pressure level. The pre-CI pure-tone average for subject N6 was not available because that subject was not implanted at our institution. Asterisks indicate the two ears for the subject with bilateral CIs. R = right; L = left. SNHL = sensorineural hearing loss. PTA = pure-tone average for 250, 500, and 1000 Hz.
| Subject | Internal device | Ear | Age at CI (yr, mos) | Time post-CI (yr, mos) | Duration of deafness (yr, mos) | Pre-CI PTA (dB HL) | Etiology and onset | CNC words | AzBio sentences |
|---|---|---|---|---|---|---|---|---|---|
| F1 | 24RE(CA) | L | 60, 7 | 4, 7 | 54, 0 | 82 | Unknown, progressive | 46% | 44% |
| F2 | 24RE(CA) | R | 60, 2 | 3, 11 | 10, 0 | 78 | Unknown, progressive | 58% | 88% |
| F4 | 24RE(CA) | L | 17, 6 | 3, 7 | 17, 0 | 92 | Ototoxicity | 36% | 41% |
| F5 | 24RE(CA) | R | 48, 3 | 2, 8 | 7, 0 | 103 | Unknown, progressive | 76% | 89% |
| F7 | 24RE(CA) | R | 39, 1 | 4, 2 | 28, 0 | 102 | Unknown, progressive | 64% | 79% |
| F10* | 24RE(CA) | R | 8, 3 | 5, 0 | 8, 3 | 95 | Waardenburg, congenital | 60% | 59% |
| F11* | 24RE(CA) | L | 1, 10 | 11, 5 | 1, 10 | 115 | Waardenburg, congenital | 54% | 67% |
| F15 | 24RE(CA) | L | 22, 10 | 2, 3 | 22, 9 | 60 | Unknown, congenital | 18% | 28% |
| N1 | CI512 | L | 58, 3 | 2, 2 | 8, 0 | 97 | Unknown, progressive | 90% | 96% |
| N5 | CI512 | R | 50, 9 | 0, 4 | 1, 0 | 55 | Unknown, sudden SNHL | 66% | 93% |
| N6 | CI512 | R | 82, 0 | 1, 11 | 4, 0 | unknown | Unknown, progressive | 40% | 64% |
| N7 | CI512 | R | 69, 9 | 0, 11 | 10+ | 103 | Unknown, progressive | 42% | 52% |
| N11 | CI512 | L | 67, 5 | 0, 3 | 6, 0 | 72 | Unknown, progressive | 61% | 81% |
| Average: | 45, 5 | 3, 5 | 13, 2 | 88 |
B. Equipment setup
The following three measures were made for each subject: (i) pitch ranking, (ii) electrode discrimination, and (iii) ECAP SOE. All measures were made using a direct-connect setup. Psychophysical measures were obtained using a laboratory Freedom speech processor and programming Pod. A custom program written in Visual Basic that implemented Nucleus Implant Communicator subroutines (NIC v. 2, Cochlear Ltd., Macquarie, New South Wales, Australia) was used for the psychophysical testing. For ECAPs, the same Freedom processor and programming Pod were used with Advanced Neural Response Telemetry and the non-FDA-approved “Dual Electrode” feature enabled within Custom Sound EP (v. 3.1 and 3.2). Impedances for all electrodes were measured for each ear prior to data collection to ensure electrodes with open or short circuits were excluded from testing. Subject F2 had an open circuit on electrode 8; therefore, the ECAP amplitude was interpolated for masker electrode 8 using the amplitude values of the adjacent electrodes in the SOE function.
C. Psychophysical stimuli and procedures
Psychophysical tasks were performed for two adjacent PEs and the corresponding DE from basal, middle, and apical regions of the array. Generally, electrodes 5 and 6 (basal), 11 and 12 (middle), and 17 and 18 (apical) were used as the PE comparison pairs. These were the same electrode sets used for ECAP testing, which was conducted first (Hughes et al., 2013). Deviations from this set were made for subjects F5 and F7 during the ECAP optimization stage to reduce stimulus artifact (for both F5 and F7, alternative pairs used were apical electrodes 18 and 19; for F5 only, middle electrodes 10 and 11 and basal electrodes 4 and 5 were used). Each PE pair was electrically coupled to create the respective DE (e.g., 5 + 6, 11 + 12, 17 + 18), resulting in three comparison pairs for each electrode region (total of nine pairs across the array). For example, in the middle region, comparison pairs were 11 versus 11 + 12 (designated as “PEB-DE” for the basal-side PE versus the DE), 11 + 12 versus 12 (“DE-PEA” for the DE versus apical-side PE) and 11 versus 12 (“PE-PE” for the comparison between adjacent PEs). Stimuli for the psychophysical tasks were 300-ms, 1000-pps, cathodic leading, 25-μsec/phase (with 8 μsec interphase gap) trains of biphasic pulses presented in monopolar mode (re: MP1 + 2). Stimulus configurations for each psychophysical task are described below. Subjects sat at a table in a sound-treated booth and indicated their responses verbally (for loudness estimates) or used a touch-screen monitor connected to the investigator's computer (for loudness balancing, pitch ranking, and electrode discrimination).
1. Loudness judgments
To establish the boundaries of each subject's behavioral dynamic range, loudness judgments were obtained for the basal-side electrode of the PE pair from each region. The 300-ms pulse trains were presented in an ascending manner while subjects provided categorical loudness estimates using a 10-point rating scale (1 = “just noticeable” to 10 = “too loud”). Current levels associated with the following loudness estimates were recorded: “just noticeable” (rating = 1), “loud but comfortable” (rating = 7), and “maximum loudness level” (rating = 9).
2. Loudness balancing
After the behavioral dynamic range was established, loudness balancing was performed for each comparison pair using the PEB in the pair as the reference electrode. For each of the three electrode sets (across regions), two comparisons were made: the PEB reference electrode versus the PEA (e.g., 11 versus 12) and the corresponding DE paired with the PEB reference electrode (11 versus 11 + 12), yielding a total of six loudness-balancing trials (two conditions for three cochlear regions). On each trial, subjects were presented with two sounds (each corresponding to a box on a visual display) and were instructed to choose the box with the louder sound. The reference stimulus on PEB was set to the current level yielding a rating of “7” from the initial loudness judgments. Balancing was achieved with an adaptive, two-alternative forced-choice (2AFC), double-staircase procedure (Jesteadt, 1980). This procedure is a subjective version of Levitt's (1971) adaptive procedure in which two independent, interleaved tracks (one starting at lower levels, one at higher levels) are interleaved at random. For each track, level is adjusted after two consecutive “correct” responses and one “incorrect” response. Each track terminated after ten reversals, with the levels corresponding to the final six reversals averaged to yield the loudness-balanced level for that track. Results from the two tracks were then averaged to yield the loudness-balanced level to be used in the pitch tasks for that electrode pair.
3. Pitch ranking
A 2IFC, non-adaptive procedure similar to Busby and Plant (2005) was used to determine whether CI users could pitch rank adjacent and intermediate electrodes. Subjects were instructed to choose which of two listening intervals (indicated by boxes on the response monitor) contained the sound that was perceived as higher in pitch. Correct-answer feedback was not provided. Because pitch ranking is a subjective task (i.e., there is no “correct” answer), it was necessary to establish a common metric for comparing performance across tasks. A “correct” response was therefore defined as a response to the most basal electrode on each trial, corresponding to the expected direction of the pitch percept based on cochlear physiology. The beginning stimulus level was determined from the loudness-balancing task, with an added random-level rove of 5% of the current level (CL) above and below the subject's “7” rating. For example, if the subject's “7” rating was 175 CL units, then loudness was roved 8.75 CL above and below 175 CL. Therefore, the change in loudness due to level rove was likely perceptible for the participants. Subjects were instructed to ignore loudness differences and focus on pitch. Stimuli were presented in blocks of 54 trials, comprised of 18 judgments per pair for each of the three contrasted electrode pairs (PEB-DE, DE-PEA, PE-PE) per region. Within a block, electrode pairs were randomized for order of presentation, and each electrode in a pair served equally often as the reference stimulus. The percentage of trials in which the basal-most electrode was chosen as higher in pitch was calculated for each comparison pair as percent correct. Four to six blocks were completed for each electrode set.
