Abstract
Chronic lymphocytic leukemia (CLL) is a rare hematologic disorder with affected patients having complications of frequent infections and possible transformation to a more aggressive malignancy. The occurrence of CLL in the bladder is a rare event, with few reported cases. As a result, its aggressiveness and the optimal course for treatment are unknown. Despite this, its presence in the bladder warrants continued surveillance, as recurrence and progression to other bladder malignancies are possible. We present a 71-year-old woman initially diagnosed with CLL who was plagued by recurrent hematuria and dysuria for over a decade, which lead to multiple negative urologic workups. However, these continued workups eventually lead to her diagnosis of bladder CLL with a subsequent finding of carcinoma in situ that was prompted by a suspicious surveillance cystoscopy performed 4 months after her initial bladder diagnosis. Hence, infiltration of CLL in the urinary bladder merits close follow up, including additional urologic procedures.
Keywords: bladder cancer, carcinoma in situ, chronic lymphocytic leukemia, gross hematuria, recurrent urinary tract infections
Introduction
Chronic lymphocytic leukemia (CLL) is a rare hematologic disorder with an annual incidence of approximately 0.0043%. More than 75% of people are diagnosed after the age of 50 years and its incidence is highest (21.1%) in those aged 55–74 [SEER, 2013]. While the exact cause of CLL is unknown, it is thought to arise from a DNA mutation resulting in the production and proliferation of abnormal lymphocytes. At the onset of the disease many patients have constitutional symptoms prompting diagnostic testing. While it has a slow and indolent course compared with other leukemias, it can have serious complications. Commonly CLL patients have frequent infections, but CLL can transform to a more aggressive hematologic malignancy. This is seen in 10–15% of cases, which includes a 1.7 times increased risk of other cancers such as skin, lung, and gastrointestinal cancer [Lishner et al. 1987]. The occurrence of hematologic malignancies such as lymphoma is well documented as the most common form of testicular cancer in those over 50 years of age [Stephenson et al. 2012]. Despite this, the incidence of hematologic malignancies in the bladder is rare, with few reported cases.
Case report
A 71-year-old African–American woman presented with a history of CLL initially diagnosed in 1996. She was treated with chlorambucil for constitutional symptoms in 2003 and developed progressive disease in 2004. She underwent additional treatments for recurrent symptoms with fludarabine in 2004, chlorambucil and prednisone in 2006, rituximab in 2007, and bendamustine and rituximab in 2010. Her last bone marrow examination in January 2012 showed no evidence of CLL. She initially presented to the urology service for evaluation of her recurrent urinary tract infections and gross hematuria. While she had no significant personal or family history of nephrolithiasis or environmental exposure risks for malignancy, she was postmenopausal and a former 25 pack/year smoker. Her urologic workup in 2003 resulted in the finding of a left middle pole calyceal mass with a normal bladder workup. These findings were confirmed intraoperatively with a retrograde pyelogram demonstrating a filling defect of the middle pole calyx, which prompted ureteroscopy where multiple papillary masses suggestive of high-grade malignancy were seen extruding from the middle pole calyx. Concerned for malignancy, the patient elected to undergo a left nephroureterectomy. However, the final pathology demonstrated chronic pyelitis with reactive changes and no evidence of malignancy. Afterwards, her urologic history was unremarkable until she presented in 2009, once again with gross hematuria, and given her clinical risks for genitourinary malignancy, she underwent an appropriate workup. While her upper tract imaging was normal, she had a suspicious urine cytology, which prompted random bladder biopsies that were negative for malignancy, but significant for chronic inflammation. After another hiatus from the urology service, she presented once again in 2012 with recurrent hematuria and dysuria. Again her upper tract imaging was normal, but her clinic cystoscopy revealed erythematous patches and a possible tumor prompting a biopsy that revealed the presence of CLL and chronic cystitis in her bladder. Of note, her preoperative cytology was atypical and suspicious for malignancy. The final specimen, shown in Figure 1, demonstrated immunohistochemical features of CLL with coexpression of human cluster of differentiation (CD) markers 20, 5, 43, and 23. It also lacked any features of urothelial atypia to suggest a urothelial cell carcinoma. A 4-month follow-up cystoscopy revealed possible recurrence and she was subsequently biopsied revealing carcinoma in situ. The specimen of carcinoma in situ was negative for CD markers 20, 5, 43, and 23, and contained significant urothelial atypia and high nuclei:cytoplasm ratios further confirming this diagnosis. As a result, she underwent weekly intravesical instillation therapy for 6 weeks and she has had no recurrence based on follow-up cystoscopy and urine cytology.
Figure 1.
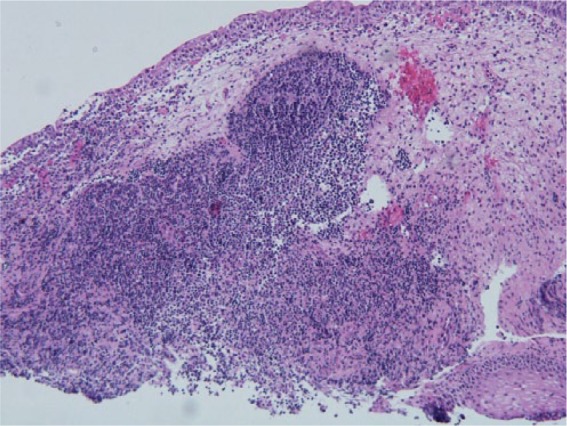
Pathology slide demonstrating chronic lymphocytic leukemia.