4. Electrode discrimination
The same stimuli and electrode pairs were used in a 3IFC, non-adaptive electrode-discrimination procedure. Selected randomly, two of the three listening intervals contained stimulation on the reference electrode in the set, and one contained stimulation on the test (most basal) electrode. The same 5% level rove was applied as in pitch ranking to further reduce loudness cues. Subjects were asked to indicate which box contained the sound that was different in pitch, while ignoring loudness changes. Correct-answer feedback was provided, defined as the interval with the basal-most electrode. Blocks of 54 trials were presented (as for pitch ranking), and percent correct was calculated for each comparison pair. Four to six blocks were completed for each electrode set.
Pitch-ranking and electrode-discrimination blocks were tested in random order within and across subjects. For both, a short training task was completed with the PE pair to ensure the subject understood the tasks without level rove, and to make certain the subject understood the concept of pitch. Chance performance for pitch ranking (2IFC) was 50%; chance for electrode discrimination (3IFC) was 33%. For training scores at or below chance, further instruction and training were completed, including the use of electrode pairs with greater spatial separation to emphasize pitch differences. One subject (not included in Table I) could not pass the training phase for any PE set and, therefore, did not participate in the remainder of the study. For this subject, even with extensive training, scores without level rove were at or near chance performance for each PE-PE set. Another subject (F7) scored significantly below chance on pitch ranking only for the middle electrode region during training. Based on subject report and the fact that reversing the instructions (“pick which interval is lower in pitch”) flipped the percentage to well above chance, we interpreted this as a pitch reversal. Instructions remained consistent for all subjects for the main pitch-ranking task; thus, scores significantly below chance on pitch-ranking are interpreted as pitch reversals. In contrast, electrode discrimination scores near chance reflect an inability to discriminate between the electrodes in the pair; however, scores well below chance would suggest a misunderstanding of how to perform the task.
D. ECAP stimuli and procedures
ECAP SOE functions were obtained for the same PEs and DEs (probe electrodes) used for the psychophysical tasks. ECAP SOE data for the subjects in this study were included in a larger data set published previously (see Hughes et al., 2013, for a comprehensive description of the methods and analyses beyond those reported here). Briefly, a standard forward-masking subtraction paradigm was used with a fixed probe electrode and varied masker (Abbas et al., 2004; Hughes and Abbas, 2006a,b). Behavioral loudness estimates for the ECAP stimulus were determined using the same 10-point loudness rating scale described above. Masker and probe stimuli were generally presented at a loudness rating of “8” (loud), except where voltage compliance limits or facial nerve stimulation occurred before that rating could be reached.
Generally, the Custom Sound (Cochlear Ltd., Macquarie, New South Wales, Australia) EP default stimulus parameters were used: 80-Hz probe rate; 7-μsec interphase gap; 400-μs masker-probe interval (MPI); stimulating reference electrode, MP1 (extracochlear monopolar ball electrode); recording reference electrode, MP2 (extracochlear monopolar case electrode); 50–100 sweeps; 50-dB gain; 122-μsec recording delay; 25-μsec/phase pulse width (or 50-μsec for F1, F5, F7, and N7 due to voltage compliance limits). Changes in delay or number of sweeps were used to maximize the ECAP response on an individual basis.
N1 and P2 peaks were marked by the automatic algorithm in Custom Sound EP, and were manually adjusted if necessary. ECAP amplitudes were calculated as the voltage difference between N1 and P2. Amplitudes for each subject were then normalized to the single highest ECAP amplitude across all three probe functions within a set (i.e., separately for each region). This ensured that spatial relationships among the probe SOEs would not change, but would still allow for comparisons across electrode sets and subjects (Hughes, 2008; Hughes and Goulson, 2011; Hughes et al., 2013). The amount of spatial separation, Σ, between each of the three functions per region (two PEs and the resulting DE) was then quantified using the formula from Hughes (2008),
where ax and ay correspond to the ECAP amplitudes of the two ECAP SOE functions compared (probe electrode, x, compared with probe electrode, y) for each masker electrode, i (summed from 1 to 22).1 A larger Σ value represents greater spatial separation between SOE functions. We hypothesized that ECAP spatial separation would be positively correlated with accuracy on the psychophysical tasks, such that larger ECAP Σ values would be associated with better pitch-ranking and electrode-discrimination scores.
III. RESULTS
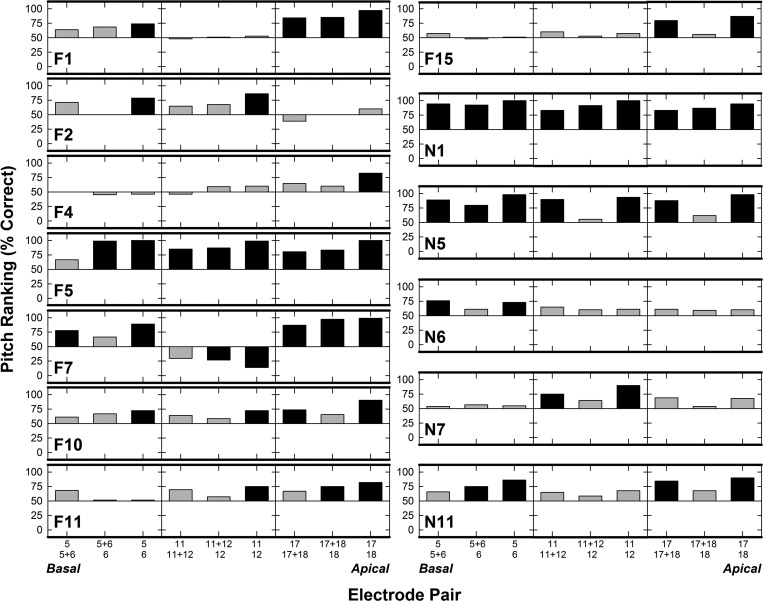
The first objective of this study was to determine whether pitch ranking and electrode discrimination yield similar perceptual outcomes for adjacent PEs and the corresponding DE. As discussed above, performance on pitch-related tasks in CIs is subjective in nature, so the meaning of percent correct for each task corresponds to responses to the most basal electrode stimulated in both pitch ranking and electrode discrimination. Individual pitch-ranking scores for the 13 ears are displayed in Fig. 1. Scores for the three comparison pairs per electrode region are plotted for each ear, with chance performance (horizontal line at 50%) serving as an anchor to show the direction of pitch. Three horizontal panels are shown for each ear: basal, middle, and apical sets are shown from left to right. Scores > 50% indicate the subject could rank the electrode pairs in the expected tonotopic order, while scores < 50% indicate the electrodes were not ranked tonotopically. To determine pitch-ranking scores significantly above or below chance performance, a two-tailed binominal probability t-test was performed (p < 0.05).2 Black bars in Fig. 1 indicate pitch-ranking scores significantly above (>72%) or below (<28%) chance performance. Results indicate that 53/117 (45%) electrode pairs were pitch ranked significantly above chance, while 2/117 (1.7%) were significantly below chance. Only one participant (N1) pitch ranked all nine comparison pairs significantly above chance, although F5 had all but one and N5 all but two. F7 was the only subject with significant pitch reversals (for two electrode comparisons: 11 versus 12 and 11 + 12 versus 12). In several individual cases, for at least part of the electrode array, only the PE-PE pairs were pitch ranked above chance performance, but not the DE pairs (e.g., F2 and F10). Further, there were instances in which subjects could not pitch rank the PEs above chance for at least part of the array (e.g., F4 and F15).
FIG. 1.
Individual pitch-ranking percent-correct for each subject. Subject number is indicated in each row. Electrode comparison pairs are delineated along the x-axis from base to apex (left to right across each grouping). The electrode with the expected higher pitch is indicated by the top number along the x-axis. Bars above 50% (chance) indicate pitch ranking was in the expected tonotopic order; bars below 50% indicate a pitch reversal. Black bars indicate pitch-ranking scores significantly above (>72%) or below (<28%) chance performance (p < 0.05).
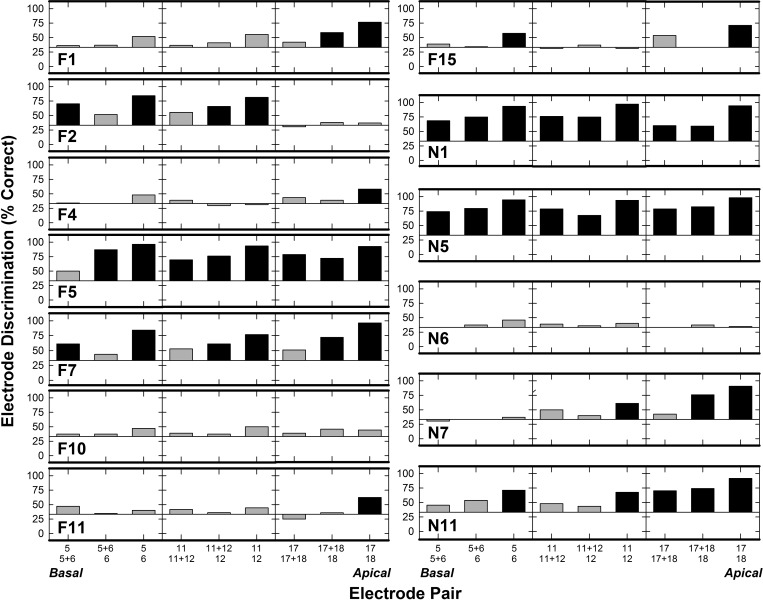
Figure 2 shows electrode-discrimination performance for each of the 13 ears; data are plotted similar to Fig. 1. Note that chance performance for electrode discrimination changes to 33% (3IFC procedure). A two-tailed binominal probability t-test indicated that 50/117 (43%) electrode pairs were discriminated significantly above chance (>56%); none of the discrimination scores were significantly below chance (<11%). Two subjects (N1 and N5) discriminated all comparison pairs significantly above chance; and F5 discriminated all but one pair above chance. Subject F7, who had significant pitch reversals for 11 versus 12 and 11 + 12 versus 12 in the pitch-ranking task, could discriminate these electrode pairs above chance performance in the electrode-discrimination task. Similar to pitch ranking, PE-PE pairs were more frequently discriminable than DE pairs (e.g., F4, F11, F15), and several subjects could not discriminate the PE pairs as “different” (e.g., F10 and N6).
FIG. 2.
Individual electrode discrimination results for the 13 ears defined by percent correct. Subject number is provided in each row. Electrode pairs are defined along the x-axis from base to apex (left to right). Bars above and below 33% indicate the electrodes were discriminated above or below chance, respectively. Black bars indicate discrimination scores significantly above (>56%) or below (<11%) chance performance (p < 0.05).
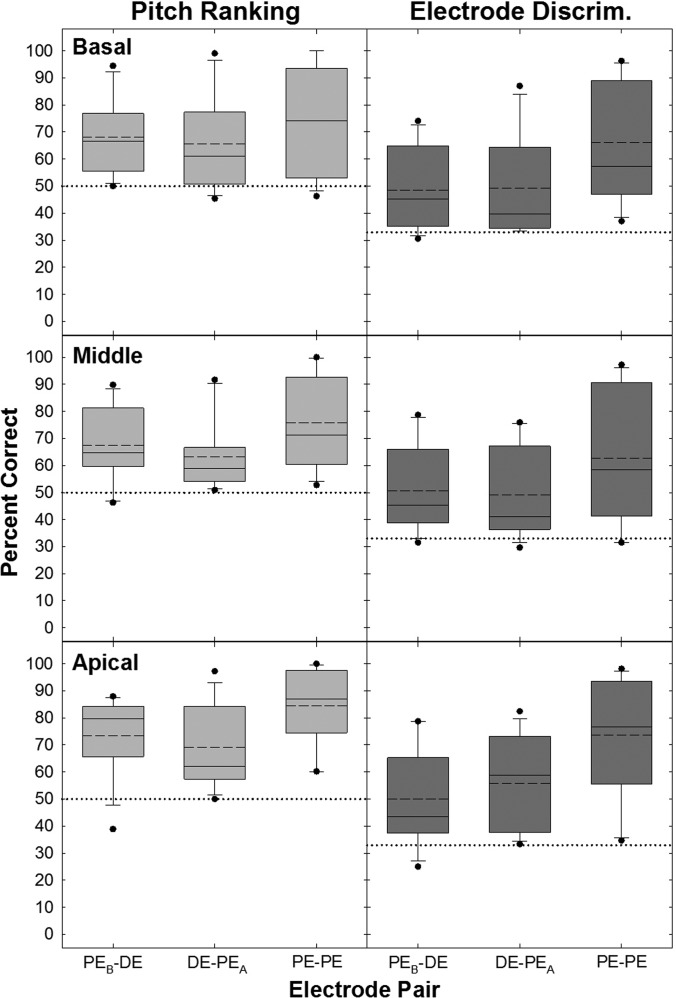
Figure 3 shows percent correct averaged across ears for pitch ranking (left column) and electrode discrimination (right column) for all electrode pairs. Box-and-whisker plots display results across the basal (top row), middle (middle row), and apical (bottom row) electrode regions. Box boundaries represent the 25th and 75th percentiles, whiskers represent the 10th and 90th percentiles, black circles represent the upper and lower outlying points, and dashed and solid horizontal lines represent means and medians, respectively. Chance performance is indicated by the horizontal dotted line across each graph. Middle-region pitch ranking and electrode discrimination data for F7 were excluded from this analysis because of the significant pitch reversals. In Fig. 3, mean performance is above chance for both pitch ranking and electrode discrimination for all electrode comparisons. A two-way repeated measures analysis of variance (RM ANOVA) was performed separately for pitch ranking and electrode discrimination (due to different levels of chance performance) to determine the effect of electrode pair (PEB-DE, DE-PEA, or PE-PE) and region (basal, middle, or apical set) for each of the tasks. A Bonferroni correction was used to adjust for multiple comparisons. There was a highly significant main effect of electrode pair for each psychophysical task. Post hoc testing showed mean values were significantly higher for the PE-PE pair than for the two DE pairs (pitch ranking: F[2,22] = 15.5, p < 0.001; electrode discrimination: F[2,22] = 52.8, p < 0.001). Performance levels for the PE-DE comparisons were not significantly different (p > 0.05). Mean percent-correct values are displayed in Table II (F7 middle set excluded); boldface indicates mean values were significantly higher for the PE-PE pair than for the two DE pairs. There was no main effect of electrode region for either task (pitch ranking: F[2,22] = 1.3, p = 0.30; electrode discrimination: F[2,22] = 0.92, p = 0.41), and no significant interaction between electrode pair and region for either task (pitch ranking: F[4,44] = 1.4, p = 0.24; electrode discrimination: F[4,44] = 1.06, p = 0.39). Although there was not a significant effect of electrode region, scores for the apical region were ∼7%–11% higher than for the basal and middle regions for each of the two tasks.