Discussion
The major cause of morbidity and mortality of CLL continues to be infection related. The pathogenesis of infections in these patients is thought to be multifactorial, related to the primary disease process as well as therapy-induced immunosuppression. Our patient underwent treatment with chlorambucil prior to her onset of recurrent urinary tract infections. While a vast majority of CLL-related infections tend to be respiratory in nature, bladder variants are possible based on similar principles. The hypogammaglobulinemia resulting from the primary disease process and medication-induced T-cell abnormalities lead to an increased susceptibility to infections in CLL patients [Baseskiogulu et al. 2013]. Normal and healthy bladders do not contain lymphoid tissue, but the presence of lymphocytic infiltrations is well described in bladder cancers. Hence, an initial conservative approach with antibiotics for patients with CLL and recurrent cystitis is logical; however, refractory symptoms should prompt further workup.
In addition to recurrent cystitis, our patient had many factors prompting an aggressive urologic workup. From her smoking history to gross hematuria, a clinic cytoscopy and computed tomography urogram were easily warranted. However, of interest is her negative hematuria workup aside from chronic bladder inflammation in 2009, and her presentation to our clinic with recurrent symptoms of cystitis and hematuria in 2012. Her repeat workup in 2012 yielded her diagnosis of CLL in the bladder. This further strengthens the notion to continue to follow these patients periodically and rescreen them when clinically indicated.
Through our extensive Medline search, the involvement of CLL in the bladder has been rarely described. The migration of lymphocytes to the bladder is thought to be a part of the normal host response where tumor infiltrating cells (TILs), composed of activated T cells, natural killer cells, and non-T and non-B lymphocytes, fend off malignant bladder cells [Ramadan et al. 2006]. Over time this response fails, as mutations within bladder tumor cells prevent the eradication of malignancy by TILs, hence, paving the way for lymphoproliferative disorders to infiltrate the urinary bladder.
The prognostic implication of CLL in the bladder is difficult to ascertain. Its rarity makes treatment decisions difficult. Chemotherapy, which leads to further immunosuppression, thus serving as a nidus for recurrent infections to a propensity for additional malignancies, makes this a difficult option to pursue universally. Infiltration of CLL in the urinary bladder is considered a progression of disease and hence consideration of chemotherapy to partial cystectomy with or without chemotherapy should be undertaken [Guthman et al. 1990].
Our patient was not the typical CLL patient based on race and gender, but did have multiple risks factors for developing malignancy, and after nearly a decade of hematuria with multiple negative workups she was found to have CLL in her bladder. We elected to manage her conservatively with local resection and active surveillance, based on the recommended management of low-grade urothelial cell carcinoma [NCCN, 2013]. While the paucity of literature on subsequent management of bladder CLL lead us to this algorithm, we continue to follow this patient vigilantly.
In conclusion, the notion of CLL developing in the bladder is not difficult to conceive and has a multifactorial basis related in part to the factors fostering CLL progression. The optimal treatment of these patients is debatable given the rarity of the disease. Nevertheless, closer follow up and routine immunohistochemical staining for CLL markers in bladder tumor specimens of these patients may result in additional cases and help to elucidate better treatment algorithms in the future.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Vikas Desai, University of Nebraska Medical Center, 984110 Nebraska Medical Center, Omaha, NE 68198-4110, USA.
Sudhir Isharwal, University of Nebraska Medical Center, Omaha, NE, USA.
Aydin Pooli, University of Nebraska Medical Center, Omaha, NE, USA.
Subodh Lele, University of Nebraska Medical Center, Omaha, NE, USA.
Michael Feloney, Omaha-VA Nebraska-Western Iowa Health Care System, Omaha, NE, USA.
References
- Baseskiogulu B., Canez F., Kaya C., Dönmez T. (2013) The type of lymphocyte infiltration near urothelial carcinoma is diagnostic for chronic lymphocytic leukemia. Urol Ann 5: 47–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthman D., Malek R., Chapman W., Farrow G. (1990) Primary malignant lymphoma of the bladder. J Urol 144: 1367–1369 [DOI] [PubMed] [Google Scholar]
- Lishner M., Prokocimer M., Ron E., Shaklai M. (1987) Primary malignant neoplasms associated with chronic lymphocytic leukaemia. Postgrad Med J 63: 253–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN (2013) National Comprehensive Cancer Network Guidelines for Treatment of Cancer by Site: Bladder Cancer. [www.nccn.org]
- Ramadan K., Kyle A., McManus D., O’Rourke D., Cuthbert R. (2006) Urinary bladder infiltration with chronic B-lymphocytic leukemia: two cases with unusual presentation. Leuk Lymphoma 47: 1184–1187 [DOI] [PubMed] [Google Scholar]
- Stephenson A., Gilligan T. (2012) Neoplasms of the testis. In: Wein A., Kavoussi L., Novick A., Partin A., Peters C. (eds), Campbell-Walsh Urology (10th edition). Philadelphia, PA: Elsevier Saunders, pp. 837–870 [Google Scholar]
- SEER (2013) Surveillance, Epidemiology, and End Results Program Research Data (1973–2010) (released April 2013, based on the November 2012. submission). National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch. [www.seer.cancer.gov] [Google Scholar]