FIG. 3.
Group percent correct for pitch ranking (left column) and electrode discrimination (right column) for basal (top row), middle (middle row), and apical (bottom row) regions. Dashed and solid lines indicate the mean and median, respectively (overlapping in basal pitch ranking PE-PE case). Box boundaries represent the 25th and 75th percentiles, whiskers represent the 10th and 90th percentiles, black circles represent the upper and lower outlying points. The horizontal dotted lines indicate chance performance levels for pitch ranking (50%) and electrode discrimination (33%).
TABLE II.
Means across subjects for pitch ranking, electrode discrimination, and normalized ECAP separation indices (Σ). Pitch-ranking and electrode-discrimination data are reported as both percent correct and d′ values. Boldface indicates mean values that statistical analyses (see text) indicated were significantly different (p = 0.05 or better) for the adjacent PE pairs (PE-PE) than for the two DE pairs (PEB-DE and DE-PEA). The two DE pairs were not significantly different.
| Basal | Middle | Apical | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PEB-DE | DE-PEA | PE-PE | PEB-DE | DE-PEA | PE-PE | PEB-DE | DE-PEA | PE-PE | ||
| Pitch ranking | % correct | 68.2% | 65.7% | 74.1% | 67.6% | 63.2% | 75.8% | 73.5% | 69.1% | 84.6% |
| d′ | 0.83 | 0.90 | 1.32 | 0.79 | 0.69 | 1.34 | 0.99 | 0.94 | 1.75 | |
| Electrode discrimination | % correct | 48.6% | 49.3% | 66.0% | 50.7% | 49.0% | 62.6% | 50.1% | 55.8% | 73.6% |
| d′ | 0.61 | 0.69 | 1.38 | 0.69 | 0.70 | 1.29 | 0.69 | 0.84 | 1.71 | |
| ECAP separation index | Σ | 1.16 | 1.36 | 1.78 | 1.31 | 1.46 | 2.22 | 0.93 | 1.10 | 1.16 |
To evaluate whether pitch ranking and electrode discrimination produce similar perceptual outcomes for PEs and corresponding DEs, a metric was needed that accounts for the differences in chance performance for the 2IFC and 3IFC tasks. Standard corrections were applied to convert percent-correct scores for each block in each task to the corresponding d′ values (Hacker and Ratcliff, 1979, Table I).3 To focus on the degree of discriminability without concern for potential pitch reversals in pitch ranking, and analyze data similarly for comparing the two tasks, the absolute value of d′ was used. These values were then averaged across blocks within each subject, and then averaged across subjects. The averaged d′ values are summarized in Table II. A three-way RM ANOVA on d′ evaluated differences across psychophysical task (pitch ranking, electrode discrimination), electrode pair (PEB-DE, DE-PEA, or PE-PE), and region (basal, middle, apical set). For this analysis, F7's middle-region data were included. A Bonferroni correction was used to adjust for multiple comparisons. There was no significant difference in mean d′ values between pitch ranking and electrode discrimination (F[1,12] = 1.36, p = 0.27). There was a significant main effect for electrode pair (F[2,24] = 22.90, p < 0.001); post hoc analyses indicated scores for the PE-PE pair were significantly higher than for both DE-PE comparisons. There was no significant difference between the two DE comparisons (PEB-DE versus DE-PEA; t = 0.07, p = 1.0), and no significant main effect for region (F[2,24] = 2.25, p = 0.13). Mean d′ values collapsed across region for pitch ranking for the PEB-DE, DE-PEA, and PE-PE comparisons were 1.02, 0.94, and 1.23, respectively. For electrode discrimination, the mean values collapsed across region were 0.89, 0.89, and 1.08 for PEB-DE, DE-PEA, and PE-PE comparisons, respectively. In sum, average results of the pitch-ranking and electrode-discrimination tasks were not significantly different; however, pitch ranking tended to be better (higher average d′ values) than electrode discrimination. Both tasks showed better performance for adjacent PEs than for the DE comparisons, as expected. Further, performance was similar between the DE and each of the flanking PEs, and overall performance was similar across electrode regions for both tasks.
The secondary goals of this study were to determine whether psychophysical results were related to measures of ECAP SOE, and whether one psychophysical task more accurately approximated the ECAP separation index (Σ). Table II lists the mean ECAP Σ values for each electrode comparison across the array (middle-region data for N7 were excluded because the middle probe set was inadvertently collected at an incorrect stimulus level). As expected, and consistent with results from our previous study investigating ECAP Σ in a larger participant group (Hughes et al., 2013), a two-way RM ANOVA revealed a statistically significant effect of electrode pair (F[2,22] = 33.08, p < 0.001) and region (F[2,22] = 5.34, p = 0.01). Post hoc analyses confirmed the amount of separation between the PE pairs (PE-PE) was significantly greater than the amount of separation between both DE pairs (PEB-DE versus PE-PE, p < 0.001; DE-PEA versus PE-PE, p = 0.003), as expected. There was no significant difference between the two DE comparison pairs (PEB-DE versus DE-PEA, t = 0.07, p = 0.07). For the effect of region, there was a significant difference in the separation index (Σ) for the middle versus apical comparison only (p = 0.02), with the middle region yielding the largest Σ value. There was a significant interaction between electrode pair and region (F[4,44] = 3.08, p = 0.03); however, post hoc testing indicated no significant difference among the comparison pairs (p = 0.52). In general, Σ was smallest for the PEB-DE pair across all three electrode regions.
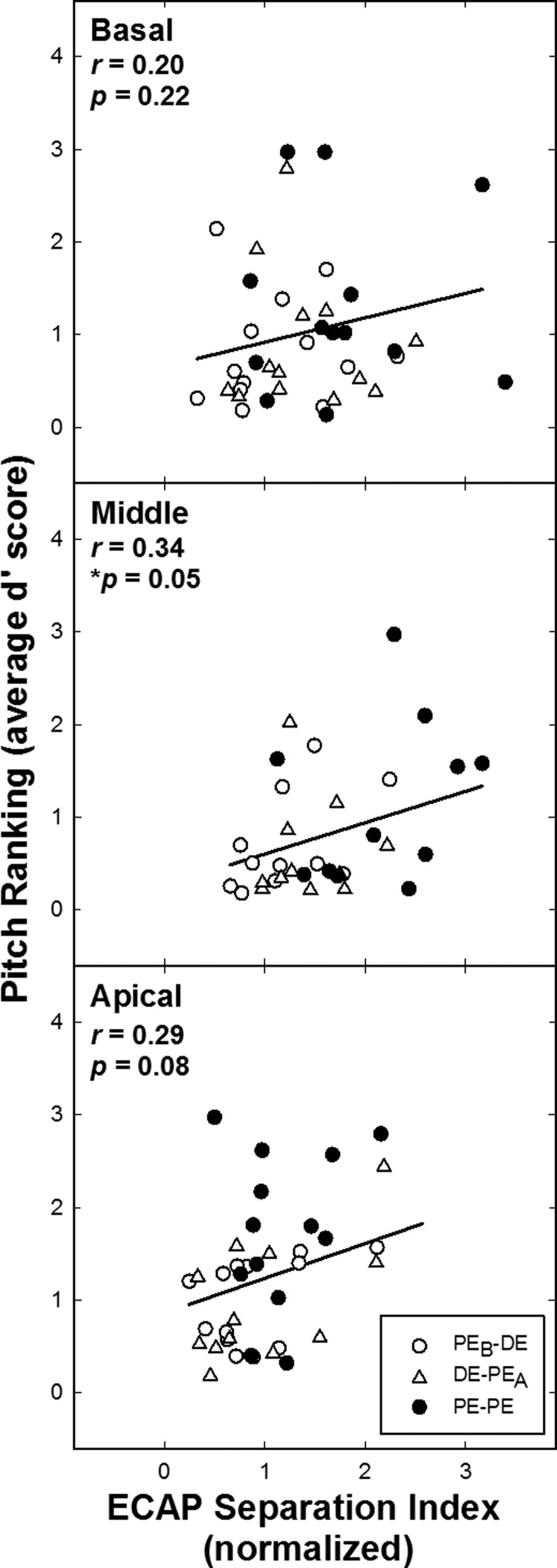
To directly assess the relation between the electrophysiological data and the psychophysical results, d′ scores for the psychophysical tasks were compared to the ECAP Σ values. Data for two subjects were excluded from all middle-region psychophysical and electrophysiological analyses: F5, because psychophysical and ECAP measures were inadvertently obtained for different electrodes, and N7, because of incorrect ECAP stimulus level as noted above. These exclusions apply for Figs. 4 and 5. Figure 4 shows data across subjects comparing pitch-ranking d′ to ECAP Σ values for all electrode pairs for the basal, middle, and apical regions (top to bottom, respectively). Within panels, each data point represents a different subject. The PE-PE comparison pairs are indicated by filled circles; open circles and triangles represent the PEB-DE and DE-PEA comparisons, respectively. If the proposed hypothesis was supported, results would show a positive correlation between ECAP Σ and pitch-ranking d′ scores. Larger ECAP Σ values and better pitch ranking were expected for the PE-PE comparisons (filled symbols expected to be clustered at the top right corner of the graph) than for the more closely spaced DE comparisons (open symbols expected to be clustered toward the bottom left corner). Results revealed no significant correlation between pitch-ranking scores and ECAP Σ for the basal (r = 0.17, p = 0.30) and apical region (r = 0.29, p = 0.08). The middle region comparison was significant (r = 0.34; p = 0.05).
FIG. 4.
Pitch-ranking d′ values versus normalized ECAP separation indices (Σ) for basal, middle, and apical regions (top to bottom, respectively). Each data point represents a different ear. Filled circles indicate the PE-PE comparison and unfilled circles and triangles indicate PEB-DE and DE-PEA, respectively. Pearson-product moment correlation values (r) with significance levels (p) are presented in each panel.
FIG. 5.
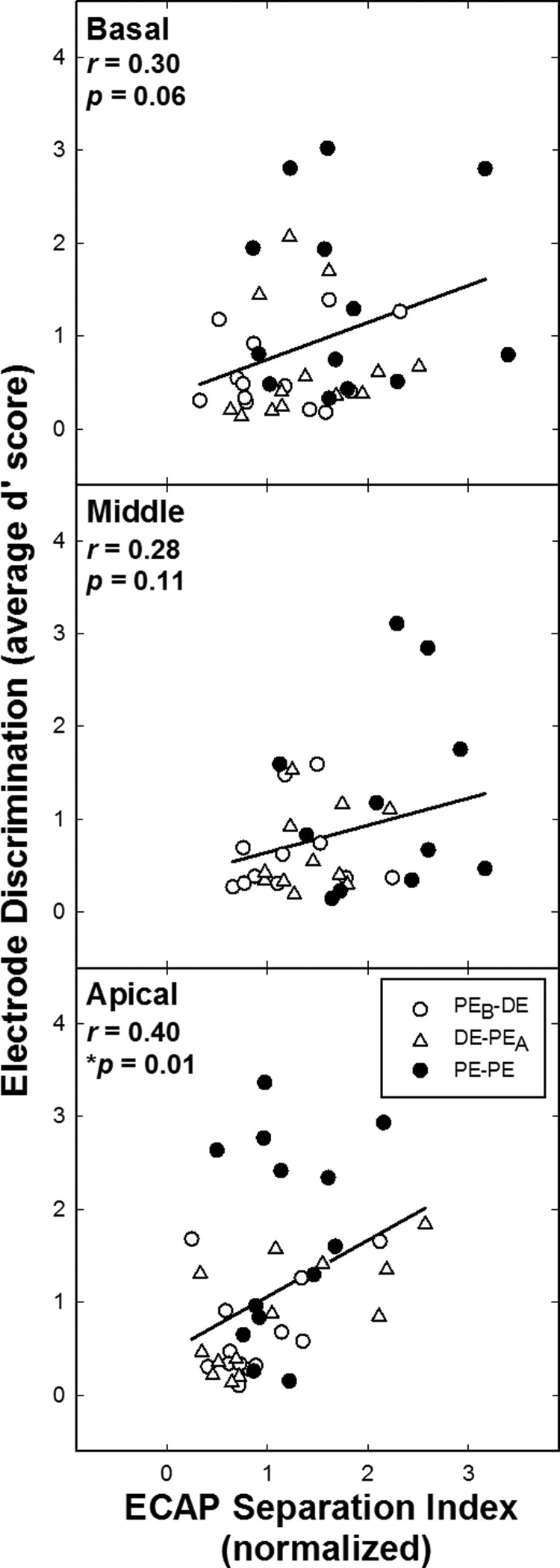
Electrode discrimination d′ values versus normalized ECAP separation indices (Σ) for basal, middle, and apical regions (top to bottom, respectively). Each data point corresponds to a different ear. Same format as for Fig. 4.
Figure 5 shows corresponding data for electrode discrimination versus ECAP Σ. There was a significant correlation for apical electrode discrimination and the ECAP separation index (r = 0.40; p = 0.01); the basal region approached significance (r = 0.30, p = 0.06); there was no significant correlation for the middle region (r = 0.28, p = 0.11). In sum, the group data for both psychophysical tasks were positively correlated with ECAP spatial separation (Σ) for all electrode regions. However, for pitch ranking, only the middle region was significantly correlated with Σ. For electrode discrimination, only the apical region was significantly correlated, with borderline significance for the basal region. Although the correlations for all regions and both tasks were in the direction hypothesized, they were not strong enough to support using ECAP measures to predict pitch perception for DE stimulation.
IV. DISCUSSION
The first objective of this study was to compare pitch-ranking and electrode-discrimination performance with Cochlear's “dual-electrode” stimulation (Cochlear Ltd., Macquarie, New South Wales, Australia) to determine whether the two tasks produce similar levels of performance for stimulation of DEs and PEs. Results indicated that the two tasks do indeed produce similar outcomes, with the important exception that pitch-ranking tasks can reveal pitch reversals. The second objective was to extend the findings of Hughes (2008) to determine whether ECAP spatial separation can be used to predict pitch perception for more closely spaced electrode pairs (i.e., DE stimulation). We hypothesized that ECAP Σ values would be positively correlated with accuracy on the psychophysical tasks. This hypothesis was not entirely supported by the results. Although there was a positive correlation between the objective and behavioral results, across-subject comparisons did not yield significant correlations for all test and region comparisons across the array.
To our knowledge, this study was the first to empirically examine electrode discrimination in CI recipients using DE stimulation and compare this to measures of pitch ranking in the same subjects. The finding of similar performance across the two pitch tasks is contradictory to previous studies by Collins et al. (1994, 1997; Collins and Throckmorton, 2000) and Zwolan et al. (1997). For all but one subject in the Collins and Zwolan studies, the electrode discrimination task produced more discriminable pitch percepts across the CI array compared with the pitch-ranking task (suggesting more difficulty with pitch ranking). In the current study, mean pitch-ranking scores were slightly better than mean electrode-discrimination scores (although not significantly different; see Table II); suggesting pitch ranking was the easier task. Several differences between the present and earlier studies likely contributed to this difference in outcomes. First, the spacing between compared electrodes was much wider in the Collins and Zwolan studies because only PEs were investigated. In contrast, the current study focused on measures for much more discrete regions localized to adjacent- and intermediate-electrode stimulation. Closer proximity of the compared pairs will make both pitch tasks more difficult, which will likely lead to larger variance in the data, thereby reducing the likelihood of observing a significant difference between the psychophysical measures.
Second, subjects in the Collins and Zwolan studies utilized older-generation Nucleus-22 devices with bipolar stimulation, whereas monopolar coupling was used in the present study. It is known that monopolar stimulation yields broader neural excitation patterns than bipolar (e.g., Zhu et al., 2012), which may degrade the ability to distinguish electrodes on the basis of pitch. Again, a more difficult task might yield greater variance in the data. However, Kwon et al. (2011) showed better place-pitch discrimination with monopolar stimulation compared to bipolar (only pitch ranking was examined). With monopolar stimulation, a neural population at the single stimulated electrode is excited; whereas with bipolar stimulation, bimodal response patterns may result (i.e., excitation at each electrode in the pair). Subjects in Kwon et al. (2011) perceived a higher pitch sensation with bipolar stimulation compared with the same site of stimulation using monopolar. Given the data from Kwon et al. (2011), it is unclear how the different modes of stimulation (monopolar versus bipolar) affect the comparison between pitch ranking and electrode discrimination at similar sites of stimulation. Finally, the studies by Collins et al. (1994, 1997; Collins and Throckmorton, 2000) did not incorporate a stimulus-level rove in their psychophysical tasks, so loudness cues could have influenced pitch judgments for each task. In sum, greater overlap between compared electrodes (whether due to close physical proximity of the electrodes or broader stimulation patterns) and reduction of potential loudness cues likely lent to greater difficulty in resolving other dimensions or added task complexity that may have influenced pitch judgments in the current study.
Henry et al. (2000) showed that electrode discrimination was significantly poorer for basal electrodes, and similar trends were reported in Zwolan et al. (1997) for electrode discrimination, and in McDermott and McKay (1994) for pitch ranking. Electrode discrimination and pitch ranking in the present data set were not significantly different across the three electrode regions tested, although both were slightly better for apical electrodes. It may be that subjects in the current study had better or more uniform nerve survival (as a group) compared with subjects in the earlier studies. Subjects in the current study had shorter durations of deafness (average of ∼13 years) compared with ∼29 years in the Collins and Zwolan studies (calculated using Table I in Collins et al., 1997 and Zwolan et al., 1997). Further, candidacy criteria have changed substantially in the past 15 years as individuals with greater amounts of residual hearing (typically in the low frequencies) are receiving standard-length CIs, and are therefore more likely to have better neuronal survival in the apical region (Nadol, 1997; Pfingst et al., 2011). Only two subjects in this study had significant residual hearing in the low frequencies before implantation [F15 and N5; see pre-CI pure-tone average (PTA) in Table I]; postoperative audiograms of the implanted ear for both individuals showed a substantial loss of residual hearing.
Not surprisingly, subjects were better at pitch ranking and discriminating the PE-PE pairs compared with the DE pairs (PEB-DE and DE-PEA); there was no difference in scores between the two DE pairs. This finding is likely the result of greater overlap of neurons recruited by DE stimulation and neurons recruited by the adjacent PE. As the physical distance between electrodes increases (e.g., PE versus PE), there is less overlap of stimulated neural populations, which should typically result in better pitch ranking and discrimination of electrodes. These findings are consistent with Hughes (2008), who found that as the spatial distance between PEs increased along the electrode array, pitch-ranking accuracy improved.
Most subjects were able to pitch rank and discriminate the PEs and corresponding DEs according to the expected tonotopic structure of the cochlea; however, only 42% of the electrode pairs were pitch ranked significantly above chance, and 40% had electrode discrimination scores above chance. When raw percent correct was converted to d′, there was no overall difference in performance across tasks. Despite the fact that correct-answer feedback was provided for electrode discrimination and not for pitch ranking, the cognitive load involved with the extra interval for the 3IFC procedure may have made the electrode discrimination task more difficult for participants. Further, with the addition of the stimulus level rove, subjects experienced more changes in perceived loudness across 3 versus 2 intervals, which may also have made the electrode-discrimination task more difficult. This interpretation is supported by the fact that when analyses intended to compensate for theoretical differences in performance based on number of intervals, the magnitude of the differences across procedures was no longer significant. Overall, however, both behavioral pitch tasks were reportedly difficult for the majority of the participants.
Results of the current study differ from Busby et al. (2008), who found that a higher percentage of electrode pairs in their sample were pitch ranked significantly above chance (76%). One possible explanation for this difference is that the current study used a more conservative approach to identify pitch reversals (upper and lower boundaries of 72% and 28% for pitch ranking, respectively), whereas Busby et al. used significance values of 65% and 35%. If the latter significance values were used in the current study, 55% of the electrode comparisons in the current study would have been significantly above chance performance rather than 42%, and there would have been three significant pitch reversals instead of two. Second, a comparison of the methodology used in Busby and Plant (2005) and Busby et al. (2008) with the current study revealed that more stimulus trials were presented for the pitch-ranking task in the present study (typically 108 total votes per condition versus 20 trials in the Busby papers), suggesting the present data set may be more accurate.
For the second goal of this study, we hypothesized that better accuracy on the psychophysical tasks would be associated with larger ECAP Σ values. Although the psychophysical measures were positively correlated with ECAP Σ for the group comparisons (Figs. 4 and 5), significant correlations were not found for all of the psychophysical task and region comparisons across the array. There were only two instances in which the electrophysiological and psychophysical group data were significantly correlated (middle-region pitch ranking and apical-region electrode discrimination). Neither psychophysical task was significantly correlated with ECAP Σ for basal-region electrodes. Although Hughes (2008) found a significant correlation using this methodology for pitch ranking versus ECAP Σ, electrode spacing in that study ranged from one to six PEs. In the current study, a much more discrete comparison region was used. The current results suggest that either the ECAP separation index (Σ) or the ECAP measures themselves are not sensitive enough to use for predicting pitch perception for more localized comparisons, such as adjacent PEs and corresponding DEs.
There may be a number of reasons why there was not a strong correlation between ECAP Σ and the psychophysical measures in the current study. First, different stimuli were used for the psychophysical and ECAP tasks. ECAP responses were evoked by single-pulse stimuli, which generally require higher current levels to obtain a response, contributing to a greater spread of excitation in the cochlea. However, psychophysical tasks were obtained with pulse trains, which require less current to reach upper comfort levels, thereby producing narrower electric fields in the cochlea. Hughes and Stille (2008) showed broader excitation patterns for ECAP measures than for psychophysical measures (using a forward-masking paradigm). Because the ECAP patterns are so broad, they are likely less sensitive at differentiating excitation regions that overlap substantially, as is the case for closely spaced (dual) electrodes versus more widely spaced PE comparisons. This difference may have directly affected the comparison between the psychophysical and ECAP measures in the current study. Although there is a difference in stimuli across the tasks, this limitation should not deter future studies from further investigating the relation between psychophysical and ECAP measures.
The non-significant correlations among the psychophysical and ECAP Σ comparisons could also be affected by truncated ECAP SOE patterns for basal and apical probes, as explained by Hughes et al. (2013). The maximum spacing between a probe in the middle of the array and the farthest masker will be half the length of the array. In contrast, the maximum separation between masker and probe electrodes is much greater for probes at the basal or apical end of the array. With forward masking, ECAP amplitudes approach zero as the separation between masker and probe electrodes increases. The Σ value for basal and apical probes will therefore encompass a greater number of zero-amplitude observations resulting in smaller Σ values as compared with a mid-array probe (as shown in Table II). We therefore might expect a stronger correlation between psychophysical and ECAP measures for middle-region electrodes. However, only pitch ranking (and not electrode discrimination) was significantly correlated with ECAP Σ for the middle region. Further, the apical comparison was significantly correlated for electrode discrimination. Therefore, limitations of the ECAP spatial separation measure do not fully explain the present results.
Second, to observe a significant correlation between two measures, there needs to be ample spread in the data. The electrophysiological and psychophysical data analyzed were confined to a discrete region in the cochlea (DEs versus flanking PE comparisons). Given the isolated comparisons, there were cases in which the data did not have sufficient distribution, which likely contributed to the non-significant findings between the two tasks. For the current hypothesis (larger Σ values associated with better performance on psychophysical tasks), we expected the PE-PE comparisons to cluster toward the upper right corner of each graph in Figs. 4 and 5 (filled symbols), and the DE comparisons to cluster toward the lower left corner (open symbols). This pattern was only seen for the middle-region pitch ranking versus ECAP Σ comparison (significantly correlated; Fig. 4, middle plot). Also significantly correlated was the apical electrode discrimination versus ECAP Σ (Fig. 5, bottom). For this comparison, generally, there was better pitch performance for PEs than DEs (filled symbols clustered at the top of the graph), which appears to be driving the correlation for this comparison (there was not ample spread among the DE and PE ECAP data). Although we might have expected the middle electrode discrimination versus ECAP Σ comparison to be correlated (Fig. 5, middle), there was better separation between PEs and DEs for the ECAP data (which is consistent with the truncated SOE issue), but there was poorer separation between PEs and DEs for the pitch data (with more PE-PE data points in the lower portion of the graph indicating poorer performance in this region). For the comparison between apical pitch ranking and ECAP Σ (Fig. 4, bottom), there was little spread for the PE and DE ECAP data (consistent with the issue of truncated SOE patterns); however, the psychophysical data did show more clustering of the DEs toward the lower left corner, as expected. For basal electrode discrimination versus Σ (Fig. 5, top), the pitch and ECAP data for the DEs tended to cluster toward the lower left corner of the graph; however, pitch performance for the PEs was not strong enough to drive a significant correlation. For the final comparison (pitch ranking for basal-region electrodes; Fig. 4, top), there was not adequate separation among the PEs versus DEs across the two measures. Poorer correlations for the basal-region data may be due to a combination of factors including potentially poorer nerve survival (Nadol, 1997), truncated ECAP SOE functions, and farther positioning of the electrode array from the modiolus (due to the larger cross section of the cochlea) in this region.
Although this study did not find a strong relationship between the psychophysical tasks and ECAP spatial separation (Σ), there were similar trends among both measures. For example, the separation index (Σ) was significantly larger for the PE-PE comparison pairs compared with the DE pairs (with no significant difference between the two DE pairs; Hughes et al., 2013). Similarly, pitch ranking and electrode discrimination were better for the PE-PE comparisons, compared with the DE pairs, which, again, were not significantly different from each other. In sum, these trends demonstrate that as the distance between electrodes increases, there is less overlap of the respective regions of excitation. More research is needed to improve correlations between behavioral and electrophysiological measures, given the continuing clinical need for an objective measure that can be used to predict pitch perception abilities in certain clinical populations.
ACKNOWLEDGMENTS
The authors thank Lisa Stille, Gina Diaz, and Katelyn Glassman for assistance with data collection and analysis, Kendra Schmid, Ryan McCreery, and Walt Jesteadt for assistance with statistics and the d′ analyses, Gail Donaldson for consultation, and Tom Creutz for development of data collection programs. This study was supported by the National Institute on Deafness and Other Communication Disorders (NIDCD) Grants No. R01DC009595 and P30DC04662. The content of this project is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or the National Institutes of Health.
Footnotes
Because ECAPs could not be recorded for masker stimuli presented to the recording electrode, amplitudes in this case were interpolated from the adjacent masker electrodes.
Binomial statistics were based on the most conservative interpretation of “independent” trials, using 18 votes for one condition in one block as our sample size, rather than the 108 total votes per condition across all replications of the condition.
Another approach was initially explored to compare the two procedures having different chance levels. Originally, percent correct was converted to z scores derived from models of binomial variables as in Thornton and Raffin (1978). The primary feature of this approach is using theoretical SDs for proportions based on different chance levels. The outcome was very similar to the d′ analysis for plots of the data in terms of relative differences across subjects (i.e., Figs. 4 and 5), and nearly identical for the statistical analysis (ANOVA, correlation values, and significance levels). The d′ analysis is presented here as the more familiar and accepted method.
REFERENCES
- 1.Abbas, P. J. , Hughes, M. L. , Brown, C. J. , Miller, C. A. , and South, H. (2004). “ Channel interaction in cochlear implant users evaluated using the electrically evoked compound action potential,” Audiol. Neurotol. 9, 203–213 10.1159/000078390 [DOI] [PubMed] [Google Scholar]
- 3.Busby, P. A. , Battmer, R. D. , and Pesch, J. (2008). “ Electrophysiological spread of excitation and pitch perception for dual and single electrodes using the Nucleus Freedom cochlear implant,” Ear Hear. 29(6 ), 853–864 10.1097/AUD.0b013e318181a878 [DOI] [PubMed] [Google Scholar]
- 2.Busby, P. A. , and Plant, K. L. (2005). “ Dual electrode stimulation using the Nucleus CI24RE cochlear implant: Electrode impedance and pitch ranking studies,” Ear Hear. 26, 504–511 10.1097/01.aud.0000179693.32989.84 [DOI] [PubMed] [Google Scholar]
- 4.Cohen, L. T., Richardson, L. M., Saunders, E., and Cowan, R. S. C. (2003). “ Spatial spread of neural excitation in cochlear implant recipients: comparison of improved ECAP method and psychophysical forward masking,” Hear. Res. 179, 72–87 10.1016/S0378-5955(03)00096-0 [DOI] [PubMed] [Google Scholar]
- 4.Collins, L. M. , and Throckmorton, C. S. (2000). “ Investigating perceptual features of electrode stimulation via a multidimensional scaling paradigm,” J. Acoust. Soc. Am. 108(5 ), 2353–2365 10.1121/1.1314320 [DOI] [PubMed] [Google Scholar]
- 5.Collins, L. M. , Zwolan, T. A. , O'Neill, J. C. , and Wakefield, G. H. (1994). “Analysis of electrode pair confusions and implications for speech recognition in cochlear implant subjects,” J. Assoc. Res. Otolaryngol. Abstracts, No. 642. Available at http://www.aro.org/archives/1994/642.html (Last viewed Jan. 7, 2014).
- 6.Collins, L. M. , Zwolan, T. A. , and Wakefield, G. H. (1997). “ Comparison of electrode discrimination, pitch ranking, and pitch scaling data in postlingually deafened adult cochlear implant subjects,” J. Acoust. Soc. Am. 101(1 ), 440–455 10.1121/1.417989 [DOI] [PubMed] [Google Scholar]
- 7.Dawson, P. W. , McKay, C. M. , Busby, P. A. , Grayden, D. B. , and Clark, G. M. (2000). “ Electrode discrimination and speech perception in young children using cochlear implants,” Ear Hear. 21(6 ), 597–607 10.1097/00003446-200012000-00007 [DOI] [PubMed] [Google Scholar]
- 8.Donaldson, G. S. , Kreft, H. A. , and Litvak, L. (2005). “ Place-pitch discrimination of single-versus dual-electrode stimuli by cochlear implant users,” J. Acoust. Soc. Am. 118(2 ), 623–626 10.1121/1.1937362 [DOI] [PubMed] [Google Scholar]
- 9.Firszt, J. B. , Koch, D. B. , Downing, M., and Litvak, L. (2007). “ Current steering creates additional pitch percepts in adult cochlear implant recipients,” Otol. Neurotol. 28(5 ), 629–636 10.1097/01.mao.0000281803.36574.bc [DOI] [PubMed] [Google Scholar]
- 10.Hacker, M. J. , and Ratcliff, R. (1979). “ A revised table of d′ for M-alternative forced choice,” Percept. Psychophys. 26(2 ), 168–170 10.3758/BF03208311 [DOI] [Google Scholar]
- 11.Henry, B. A. , McKay, C. M. , McDermott, H. J. , and Clark, G. M. (2000). “ The relationship between speech perception and electrode discrimination in cochlear implantees,” J. Acoust. Soc. Am. 108(3 ), 1269–1280 10.1121/1.1287711 [DOI] [PubMed] [Google Scholar]
- 12.Hughes, M. L. (2008). “ A re-evaluation of the relation between physiological channel interaction and electrode pitch ranking in cochlear implants,” J. Acoust. Soc. Am. 124(5 ), 2711–2714 10.1121/1.2990710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes, M. L. , and Abbas, P. J. (2006a). “ The relation between electrophysiological channel interaction and electrode pitch ranking in cochlear implant recipients,” J. Acoust. Soc. Am. 119, 1527–1537 10.1121/1.2163273 [DOI] [PubMed] [Google Scholar]
- 14.Hughes, M. L. , and Abbas, P. J. (2006b). “ Electrophysiologic channel interaction, electrode pitch ranking, and behavioral threshold in straight versus periomodiolar cochlear implant electrode arrays,” J. Acoust. Soc. Am. 119, 1538–1547 10.1121/1.2164969 [DOI] [PubMed] [Google Scholar]
- 15.Hughes, M. L. , and Goulson, A. (2011). “ Electrically evoked compound action potential measures for virtual channels versus physical electrodes,” Ear Hear. 32(3 ), 323–330 10.1097/AUD.0b013e3182008c56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes, M. L. , and Stille, L. J. (2008). “ Psychophysical versus physiological spatial forward masking and the relation to speech perception in cochlear implants,” Ear Hear. 29(3 ), 435–452 10.1097/AUD.0b013e31816a0d3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes, M. L. , Stille, L. J. , Baudhuin, J. L. , and Goehring, J. L. (2013). “ ECAP spread of excitation with virtual channels and physical electrodes,” Hear. Res. 306, 93–103 10.1016/j.heares.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jesteadt, W. (1980). “ An adaptive procedure for subjective judgments,” Percept. Psychophys. 28(1 ), 85–88 10.3758/BF03204321 [DOI] [PubMed] [Google Scholar]
- 19.Kwon, B. J. , Perry, T. T. , and Olmstead, V. (2011). “ Effects of stimulation configurations on place pitch discrimination in cochlear implants,” J. Acoust. Soc. Am. 129(6 ), 3818–3826 10.1121/1.3586786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levitt, H. (1971). “ Transformed up-down methods in psychoacoustics,” J. Acoustic. Soc. Am. 49(2 ), 467–477 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- 21.McDermott, H. J. , and McKay, C. M. (1994). “ Pitch ranking with nonsimultaneous dual-electrode electrical stimulation of the cochlea,” J. Acoust. Soc. Am. 96(1 ), 155–162 10.1121/1.410475 [DOI] [PubMed] [Google Scholar]
- 22.Nadol, J. B. , Jr. (1997). “ Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation,” Otol. Head Neck Surg. 117(3 ), 220–228 10.1016/S0194-5998(97)70178-5 [DOI] [PubMed] [Google Scholar]
- 23.Peterson, G. E. , and Lehiste, I. (1962). “ Revised CNC lists for auditory tests,” J. Speech Hear. Disord. 27, 62–70 [DOI] [PubMed] [Google Scholar]
- 24.Pfingst, B. E. , Colesa, D. J. , Hembrador, S., Kang, S. Y. , Middlebrooks, J. C. , Raphael, Y., and Su, G. L. (2011). “ Detection of pulse trains in the electrically stimulated cochlea: Effects of cochlear health,” J. Acoust. Soc. Am. 130(6 ), 3954–3968 10.1121/1.3651820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saoji, A. A. , Litvak, L. M. , and Hughes, M. L. (2009). “ Excitation patterns of simultaneous and sequential dual-electrode stimulation in cochlear implant recipients,” Ear Hear. 30(5 ), 559–567 10.1097/AUD.0b013e3181ab2b6f [DOI] [PubMed] [Google Scholar]
- 26.Shannon, R. V. (1983). “ Multichannel electrical stimulation of the auditory nerve in man. I. Basic psychophysics,” Hear. Res. 11, 157–189 10.1016/0378-5955(83)90077-1 [DOI] [PubMed] [Google Scholar]
- 27.Snel-Bongers, J., Briaire, J. J. , Vanpoucke, F. J. , and Frijns, J. H. M. (2012). “ Spread of excitation and channel interaction in single- and dual-electrode cochlear implant stimulation,” Ear Hear. 33(3 ), 367–376 10.1097/AUD.0b013e318234efd5 [DOI] [PubMed] [Google Scholar]
- 28.Spahr, A. J. , Dorman, M. F. , Litvak, L. M. , Van Wie, S., Gifford, R. H. , Loizou, P. C. , Loiselle, L. M. , Oakes, T., and Cook, S. (2012). “ Development and validation of the AzBio sentence lists,” Ear Hear. 33(1 ), 112–117 10.1097/AUD.0b013e31822c2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornton, A. R. , and Raffin, M. J. M. (1978). “ Speech-discrimination scores modeled as a binomial variable,” J. Speech Lang. Hear. 21, 507–518 [DOI] [PubMed] [Google Scholar]
- 30.Townshend, B., Cotter, N., Van Compernolle, D., and White, R. L. (1987). “ Pitch perception by cochlear implant subjects,” J. Acoust. Soc. Am. 82(1 ), 106–115 10.1121/1.395554 [DOI] [PubMed] [Google Scholar]
- 31.Wilson, B. S. , Schatzer, R., Lopez-Poveda, E. A. , Sun, X., Lawson, D. T. , and Wolford, R. D. (2005). “ Two new directions in speech processor design for cochlear implants,” Ear Hear. 26, 73S–81S 10.1097/00003446-200508001-00009 [DOI] [PubMed] [Google Scholar]
- 32.Zhu, Z., Tang, Q., Zeng, F. G. , Guan, T., and Ye, D. (2012). “ Cochlear-implant spatial selectivity with monopolar, bipolar and tripolar stimulation,” Hear. Res. 283, 45–58 10.1016/j.heares.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwolan, T. A. , Collins, L. M. , and Wakefield, G. H. (1997). “ Electrode discrimination and speech perception in postlingually deafened adult cochlear implant subjects,” J. Acoust. Soc. Am. 102, 3673–3685 10.1121/1.420401 [DOI] [PubMed] [Google Scholar]